Abstract
Capnography continues to be an important tool in measuring expired carbon dioxide (CO2). Most recent Advanced Cardiac Life Support (ACLS) guidelines now recommend using capnography to ascertain the effectiveness of chest compressions and duration of cardiopulmonary resuscitation (CPR). Based on an extensive review of available published literature, we selected all available peer-reviewed research investigations and case reports. Available evidence suggests that there is significant correlation between partial pressure of end-tidal CO2 (PETCO2) and cardiac output that can indicate the return of spontaneous circulation (ROSC). Additional evidence favoring the use of capnography during CPR includes definitive proof of correct placement of the endotracheal tube and possible prediction of patient survival following cardiac arrest, although the latter will require further investigations. There is emerging evidence that PETCO2 values can guide the initiation of extracorporeal life support (ECLS) in refractory cardiac arrest (RCA). There is also increasing recognition of the value of capnography in intensive care settings in intubated patients. Future directions include determining the outcomes based on capnography waveforms PETCO2 values and determining a reasonable duration of CPR. In the future, given increasing use of capnography during CPR large databases can be analyzed to predict outcomes.
Keywords: Capnography, cardiac arrest, cardiopulmonary resuscitation, end-tidal carbon dioxide
INTRODUCTION
Recent changes in Advanced Cardiac Life Support (ACLS) guidelines recommending the use of capnography during cardiopulmonary resuscitation (CPR) have led to many institutions revisiting their policies on CPR.[1] Currently, terminating resuscitative efforts in patients with cardiopulmonary arrest is an intuitive and personal call. Although capnography has now been incorporated into CPR to judge the effectiveness of chest compressions, increasing evidence suggests that it may offer an objective basis for determining patient prognosis or terminating CPR. The objective of this commentary is to review the underlying physiology of capnography during CPR, analyze evidence favoring capnography use during CPR, and outline future directions.
Time capnography
The carbon dioxide (CO2) concentration can be plotted against time (time capnography) or against expired volume (volume capnography). In clinical practice, time capnography is the method that is commonly used. Infrared (IR) technology is the method of CO2 analysis in practice. CO2 is an asymmetric and polyatomic gas molecule that absorbs IR light at 4.3 mμ. The concentration of CO2 in a gas sample can be measured by shining IR light on the respiratory gas sample and comparing the intensity of light that passes through the sample with the original light intensity.[2] The remaining IR light is detected by the IR detector and computed to display CO2 waveform and CO2 concentration. The CO2 concentration can be also displayed as mmHg if atmospheric pressure is known.
Sidestream capnography
Sidestream capnography is the most widely used method for continuous CO2 monitoring [Figure 1].[3] It involves the use of disposable tubing (usually several feet long) and a T-piece adapter, which is inserted between the breathing circuit and endotracheal tube or other airway device. The tubing is connected from the side port on the adapter to a separate unit which contains the CO2 sensor, and a sample of gas is aspirated through this disposable tubing during the respiratory cycle into the capnograph for measurement. Because this gas sample must travel through the tubing to the CO2 sensor before it is processed, there is a slight delay in the display of the CO2 waveform. One of the main advantages of sidestream capnography is that it can be used in nonintubated patients. For example, there are modified nasal cannulas which allow the sampling of expired gases even while administering supplemental oxygen. A drawback of sidestream capnography is that the tubing may become blocked from water vapor or secretions. The use of a filter between the tubing and the unit containing the CO2 sensor minimizes this problem. Keeping the sample tubing antigravity can also minimize contamination from water vapor or secretions. A sidestream capnograph with a display of waveforms and end-tidal CO2 (ETCO2) values is shown in Figure 2.
Figure 1.
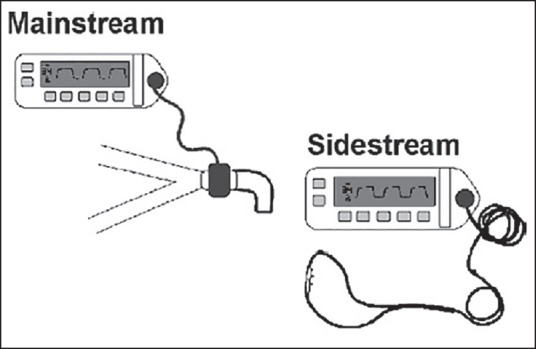
Schematic representation of sidestream and mainstream capnography devices. Source: Puente EG, Antor MA, Bergese SD. Patient monitoring, equipment, and intravenous fluids. In: Urman RD, Kaye AD. Moderate and deep sedation in clinical practice, Cambridge University Press, 2012, with permission
Figure 2.
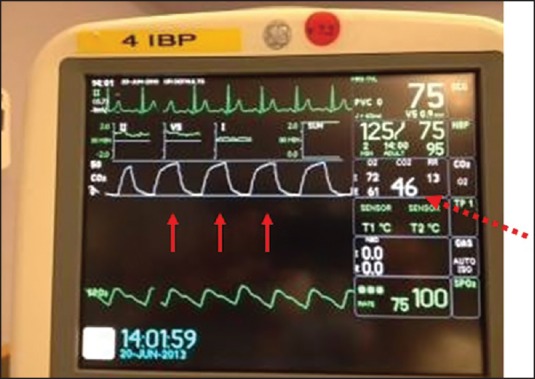
A sidestream capnograph with CO2 display showing waveforms (solid arrows) and end-tidal CO2 value (dashed arrow)
Mainstream capnography
Mainstream capnography involves the use of an adapter between the breathing circuit and endotracheal tube [Figure 1]. In this method, a lightweight IR sensor is attached directly to the adapter. The sensor emits IR light to a photodetector on the other side of the adapter. During the respiratory cycle, respiratory gases flow through the adapter and the amount of CO2 in the sample is measured immediately. There is no extra tubing, and there is no delay in the display of the waveform. The mainstream sensor is heated above body temperature, which prevents condensation and allows the sensor to function in high moisture environments. Condensation of moisture can interfere with the functioning of the unit. The earlier generations of mainstream capnometers had significant disadvantages, including bulky sensors requiring sterilization after each use and a risk of facial burns from the heated sensor. Newer versions, however, are more lightweight and have disposable adaptors. The temperature of the sensors is also decreased with better shielding to minimize facial burns. Further advances in mainstream capnography have produced smaller, light-weighted adapters for use in nonintubated patients. These units attach directly to the oxygen facemask or nasal cannula, allowing for CO2 monitoring in a spontaneously breathing patient receiving sedation.
A typical time capnogram is divided into an inspiratory and expiratory segment [Figure 3]. The expiratory segment is further divided into three phases. Phase I represents dead space gases containing no CO2. Phase II is the mixture of dead space gases and alveolar gases. Phase III (alveolar plateau) represents alveolar gases. At the end of phase III, the CO2 concentration decreases abruptly to zero representing the onset of next inhalation of CO2-free gases. The maximum value of CO2 at the end of the breath is designated as end-tidal partial pressure of CO2 (PETCO2). It is generally lower than arterial partial pressure of CO2 (PaCO2) by about 5 mmHg in healthy subjects. The slope and the height of phase III is dependent on the CO2 concentration of alveoli and their emptying patterns. In turn, the CO2 concentration in the alveoli is dependent on the ventilation and perfusion characteristics of the alveoli. The caliber of respiratory conduits determines the ventilation to the alveoli; whereas, cardiac output alters perfusion of the alveoli. Therefore, it can be inferred that the height of phase III is predominantly dependent on the cardiac output, and the slope of phase II and III is dependent on the emptying patterns of the alveoli as well as ventilation-perfusion ratio [Figure 4].[4] The angle between phase II and III, which is referred to as the alpha angle (α) and is generally 100 degrees, is increased. The alpha angle (primarily representing variations in time constants within the lung) is thus an indirect indication of V/Q status of the lung [Figure 4].[4,5] The angle between phase III and the descending limb is referred to as beta (β) angle, which is generally 90 degrees. The increase in this angle from 90 degrees suggests rebreathing of CO2.[4,6]
Figure 3.
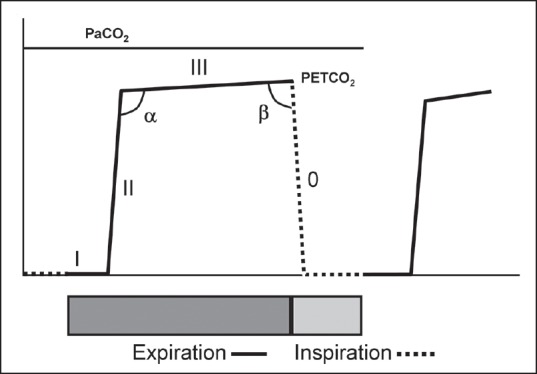
Time capnogram showing segments, phases, and angles. Inspiratory segment is phase 0, expiratory segment is divided into three phases: I, II, and III. Maximum value of carbon dioxide at the end of the expiration is designated as end-tidal partial pressure of carbon dioxide (PETCO2). The angle between phase II and III is α angle and between phase III and the inspiratory limb is β angle
Figure 4.
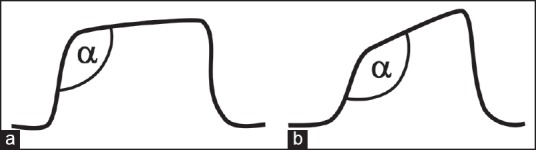
Capnogram (a) During normal breathing, α angle is 108 degrees. Capnogram (b) Recorded during bronchospasm. The angle is increased to 140 degrees
Physiology of capnography during CPR
During the administration of anesthesia, there is a good correlation between PETCO2 values and cardiac index.[6,7] Several studies showed that this relationship between cardiac output and PETCO2 values is also valid during CPR.[8,9,10,11,12] This principle formed the basis for the induction of capnography into current ACLS guidelines.[1] In a study by Jin et al., hemorrhagic shock was induced in five pigs by bleeding them followed by reinfusion of shed blood.[8] End-tidal CO2 values were measured throughout this process. The authors determined that there was good correlation between cardiac output and PETCO2 values during hemorrhage and reinfusion. Shibutani et al., replicated these results in patients undergoing aortic aneurysm surgery.[7] The authors demonstrated a significant correlation between the percent decreases of PETCO2 versus the percent decreases in cardiac output in patients with constant ventilation.[7] Two mechanisms have been postulated to explain this relationship: firstly, decreased delivery of CO2 to the lung and, secondly, increase in the alveolar dead space consequent to relatively high ventilation to perfusion rate, resulting in high ventilation/perfusion ratio. Both of these factors can result in the overall decrease in CO2 output from the lungs during decreased cardiac output states. The correlation between PETCO2 and cardiac output has two important implications during CPR. First, the effectiveness of CPR in producing adequate cardiac output can be monitored based on the PETCO2 values. Secondly, abrupt increases in PETCO2 values suggest concomitant increases in cardiac output and are indicative of the return of spontaneous circulation (ROSC) as shown in Figure 5.
Figure 5.
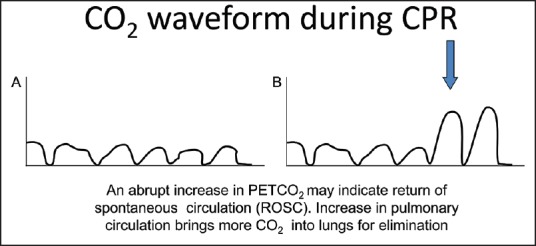
Return of spontaneous circulation is indicated by an abrupt and sustained increase in the height of capnogram
Evidence favoring capnography use during CPR
Methods
We conducted an extensive literature search of peer-reviewed articles consisting of primary research studies, review papers, and case reports published between 1960 and 2014. We considered published research from all years. Keywords such as capnography, cardiopulmonary resuscitation, cardiopulmonary arrest survival, and end-tidal CO2 were used for the initial search, and then we selected articles specifically related to the use of capnography during cardiopulmonary resuscitation and outcomes for our literature review.
Capnography is the most definitive evidence of correct endotracheal tube placement
In the past, it was believed that capnography would not be able to detect correct placement of an endotracheal tube due to decreased evolution of CO2 from the lungs consequent to decreased cardiac output generated during CPR. This may be true with the use of colorimetric devices where the sensitivity of color change is not appreciable at low concentrations. Increasing use of waveform capnography for over 3 decades has helped to improve our understanding of its use in CPR. It is now well-appreciated that presence of small obtunded CO2 waveforms during CPR reinforces tracheal location of an endotracheal tube. There is strong evidence of data demonstrating 100% sensitivity and 100% specificity in identifying correct endotracheal tube placements during CPR.[13] Studies of other devices such as colorimetric end-tidal CO2 detectors, self-inflating esophageal detectors, and non-waveform end-tidal capnometers do not match up the accuracy of waveform capnography.[14,15,16] Based on the results of the Fourth National Audit Project of the Royal College of Anaesthetists and Difficult Airway Society, a strong recommendation was made to recognize the presence of abnormal (but not flat) capnogram trace during CPR.[17] Among cases reported in this study, where capnography might have altered the course of clinical events, there was an instance with ‘flat capnogram’ during prolonged CPR and this appeared to be a case of unrecognized esophageal intubation. Absence of CO2 tracings during CPR should alert the physician in charge of CPR code team to reevaluate the location of endotracheal tube.[6] A recent study analyzed the data from Get With the Guidelines-Resuscitation (GWTG-R), which is a prospective observational registry of in-hospital cardiac arrest and resuscitation. In this study, patients whose endotracheal tube during CPR was confirmed by capnography were more likely to have ROSC.[18] Of the 176,054 patients entered into the GWTG-R database, 75,777 had an endotracheal tube placed. Confirmation of endotracheal tube position by capnography was documented in 43,034 (56%) cases. ROSC occurred in 39,063 (51.6%), and 13,474 (17.8%) survived to discharge. Patients whose endotracheal tube was confirmed by capnography or esophageal detector device were more likely to have ROSC (odds ratio 1.229, 1.179-1.282).[18] Occasionally, if the operator had provided mouth-to-patient ventilation (bystander mouth-to-mouth ventilation), the stomach contained CO2.[19] However, after seven waveforms, the expiratory gases should not contain CO2 and consistency of CO2 waveform height would not be maintained.[20]
Capnography as measure of cardiac output generated during CPR and ROSC
The height of the CO2 waveform during CPR, which is a function of cardiac output during chest compression, should be monitored, and every effort should be made to achieve PETCO2 values of at least 20 mmHg.[21] Usually during CPR, the cardiac index generated varies from 1.6 to 1.9 L/min/m2.[22] This generally corresponds to PETCO2 values of over 20 mmHg.[7] Therefore, it seems reasonable to recommend attaining a PETCO2 of 20 mmHg and above during CPR. Consistency of CPR is an important factor that may result in successful resuscitation, and continuous capnography helps to maintain consistent chest compression. Inadequate capnography waveforms can help the code team to explore factors impeding successful CPR. Such factors may include fatigue of the CPR performer, suboptimal chest compressions, ongoing hemorrhage, cardiac tamponade, and pneumothorax, among others. Capnography has been used to monitor effectiveness of various hand positions during chest compressions to determine the most optimum hand position facilitating effective compressions. Qvigstad et al.,[23] studied PETCO2 values during CPR in cardiac arrest patients treated by the physician-manned ambulances. One minute of chest compressions at the internipple line (INL) was followed by four 30-s intervals with compressions at four different levels: 2 cm below the INL, 2 cm below and to the left of INL, and 2 cm below and to the right of INL. At the end of 30-s interval, median PETCO2 was 23 mmHg at INL, 26 mmHg at 2 cm below INL, 26 mmHg and to the left of INL, and 28 mmHg at 2 cm below and to the right of INL. There were no significant differences in the PETCO2 values between the various levels of hand positions. However, a noteworthy feature in this study was substantial interindividual variation among operators. It appears that capnography can help optimize hand positions to facilitate effective compressions during CPR.[23] Capnography has also been used to study the effectiveness of mechanical chest compression devices for CPR. Axelsson et al., studied the effectiveness of a mechanical active compression CPR device (ACD-CPR) in a group of patients 18 years of age and above who suffered out-of-hospital cardiac arrest.[24] Results were compared to a control group who underwent conventional CPR. The authors found higher PETCO2 values in the ACD-CPR group than those in the conventional group. However, there is no evidence yet that mechanical device-assisted CPR contributes to better outcomes compared to conventional CPR.[25,26]
Abrupt increase in PETCO2 values during CPR suggests ROSC. Pokorna et al., observed sudden increase in PETCO2 values at the moment of ROSC.[27] Based on the premise that a PETCO2 level of 10 mmHg during CPR divides patients into those likely to be resuscitated (values > 10 mmHg) and those likely to die during CPR (values < 10 mmHg), the authors of this study tested the significance of a sudden increase in the PETCO2 values in signaling the ROSC during CPR. One hundred and eight patients representing two extreme outcomes of CPR, were subdivided into two groups. The first group included 59 patients with ROSC followed by a stable spontaneous circulation, and the second group included 49 patients with no signs of ROSC. ROSC was associated with a sudden increase in PETCO2 values that remained significantly higher than those before ROSC. PETCO2 did not rise during the entire CPR in the second group of patients without ROSC and was lower than in the first group of patients. This study concluded that during unchanged ventilation in patients undergoing CPR, PETCO2 values were significantly higher (about 10 mmHg) after ROSC than before ROSC. This implies that a sudden increase in PETCO2 exceeding 10 mmHg may indicate ROSC.[27]
Prediction of survival based on capnography
In the last few years, considerable data has emerged supporting the view that capnography can predict and improve outcome following CPR. Pokorna et al. observed that patients undergoing CPR with PETCO2 values greater than 10 mmHg during CPR were more likely to return to spontaneous circulation.[27] Grmec and Klemin[28] prospectively studied PETCO2 trends during CPR in 139 adult victims of out-of-hospital nontraumatic cardiac arrests. The initial, final, average, minimal, and maximal PETCO2 values were significantly higher in resuscitated patients than in nonresuscitated patients. Using initial, average, and final PETCO2 values of 10 mmHg correctly identified 100% of the patients who were subsequently resuscitated (specificity 74.1, 90, and 81.4%, respectively). An important observation made by the authors of this study was that none of the patients with average, initial, and final PETCO2 values of less than 10 mmHg was successfully resuscitated.[28] On the other hand, Callaham and Barton reported, based on the results of their study that patients who developed a pulse had a mean PETCO2 of 19 ± standard deviation (SD) 14 mmHg at the start of resuscitation, and those who did not, had a mean PETCO2 of 5 (±SD 4) mmHg (P < 0.0001).[29] An initial PETCO2 value of at least 15 mmHg correctly predicted eventual return of pulse (sensitivity 71%, specificity 98%, positive predictive value of 91%, and a negative predictive value of 91%).[29] The observed variation in PETCO2 values predicting successful outcome in the above studies is better explained by the observations made by Heradstveit et al. These authors also confirmed that patients who attained ROSC had significantly higher PETCO2 values compared to those who did not have ROSC regardless of the initial cardiac rhythm or cause of the cardiac arrest.[30] However, an important additional finding of this study was that despite patients with ROSC having higher PETCO2 values than those without ROSC, the actual PETCO2 values were dependent on the cause of cardiac arrest. The respective PETCO2 values with ROSC versus no ROSC, depending on the cause of cardiac arrest were as follows: cardiac 25.5 and 18 mmHg, pulmonary 33.7 and 17 mmHg, pulmonary embolus 16 and 6.7 mmHg, and unknown cause 20.5 and 9.7 mmHg.[30] Therefore, it is important to understand the limitation of using one absolute value of PETCO2 to predict the prognosis as the cause of cardiac arrest can influence the PETCO2 values, particularly if the cause is related to pulmonary function.
Einav et al. used mathematical modeling to predict survival from resuscitation based on computerized continuous capnography.[21] In this prospective cohort study of 30 patients who underwent CPR, the capnography data was collected during CPR and analyzed by a mathematician blinded to patient outcome. The primary outcome studied was to determine whether a meaningful relationship exists between the computerized PETCO2 values and ROSC. Ten minutes after intubation, patients with ROSC had higher peak PETCO2 values and a larger area under PETCO2 curves when compared to patients without ROSC. Furthermore, cumulative max PETCO2 > 20 mmHg at all-time points measured between 5 and 10 min postintubation best predicted ROSC (sensitivity = 88%, specificity = 77%). The authors of this study concluded that computerized PETCO2 values could be a potentially valuable tool for early, real-time decision-making during resuscitation.
When to terminate cardiac resuscitation in a patient is difficult to answer at this stage. This may depend on the cause of cardiac arrest, age and prior morbidity, location where cardiac arrest occurs, and the personnel who are conducting the CPR.[31] Not surprisingly, resuscitation of cardiac arrests occurring in the operating room or post-anesthesia care unit shows better survival outcomes.[31] The following case report illustrates the complexity of decision making. This case, a 54-year-old man with no known cardiac disease who collapsed outdoors in a small rural community, may be an example of the longest known duration of CPR resulting in a favorable outcome.[32] This cardiac arrest was witnessed, and immediate cardiopulmonary resuscitation efforts were initiated by a bystander and a trained first responder nearby. After moving the patient inside a building and continuing resuscitation, first responders arrived with an automated external defibrillator, and ventricular fibrillation (VF) was documented. Defibrillation shocks were delivered, transiently restoring an organized electrocardiographic rhythm, but with no pulse at any time. The trachea was intubated, ventilation-controlled, and end-tidal CO2 tension continuously monitored. Defibrillation continued and antiarrhythmic and inotropic drugs were administered intravenously. End-tidal CO2 measurements confirmed good pulmonary blood flow with chest compressions, and resuscitation was continued until a stable cardiac rhythm was achieved after 96 min of pulselessness. During helicopter transport to the hospital, the patient was in cardiogenic shock but maintained a spontaneous circulation. Coronary angiography documented a left anterior descending coronary artery thrombotic occlusion that was treated successfully. Once in the hospital, the patient required circulatory and ventilatory support and hemodialysis for acute renal failure. He experienced a complete neurologic recovery to his pre-cardiac arrest state and was discharged from the hospital. An earlier case report described CPR administration for 107 min also resulting in patient survival. However, this patient, contrary to the first case, had a subnormal body temperature of 26.2°C after retrieval from cold water.[33]
In an effort to more accurately predict resuscitation outcomes, Salen et al. attempted to pair capnography with sonography at two community hospital emergency departments.[34] Over a 12-month period, 102 patients were enrolled, all undergoing cardiac sonography evaluations, ranging from one to five scans during cardiac resuscitation. The presence of sonographically identified cardiac activity at any point during the resuscitation was associated with survival to hospital admission of 27% in contrast to only 3% of those without cardiac activity. Higher median PETCO2 levels (35 mmHg) were associated with improved chances of survival as compared to the median PETCO2 levels for nonsurvivors (13.7 mmHg). Both the sonographic detection of cardiac activity and PETCO2 levels higher than 16 mmHg were significantly associated with survival from resuscitation in the emergency department. However, logistic regression analysis demonstrated that prediction of survival using capnography was not enhanced by the addition of cardiac sonography.
While the use of capnography data during CPR shows promise in predicting survival of patients following cardiac arrest, more robust data is required to formulate more assertive recommendations in the future.
Role of capnography in therapeutic hypothermia and extracorporeal life support (ECLS)
Cooling strategies to improve patient outcomes following ROSC have been described in recent literature, showing tangible benefit in terms of neurologic outcome in patients who suffered out-of-hospital cardiac arrest.[35] Although evidence points to benefit from an early initiation of therapeutic cooling in post-cardiac arrest patients who were initially found to be in VF, it is still unclear when, how, and for what duration of time it should be applied.[36] Investigation is needed to help determine the role of PETCO2 during CPR to guide initiation, method, and duration of this emerging neuroprotective strategy. Likewise, there is encouraging evidence for the use of ECLS strategies in refractory cardiac arrest (RCA).[37,38,39] A 2009 French guideline addressing indications for ECLS suggests using a PETCO2 value of ≥10 mmHg to guide the initiation of ECLS following out-of-hospital cardiac arrest.[40] The most recent study by Le Guen et al., that evaluated patients who experienced a witnessed out-of-hospital RCA confirmed the validity of PETCO2 level ≥10 mmHg for the initiation of ECLS, although this value was thought to be possibly too liberal given lower values in patients who survived less than 24 h.[41]
Capnography in the intensive care setting
Currently, capnography is not the standard of care in some intensive care units. The value of capnography is underrecognized in this area unlike in the operating rooms.[6] There are many advantages of capnography in a ventilated patient and these include its value in CPR. According to the US National Registry of Cardiopulmonary Resuscitation, 46% of cardiac arrests (40,050 out of 86,748) occur in intensive care units.[42] Many of these cardiac arrests have worst outcome (15.5 survived to discharge) compared to other settings. Therefore, it is logical that all intensive care units should consider using capnography to monitor ventilation, cardiac output, and will serve as a valuable tool in the event of cardiac arrest. This recognition of capnography by the intensivists is on the rise, and a demand for ventilators with capnography capability has arisen nationally.
Capnography and ACLS guidelines
Anesthesiologists have incorporated capnography into clinical practice approximately 25 years ago. The reason for this change of practice was in large part attributed to American Society of Anesthesiologists (ASA) closed claim analysis which revealed that 93% of anesthesia mishaps could have been prevented if capnography was used together with pulse oximetry.[43,44,45,46] Similarly, the recent National Airway Project of Royal College of Anaesthetists and Difficult Airway Society revealed that capnography could have prevented 74% of airway-related deaths in the intensive care units.[17,47] Furthermore, capnography is the most reliable method of determining the correct position of the endotracheal tube during CPR when the pulmonary circulation is considerably low. The National Airway Project emphasized that if no CO2 waveform is obtained during CPR, the endotracheal tube is most likely not in the trachea. The pulmonary circulation produced by the chest compressions is enough to produce visible CO2 waveforms, thus confirming the position of the endotracheal tube. This is probably one of the main reasons why American Heart Association (AHA) strongly recommended the use of capnography during CPR. The AHA also recognized the value of capnography waveform height/CO2 values as an indirect measure of pulmonary circulation generated by the chest compressions. Another reason for inclusion of capnography during CPR is the abrupt rise in CO2 suggesting ROSC. International Liaison Committee on Resuscitation conferences are periodically held to evaluate resuscitation science and generate conclusions and recommendations which are published as consensus science statements and treatment recommendations. This review of science and guidance documents has been occurring on a 5-year cycle. The most recent guidelines from the AHA and European Resuscitation Council (ERC) resulted from the 2010 International Consensus Conference in February 2010, attended by 313 experts from 30 countries.[1] Part 8.1 of the guidelines states that “Continuous waveform capnography is recommended in addition to clinical assessment as the most reliable method of confirming and monitoring correct placement of an endotracheal tube”. This statement is based on Class 1 level of evidence (LOE A), meaning that it is based on the data derived from multiple randomized clinical trials or meta-analyses. In addition, the guideline states that “providers should observe a persistent capnographic waveform with ventilation to confirm and monitor endotracheal tube placement in the field, in the transport vehicle, or arrival at the hospital and after any patient transfer to reduce the risk of unrecognized tube misplacement or displacement”.[1] The introduction of these recommendations and guidelines will generate substantial data in the future to accurately predict the course and outcome of CPR. As mentioned previously, the value of capnography during CPR has been highlighted in case reports and public media. As previously discussed, a recent case report highlighted the value of capnography during CPR in a patient who sustained cardiac arrest and collapsed in a grocery store.[32] Capnography assured that effective CPR was being performed and the resuscitation team continued with resuscitation efforts for 96 min when sustained spontaneous heart beat and circulation were finally restored. Throughout the resuscitation, end-tidal CO2 was consistently in the 28-36 mmHg range during VF/CPR. These levels of CO2 were consistent with effective chest compression generating reasonable pulmonary blood flow, justifying continuation of resuscitation. When the 12th shock restored VF to an organized rhythm, there was no palpable pulse, but a PETCO2 of 37 mmHg suggested that spontaneous circulation had resumed, and CPR was terminated. The patient underwent coronary stent placement and was discharged on the 10th day with no neurologic or cognitive deficit.
Based on the current guidelines of AHA/ACLS, our institution has mounted a capnography unit on a movable code stand [Figure 6]. The unit is turned on at the first notification of the code so that it undergoes the calibration process by the time the code team arrives at the code location. In addition, the stand has a video laryngoscope for an unanticipated difficult intubation.
Figure 6.
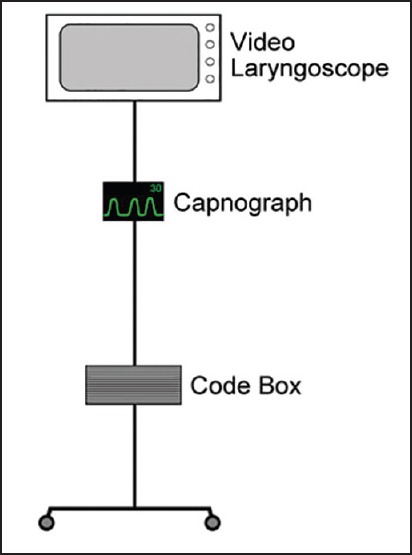
Example of a capnography unit mounted on a movable code stand
Limitations of capnography in CPR
Lack of availability of equipment is a limitation in the majority of situations. However, it is likely that, in due course, institutional guidelines will require capnography to be used during every CPR regardless of the location. The National Airway Project strongly recommends this approach and asks clinicians to use capnography during CPR. If the equipment is not available, the cause of unavailability must be stated.[17,47] It is conceivable that, in due course, capnography equipment use will become standard during CPR. Although older capnographs were bulky and marketed as stand-alone units, many portable capnograph unit types are now available. These can be mounted on a mobile stand as described above or can be carried. Many capnographs require a calibration period for several minutes before functionality could be achieved. Consequently, the monitor can be switched on as soon as a call for CPR is received. This time frame should be adequate enough for calibration, and the device should be ready to show CO2 waveforms. Use of a capnometer that only displays CO2 values but not waveforms is not acceptable during CPR. It is important to observe small but consistent CO2 waveforms during CPR not only to confirm the endotracheal tube placement but also to monitor the efficacy of CPR. The functionality of the capnograph unit can be easily tested by the operator blowing into the capnograph sampling tube after calibration. A good waveform and end-tidal CO2 value of about 35 mmHg demonstrates functionality of the unit. Blockage of the sampling tube by the secretions from the patient can occur, but this should not be a major predicament. If this happens, the sampling tube can be easily changed. Personnel using capnograph units will require training and interpretation of CO2 waveforms. In our institution, we have developed an online course for all physicians who are likely to be called for CPR. In the intensive care units, the capnographs should be used continuously so that personnel become familiar with its use so that they are more adept at interpreting capnography during a crisis.
CONCLUSION AND FUTURE DIRECTIONS
The AHA and Association of Anaesthetists of Great Britain and Ireland have rightfully endorsed the use of capnography during CPR. Other regional associations in many countries are inducting capnography into CPR guidelines. In our institution, the physicians are required to undergo an online training module that explains the physiology, functionality, and guidelines aimed towards successful resuscitation. The respiratory therapist is responsible for bringing the capnography units to all codes. Several intensive care units are upgrading their ventilators to enable the use of capnography.
Presence of consistent blunted waveforms reinforces tracheal placement of the endotracheal tube. Furthermore, the effectiveness of CPR can be monitored by consistently producing PETCO2 values greater than 10-20 mmHg. Although there are 10 mmHg variations in PETCO2 values from various studies that predict positive outcome, the aim should be to maintain consistently higher PETCO2 values to generate optimum cardiac output. This may necessitate a change in chest compressions or change in the operator performing CPR. An abrupt increase in PETCO2 values suggests ROSC.
The future focus of capnography in CPR will be in determining the outcomes based on capnography waveform PETCO2 values. With the increasing use of capnography during CPR, an opportunity to analyze large databases to predict outcomes of CPR would be possible. Another important focus point will be the determination of reasonable duration of CPR before a decision to terminate it is made. Currently, this responsibility rests with the CPR code leader. With more evidence emerging, the anecdotal positive outcomes following capnography use during CPR reported in a few well-known cases will transform into more definitive recommendations in the future. In the meantime, manufacturers of capnography devices will direct their efforts in producing cost-effective, portable devices that are essential for use in CPR given the nature of occurrence at unexpected locations.
ACKNOWLEDGMENT
We would like to acknowledge Mr. James Bell for creating figure graphics.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, et al. CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and, Resuscitation. Cardiopulmonary resuscitation quality: [Corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the American Heart Association. Circulation. 2013;128:417–35. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe MB. Infrared measurement of carbon dioxide in the human breath: “Breathe-through” devices from tyndall to the present day. Anesth Analg. 2008;107:890–904. doi: 10.1213/ane.0b013e31817ee3b3. [DOI] [PubMed] [Google Scholar]
- 3.Puente EG, Bergese SD. Patient monitoring, equipment, and intravenous fluids. In: Urman RD, editor. Moderate and Deep Sedation in Clinical Practice. New York: Cambridge University Press; 2012. pp. 57–76. [Google Scholar]
- 4.Bhavani-Shankar K, Moseley H, Kumar AY, Delph Y. Capnometry and anaesthesia. Can J Anaesth. 1992;39:617–32. doi: 10.1007/BF03008330. [DOI] [PubMed] [Google Scholar]
- 5.Bhavani-Shankar K, Kumar AY, Moseley HS, Ahyee-Hallsworth R. Terminology and the current limitations of time capnography: A brief review. J Clin Monit. 1995;11:175–82. doi: 10.1007/BF01617719. [DOI] [PubMed] [Google Scholar]
- 6.Kodali BS. Capnography outside the operating rooms. Anesthesiology. 2013;118:192–201. doi: 10.1097/ALN.0b013e318278c8b6. [DOI] [PubMed] [Google Scholar]
- 7.Shibutani K, Muraoka M, Shirasaki S, Kubal K, Sanchala VT, Gupte P. Do changes in end-tidal PCO2 quantitatively reflect changes in cardiac output? Anesth Analg. 1994;79:829–33. doi: 10.1213/00000539-199411000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Jin X, Weil MH, Tang W, Povoas H, Pernat A, Xie J, et al. End-tidal carbon dioxide as a noninvasive indicator of cardiac index during circulatory shock. Crit Care Med. 2000;28:2415–9. doi: 10.1097/00003246-200007000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Isserles SA, Breen PH. Can changes in end-tidal PCO2 measure changes in cardiac output? Anesth Analg. 1991;73:808–14. doi: 10.1213/00000539-199112000-00023. [DOI] [PubMed] [Google Scholar]
- 10.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–9. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Trevino RP, Bisera J, Weil MH, Rackow EC, Grundler WG. End-tidal CO2 as a guide to successful cardiopulmonary resuscitation: A preliminary report. Crit Care Med. 1985;13:910–1. doi: 10.1097/00003246-198511000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pernat A, Weil MH, Sun S, Tang W. Stroke volumes and end-tidal carbon dioxide generated by precordial compression during ventricular fibrillation. Crit Care Med. 2003;31:1819–23. doi: 10.1097/01.CCM.0000069538.12447.82. [DOI] [PubMed] [Google Scholar]
- 13.Silvestri S, Ralls GA, Krauss B, Thundiyil J, Rothrock SG, Senn A, et al. The effectiveness of out-of-hospital use of continuous end-tidal carbon dioxide monitoring on the rate of unrecognized misplaced intubation within a regional emergency medical services system. Ann Emerg Med. 2005;45:497–503. doi: 10.1016/j.annemergmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Scarth E, Cook T. Capnography during cardiopulmonary resuscitation. Resuscitation. 2012;83:789–90. doi: 10.1016/j.resuscitation.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ornato JP, Shipley JB, Racht EM, Slovis CM, Wrenn KD, Pepe PE, et al. Multicenter study of a portable, hand-size, colorimetric end-tidal carbon dioxide detection device. Ann Emerg Med. 1992;21:518–23. doi: 10.1016/s0196-0644(05)82517-x. [DOI] [PubMed] [Google Scholar]
- 16.Bozeman WP, Hexter D, Liang HK, Kelen GD. Esophageal detector device versus detection of end-tidal carbon dioxide level in emergency intubation. Ann Emerg Med. 1996;27:595–9. doi: 10.1016/s0196-0644(96)70162-2. [DOI] [PubMed] [Google Scholar]
- 17.Cook TM, Woodall N, Frerk C. Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br J Anaesth. 2011;106:617–31. doi: 10.1093/bja/aer058. [DOI] [PubMed] [Google Scholar]
- 18.Phelan MP, Ornato JP, Peberdy MA, Hustey FM American Heart Association's get with the Guidelines-Resuscitation Investigators. Appropriate documentation of confirmation of endotracheal tube position and relationship to patient outcome from in-hospital cardiac arrest. Resuscitation. 2013;84:31–6. doi: 10.1016/j.resuscitation.2012.08.329. [DOI] [PubMed] [Google Scholar]
- 19.Kramer-Johansen J, Dorph E, Steen PA. Detection of carbon dioxide in expired air after oesophageal intubation; the role of bystander mouth-to-mouth ventilation. Acta Anaesthesiol Scand. 2008;52:155–7. doi: 10.1111/j.1399-6576.2007.01503.x. [DOI] [PubMed] [Google Scholar]
- 20.Ornato JP. Hemodynamic monitoring during CPR. Ann Emerg Med. 1993;22:289–95. doi: 10.1016/s0196-0644(05)80458-5. [DOI] [PubMed] [Google Scholar]
- 21.Einav S, Bromiker R, Weiniger CF, Matot I. Mathematical modeling for prediction of survival from resuscitation based on computerized continuous capnography: Proof of concept. Acad Emerg Med. 2011;18:468–75. doi: 10.1111/j.1553-2712.2011.01067.x. [DOI] [PubMed] [Google Scholar]
- 22.Zuercher M, Hilwig RW, Ranger-Moore J, Nysaether J, Nadkarni VM, Berg MD, et al. Leaning during chest compressions impairs cardiac output and left ventricular myocardial blood flow in piglet cardiac arrest. Crit Care Med. 2010;38:1141–6. doi: 10.1097/CCM.0b013e3181ce1fe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qvigstad E, Kramer-Johansen J, Tomte O, Skalhegg T, Sorensen O, Sunde K, et al. Clinical pilot study of different hand positions during manual chest compressions monitored with capnography. Resuscitation. 2013;84:1203–7. doi: 10.1016/j.resuscitation.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Axelsson C, Karlsson T, Axelsson AB, Herlitz J. Mechanical active compression-decompression cardiopulmonary resuscitation (ACD-CPR) versus manual CPR according to pressure of end tidal carbon dioxide (P(ET)CO2) during CPR in out-of-hospital cardiac arrest (OHCA) Resuscitation. 2009;80:1099–103. doi: 10.1016/j.resuscitation.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Schwab TM, Callaham ML, Madsen CD, Utecht TA. A randomized clinical trial of active compression-decompression CPR vs standard CPR in out-of-hospital cardiac arrest in two cities. JAMA. 1995;273:1261–8. [PubMed] [Google Scholar]
- 26.Brooks SC, Hassan N, Bigham BL, Morrison LJ. Mechanical versus manual chest compressions for cardiac arrest. Cochrane Database Syst Rev. 2011:CD007260. doi: 10.1002/14651858.CD007260.pub2. [DOI] [PubMed] [Google Scholar]
- 27.Pokorna M, Necas E, Kratochvil J, Skripsky R, Andrlik M, Franek O. A sudden increase in partial pressure end-tidal carbon dioxide (P(ET)CO(2)) at the moment of return of spontaneous circulation. J Emerg Med. 2010;38:614–21. doi: 10.1016/j.jemermed.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 28.Grmec S, Klemen P. Does the end-tidal carbon dioxide (EtCO2) concentration have prognostic value during out-of-hospital cardiac arrest? Eur J Emerg Med. 2001;8:263–9. doi: 10.1097/00063110-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Callaham M, Barton C. Prediction of outcome of cardiopulmonary resuscitation from end-tidal carbon dioxide concentration. Critical Care Med. 1990;18:358–62. doi: 10.1097/00003246-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Heradstveit BE, Sunde K, Sunde GA, Wentzel-Larsen T, Heltne JK. Factors complicating interpretation of capnography during advanced life support in cardiac arrest — a clinical retrospective study in 575 patients. Resuscitation. 2012;83:813–8. doi: 10.1016/j.resuscitation.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Krishna Ramachandran S, Mhyre J, Kheterpal S, Christensen RE, Tallman K, Morris M, et al. American Heart Association's Get With The Guidelines-Resuscitation Investigators. Predictors of survival from perioperative cardiopulmonary arrests: A retrospective analysis of 2,524 events from the get with the guidelines-resuscitation registry. Anesthesiology. 2013;119:1322–39. doi: 10.1097/ALN.0b013e318289bafe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White RD, Goodman BW, Svoboda MA. Neurologic recovery following prolonged out-of-hospital cardiac arrest with resuscitation guided by continuous capnography. Mayo Clinic Proc. 2011;86:544–8. doi: 10.4065/mcp.2011.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmaas G, Vikenes BH. Survival after 48 min submersion and 107 min cardiopulmonary resuscitation. Resuscitation. 2011;82:494–5. doi: 10.1016/j.resuscitation.2010.09.482. [DOI] [PubMed] [Google Scholar]
- 34.Salen P, O’Connor R, Sierzenski P, Passarello B, Pancu D, Melanson S, et al. Can cardiac sonography and capnography be used independently and in combination to predict resuscitation outcomes? Acad Emerg Med. 2001;8:610–5. doi: 10.1111/j.1553-2712.2001.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 35.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 36.Stub D, Bernard S, Duffy SJ, Kaye DM. Post cardiac arrest syndrome: A review of therapeutic strategies. Circulation. 2011;123:1428–35. doi: 10.1161/CIRCULATIONAHA.110.988725. [DOI] [PubMed] [Google Scholar]
- 37.Dembitsky WP, Moreno-Cabral RJ, Adamson RM, Daily PO. Emergency resuscitation using portable extracorporeal membrane oxygenation. Ann Thoracic Surg. 1993;55:304–9. doi: 10.1016/0003-4975(93)90542-p. [DOI] [PubMed] [Google Scholar]
- 38.Megarbane B, Leprince P, Deye N, Resiere D, Guerrier G, Rettab S, et al. Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med. 2007;33:758–64. doi: 10.1007/s00134-007-0568-4. [DOI] [PubMed] [Google Scholar]
- 39.Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, et al. Back from irreversibility: Extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg. 2005;79:178–83. doi: 10.1016/j.athoracsur.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 40.Adnet F, Baud F, Cariou A, Carli P, Combes A, Devictor D, et al. Guidelines for indications for the use of extracorporeal life support in refractory cardiac arrest. French Ministry of Health. Annales francaises d’anesthesie et de reanimation. 2009;28:182–90. doi: 10.1016/j.annfar.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Le Guen M, Nicolas-Robin A, Carreira S, Raux M, Leprince P, Riou B, et al. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 2011;15:R29. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, et al. National Registry of Cardiopulmonary Resuscitation Investigators. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–92. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 43.Caplan RA, Posner KL, Ward RJ, Cheney FW. Adverse respiratory events in anesthesia: A closed claims analysis. Anesthesiology. 1990;72:828–33. doi: 10.1097/00000542-199005000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Cooper JB, Newbower RS, Kitz RJ. An analysis of major errors and equipment failures in anesthesia management: Considerations for prevention and detection. Anesthesiology. 1984;60:34–42. doi: 10.1097/00000542-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Eichhorn JH. Prevention of intraoperative anesthesia accidents and related severe injury through safety monitoring. Anesthesiology. 1989;70:572–7. doi: 10.1097/00000542-198904000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Tinker JH, Dull DL, Caplan RA, Ward RJ, Cheney FW. Role of monitoring devices in prevention of anesthetic mishaps: A closed claims analysis. Anesthesiology. 1989;71:541–6. doi: 10.1097/00000542-198910000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Cook TM, Woodall N, Harper J, Benger J Fourth National Audit Project. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive care and emergency departments. Br J Anaesth. 2011;106:632–42. doi: 10.1093/bja/aer059. [DOI] [PubMed] [Google Scholar]


