Abstract
Nitrogen (N) cycling microbial communities in marine sediments are extremely diverse, and it is unknown whether this diversity reflects extensive functional redundancy. Sedimentary denitrifiers remove significant amounts of N from the coastal ocean and diazotrophs are typically regarded as inconsequential. Recently, N fixation has been shown to be a potentially important source of N in estuarine and continental shelf sediments. Analysis of expressed genes for nitrite reductase (nirS) and a nitrogenase subunit (nifH) was used to identify the likely active denitrifiers and nitrogen fixers in surface sediments from different seasons in Narragansett Bay (Rhode Island, USA). The overall diversity of diazotrophs expressing nifH decreased along the estuarine gradient from the estuarine head to an offshore continental shelf site. Two groups of sequences related to anaerobic sulphur/iron reducers and sulphate reducers dominated libraries of expressed nifH genes. Quantitative polymerase chain reaction (qPCR) and quantitative reverse transcription polymerase chain reaction (qRT-PCR) data shows the highest abundance of both groups at a mid bay site, and the highest nifH expression at the head of the estuary, regardless of season. Several potential environmental factors, including water temperature, oxygen concentration and metal contamination, may influence the abundance and nifH expression of these two bacterial groups.
Introduction
Estuaries and continental shelves are dynamic ecosystems that receive and process large inputs of nutrients including those resulting from anthropogenic activities (Pinckney et al., 2001; Liu et al., 2010). Most of the nitrogen (N) is removed by denitrification in sediments in these coastal regions (Nixon et al., 1996; Seitzinger and Giblin, 1996). Denitrification is an anaerobic microbially mediated process in which oxidized forms of N are sequentially reduced to N2 gas. This pathway is responsible for the major loss of fixed N in coastal margins, which in turn drives the marine N deficit (Codispoti, 2007).
Biological N fixation, the conversion of N2 gas into ammonia, is usually regarded as an inconsequential component in most estuarine N budgets (Howarth et al., 1988b; Galloway et al., 2004). However, N fixation is being considered increasingly important in several benthic habitats, particularly in areas associated with the photic zone including photosynthetic microbial mats (Capone, 1983; Paerl et al., 1996) and sediments vegetated by sea grasses (McGlathery et al., 1998; Herbert, 1999) and salt marsh plants (Welsh et al., 1996; Herbert, 1999). Non-vegetated estuarine sediments are generally considered net sinks for N because of high rates of denitrification (Nixon et al., 1996; Seitzinger and Giblin, 1996), and it has been thought that N fixation is a negligible process (Capone, 1983). Measurements of N fixation in the benthic sediments of the temperate estuary Narragansett Bay (Rhode Island, USA) during the 1970s show that it accounted for < 1% of the total annual influx of N into the system (Seitzinger, 1987). Recent measurements of N2 gas flux in sediments from the Narragansett Bay shows a seasonal switch in N cycling with high rates of episodic net N2 fixation, challenging the denitrification-dominated paradigm (Fulweiler et al., 2007).
Functional genes encoding cellular proteins that mediate biogeochemical transformations not only provide insight into the ecology of a system, but also can be used to investigate the diversity of specific groups of micro-organisms (e.g., denitrifiers and N fixers) in the environment. The key intermediate step in the denitrification pathway, reduction of nitrite to nitric oxide, is catalyzed by the NirS and NirK proteins, two known forms of dissimilatory nitrite reductase. Bacteria harbour copies of either nirS or nirK genes and both have been used as gene markers used for ecological studies to follow denitrifier community composition (Braker et al., 2001; Avrahami et al., 2003). nirS was targeted for this study as the gene is preferentially found in marine sediments through polymerase chain reaction (PCR)-based methods, while nirK is detected more readily in soil (Braker et al., 2000). The study of diazotroph diversity has been largely based on the phylogenetic analysis of nifH, a reliable genetic marker for microbes capable of fixing N (Zehr and McReynolds, 1989; Zehr et al., 2003; Jenkins and Zehr, 2008). The nifH gene encodes the nitrogenase iron protein component of the conserved nitrogenase protein complex, an enzyme-catalyzing N fixation in these microbes (Howard and Rees, 1996).
The capability to denitrify or fix N is distributed through diverse prokaryotic taxa throughout the bacteria and archaea (Young, 1992; Zumft, 1997). Several studies in Chesapeake Bay (Maryland and Virginia, USA) have sought to understand the mechanisms driving the distribution of denitrifiers (Bulow et al., 2008) and diazotrophs (Burns et al., 2002; Jenkins et al., 2004; Short et al., 2004; Steward et al., 2004; Moisander et al., 2007). These studies have highlighted that diverse communities of microbes containing the nirS or nifH gene are present in the estuarine ecosystem, but it remains unclear what factors control their diversity and what fraction of these microbes are metabolically active. In this study, we follow gene expression to identify the likely active groups driving denitrification and N fixation. Determining the functional groups can help elucidate the environmental controls regulating these two processes. Very few studies have used gene expression as a method to examine biodiversity of the most potentially active N-cycling microbes. Only a few groups have detected nirS expression (Nogales et al., 2002; Bulow et al., 2008), and to our knowledge, there has been only one report of nifH expression in non-vegetated estuarine sediments (Fulweiler et al., 2013). Our purpose is to go beyond deoxyribonucleic acid (DNA) diversity studies, targeting messenger ribonucleic acid (mRNA) from sediments to understand the diversity of the assemblages of denitrifiers and N fixers expressing the nirS and nifH genes respectively.
In this study, we examined the active nirS- and nifH-transcribing microbial populations to determine the likely functional denitrifiers and diazotrophs in benthic sediment samples collected along the estuarine gradient from the head of Narragansett Bay to an offshore continental shelf site. One of our aims was to determine if the expressed nirS and nifH sequence diversity patterns resembled the unique distribution of denitrifiers and N fixers in Chesapeake Bay, in which the diversity of nirS and nifH decreased along the estuarine gradient from the freshwater end to the more saline mouth (Moisander et al., 2007; Bulow et al., 2008). N fixation in bare estuarine sediments is recently becoming recognized as an important process occurring in coastal systems (Fulweiler et al., 2007; 2013,; Bertics et al., 2010; 2012a,b,,), so for the remainder of the study we focused on quantifying the transcriptional activity of bacterial populations actively expressing nifH. Predominant expressed nifH sequences were used to develop primers and probes for quantitative PCR to follow the changes in abundance, distribution and nifH expression of the microbial groups along the estuarine gradient over an annual temporal cycle. To elucidate how the environment potentially impacts the biodiversity of genetically active diazotrophs, we also considered possible mechanisms (e.g., oxygen, temperature and salinity) driving shifts in these diazotroph communities in the benthic sediments.
Results
Expression of functional genes associated with N fixation (nifH) and denitrification (nirS) were analyzed at four stations [head of the estuary (PRE), mid bay site (MNB), Rhode Island Sound (RIS2) and the Mud Patch (MP1)] along the estuarine gradient of Narragansett Bay to an offshore continental shelf site over a temporal cycle (Fig. 1, Supporting Information Table S1). (Refer to Experimental Procedures for more in-depth collection and site description.)
Figure 1.
Map of Narragansett Bay and the southern coast of Rhode Island and Massachusetts. Sampling sites are represented by black dots.
Phylogenetic relationships of expressed nirS and nifH sequences
Expression of nifH was detected at all four sites throughout the temporal cycle (Fig. 2). The spatial distribution of nifH mRNA transcripts was variable along the sediment depth gradient and did not appear to be impacted by location or season of collection (Fig. 2). nirS expression was also observed at all four stations, however it was usually detected in the warmer sampling months (May through October) (Fig. 2). nirS expression was localized to the top 2 cm except at station MP1 (Fig. 2). The expression of nirS was rarely detected without concurrent nifH expression (Fig. 2).
Figure 2.
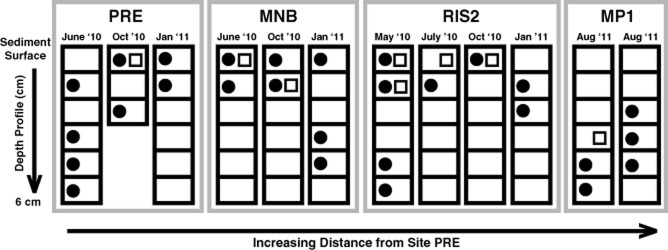
Downcore nifH (closed circles) and nirS (open squares) gene expression in 1 cm intervals in the sediment as a function of increasing distance from site PRE. Blank spaces indicate that no gene expression was detected. Sampling month is indicated above each profile.
Phylogenetic analysis of nirS mRNA transcript sequences shows that they are distributed throughout several diverse groups amongst nirS phylogeny (Supporting Information Fig. S1, Supporting Information Table S2). The majority of expressed nirS sequences group close (> 70% sequence identity) to Azoarcus tolulyticus, a bacterium notable for its ability to both denitrify and fix N (Zhou et al., 1995) (Supporting Information Fig. S1). Both spatially and seasonally, a major shift was not detected in the distribution of denitrifiers expressing nirS (data not shown).
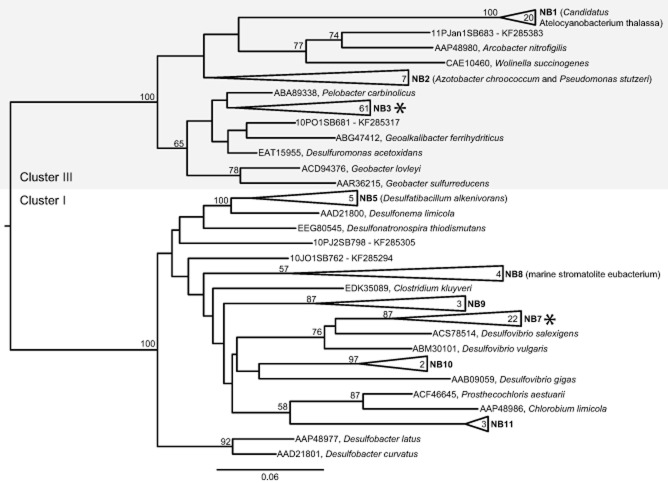
Phylogenetic analysis of the expressed nifH sequences from these sites shows that they are restricted to two main nifH phylogenetic groups [nifH clusters I and III, as previously defined (Zehr et al., 2003)] and group with known sulphate, sulphur and iron-reducing bacteria (Fig. 3, Supporting Information Table S3). Most of these nifH expressing groups (designated as NB), with the exception of NB1, are classified as Mo-dependent nitrogenase group II by (Raymond et al., 2004). The majority of expressed nifH sequences (61) are within group NB3, which has as its most closely related (> 94% sequence identity) cultivated species Pelobacter carbinolicus, an anaerobe known to reduce sulphur and iron compounds (Lovley et al., 1995) (Fig. 3). The second most prevalent expressed nifH sequence groups were NB7 and NB1, which contained 22 and 20 nifH sequences respectively (Fig. 3). Group NB7 is most closely related (> 88% sequence identity) to the sulphate reducers Desulfovibiro salexigens and Desulfovibrio vulgaris. Group NB1 clustered with > 98% sequence identity to an uncultivated marine cyanobacterium, [UCYN-A (Tripp et al., 2010)], recently renamed Candidatus Atelocyanobacterium thalassa (Thompson et al., 2012) (Fig. 3). Even though the expressed nifH sequences we recovered are constrained to a few broad taxonomic groups (clusters I and III), there is microdiversity (groups NB1–3, NB5 and NB7–11) detected among the different sites.
Figure 3.
Maximum likelihood protein tree of expressed nifH sequences obtained from sediment samples and nifH sequences from cultivated representatives. nifH cluster designations are denoted according to Zehr and colleagues (2003). Groups NB1-NB7 were previously described (Fulweiler et al., 2013), while groups NB9-NB11 are novel to this study. The number inside the group indicates the total number of sequences within the grouping. Supporting Information Table S3 describes all sequences within a collapsed group. Bootstrap values (1000 replicates) > 50% are shown at the respective nodes. Asterisk indicates groups targeted for quantitative PCR.
Diazotroph diversity shifts along the estuarine gradient
The diversity of microbes expressing nifH in the sediment decreases along the estuarine gradient, from 10 groups identified at the head of Narragansett Bay (PRE) to three groups at RIS2 (Fig. 4). Even though we only sampled site MP1 once, the trend continues and three groups were detected to be expressing nifH at the most offshore station (Fig. 4). Fisher's alpha diversity index supported these findings and decreased along estuarine gradient with the highest values at site PRE and lower values at the offshore sites RIS2 and MP1 (Supporting Information Table S4). Principle component analysis (PCA) revealed that the community composition at site PRE was most dissimilar to site MP1 (Supporting Information Fig. S2). Group NB3, related to Pelobacter carbinolicus was detected at all four sites and every time point, except in January 2011 at site RIS2. The community composition of the remaining diazotroph shifts from being comprised by microbial groups with nifH sequences related to those from several different sulphate and sulphur reducers at sites PRE and MNB to a group of sequences related to UCYN-A (Tripp et al., 2010) at the offshore sites (Fig. 4). Seasonality and depth in the sediment column did not appear to impact the overall diversity of the bacterial populations expressing nifH at each site (Supporting Information Figs S3 and S4).
Figure 4.
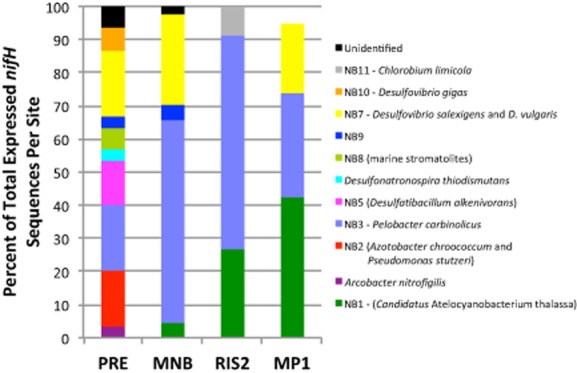
Percent of total expressed nifH sequences per site as a function of increasing distance from site PRE. Each color represents a cultivated species our environmental expressed sequences are related to as depicted in the nifH maximum likelihood tree (Fig. 3). Organisms listed in parentheses are contained within the grouping.
Quantitative polymerase chain reaction (qPCR) targeting specific diazotroph groups related to anaerobic bacteria
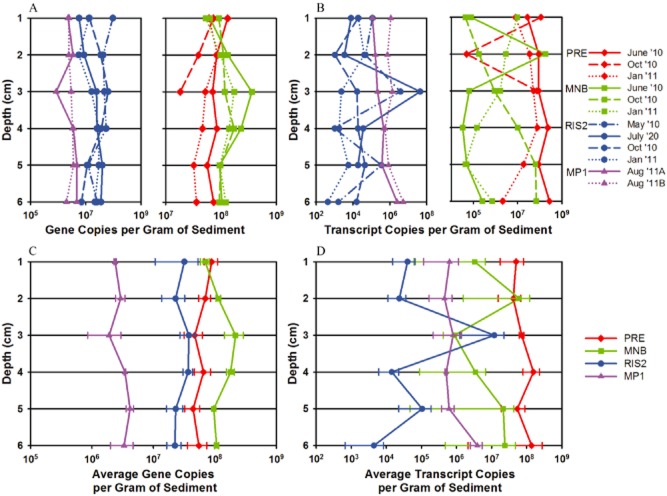
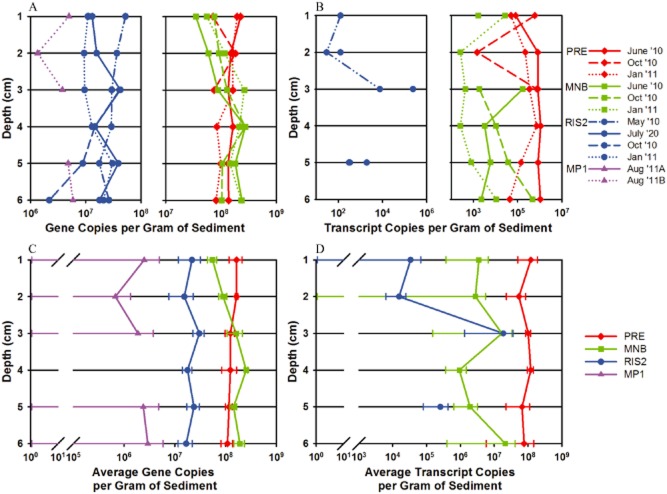
Changes in abundance, distribution and levels of expression of the two dominant microbial groups related to anaerobic bacteria expressing nifH, groups NB3 and NB7, were determined by qPCR. The greatest overall abundance of group NB3 was detected at site MNB at 3 to 4 cm in depth (Fig. 5A and C). Site PRE maintains the next highest levels of group NB3, with the lowest levels detected at the offshore sites RIS2 and MP1 (Fig 5A and C). One-way analysis of variance (anova) tests revealed the abundance of NB3 differed significantly among sites [F(3,20) = 20.98, P < 0.0001], with the maximum difference between site MNB and the other three sites as determined by the Tukey–Kramer honest significant difference (HSD) test (Supporting Information Table S5). The highest nifH expression of group NB3 is observed at the head of the Bay (site PRE), with a peak from 4 to 6 cm in depth during June 2010 (Fig. 5B and D). Both the abundance and nifH expression of group NB3 are lowest at the offshore sites, RIS2 and MP1 (Fig. 5). Group NB7 followed a similar distribution in the depth profile, with the greatest abundance between 3 and 6 cm in depth at site MNB (Fig. 6A and C). The abundance of group NB7 differed significantly between sites [F(3,20) = 24.1, P < 0.0001], with the greatest difference between the sites enclosed by land, PRE and MNB, versus the offshore locations, RIS2 and MP1 (Supporting Information Table S6). Highest nifH expression by group NB7 was also detected at lower depths at site PRE, with the exception of peak nifH transcripts detected at the sediment–water interface in October 2010 (Fig. 6B and D). Even though both groups NB3 and NB7 established highest abundances at site MNB, expression of nifH by these groups follows the estuarine gradient, with maximum levels observed at site PRE decreasing out to the continental shelf station, MP1 (Fig. 7). The expression of both microbial groups, NB3 and NB7, is significantly higher at site PRE compared with the other three sites [F(3,20) = 12.74, P < 0.0001 and F(3,20) = 48.19, P < 0.0001 respectively] (Supporting Information Tables S5 and S6). No statistical differences were detected in abundance or expression of groups NB3 and NB7 over the seasonal cycle or along the depth gradient at the sampling locations.
Figure 5.
Downcore abundance and nifH expression of group NB3 (related to P.carbinolicus) enumerated by quantitative PCR in 1 cm intervals from sediment samples collected at four sites, PRE (red), MNB (green), RIS2 (blue) and MP1 (purple). NB3 (A) abundance and (B) nifH expression during each sampling month as indicated by different line styles. NB3 (C) average abundance and (D) average nifH expression over the sampling time points. Graphs are plotted on a log scale and the standard error of the mean indicated by the error bars.
Figure 6.
Downcore abundance and nifH expression of group NB7 (related to D. salexigens and D. vulgaris) enumerated by quantitative PCR in 1 cm intervals from sediment samples collected at four sites, PRE (red), MNB (green), RIS2 (blue) and MP1 (purple). NB7 (A) abundance and (B) nifH expression during each sampling month as indicated by different line styles. NB7 (C) average abundance and (D) average nifH expression over the sampling time points. Graphs are plotted on a log scale and the standard error of the mean indicated by the error bars.
Figure 7.
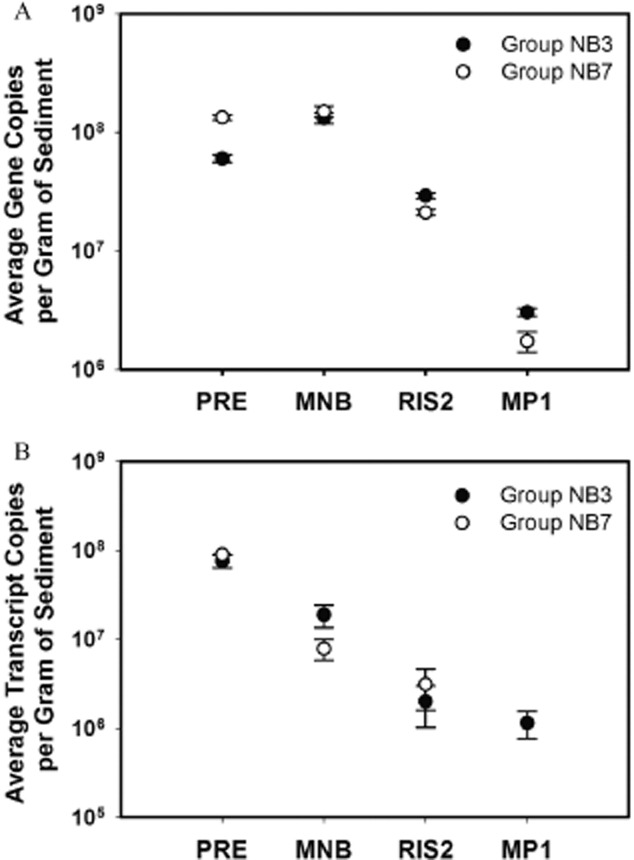
NB3 (closed circles) and NB7 (open circles) (A) abundance and (B) nifH expression integrated over depth and sampling time at the four sites as a function of increasing distance from site PRE. Graphs are plotted on a log scale and the standard error of the mean indicated by the error bars.
Discussion
Recently, benthic sediments from several locations in upper Narragansett Bay, including sites PRE and MNB, were shown to exhibit a seasonal switch in N cycling with high rates of net N fixation (up to −650 μmol N2-N m−2 h−1) during the summer months (Fulweiler et al., 2007). These findings challenge the denitrification-dominated paradigm (Christensen et al., 1987; Hulth et al., 2005), in which N fixation was thought to be a negligible process occurring in coastal systems due to the high input of N into the system (Howarth et al., 1988a,b,) and the process being repressed by combined N (Postgate, 1982). Numerous studies have reported the importance of N fixation in coastal habitats, specifically in photosynthetic microbial mats and in sediments vegetated by salt marsh plants and seagrass (e.g., Capone, 1983; Paerl et al., 1996; Welsh et al., 1996; McGlathery et al., 1998; Herbert, 1999). However, estuaries are still considered a net sink for N. More recently, N fixation (Bertics et al., 2010; 2012a,) as well as nifH expression (Fulweiler et al., 2013) has been detected in non-vegetated, bare sediments. We present gene expression data distinguishing and targeting the likely active microbes driving N fixation in a background of diverse genetic potential in marine sediments (Torsvik et al., 1996). We hypothesize that anthropogenically influenced changing environmental conditions including increases in water temperatures (Scavia et al., 2002) and eutrophication-induced hypoxia (Diaz and Rosenberg, 2008; Zhang et al., 2010) may be driving active heterotrophic N fixation in coastal and shelf sediments.
Variable nifH and nirS expression
We detected nifH expression at all sites and time points, however the expression varied throughout the depth profile with no apparent seasonal correlation. These results were not surprising as variable nifH expression has been recently reported in mesocosm experiments from sediments collected at site MNB (Fulweiler et al., 2013) and N fixation has been detected in sediments at depths > 5 cm (Bertics et al., 2010). nirS expression was likely only detected in the top 2 cm because in coastal sediments denitrification is typically coupled to nitrification (Seitzinger et al., 1984; Nowicki, 1994), an oxygen requiring process and therefore needs to occur in the surface sediments. nirS expression was detected in deeper sediments at site MP1, an area that has not been well studied. The horizon of observed expression may be attributed to dissolved oxygen penetrating deeper in open-ocean sediments (Glud, 2008) and a deeper niche for bacteria that can use alternatives to oxygen for respiration. Slightly increased rates of direct denitrification, reduction of water column nitrate that diffuses into the anoxic sediment, occurring on the continental shelf may also explain the observed nirS expression deeper in the most offshore sediments. For example, on the mid-Atlantic Bight, 9% of N removed was accounted for by direct denitrification (Laursen and Seitzinger, 2002).
Fluctuating environmental conditions may promote diazotroph diversity
The diversity of major N cycling organisms, including denitrifiers, nitrifiers and N fixers, have been well studied in Chesapeake Bay by detecting the functional genes nirS (Bulow et al., 2008), amoA (Francis et al., 2003; Ward et al., 2007) and nifH (Moisander et al., 2007) respectively. In the Cheasapeake Bay, the diversity pattern was similar for all genes studied, with the greatest diversity observed at the freshwater head decreasing to the mouth. We see a similar pattern in Narragansett Bay for the potentially active N fixers, in which the highest diversity of microbes expressing nifH is detected at site PRE, near the head of the Bay and decreasing out to site MP1 on the continental shelf. Narragansett Bay, like Chesapeake Bay, exhibits an estuarine gradient with respect to temperature, salinity and nutrients (Kremer and Nixon, 1978; Oviatt, 2008). The northernmost area, site PRE, is a dynamic region with large fluctuations in temperature, salinity, oxygen and nutrients over a temporal cycle (Granger, 1994). Because of these wide ranges in environmental conditions, microbes need to adapt to a continuously changing ecosystem. Conversely, the offshore sites (RIS2 and MP1) remain relatively stable because of exchange with the Atlantic Ocean and the deeper waters. For example, the bottom water at Rhode Island Sound ranges from 4.5 to 14°C over the course of a year and salinity remains stable between 29.75 to 33.5 ppt (Codiga and Ullman, 2010). The intermediate disturbance hypothesis (IDH) suggests that species diversity is maximized when ecological disturbance is neither too rare nor too frequent (Connell, 1978; Huston, 1979). At low levels of disturbance, more competitive organisms will dominate the ecosystem while at high levels of disturbance, organisms may not be able to adapt to their surroundings. IDH was originally developed for tropical rainforests and coral reefs (Connell, 1978), and has recently been applied to plankton communities (Floder and Sommer, 1999). We may be detecting the greatest diversity of diazotrophs expressing nifH at the head of Narragansett Bay because the microbes are competing to adapt to the intermediate fluctuating environmental conditions, while at the more stable offshore sites, the benthos is dominated by the more competitive microbes.
Dominant active diazotrophs may be influenced by oxygen, temperature and metals
Sequence analysis revealed that the majority of nifH mRNA transcripts grouped with anaerobic bacteria (Fig. 3). The two dominant anaerobic microbial groups, NB3 and NB7, were related to the iron/sulphur reducer Pelobacter carbinolicus, and the sulphate reducers D. salexigens and D. vulgaris respectively. nifH expression at the offshore sites, RIS2 and MP1, was dominated by aerobic organisms related to the unicellular cyanobacterium Candidatus Atelocyanobacterium thalassa (NB1). Interestingly, the majority of our nifH ribonucleic acid (RNA) sequences phylogenetically group with DNA sequences reported from sediments in coastal California and Eckernförde Bay, Baltic Sea (Bertics et al., 2010; 2012a). In both studies, nifH sequence types were identified that were related to various sulphur- and sulphate-reducing bacteria, including Desulfovibrio spp. and Desulfobacter spp., which are microbes that have been shown to fix N in culture (Sisler and ZoBell, 1951; Widdel, 1987). Based on acetylene reduction and sulphate reduction inhibition assays, Bertics et al. attributed the N fixation rates to sulphur- and sulphate-reducing bacteria (Bertics et al., 2010), corroborating our findings that these microbes are likely to be driving N fixation in these sediments. Our expressed nifH sequences also group with nifH RNA sequences recently reported from mesocosm experiments with sediment collected at site MNB (Fulweiler et al., 2013). N fixation by these anaerobic microbial communities could provide unanticipated inputs of N into ecosystems already stressed by eutrophication, including Narragansett Bay. Denitrification may not balance the anthropogenic inputs of N to the extent previously believed, and the sediments could instead become a net source of N exacerbating the nutrient loading into the system.
To better understand controls on the activity of the anaerobic diazotrophs, we followed the dominant anaerobic nifH-expressing groups, NB3 and NB7. Both microbial groups are more abundant and have increased nifH expression in the sediment at sites PRE and MNB in upper Narragansett Bay compared with the offshores sites (Fig. 7). Within Narragansett Bay, environmental conditions may be shifting to those that promote the increased proliferation and activity of these N fixing anaerobes. During the summer of 2006 when high rates of net N fixation were recorded at sites PRE and MNB (Fulweiler et al., 2007), there were several bouts of widespread hypoxia that severely impacted regions of upper Narragansett Bay (Codiga et al., 2009). For the last several decades, episodic hypoxia has been documented in Narragansett Bay (Oviatt et al., 1984; Bergondo et al., 2005; Deacutis et al., 2006; Melrose et al., 2007; Deacutis, 2008; Saarman et al., 2008). The severity of hypoxia generally decreases in intensity with distance from site PRE, in the Providence River Estuary, following the north–south gradient of nutrients, phytoplankton and freshwater influence (Oviatt et al., 2002; Prell et al., 2004; Melrose et al., 2007; Deacutis, 2008; Oviatt, 2008; Saarman et al., 2008). The occurrence of these low-oxygen events may be stimulating the growth and activity of groups NB3 and NB7. We observed the highest bulk expression of nifH at site PRE, which is an area that usually experiences severe hypoxia during the summer months (Saarman, 2002). In some years, hypoxia can reach as far south as site MNB (Deacutis et al., 2006; Melrose et al., 2007). Anoxic or hypoxic conditions have not been reported for waters in Rhode Island and Block Island Sounds. Perhaps these persistent hypoxic conditions in Narragansett Bay are selecting for the proliferation of anaerobic sulphur/iron and sulphate reducers over time.
Along with dissolved oxygen concentrations, temperature may be another key driver of growth and activities of NB3 and NB7 as these groups are related to mesophilic anaerobes. We detected the highest nifH expression along the entire depth profile during the summer months when the water temperature was the warmest (18°C) at site PRE. The water temperatures in Narragansett Bay can reach up to 24°C during the summer (Kremer and Nixon, 1978). Bottom water temperature at site RIS2 during July 2010 was 13°C and at site MP1 during August 2011 only reached 8°C. We detect the lowest abundance and nifH expression of NB3 and NB7 at sites RIS2 and MP1, so the offshore regions may not provide an optimal temperature range for these microbial populations.
Narragansett Bay waters also exhibit a strong north to south gradient with regard to several environmental factors, including temperature, salinity, nutrients and phytoplankton (Kremer and Nixon, 1978; Oviatt, 2008). This gradient is also observed in the sediment. For example, both organic carbon and metal concentrations, such as iron, decrease along the estuarine gradient (King et al., 1995; Nixon, 1995; Murray et al., 2007). Several of the potentially active N fixers are related to heterotrophic anaerobes that have the ability to also reduce iron, including Pelobacter carbinolicus (Nealson and Saffarini, 1994; Lovley et al., 1995). The energy gained from respiring iron, a fairly energy-yielding electron acceptor, and consuming organic carbon may be promoting the growth and activity of these diazotrophs in upper Narragansett Bay sediments.
From our study, it is more difficult to conclude whether there is greater N fixation potential in offshore sediments. Prior to the measured net N fixation rates during the summer of 2006, sediments in Narragansett Bay, including sites PRE and MNB, were shown to be dominated by denitrification (Seitzinger et al., 1984; Fulweiler et al., 2007). Offshore sediments continue to be a sink for N as recent measurements of net sediment denitrification rates at several locations off the coast of southern Rhode Island, including RIS2, ranged from 20 to 75 μmol N2-N m−2 h−1 (Heiss et al., 2012) and were not significantly different between sites or over a temporal cycle. These measurements are within the range of values reported from nearby continental shelf areas, including the Mid-Atlantic Bight and South Atlantic Bight (Devol, 1991; Devol et al., 1997). The gradient and conditions in upper Narragansett Bay may be more optimal for growth and activity of groups NB3 and NB7, while offshore sediments may not provide an appropriate niche for these microbes to thrive.
Conclusion
Benthic N-cycling processes are influenced by changes in environmental conditions, including temperature, dissolved oxygen concentrations, salinity and organic-matter loading. Climate change is predicted to increase seawater temperatures (Scavia et al., 2002) and exacerbate eutrophication-driven hypoxia (Diaz and Rosenberg, 2008; Zhang et al., 2010). Since the 1960s, the number of hypoxic zones has approximately doubled each decade (Diaz and Rosenberg, 2008) and these expanding low-oxygen events have the potential to perturb the functioning of the N cycle in estuarine ecosystems. Narragansett Bay, like many coastal ecosystems, is exposed to elevated water temperatures and exhibits seasonal hypoxia (Diaz and Rosenberg, 2008; Zhang et al., 2010). Microbes related to iron/sulphur and sulphate reducers that express nifH are highly abundant and have increased levels of nifH mRNA transcripts in Narragansett Bay sediments compared with the offshore sites. N fixation by these anaerobic micro-organisms in coastal sediments could provide unanticipated inputs of N into environments already stressed by eutrophication, significantly altering the N cycle in unprecedented ways.
Experimental procedures
Study sites
We sampled for sediment at four sites in southern New England coastal waters from May 2010 to August 2011. Sites PRE (41°46.7′, 71°22.8′) and MNB (41°35.3′, 71°22.4′) are located within the temperate estuary, Narragansett Bay, Rhode Island. The offshore sites, RIS2 (41°17.1′, 71°18.2′) and MP1 (40°26.1′, 70°28.9′), are located in Rhode Island Sound and on the continental shelf 110 km south of Cape Cod, Massachusetts respectively. Sites PRE and MNB were sampled in June and October 2010 and January 2011. Site RIS2 was sampled in May, July and October 2010 and January 2011. The most offshore site, MP1, was only sampled in August 2011 (Fig. 1, Supporting Information Table S1).
Field methods
Intact sediment cores (10 cm inner diameter and 30.5 cm long) were collected at site PRE using a 5 m pull corer and at site MNB by scuba divers. At sites RIS2 and MP1, sediment cores were collected using a box corer (0.25 m2) and pre-mounted polyvinyl chloride (PVC) cores. All cores were transported to and stored in the dark at field bottom-water temperature in a walk-in environmental chamber at the Graduate School of Oceanography at the University of Rhode Island. The cores were left uncapped with air gently bubbling through the overlying water for about 8–12 h prior to net N2 flux incubations (Heiss et al., 2012) and subsequent molecular analysis.
Subsampling and nucleic acid extractions
The cores were subsampled using a 60 ml syringe. The subcores were flash frozen in liquid N2 and sectioned into 1 cm segments from the sediment water interface to 6 cm in depth. The frozen sediment cross-sections were cut up to yield 0.25 g and 0.5 g of wet sediment for DNA and RNA isolation respectively. Total DNA was extracted using the MO Bio Powersoil DNA Isolation Kit (Carlsbad, CA, USA) and quantified using Invitrogen's Qubit dsDNA HS Assay Kit. All DNA samples were diluted to a concentration of 1 ng μl−1 for qPCR analysis. Total RNA was extracted using the MO Bio Powersoil RNA Isolation Kit (Carlsbad, CA, USA); however, the kit was designed to extract RNA from 2 g of soil. To accommodate this reduction in reaction scale, a quarter of the volumes of the Bead, SR1, SR2, SR3 and SR4 solutions and phenol-chloroform-isoamyl alcohol were used. After the RNA precipitation step, the dried pellet was resuspended with 100 μl of nuclease-free water, 10 μl of 10X TURBO DNase buffer and 1 μl of TURBO DNase from the TURBO DNase-free Kit (Ambion, Austin, TX, USA) and incubated at 37°C for 30 min. To inactivate the reaction, 10 μl of DNase Inactivation reagent was added and incubated at room temperature for 5 min. The remaining RNA purification steps were carried out using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Total RNA was quantified using Invitrogen's Qubit RNA Assay Kit and all RNA samples were diluted to 4 ng μl−1. Complementary DNA (cDNA) copies of RNA were generated with Invitrogen's SuperScript First-Strand Synthesis System for reverse transcription polymerase chain reaction. For all samples, 8 μl of DNase-treated RNA at a concentration of 4 ng μl−1 was added to the reaction. Each reaction was primed with 1 μl of 2 μM outer reverse primers for both our genes of interest, nifH3 and nirS6R (Supporting Information Tables S7 and S8). After the reverse transcriptase was added, the mixture was incubated at 50°C for 50 min. All the other steps followed the instructions of the manufacturer. For every sample, we also included controls that did not contain reverse transcriptase to confirm there was no DNA contamination in the subsequent PCR amplification.
Functional gene sequence analysis
The nifH gene from environmental cDNA was isolated using nested PCR with degenerate outer primers nifH4-nifH3 and inner primers nifH1-nifH2 (Supporting Information Table S7). Both rounds of PCR consisted of an initial denaturation step of 2 min at 94°C, cycling steps that included: a denaturation step of 30 s at 94°C, an annealing step of 30 s at 50°C, and an extension step of 1 min at 72°C. All reactions had a final extension step of 7 min at 72°C. First round reactions had 25 cycles and the second round reactions had 30 cycles (Zehr and McReynolds, 1989; Kirshtein et al., 1991). nirS was amplified using the primer pair nirS1F-nirS6R (Supporting Information Table S8). After a 2 min initial denaturation step 94°C, a touchdown PCR was performed that consisted of a denaturation step of 30 s at 94°C, an annealing step of 30 s, and an extension step of 1 min at 72°C. During the first 11 cycles, the annealing temperature decreased 0.5°C every cycle starting at 56°C. For the last 25 cycles, the annealing temperature was 54°C. A final extension step was performed for 7 min at 72°C (Braker et al., 1998; 2000,).
After amplification, the PCR products were loaded on to a 1% agarose (wt/vol) TAE gel. Bands of the correct size were purified using the QIAquick Gel Extraction Kit according to the manufacturer's protocol (Qiagen Valencia, CA, USA). The purified products were cloned into pGEM-T vectors (Promega, Madison, WI, USA), transformed into JM109 Escherichia coli competent cells (Zymo Research, Irvine, CA, USA) and identified by blue–white screening. The plasmids were purified using the QIAprep Spin Miniprep Kit (Qiagen) and sequenced on the Applied Biosystems 3130 xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) at the RI Genomic Sequencing Center at the University of Rhode Island. For every expressed nifH and nirS amplicon we collected 4–5 sequences. The total number of nifH clones per site was 30 for site PRE, 31 for site MNB, 34 for site RIS2 and 19 for site MP1. The total number of nirS clones per site was 5 for site PRE, 10 for site MNB, 15 for site RIS2 and 4 for site MP1.
Expressed environmental nifH sequences from sites PRE, MNB, RIS2 and MP1, accession numbers KF285284-KF285397, were combined with nifH sequences (23) from closely related cultivated species in GenBank (Benson et al., 2009) as determined by top blastn hits (Altschul et al., 1990). Translated NifH protein sequences were aligned using the multiple sequence alignment tool, muscle (Edgar, 2004) within the geneious software package. A nifH maximum likelihood tree of aligned protein sequences was constructed in geneious (Biomatters, Auckland, New Zealand) using the PhyML plugin (Guindon et al., 2010) with 1000 bootstrap replicates.
A nirS database was created by collecting all the nirS sequences from the National Center for Biotechnology Information (NCBI) (Benson et al., 2009) and importing the GenBank files into arb (Ludwig et al., 2004). Translated NirS protein sequences were aligned using the multiple sequence alignment tool, muscle (Edgar, 2004) within the geneious software package. A NirS protein maximum likelihood tree including the Narragansett Bay expressed nirS sequences, accession numbers KF285398-KF285429 was constructed using the PhyML algorithm in geneious with 1000 bootstrap replicates.
Quantitative real-time PCR
qPCR was conducted on all environmental DNA and cDNA samples using the Roche's LightCycler 480 Probes Master Mix and were analyzed using Stratagene's Mx3005 qPCR System. Sets of degenerate qPCR primers and dual-labeled TaqMan probes (Sigma-Aldrich Corporation, The Woodlands, TX, USA) were designed to target the nifH gene specifically related to nifH groups NB3 and NB7 (Fig. 3, Supporting Information Table S9). Both probes were labelled with a fluorophore FAM (Sigma-Aldrich Corporation, The Woodlands, TX, USA) on the 5′ end and BHQ1 quencher (Sigma-Aldrich Corporation) on the 3′ end. A standard curve was produced with triplicate 10-fold dilution series ranging from 1 ng to 1 ag of linearized plasmid containing a sequenced nifH clone from group NB3 and NB7 respectively. The qPCR reactions consisted of 10 μl of the Roche LightCycler 480 Probes Master mix (Roche), 5.7 μl of water, 2 μl of a primers/probe mix (at concentrations of 0.4 μM and 0.2 μM respectively), and 0.3 μl of Stratagene Brilliant II qPCR reference dye (ROX; Agilent Technologies, Santa Clara, CA, USA). A saturation test was used to determine the optimal concentration of DNA and cDNA template going into the reaction. The optimal amount was 2 μl of 1 ng μl−1 DNA sample or 2 μl of 1.5 ng μl−1 cDNA sample to be added to the reaction, totalling 20 μl. The qPCR thermocycling conditions were identical for both targeted groups, except for the annealing temperature: 1 cycle of 95°C for 10 min, followed by 45 cycles of 95°C for 30 s and 60°C (NB3) or 55°C (NB7) for 1 min. The standard curve was used to determine groups NB3 and NB7's absolute nifH gene copy or transcript copy number in the environmental samples. The efficiency of the qPCR assays targeting groups NB3 and NB7 was 93% and 101%, respectively, and the detection limit was 1 × 10−8 ng.
Statistical analyses
Diversity index
Fisher's alpha diversity index was calculated using EstimateS 9.1.0 to compare diversity of nifH expressing groups between sites (Colwell, 2013). The following parameters were used: 100 runs (number of randomizations), extrapolate rarefaction covers by a factor of three, and estimate at every sample. The alpha mean values are reported.
PCA
Sequences recovered for each season at a given site were summed and normalized to the number of total sequences recovered. PCA were performed using the r and ade4tkgui software packages (Thioulouse et al., 1997). Box plots were generated using r. Pairwise compositional similarity between samples was performed in EstimateS 9.1.0 (Colwell, 2013) and Bray–Curtis values supported PCA results.
Analysis of variance
One-way anova tests were conducted using JMP 10.0.2 to determine statistically significant differences among samples for qPCR and quantitative reverse transcription polymerase chain reaction. If the P value was deemed significant (< 0.05), a Tukey–Kramer HSD post-hoc test was performed to distinguish statistical significance between samples compared.
Acknowledgments
The work presented in this manuscript was supported by funding from the following grants to B.D.J. (NSF Chemical Oceanography OCE 0926875; RI Sea Grant/NOAA NA08OAR4170691 and NA 10OAR4170076, RI Research Alliance 2012 Collaborative Grant, URI Council for Research Proposal Development Grant 2011–2012). Funds for S.M.B, sequencing costs and costs associated with the Marine Life Science Facility were supported on funds from NSF RI EPSCoR 1004057). We gratefully acknowledge helpful comments on this manuscript and sampling assistance from Dr. Robinson Fulweiler (Boston University) and the helpful comments from two anonymous reviewers. We also acknowledge Dr. Christopher Deacutis (RI DEM) for many helpful conversations. We also are grateful to the larger collaborative team for discussions and sampling assistance: Dr. Scott Nixon, Dr. Lindsey Fields and Steve Granger (University of Rhode Island Graduate School of Oceanography), Elise Heiss and Sarah Foster (Boston University), Dr. Anne Giblin, Jane Tucker and Sam Kelsey (Marine Biological Laboratory), and Dr. Jeremy Rich, Dr. Amber Hardison and Dr. Lindsay Brin (Brown University). We would also like to thank Ed Baker in the RI EPSCoR Marine Life Science Facility (MLSF) at the URI Graduate School of Oceanography for his assistance and Paul Johnson in the RI EPSCoR RI Genomic Sequencing Center at the University of Rhode Island. We also acknowledge Dr. Tom Delmont at the Marine Biological Laboratory for his help with the principle component analysis.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Fig. S1. Maximum likelihood protein tree of expressed nirS sequences from sediment samples and nirS sequences observed from cultured organisms. The number inside the group indicates the number of total sequences within the grouping. Supporting Information Table S3 describes all sequences within a collapsed group. Bootstrap values (1000 replicates) > 50% are shown at respective nodes.
Fig. S2. Principal component analysis (PCA) of the relative distribution of OTUs, as designated by the nifH Maximum Likelihood tree (Fig. 3), for each site and season. These results are supported by Bray–Curtis values. The highest value (0.909) is for RIS2 Oct and RIS2 July, while the lowest value (0.087) is for PRE Oct and MP1 August.
Fig. S3. Percent of total expressed nifH sequences per site as a function increasing distance from site PRE separated by month sampled. Each color represents a cultivated species our environmental expressed sequences are related to as depicted in the nifH Maximum Likelihood tree (Fig. 3).
Fig. S4. Percent of total expressed nifH sequences per site as a function of depth and increasing distance from site PRE. Each color represents a cultivated species our environmental expressed sequences are related to as depicted in the nifH Maximum Likelihood tree (Fig. 3).
Table S1. Coordinates and water column depth of study sites with bottom water temperature during collection.
Table S2. List of nirS sequences (GenBank accession numbers) from mRNA in this study, closely related cultivated species and uncultivated species (Bulow et al., 2008) contained within groups NB1-NB9 (Supporting Information Fig. S1). Numbers in the parentheses indicate number of sequences collected at that time point.
Table S3. List of nifH sequences (GenBank accession numbers) from mRNA in this study and closely related cultivated species contained within groups NB1-NB3, NB5 and NB7-NB11 (Fig. 3).
Table S4. Fisher's alpha diversity index comparing diversity of nifH expressing groups, as designated by the nifH Maximum Likelihood tree (Fig. 3), between sites and month sampled.
Table S5. Statistical analyses of group NB3 abundance and expression using one-way anova and Tukey–Kramer HSD post-hoc tests. F ratio (df1 = degrees of freedom between groups, df2 = degrees of freedom within groups) and P values are denoted. Asterisk (*) indicates statistically significant P values (< 0.05). If the one-way anova test revealed statistical significance between samples compared, a Tukey–Kramer HSD post-hoc test was performed. Only the resultswitha P value < 0.05 are included underneath the corresponding anova test.
Table S6. Statistical analyses of group NB7 abundance and expression using one-way anova and Tukey–Kramer HSD post-hoc tests. F ratio (df1 = degrees of freedom between groups, df2 = degrees of freedom within groups) and P valuesare denoted. Asterisk (*) indicates statistically significant P values (< 0.05). If the one-way anova test revealed statistical significance between samples compared, a Tukey–Kramer HSD post-hoc test was performed. Only the results with a P value < 0.05 are included underneath the corresponding anova test.
Table S7. Primers and cycling conditions for PCR of nifH from environmental samples. All thermocycles included an initial 2 min denaturation at 94°C, and a final extension for 7 min at 72°C. *The outer reverse primer nifH3 was used to prime the RT reactions. **First round cycling conditions for RT products included 3 additional initial cycles with annealing steps at 44°C, 46°C, and 48°C.
Table S8. Primers and cycling conditions for PCR of nirS from environmental samples. All thermocycles included an initial 2 min denaturation at 94°C, and a final extension for 7 min at 72°C. *For the first 11 cycles the temperature decreased 0.5°C every cycle.
Table S9. Primers, probe and cycling conditions for quantitative PCR targeting the nifH gene of group NB3 and NB7 (Fig. 3). The quantitative PCR cycling conditions for both target groups included an initial 10 min denaturation at 95°C followed by 45 cycles of 95°C for 30 s and 60°C for 1 min.
References
- Altschul SF, Gish W, Miller W, Myers EW. Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Avrahami S, Conrad R. Braker G. Effect of ammonium concentration on N2O release and on the community structure of ammonia oxidizers and denitrifiers. Appl Environ Microbiol. 2003;69:3027–3027. doi: 10.1128/AEM.68.11.5685-5692.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J. Sayers EW. GenBank. Nucleic Acids Res. 2009;37:D26–D31. doi: 10.1093/nar/gkn723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergondo DL, Kester DR, Stoffel HE. Woods WL. Time-series observations during the low sub-surface oxygen events in Narragansett Bay during summer 2001. Mar Chem. 2005;97:90–103. [Google Scholar]
- Bertics VJ, Sohm JA, Treude T, Chow CET, Capone DG, Fuhrman JA, et al. Burrowing deeper into benthic nitrogen cycling: the impact of bioturbation on nitrogen fixation coupled to sulfate reduction. Mar Ecol Prog Ser. 2010;409:1–15. [Google Scholar]
- Bertics VJ, Löscher DR, Salonen I, Dale AW. Schmitz RA. Occurrence of benthic microbial nitrogen fixation coupled to sulfate reduction in the seasonally hypoxic Eckernförde Bay, Baltic Sea. Biogeosci Discuss. 2012a;10:1243–1258. [Google Scholar]
- Bertics VJ, Sohm JA, Magnabosco C. Ziebis W. Denitrification and nitrogen fixation dynamics in the area surrounding an individual ghost shrimp (Neotrypaea californiensis) burrow system. Appl Environ Microbiol. 2012b;78:3864–3872. doi: 10.1128/AEM.00114-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braker G, Fesefeldt A. Witzel KP. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol. 1998;64:3769–3775. doi: 10.1128/aem.64.10.3769-3775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braker G, Zhou JZ, Wu LY, Devol AH. Tiedje JM. Nitrite reductase genes (nirK and nirS) as functional markers to investigate diversity of denitrifying bacteria in Pacific northwest marine sediment communities. Appl Environ Microbiol. 2000;66:2096–2104. doi: 10.1128/aem.66.5.2096-2104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braker G, Ayala-del-Rio HL, Devol AH, Fesefeldt A. Tiedje JM. Community structure of denitrifiers, bacteria, and archaea along redox gradients in pacific northwest marine sediments by terminal restriction fragment length polymorphism analysis of amplified nitrite reductase (nirS) and 16S rRNA genes. Appl Environ Microbiol. 2001;67:1893–1901. doi: 10.1128/AEM.67.4.1893-1901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulow SE, Francis CA, Jackson GA. Ward BB. Sediment denitrifier community composition and nirS gene expression investigated with functional gene microarrays. Environ Microbiol. 2008;10:3057–3069. doi: 10.1111/j.1462-2920.2008.01765.x. [DOI] [PubMed] [Google Scholar]
- Burns JA, Zehr JP. Capone DG. Nitrogen-fixing phylotypes of Chesapeake Bay and Neuse River estuary sediments. Microbial Ecol. 2002;44:336–343. doi: 10.1007/s00248-002-1000-9. [DOI] [PubMed] [Google Scholar]
- Capone DG. Benthic nitrogen fixation. In: Capone DG, editor; Carpenter EJ, editor. Nitrogen in the Marine Environment. New York, USA: Academic Press; 1983. pp. 105–137. [Google Scholar]
- Christensen JP, Smethie WM. Devol AH. Benthic nutrient regeneration and denitrification on the Washington continental-shelf. Deep-Sea Res. 1987;34:1027–1047. [Google Scholar]
- Codiga DL. Ullman DS. 2010. and Characterizing the physical oceanography of coastal waters off Rhode Island, part 1: literature review, available observations and a representative model simulation . In Rhode Island Ocean Special Area Management Plan(eds), Rhode Island Coastal Resources Management Council. 2: 14–184.
- Codiga DL, Stoffel HE, Deacutis CF, Kiernan S. Oviatt CA. Narragansett Bay hypoxic event characteristics based on fixed-site monitoring network time series: intermittency, geographic distribution, spatial synchronicity, and interannual variability. Estuar Coast. 2009;32:621–641. [Google Scholar]
- Codispoti L. An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences. 2007;4:233–253. [Google Scholar]
- Colwell RK. 2013. EstimateS: Statistical estimation of species richeness and shared species from samples. Version 9. User's Guide and application published URL http://purl.oclc.org/estimates.
- Connell JH. Diversity in tropical rain forests and coral reefs – high diversity of trees and corals is maintained only in a non-equilibrium state. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Deacutis CF. Evidence of ecological impacts from excess nutrients in upper Narragansett Bay. In: Costa-Pierce BA, editor; Desbonnet A, editor. Science for Ecosystem-Based Management. New York, USA: Springer; 2008. pp. 349–381. [Google Scholar]
- Deacutis CF, Murray D, Prell W, Saarman E. Korhun L. Hypoxia in the upper half of Narragansett Bay, RI, during August 2001 and 2002. Northeast Nat. 2006;13:173–198. [Google Scholar]
- Devol AH. Direct measurement of nitrogen gas fluxes from continental-shelf sediments. Nature. 1991;349:319–321. [Google Scholar]
- Devol AH, Codispoti LA. Christensen JP. Summer and winter denitrification rates in western Arctic shelf sediments. Cont Shelf Res. 1997;17:1029–1050. [Google Scholar]
- Diaz RJ. Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floder S. Sommer U. Diversity in planktonic communities: an experimental test of the intermediate disturbance hypothesis. Limnol Oceanogr. 1999;44:1114–1119. [Google Scholar]
- Francis CA, O'Mullan GD. Ward BB. Diversity of ammonia monooxygenase (amoA) genes across environmental gradients in Chesapeake Bay sediments. Geobiology. 2003;1:129–140. [Google Scholar]
- Fulweiler RW, Nixon SW, Buckley BA. Granger SL. Reversal of the net dinitrogen gas flux in coastal marine sediments. Nature. 2007;448:180–182. doi: 10.1038/nature05963. [DOI] [PubMed] [Google Scholar]
- Fulweiler RW, Brown SM, Nixon SW. Jenkins BD. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Mar Ecol Prog Ser. 2013;482:57–68. [Google Scholar]
- Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, et al. Nitrogen cycles: past, present, and future. Biogeochemistry. 2004;70:153–226. [Google Scholar]
- Glud RN. Oxygen dynamics of marine sediments. Marine Biology Research. 2008;4:243–289. [Google Scholar]
- Granger S. 1994. The basic hydrography and mass transport of dissolved oxygen in the Providence and Seekonk River estuaries. MS Thesis. Narragansett, RI, USA: Graduate School of Oceanography, University of Rhode Island.
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W. Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Heiss EM, Fields L. Fulweiler RW. Directly measured net denitrification rates in offshore New England sediments. Cont Shelf Res. 2012;45:78–86. [Google Scholar]
- Herbert RA. Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev. 1999;23:563–590. doi: 10.1111/j.1574-6976.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Howard JB. Rees DC. Structural basis of biological nitrogen fixation. Chem Rev. 1996;96:2965–2982. doi: 10.1021/cr9500545. [DOI] [PubMed] [Google Scholar]
- Howarth RW, Marino R. Cole JJ. Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems .2. biogeochemical controls. Limnol Oceanogr. 1988a;33:688–701. [Google Scholar]
- Howarth RW, Marino R, Lane J. Cole JJ. Nitrogen-fixation in fresh-water, estuarine, and marine ecosystems .1. rates and importance. Limnol Oceanogr. 1988b;33:669–687. [Google Scholar]
- Hulth S, Aller RC, Canfield DE, Dalsgaard T, Engstrom P, Gilbert F, et al. Nitrogen removal in marine environments: recent findings and future research challenges. Mar Chem. 2005;94:125–145. [Google Scholar]
- Huston M. General hypothesis of species-diversity. Am Nat. 1979;113:81–101. [Google Scholar]
- Jenkins BD. Zehr JP. Molecular approaches to the nitrogen cycle. In: Carpenter EJ, editor; Bronk DA, Mulholland MR, Capone D, editors. Nitrogen in the Marine Environment. Amsterdam, The Netherlands: Elsevier; 2008. pp. 1303–1344. [Google Scholar]
- Jenkins BD, Steward GF, Short SM, Ward BB. Zehr JP. Fingerprinting diazotroph communities in the Chesapeake Bay by using a DNA macroarray. Appl Environ Microbiol. 2004;70:1767–1776. doi: 10.1128/AEM.70.3.1767-1776.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, Corbin J, McMaster R, Quinn J, Gangemi P, Cullen D, et al. 1995. A Study of the Sediments in Narragansett Bay, Volume 1- The Surface Sediments of Narragansett Bay . In Final Report submitted to the Narragansett Bay Project. Graduate School of Oceanography, University of Rhode Island. Narragansett, Rhode Island, USA: p. 29.
- Kirshtein JD, Paerl HW. Zehr J. Amplification, cloning, and sequencing of a nifH segment from aquatic microorganisms and natural communities. Appl Environ Microbiol. 1991;57:2645–2650. doi: 10.1128/aem.57.9.2645-2650.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer JN. Nixon SW. A Coastal Marine Ecosystem: Simulation and Analysis. New York, NY, USA: Springer-Verlag; 1978. [Google Scholar]
- Laursen AE. Seitzinger SP. The role of denitrification in nitrogen removal and carbon mineralization in Mid-Atlantic Bight sediments. Cont Shelf Res. 2002;22:1397–1416. [Google Scholar]
- Liu KK, Rabalais NN. Middelburg JJ. A guide to future research on continental marine biogeochemistry. In: Talaue-McManus L, editor; Liu K-K, Atkinson L, Quinones R, editors. Carbon and Nutrient Fluxes in Continental Margins. Berlin, Germany: Spring-Verlag; 2010. pp. 625–626. [Google Scholar]
- Lovley DR, Phillips EJP, Lonergan DJ. Widman PK. Fe(Iii) and S-0 Reduction by Pelobacter-Carbinolicus. Appl Environ Microbiol. 1995;61:2132–2138. doi: 10.1128/aem.61.6.2132-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlathery KJ, Risgaard-Petersen N. Christensen PB. Temporal and spatial variation in nitrogen fixation activity in the eelgrass Zostera marina rhizosphere. Mar Ecol Prog Ser. 1998;168:245–258. [Google Scholar]
- Melrose DC, Oviatt CA. Berman MS. Hypoxic events in Narragansett Bay, Rhode Island, during the summer of 2001. Estuar Coast. 2007;30:47–53. [Google Scholar]
- Moisander PH, Morrison AE, Ward BB, Jenkins BD. Zehr JP. Spatial-temporal variability in diazotroph assemblages in Chesapeake Bay using an oligonucleotide nifH microarray. Environ Microbiol. 2007;9:1823–1835. doi: 10.1111/j.1462-2920.2007.01304.x. [DOI] [PubMed] [Google Scholar]
- Murray DW, Prell WL, Rincon CE. Saarman E. 2007. and Physical property and chemical characteristics of surface sediment grab samples from Narragansett Bay and the Providence and Seekonk Rivers, a summary of the Brown Univeristy Narragansett Bay Sediment Project (BUNBSP) . In Narragansett Bay Estuary Program Report.
- Nealson KH. Saffarini D. Iron and manganese in anaerobic respiration – environmental significance, physiology, and regulation. Annu Rev Microbiol. 1994;48:311–343. doi: 10.1146/annurev.mi.48.100194.001523. [DOI] [PubMed] [Google Scholar]
- Nixon SW. Metal inputs to Narragansett Bay: A history and assessment of recent conditions. Narragansett, RI, USA: Rhode Island Sea Grant; 1995. [Google Scholar]
- Nixon SW, Ammerman JW, Atkinson LP, Berounsky VM, Billen G, Boicourt WC, et al. The fate of nitrogen and phosphorus at the land sea margin of the North Atlantic Ocean. Biogeochemistry. 1996;35:141–180. [Google Scholar]
- Nogales B, Timmis KN, Nedwell DB. Osborn AM. Detection and diversity of expressed denitrification genes in estuarine sediments after reverse transcription-PCR amplification from mRNA. Appl Environ Microbiol. 2002;68:5017–5025. doi: 10.1128/AEM.68.10.5017-5025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki BL. The effect of temperature, oxygen, salinity, and nutrient enrichment on estuarine denitrification rates measured with a modified nitrogen gas flux technique. Estu Coast Shelf Sci. 1994;38:137–156. [Google Scholar]
- Oviatt CA. Impacts of nutrients on Narragansett Bay productivity: a gradient approach. In: Costa-Pierce BA, editor; Desbonnet A, editor. Science for Ecosystem-Based Management. New York, USA: Springer; 2008. pp. 523–543. [Google Scholar]
- Oviatt CA, Pilson MEQ, Nixon SW, Frithsen JB, Rudnick DT, Kelly JR, et al. Recovery of a polluted estuarine system – a mesocosm experiment. Mar Ecol Prog Ser. 1984;16:203–217. [Google Scholar]
- Oviatt CA, Keller A. Reed L. Annual primary production in Narragansett Bay with no bay-wide winter-spring phytoplankton bloom. Estu Coast Shelf Sci. 2002;54:1013–1026. [Google Scholar]
- Paerl HW, Fitzpatrick M. Bebout BM. Seasonal nitrogen fixation dynamics in a marine microbial mat: potential roles of cyanobacteria and microheterotrophs. Limnol Oceanogr. 1996;41:419–427. [Google Scholar]
- Pinckney JL, Paerl HW, Tester P. Richardson TL. The role of nutrient loading and eutrophication in estuarine ecology. Environ Health Persp. 2001;109:699–706. doi: 10.1289/ehp.01109s5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate JR. Biological nitrogen-fixation – fundamentals. Philos T Roy Soc B. 1982;296:375–385. [Google Scholar]
- Prell W, Saarman E, Murray DW. Deacutis CF. 2004. Summer season, nightime surveys of dissolved oxygen in upper Narragansett Bay (1999–2003) . URL http://www.geo.brown.edu/georesearch/insomniacs.
- Raymond J, Siefert JL, Staples CR. Blankenship RE. The natural history of nitrogen fixation. Mol Biol Evol. 2004;21:541–554. doi: 10.1093/molbev/msh047. [DOI] [PubMed] [Google Scholar]
- Saarman E, Prell W, Murray DW. Deacutis CF. Summer bottom water dissolved oxygen in upper Narragansett Bay. In: Costa-Pierce BA, editor; Desbonnet A, editor. Science for Ecosystem-Based Management. New York, USA: Springer; 2008. pp. 325–347. [Google Scholar]
- Saarman ET. 2002. Hypoxic conditions in Narragansett Bay during the summer of 2001. Senior Thesis, Brown University.
- Scavia D, Field JC, Boesch DF, Buddemeier RW, Burkett V, Cayan DR, et al. Climate change impacts on US coastal and marine ecosystems. Estuaries. 2002;25:149–164. [Google Scholar]
- Seitzinger SP. Nitrogen biogeochemistry in an unpolluted estuary: the importance of benthic denitrification. Mar Ecol Prog Ser. 1987;41:177–186. [Google Scholar]
- Seitzinger SP. Giblin AE. Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry. 1996;35:235–260. [Google Scholar]
- Seitzinger SP, Nixon SW. Pilson MEQ. Denitrification and nitrous-oxide production in a coastal marine ecosystem. Limnol Oceanogr. 1984;29:73–83. [Google Scholar]
- Short SM, Jenkins BD. Zehr JP. Spatial and temporal distribution of two diazotrophic bacteria in the Chesapeake Bay. Appl Environ Microbiol. 2004;70:2186–2192. doi: 10.1128/AEM.70.4.2186-2192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler FD. ZoBell CE. Nitrogen fixation by sulfate-reducing bacteria indicated by nitrogen/argon ratios. Science. 1951;113:511–513. doi: 10.1126/science.113.2940.511. [DOI] [PubMed] [Google Scholar]
- Steward GF, Jenkins BD, Ward BB. Zehr JP. Development and testing of a DNA macroarray to assess nitrogenase (nifH) gene diversity. Appl Environ Microbiol. 2004;70:1455–1465. doi: 10.1128/AEM.70.3.1455-1465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thioulouse J, Chessel D, Dole S. Olivier J-M. ADE-4: a multivariate analysis and graphical display software. Stat Comput. 1997;7:75–83. [Google Scholar]
- Thompson AW, Foster RA, Krupke A, Carter BJ, Musat N, Vaulot D, et al. Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science. 2012;337:1546–1550. doi: 10.1126/science.1222700. [DOI] [PubMed] [Google Scholar]
- Torsvik V, Sorheim R. Goksoyr J. Total bacterial diversity in soil and sediment communities – A review. J Ind Microbiol. 1996;17:170–178. [Google Scholar]
- Tripp HJ, Bench SR, Turk KA, Foster RA, Desany BA, Niazi F, et al. Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature. 2010;464:90–94. doi: 10.1038/nature08786. [DOI] [PubMed] [Google Scholar]
- Ward BB, Eveillard D, Kirshtein JD, Nelson JD, Voytek MA. Jackson GA. Ammonia-oxidizing bacterial community composition in estuarine and oceanic environments assessed using a functional gene microarray. Environ Microbiol. 2007;9:2522–2538. doi: 10.1111/j.1462-2920.2007.01371.x. [DOI] [PubMed] [Google Scholar]
- Welsh DT, Bourgues S, deWit R. Herbert RA. Seasonal variations in nitrogen-fixation (acetylene reduction) and sulphate-reduction rates in the rhizosphere of Zostera noltii: nitrogen fixation by sulphate reducing bacteria. Mar Biol. 1996;125:619–628. [Google Scholar]
- Widdel F. New types of acetate-oxidizing, sulfate-reducing Desulfobacter species, D-Hydrogenophilus sp-nov, D-Latus sp-nov, and D-Curvatus sp-nov. Arch Microbiol. 1987;148:286–291. [Google Scholar]
- Young JPW. Phylogenetic classification of nitrogen-fixing organisms. In: Evans HJ, editor; Stacey G, Burris RH, editors. Biological Nitrogen Fixation. New York, NY, USA: Chapman and Hall; 1992. pp. 43–86. [Google Scholar]
- Zehr JP. McReynolds LA. Use of degenerate oligonucleotides for amplification of the nifH gene from the marine cyanobacterium Trichodesmium-thiebautii. Appl Environ Microbiol. 1989;55:2522–2526. doi: 10.1128/aem.55.10.2522-2526.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehr JP, Jenkins BD, Short SM. Steward GF. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol. 2003;5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Gilbert D, Gooday AJ, Levin L, Naqvi SWA, Middelburg JJ, et al. Natural and human-induced hypoxia and consequences for coastal areas: synthesis and future development. Biogeosciences. 2010;7:1443–1467. [Google Scholar]
- Zhou JZ, Fries MR, Cheesanford JC. Tiedje JM. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol. 1995;45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]
- Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol R. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Maximum likelihood protein tree of expressed nirS sequences from sediment samples and nirS sequences observed from cultured organisms. The number inside the group indicates the number of total sequences within the grouping. Supporting Information Table S3 describes all sequences within a collapsed group. Bootstrap values (1000 replicates) > 50% are shown at respective nodes.
Fig. S2. Principal component analysis (PCA) of the relative distribution of OTUs, as designated by the nifH Maximum Likelihood tree (Fig. 3), for each site and season. These results are supported by Bray–Curtis values. The highest value (0.909) is for RIS2 Oct and RIS2 July, while the lowest value (0.087) is for PRE Oct and MP1 August.
Fig. S3. Percent of total expressed nifH sequences per site as a function increasing distance from site PRE separated by month sampled. Each color represents a cultivated species our environmental expressed sequences are related to as depicted in the nifH Maximum Likelihood tree (Fig. 3).
Fig. S4. Percent of total expressed nifH sequences per site as a function of depth and increasing distance from site PRE. Each color represents a cultivated species our environmental expressed sequences are related to as depicted in the nifH Maximum Likelihood tree (Fig. 3).
Table S1. Coordinates and water column depth of study sites with bottom water temperature during collection.
Table S2. List of nirS sequences (GenBank accession numbers) from mRNA in this study, closely related cultivated species and uncultivated species (Bulow et al., 2008) contained within groups NB1-NB9 (Supporting Information Fig. S1). Numbers in the parentheses indicate number of sequences collected at that time point.
Table S3. List of nifH sequences (GenBank accession numbers) from mRNA in this study and closely related cultivated species contained within groups NB1-NB3, NB5 and NB7-NB11 (Fig. 3).
Table S4. Fisher's alpha diversity index comparing diversity of nifH expressing groups, as designated by the nifH Maximum Likelihood tree (Fig. 3), between sites and month sampled.
Table S5. Statistical analyses of group NB3 abundance and expression using one-way anova and Tukey–Kramer HSD post-hoc tests. F ratio (df1 = degrees of freedom between groups, df2 = degrees of freedom within groups) and P values are denoted. Asterisk (*) indicates statistically significant P values (< 0.05). If the one-way anova test revealed statistical significance between samples compared, a Tukey–Kramer HSD post-hoc test was performed. Only the resultswitha P value < 0.05 are included underneath the corresponding anova test.
Table S6. Statistical analyses of group NB7 abundance and expression using one-way anova and Tukey–Kramer HSD post-hoc tests. F ratio (df1 = degrees of freedom between groups, df2 = degrees of freedom within groups) and P valuesare denoted. Asterisk (*) indicates statistically significant P values (< 0.05). If the one-way anova test revealed statistical significance between samples compared, a Tukey–Kramer HSD post-hoc test was performed. Only the results with a P value < 0.05 are included underneath the corresponding anova test.
Table S7. Primers and cycling conditions for PCR of nifH from environmental samples. All thermocycles included an initial 2 min denaturation at 94°C, and a final extension for 7 min at 72°C. *The outer reverse primer nifH3 was used to prime the RT reactions. **First round cycling conditions for RT products included 3 additional initial cycles with annealing steps at 44°C, 46°C, and 48°C.
Table S8. Primers and cycling conditions for PCR of nirS from environmental samples. All thermocycles included an initial 2 min denaturation at 94°C, and a final extension for 7 min at 72°C. *For the first 11 cycles the temperature decreased 0.5°C every cycle.
Table S9. Primers, probe and cycling conditions for quantitative PCR targeting the nifH gene of group NB3 and NB7 (Fig. 3). The quantitative PCR cycling conditions for both target groups included an initial 10 min denaturation at 95°C followed by 45 cycles of 95°C for 30 s and 60°C for 1 min.