Abstract
Youth with high callous-unemotional traits (CU) are at risk for early-onset and persistent conduct problems. Research suggests that there may be different developmental pathways to CU (genetic/constitutional vs environmental), and that the absence or presence of co-occurring internalizing problems is a key marker. However, it is unclear whether such a distinction is valid. Intermediate phenotypes such as DNA methylation, an epigenetic modification regulating gene expression, may help to clarify aetiological pathways. This is the first study to examine prospective inter-relationships between environmental risk (prenatal/postnatal) and DNA methylation (birth, age 7, age 9) in the prediction of CU (age 13), for youth low vs. high in internalizing problems. We focused on DNA methylation in the vicinity of the oxytocin receptor (OXTR) gene as it has been previously implicated in CU. Participants were 84 youth with early-onset and persistent conduct problems drawn from the Avon Longitudinal Study of Parents and Children. For youth with low internalizing problems (46%), we found that: (i) OXTR methylation at birth associated with higher CU (age 13) as well as decreased experience of victimization during childhood (birth – age 9), (ii) higher prenatal parental risks (maternal psychopathology, criminal behaviors, substance use) associated with higher OXTR methylation at birth, and (iii) OXTR methylation levels were more stable across time (birth – age 9). In contrast, for youth with high internalizing problems, CU was associated with prenatal risks of an interpersonal nature (i.e., intimate partner violence, family conflict) but not OXTR methylation. Findings support the existence of distinct developmental pathways to CU.
Keywords: DNA Methylation, Oxytocin Receptor gene, callous-unemotional traits, environmental risk, internalizing problems, ALSPAC
Introduction
In DSM 5, low prosocial emotions (i.e., callous-unemotional traits [CU] – lack of guilt, empathy), designate youth at risk for early-onset, persistent conduct problems (1, 2), and adult psychopathy (3). Yet, little is currently known about how CU traits develop. Research suggests that similar levels of CU may arise from distinct developmental circumstances (4, 5), and that the presence of co-occurring internalizing problems (anxiety/depression) is a key marker. More specifically, CU youth with low internalizing problems have been shown to experience less environmental risk (e.g. stressful life events) during childhood than those with high internalizing problems, despite showing similar levels of CU. This has led to speculations that CU may arise primarily from heritable/constitutional influences in the former group vs environmental influences in the latter group (6, 7). However, to date, few published biologically-informed longitudinal studies have tested whether such a distinction is valid.
Intermediate phenotypes, such as DNA methylation, may help clarify aetiological pathways to CU, for youth with low vs. high internalizing problems. DNA methylation is an epigenetic mechanism involved in transcriptional regulation (8) that can be influenced by the social environment (9). Recently, Dadds et al (10) reported that elevated DNA methylation in the vicinity of the oxytocin receptor gene (OXTR) is associated with (i) lower levels of circulating oxytocin and (ii) higher levels of CU. This is noteworthy, given that oxytocin (a hormone and neuropeptide) is known to modulate prosocial and affiliative behaviours that are impaired in CU youth, including empathy, emotional recognition, attachment and bonding (11–13). Also of interest, Dadds et al reported no association between levels of OXTR methylation and environmental risk exposure (measured as quality of family environment). The study, however, did not assess levels of co-occurring internalizing problems. As a result, it is not known whether OXTR methylation associates with CU and/or is influenced by environmental risks similarly for youth with low vs. high internalizing problems. Moreover, because the study was cross-sectional, it was not possible to pinpoint when in development environmental risks and/or alterations in OXTR DNA methylation may contribute to CU.
The present study is the first, to our knowledge, to make use of an integrative developmental model to examine, for youth low vs high in internalizing problems: (i) prospective interrelationships between environmental risk exposure (prenatal, postnatal) and OXTR methylation (birth, age 7, age 9); and (ii) the unique contribution of these factors to CU (age 13). Such a model not only has the capacity to further our understanding of how CU develop, but is also important for identifying the timing and targets of intervention to prevent the emergence of CU. We focussed specifically on youth following an early-onset, persistent trajectory of conduct problems, as these typically present with the highest CU levels (14, 15). Based on previous literature, we expected that youth with low vs high internalizing problems would show similar levels of CU, but that youth with low internalizing problems would experience less environmental risk. In order to clarify aetiological pathways to CU, we then tested whether associations between OXTR methylation, environmental risk and CU differed for youth with low vs high internalizing problems.
Materials and Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is an ongoing epidemiological study of children born from 14,541 pregnant women residing in Avon, UK, with an expected delivery date between April 1991 and December 1992 (85% of eligible population;16). Ethical approval was obtained from the ALSPAC Law and Ethics Committee as well as Local Research Committees. The sample is representative of the general population (17). Please note that the study website contains details of all the data that is available through a fully searchable data dictionary: http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/.
The Epigenetic Pathways to Conduct Problems Study consists of a subsample of youth (n = 339, 50% female) nested within a larger study of DNA methylation in ALSPAC (www.ariesepigenomics.org) who follow previously established conduct problem trajectories and have epigenetic data at two or more time points (birth, age 7, age 9). The trajectories have been identified and validated using General Mixture Model based on data drawn from the Strengths and Difficulties Questionnaire ‘Conduct Problem’ subscale (4–13 years; 18). The conduct problem trajectories are: (i) Low (25.4%), Childhood-limited (24.8%), (iii) Adolescent-onset (20.4%), and (iv) Early-onset persistent (29.5%). This subsample is comparable to the full trajectory sample (n = 7,218) in terms of environmental risk and psychiatric comorbidity (14). DNA methylation was available for 326 youth at birth, 332 at age 7, and 339 at age 9. Except for factor analyses, in which we used data from all youth, the present study only included youth following the early-onset, persistent conduct problem trajectory, who had complete data for CU and internalizing problems (total n = 84). Consistent with prior research (15), these youth showed the highest CU levels (M = 2.52, SD = .53) compared to youth in other trajectories (see Supplementary Information, SI1).
Measures
DNA methylation data (birth, age 7, age 9)
500ng genomic DNA from cord blood (birth) or peripheral blood (age 7 and 9) was bisulfite-converted using the EZ-DNA methylation kit (Zymo Research, Orange, CA, USA). The protocol followed manufacturer instructions using the recommended alternative incubation conditions for use with Illumina Infinium arrays. Illumina HumanMethylation450 BeadChips (Illumina, USA) were run following the manufacturer’s protocol with no modifications and arrays were scanned using an Illumina iScan (software version 3.3.28). Initial quality control of data generated was conducted using GenomeStudio (version 2011.1) to determine the status of staining, extension, hybridization, target removal, bisulfite conversion, specificity, non-polymorphic and negative controls. DNA methylation data was only available on samples that passed this stage. Samples were quantile normalised using the dasen function within the wateRmelon package (wateRmelon_1.0.3; 19) in R and batch corrected using the ComBat package (20).
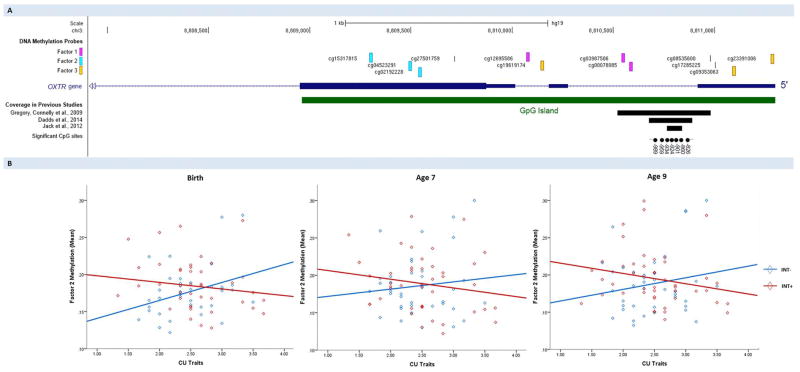
We extracted probes located within the OXTR CpG island (n = 12), as this area has been previously investigated by Dadds et al (10; see Figure 1, Panel A) and others (13, 21, 22), and shown to play a key role in modulating the transcriptional activity of OXTR (23). For each probe, methylation levels were indexed by beta values (corresponding to the ratio of methylated signal divided by the sum of the methylated and unmethylated signal). Factor analysis was used to reduce the 12 OXTR probes into a smaller set of factors, which accounted for shared variance between them (24). A 3-factor solution (see Figure 1A) showed the best fit to the data as well as good temporal stability. Full details of the factor analysis procedure and results are provided as an online supplement (SI 2–5). We present findings relating specifically to Factor 2 (3 probes located on Exon 2), because (i) Factor 1 did not associate with CU at any time point in either youth with low or high internalizing problems, and (ii) Factor 3 variance was not significant at any time point, thereby precluding the examination of associations with environmental risk and CU traits.
Figure 1.
Location of OXTR methylation probes and associations with CU traits. Panel A: Location of methylation probes within the CpG island (hg19; chr3:8808962–8811280) included in the study, and how these are grouped into factors (i.e. Factor 1: purple; Factor 2: blue; Factor 3: yellow). Black rectangles indicate the area of the OXTR CpG island investigated by previous research on DNA methylation. Significant CpG sites identified by these studies are shown as black circles. Numbering is relative to the translation start site (+1). Panel B: Association between Factor 2 methylation and CU traits for youth with low (INT−) vs high (INT+) co-occurring levels of internalizing problems, across time points.
Environmental risk (prenatal – age 9)
Cumulative environmental risk scores were created based on maternal reports. Risk items were organized into one of three developmental eras so as to coincide with the OXTR methylation data: (i) prenatal risks, (ii) early childhood risks (birth – age 7), and (iii) mid-childhood risks (age 8–9). For each developmental period, items were summed to create five conceptually distinct but related risk domains: (i) Life events (e.g. death in family, accident, illness), (ii) Contextual risks (e.g. poor housing conditions, financial problems), (iii) Parental risks (e.g. parental psychopathology, criminal involvement and substance use), (iv) Interpersonal risks (e.g. intimate partner violence, family conflict), and (v) Direct victimization (e.g. child bullied by peers or physically hurt ; available postnatally). Risk domains were positively and significantly correlated, both within and between developmental periods. We used confirmatory factor analyses (CFAs) to assess the internal reliability of the risk domains and to extract one global cumulative risk score for each developmental period, showing good model fit. Higher scores indicate greater environmental risk exposure. See SI 6–9 for full item descriptions, details of inter-correlations between risk domains and factor analysis fit indices.
Internalizing Problems (age 13)
Internalizing problems were assessed via maternal ratings using the well-validated Development and Well-Being Assessment (DAWBA). Computer-generated diagnostic ‘probability bands’ for anxiety and depression (six-level ordered-categorical variables ranging from <0.1% to >70% probability of DSM-IV psychiatric diagnosis) were averaged to create an interval level composite score. Description and validation of DAWBA bands are available elsewhere (25).
CU traits (age 13)
CU traits were measured using a 6-item questionnaire completed by mothers (26). Items were rated on a 3-point scale ranging from ‘not true’ to ‘certainly true’ (α = .79). These included: (i) Makes a good impression at first but people tend to see through him/her after they get to know him/her, (ii) Shallow or fast-changing emotions; (iii) Is usually genuinely sorry if s/he has hurt someone or acted badly (R); (iv) Can seem cold-blooded or callous; (v) Keeps promises (R); and (vi) Genuine in his/her expression of emotions (R). This measure has been shown to correlate highly with the CU scale of the Antisocial Process Screening Device (27).
Data Analysis
Youth were separated into two groups based on a median split on internalizing problems (median value = 1.00, range = 0 – 5), resulting in a low internalizing (INT−; n = 39) and a high internalizing (INT+; n = 45) group.
Step 1: Group characteristics
We compared INT− and INT+ on CU levels to ascertain that, consistent with previous research, they would show similar levels of CU even though different in levels of internalizing problems. We also compared the groups on environmental risk exposure (prenatal, early and mid-childhood), to establish whether INT− experience less environmental risk than INT+. We then assessed partial correlations (controlling for sex) between OXTR methylation, risk exposure and CU levels separately for each group, to assess whether associations between these variables differ for INT− vs INT+.
Step 2: Integrative developmental model
We estimated a path analytic model that incorporated environmental risk exposure, OXTR methylation and CU. This analytic strategy enabled us to establish for each group:
Longitudinal inter-relationships between environmental risk exposure and OXTR methylation (controlling for previous and subsequent measurements of each);
The unique timing of environmental risk and OXTR methylation effects on CU.
Sex was included as a covariate.
Analyses were performed in Mplus version 6.1.1 (28) using maximum likelihood estimation and bootstrapping. Bootstrapping is advantageous with small samples as it derives an approximation of the sampling distribution via repeated resampling of the available data to yield bias-corrected 95% confidence intervals. Significant associations were only presented if they survived bootstrapped confidence intervals. Model fit was first established using the chi-square statistic, which tests the difference between observed and expected covariance matrices, producing a non-significant value if this difference is close to zero (29). In the event of a significant chi-square value, we further examined relative fit indices that also test the discrepancy between the estimated model and the data, including the mean square error of approximation (RMSEA; acceptable fit =< .08), the Comparative Fit Index and Tucker-Lewis Index (CFI & TLI; acceptable fit => .90) (30). Nested model comparisons – using the Satorra-Bentler Scaled Chi-Square test (31) – were used to assess differences between the INT− and INT+.
Youth with scores greater than 3 standard deviations from the mean on any study variable were treated as outliers (n = 2) and their scores winsorized (i.e. transformed to match next highest value). This method uses censoring rather than exclusion, which is preferable with small sample size (32).
Results
Step 1: Group characteristics
Three results are highlighted. First, as seen in Figure 2A, INT − and INT + did not differ in CU. Second, the INT− group (vs INT+) experienced significantly lower levels of cumulative environmental risk (Figure 2B) during early childhood (birth – age 7; p = .008). Third, as seen in Table 1, correlations between environmental risk, CU and OXTR methylation showed that (a) Parental risks were positively correlated with DNA methylation across both INT− (at birth) and INT+ (at age 7) youth; and (b) OXTR methylation at birth was significantly correlated with higher CU, but only for INT−. This result is visually presented in Figure 1B, where it can be seen that, for INT− youth, OXTR methylation levels increased as CU traits became more severe (≤ 6% beta change). Post-hoc analysis confirmed that the association between OXTR methylation at birth and CU differed for the INT− vs INT+ group (i.e. significant interaction effect; p = .034). Interactions were not significant at age 7 and 9.
Figure 2.
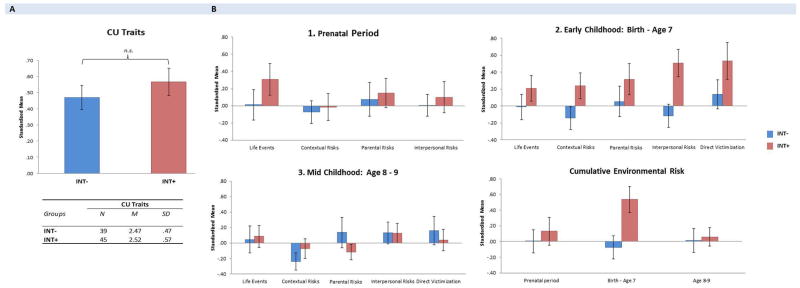
Levels of CU traits and environmental risk exposure across groups. Panel A: Standardized mean levels of CU and descriptives for INT− vs INT+. Panel B: Standardized mean levels of environmental risk exposure across individual domains and developmental eras for INT− vs INT+.
Table 1.
Associations between environmental risk exposure, CU traits and OXTR methylation across groups
| Environmental risk exposure |
OXTR Methylation
|
|||||
|---|---|---|---|---|---|---|
| INT−
|
INT+
|
|||||
| Birth | Age 7 | Age 9 | Birth | Age 7 | Age 9 | |
| Individual Risk Domains | ||||||
| Prenatal period | ||||||
| Life Events | −.01 | −.14 | −.09 | −.13 | −.06 | .13 |
| Contextual Risks | .14 | .06 | .18 | −.03 | −.08 | .20 |
| Parental Risks | .40* | .00 | .24 | .19 | .32* | .25 |
| Interpersonal Risks | .05 | −.15 | −.18 | −.13 | −.13 | −.01 |
| Early childhood: Birth - Age 7 | ||||||
| Life Events | --- | −.13 | −.24 | --- | −.03 | .08 |
| Contextual Risks | --- | −.30 | −.09 | --- | −.04 | .01 |
| Parental Risks | --- | .04 | .17 | --- | .25 | .29 |
| Interpersonal Risks | --- | −.18 | −.11 | --- | .04 | .02 |
| Direct Victimization | --- | −.18 | −.24 | --- | .06 | .02 |
| Mid childhood: Age 8 – 9 | ||||||
| Life Events | --- | --- | −.14 | --- | --- | .09 |
| Contextual Risks | --- | --- | .15 | --- | --- | −.15 |
| Parental Risks | --- | --- | .19 | --- | --- | .45** |
| Interpersonal Risks | --- | --- | .11 | --- | --- | −.45 |
| Direct Victimization | --- | --- | .11 | --- | --- | −.08 |
| Cumulative Risk Composites | ||||||
| Prenatal period | .23 | −.07 | .07 | −.02 | −.08 | .04 |
| Early childhood: Birth - Age 7 | --- | −.21 | −.14 | --- | .07 | .09 |
| Mid childhood: Age 8 – 9 | --- | --- | −.02 | --- | --- | .01 |
N.b.
p < .001;
p < .01;
p < .05. All associations control for sex.
Step 2: Integrative developmental model
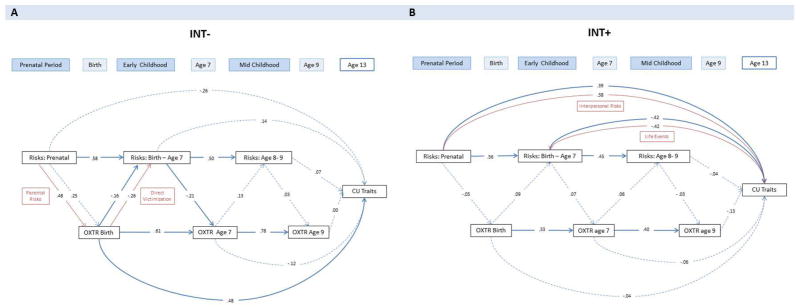
Figure 3 illustrates the integrative developmental model for INT− vs INT+. Model fit was good: X2 (12) =20.78, p = .06.
Figure 3.
Integrative developmental model. Panel A: INT− group. Panel B: INT+ group. Single arrowed lines indicate standardized path coefficients that survived bootstrap-corrected confidence intervals (i.e. significant path). Red arrows show significant individual risk domains based on post-hoc analyses. Dotted arrowed lines indicate non-significant paths. Population effect sizes are interpreted using the standardized estimates (Std. B) following Cohen’s guidelines: an effect of .10 is small effect, an effect of .24 is a medium effect, and an effect of .37 is a large effect.
For the INT− (Figure 3A), three results are highlighted. First, higher OXTR methylation at birth was prospectively associated with higher CU at age 13, and this association was stronger for INT− vs INT+ (Δχ2[1] = 4.78, p = .03). Higher OXTR methylation at birth also associated with lower risk exposure during early childhood (birth – age 7) – specifically direct victimization (e.g., bullying by peers, physical harm by adults) – and this association was stronger for INT− vs INT+ (3 Δχ2[1] = 7.11, p = .01). In other words, for INT−, higher OXTR methylation at birth associated with both (i) higher CU and (ii) less subsequent victimization, suggestive of what we refer to as an ‘evocative epigenetic-environment correlation’. Second, although no effect of overall prenatal environmental risk on OXTR methylation at birth was observed, one specific risk domain, prenatal parental risks, was found to be significantly associated with OXTR methylation levels at birth. This association was stronger for INT− vs INT+ (Δχ2[1] = 4.07, p = .04). Third, temporal stability between birth and age 9 was greater in magnitude for the INT− vs. INT+ (Δχ2[2] = 12.00, p = .001).
For the INT+ (Figure 3B), three results are highlighted. First, OXTR methylation did not significantly associate with CU (see above difference test). Second, higher prenatal risks associated with higher CU. This association was specific to interpersonal risks (e.g., intimate partner violence, family conflict), and was stronger for the INT+ vs. INT− (Δχ2 [1] = 7.89, p = .005). Third, postnatal risks – specifically life events (e.g., death of relative, accidents, illness) – associated with lower CU, and this association was stronger for INT+ vs. INT− (Δχ2 [1] = 1.48, p = .001).
Follow up analyses
We conducted two follow-up analyses in light of the findings above. First, we unpacked the Factor 2 methylation-CU association at birth in the INT− group. We examined the three probes included in Factor 2 individually. DNA methylation at birth prospectively associated with CU traits for probe cg04523291 (Std B = .46, B = 4.61; bootstrapped 95% CI = .32 – 8.52) and probe cg15317815 (Std B = .46, B = 3.97; bootstrapped 95% CI = .76 – 8.05), but not for probe cg02192228 (Std B = .34, B = 4.10; bootstrapped 95% CI = −2.60 – 10.46).
Second, we investigated the potential influence of genetic variation on DNA methylation and CU (33, 34). We selected 4 SNPs (rs2301261, rs237915, rs4564970, and rs4686302) that were (a) available in ALSPAC via GWAS (for details see 35), (ii) located within the CpG island examined in the present study, and (iii) have been examined in previous studies in relation to phenotypes relevant to CU (36–39). None of these SNPs were found to associate with DNA methylation levels, CU or the INT−/INT+ groups (full details provided in SI9–10).
Discussion
Our goal was to examine whether aetiological pathways to CU differed for INT − vs INT + youth, using repeated measures of environmental risk exposure and OXTR methylation. Consistent with our hypothesis, INT− and INT+ did not differ in CU levels, but INT-experienced lower risk exposure than INT+. Our integrative developmental model showed that, for INT− youth: (i) OXTR methylation at birth associated with higher CU (age 13), as well as decreased experience of victimization during childhood (birth – age 7); (ii) higher prenatal parental risks (e.g. maternal psychopathology, criminal behavior, substance use) associated with higher OXTR methylation at birth; and (iii) temporal stability of OXTR methylation was greater (birth – age 9) than for INT+. In contrast, for INT+ youth, OXTR methylation did not associate with CU. Rather, prenatal risks of an interpersonal nature (e.g., intimate partner violence and family conflict) associated with higher CU levels. Findings support the existence of distinct pathways to CU.
For INT− youth, we highlight three main findings. First, we support Dadds et al’s (10) study showing an association between higher OXTR methylation and higher CU. Of interest, we show that this association is specific to INT− youth, and occurred at birth, whereas Dadds et al found an association in older youth. Together, these findings may suggest that there are multiple developmental periods of vulnerability for CU via OXTR methylation. In addition, it is worth noting that our methylation probes were different from Dadds et al’s, which raises the possibility that multiple sites across the CpG island mat be associated with CU. We also found that, in INT− youth, higher OXTR methylation at birth was associated with lower levels of direct victimization during childhood, suggestive of an ‘evocative epigenetic-environment correlation’. More specifically, this finding parallels evocative gene-environment correlation studies showing that a child’s genetic characteristics can influence his or her environment (40–43). Importantly, this finding may lend insight into how INT− youth experience less victimization than INT+ youth (6, 7). In light of prior evidence showing that (i) higher OXTR methylation is associated with lower circulating levels of oxytocin (10) and (ii) lower levels of oxytocin are associated with deficits in prosocial and affiliative behaviours (12, 13), it is possible that INT− youth develop socio-emotional characteristics that actively discourage victimizing behaviour from others (e.g. showing lower empathy and trust), and potentially fit more of a ‘bullying-only’ profile (44, 45).Moreover, it is possible that higher OXTR methylation ‘protects’ INT− youth from developing internalizing problems, which act as both an antecedent and maintaining factor for victimization (46). More generally, this effect may lend support to the notion that epigenetic mechanisms can prepare children to cope with forthcoming environmental challenges (47). The second finding is that one specific domain of environmental risk, prenatal parental risks, are associated with higher OXTR methylation at birth. It is unclear whether this association is causal (e.g., a direct impact of parental risks on fetal development) or correlational (e.g., reflecting genetic confounding; 48, 49). Third, OXTR methylation in this group was found to have greater temporal stability (vs. INT+) from birth to age 9. The finding that OXTR methylation is more stable and associated with CU exclusively in INT− raises the question of whether this group may respond differently to interventions, such as intranasal oxytocin administration, compared to the INT+.
A different pattern of results emerged for INT+ youth. First and foremost, OXTR methylation did not associate with CU (at any time point). In addition, consistent with previous findings (6, 7, 50), this group experienced higher levels of environmental risk exposure than the INT−. Of interest, prenatal interpersonal risks (e.g. intimate partner violence, family conflict) prospectively associated with higher CU. Although speculative, this effect may be mediated by maternal stress, which has been associated with both internalizing problems (51) and CU (15) within ALSPAC. A counter-intuitive finding was that postnatal risks (i.e., adverse life events) associated with lower CU. As with the above results, this finding will need replication, particularly within high-risk samples.
The present findings should be interpreted in light of a number of limitations. First, the study focussed specifically on DNA methylation of annotated probes located within the CpG island of OXTR and it is likely that differences across groups may be found in other genes (e.g. glucocorticoid or serotonergic pathways; 52). While an epigenome-wide approach would have enabled us to examine group differences in DNA methylation across the genome (53), at present it is not plausible to build integrative developmental models, such as the one used here (i.e., with repeated methylation data included both as an independent variable and as a dependent variable), using a genome-wide approach, due to the computational burden of applying this method to hundreds of thousands of variables. Second, findings are based on a relatively small sample of youth with limited statistical power in view of the complex longitudinal models tested. Consequently, the present findings should be interpreted as well-grounded hypotheses for further examination in larger longitudinal studies. The use of larger studies may help to clarify the present findings, including (i) which aspects of prenatal parental risks associate with OXTR methylation at birth (e.g. parental criminality vs substance use) in INT− youth; (ii) what biological mechanisms mediate the effect of prenatal environmental risk exposure on CU for INT+ youth; and (iii) why we find a negative association between postnatal environmental risks and CU traits in the INT+ group. Third, the current study was based on a community sample of youth with relatively low rates of internalizing problems. This resulted in the classification of groups based on a median split. In future, it will be important to replicate developmental pathways to CU in high-risk youth who show more severe internalizing problems (e.g. young offenders, psychiatric inpatients). Finally, although the current study did not find that DNA sequence-based variation in OXTR influenced DNA methylation or CU levels, analyses were limited to four SNPs. In future, it will be necessary to examine additional local (cis) and distant (trans) SNPs to better establish the relationship between genetic variation across the genome and methylation at the OXTR locus. More generally, the inclusion of genetic information may help further elucidate aetiological pathways to CU, for INT− vs. INT+ youth.
In summary, this is the first longitudinal study to examine the role of environmental risk exposure and OXTR methylation in the development of CU for INT− vs INT+ youth. Our findings show that: (i) OXTR methylation (at birth) prospectively associated with CU only for INT− youth; (ii) for this group, OXTR methylation (at birth) also associated with reduced experience of direct victimization (by peers and adults), which, to our knowledge, provides the first example (in humans) of what we refer to as an ‘evocative epigenetic-environment association’; (iii) OXTR methylation had greater temporal stability for the INT− vs. INT+ youth; and (iv) environmental risk (prenatal and postnatal) associated with CU only for the INT+ youth. As a result, the present study supports the existence of distinct developmental pathways to CU. Findings also identify targets and windows of opportunity for prevention as well as highlighting the salience of the prenatal period, both for INT− and INT+. More generally, the present study illustrates an innovative way to integrate longitudinal environmental and epigenetic information in the study of psychiatric disorders.
Supplementary Material
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. With regard to the ALSPAC DNA methylation, we thank all involved, particularly the laboratory scientists and bioinformaticians who contributed considerable time and expertise to the data in this paper. We also thank Prof Dieter Wolke for his helpful comments on a previous version of this manuscript. The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This work was funded by the National Institute of Child and Human Development grant (R01HD068437). JBP was supported by a Marie Curie Intra-European Fellowship (N° 330699).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Dandreaux DM, Frick PJ. Developmental pathways to conduct problems: a further test of the childhood and adolescent-onset distinction. Journal of abnormal child psychology. 2009 Apr;37(3):375–85. doi: 10.1007/s10802-008-9261-5. [DOI] [PubMed] [Google Scholar]
- 2.Rowe R, Maughan B, Moran P, Ford T, Briskman J, Goodman R. The role of callous and unemotional traits in the diagnosis of conduct disorder. Journal of Child Psychology and Psychiatry. 2010;51(6):688–95. doi: 10.1111/j.1469-7610.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 3.Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology. 2009;21(Special Issue 04):1111–31. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- 4.Kahn RE, Frick PJ, Youngstrom EA, Kogos Youngstrom J, Feeny NC, Findling RL. Distinguishing primary and secondary variants of callous-unemotional traits among adolescents in a clinic-referred sample. Psychological assessment. 2013 Sep;25(3):966–78. doi: 10.1037/a0032880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimonis ER, Frick PJ, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Dev Psychopathol. 2012 Aug;24(3):1091–103. doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- 6.Tatar JR, Cauffman E, Kimonis ER, Skeem JL. Victimization History and Posttraumatic Stress: An Analysis of Psychopathy Variants in Male Juvenile Offenders. Journal of Child & Adolescent Trauma. 2012 Apr 01;5(2):102–13. [Google Scholar]
- 7.Vaughn MG, Edens JF, Howard MO, Smith ST. An Investigation of Primary and Secondary Psychopathy in a Statewide Sample of Incarcerated Youth. Youth Violence and Juvenile Justice. 2009 Jul 1;7(3):172–88. [Google Scholar]
- 8.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33:245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 9.Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental psychobiology. 2010 May;52(4):299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- 10.Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, et al. Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Dev Psychopathol. 2014 Feb;26(1):33–40. doi: 10.1017/S0954579413000497. [DOI] [PubMed] [Google Scholar]
- 11.Frick PJ, Ray JV, Thornton LC, Kahn RE. Annual Research Review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of child psychology and psychiatry, and allied disciplines. 2013 Oct 9; doi: 10.1111/jcpp.12152. [DOI] [PubMed] [Google Scholar]
- 12.Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010 Mar 25;65(6):768–79. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumsta R, Hummel E, Chen FS, Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Frontiers in neuroscience. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker ED, Oliver BR, Maughan B. Co-occurring problems of early onset persistent, childhood limited, and adolescent onset conduct problem youth. Journal of child psychology and psychiatry, and allied disciplines. 2010 Nov;51(11):1217–26. doi: 10.1111/j.1469-7610.2010.02240.x. [DOI] [PubMed] [Google Scholar]
- 15.Barker ED, Oliver BR, Viding E, Salekin RT, Maughan B. The impact of prenatal maternal risk, fearless temperament and early parenting on adolescent callous-unemotional traits: a 14-year longitudinal investigation. Journal of child psychology and psychiatry, and allied disciplines. 2011 Aug;52(8):878–88. doi: 10.1111/j.1469-7610.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 16.Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, et al. Cohort Profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. International journal of epidemiology. 2013 Feb;42(1):97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, et al. Cohort Profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. International journal of epidemiology. 2013 Feb;42(1):111–27. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker ED, Maughan B. Differentiating early-onset persistent versus childhood-limited conduct problem youth. The American journal of psychiatry. 2009 Aug;166(8):900–8. doi: 10.1176/appi.ajp.2009.08121770. [DOI] [PubMed] [Google Scholar]
- 19.Pidsley R, CCYW, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC genomics. 2013;14:293. doi: 10.1186/1471-2164-14-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007 Jan;8(1):118–27. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 21.Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC medicine. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jack A, Connelly JJ, Morris JP. DNA methylation of the oxytocin receptor gene predicts neural response to ambiguous social stimuli. Frontiers in human neuroscience. 2012;6:280. doi: 10.3389/fnhum.2012.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusui C, Kimura T, Ogita K, Nakamura H, Matsumura Y, Koyama M, et al. DNA methylation of the human oxytocin receptor gene promoter regulates tissue-specific gene suppression. Biochemical and biophysical research communications. 2001 Dec 7;289(3):681–6. doi: 10.1006/bbrc.2001.6024. [DOI] [PubMed] [Google Scholar]
- 24.Thompson B. Exploratory and confirmatory factor analysis: Understanding concepts and applications. Washington, DC, US: American Psychological Association; 2004. [Google Scholar]
- 25.Goodman A, Heiervang E, Collishaw S, Goodman R. The ‘DAWBA bands’ as an ordered-categorical measure of child mental health: description and validation in British and Norwegian samples. Social psychiatry and psychiatric epidemiology. 2011 Jun;46(6):521–32. doi: 10.1007/s00127-010-0219-x. [DOI] [PubMed] [Google Scholar]
- 26.Moran P, Ford T, Butler G, Goodman R. Callous and unemotional traits in children and adolescents living in Great Britain. The British journal of psychiatry : the journal of mental science. 2008 Jan;192(1):65–6. doi: 10.1192/bjp.bp.106.034876. Epub 2008/01/05. eng. [DOI] [PubMed] [Google Scholar]
- 27.Moran P, Rowe R, Flach C, Briskman J, Ford T, Maughan B, et al. Predictive value of callous-unemotional traits in a large community sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2009 Nov;48(11):1079–84. doi: 10.1097/CHI.0b013e3181b766ab. [DOI] [PubMed] [Google Scholar]
- 28.Muthen LK, Muthen BO. MPLUS user’s guide, 1998–2010. 6. Los Angeles, CA: Muthen & Muthen; 2011. [Google Scholar]
- 29.Kline RB. Principles and practice of structural equation modeling. 3. New York, NY: The Guildford Press; 2010. [Google Scholar]
- 30.Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999 Jan 01;6(1):1–55. [Google Scholar]
- 31.Satorra A. Scaled and Adjusted Restricted Tests in Multi-Sample Analysis of Moment Structures. In: Heijmans RDH, Pollock DSG, Satorra A, editors. Innovations in Multivariate Statistical Analysis. Advanced Studies in Theoretical and Applied Econometrics. Vol. 36. Springer; US: 2000. pp. 233–47. [Google Scholar]
- 32.Sheskin D. The handbook of parametric and nonparametric statistical procedures. Boca Raton, FL: CRC Press; 2003. [Google Scholar]
- 33.Meaburn EL, Schalkwyk LC, Mill J. Allele-specific methylation in the human genome: implications for genetic studies of complex disease. Epigenetics : official journal of the DNA Methylation Society. 2010 Oct 1;5(7):578–82. doi: 10.4161/epi.5.7.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schalkwyk LC, Meaburn EL, Smith R, Dempster EL, Jeffries AR, Davies MN, et al. Allelic skewing of DNA methylation is widespread across the genome. American journal of human genetics. 2010 Feb 12;86(2):196–212. doi: 10.1016/j.ajhg.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eicher JD, Powers NR, Miller LL, Akshoomoff N, Amaral DG, Bloss CS, et al. Genome-wide association study of shared components of reading disability and language impairment. Genes, brain, and behavior. 2013 Nov;12(8):792–801. doi: 10.1111/gbb.12085. Epub 2013/09/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson A, Westberg L, Sandnabba K, Jern P, Salo B, Santtila P. Associations between oxytocin receptor gene (OXTR) polymorphisms and self-reported aggressive behavior and anger: Interactions with alcohol consumption. Psychoneuroendocrinology. 2012 Sep;37(9):1546–56. doi: 10.1016/j.psyneuen.2012.02.009. Epub 2012/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 37.Nyffeler J, Walitza S, Bobrowski E, Gundelfinger R, Grunblatt E. Association study in siblings and case-controls of serotonin- and oxytocin-related genes with high functioning autism. Journal of Molecular Psychiatry. 2014;2(1):1. doi: 10.1186/2049-9256-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loth E, Poline JB, Thyreau B, Jia T, Tao C, Lourdusamy A, et al. Oxytocin Receptor Genotype Modulates Ventral Striatal Activity to Social Cues and Response to Stressful Life Events. Biol Psychiatry. 2013 Oct 8; doi: 10.1016/j.biopsych.2013.07.043. Epub 2013/10/15. Eng. [DOI] [PubMed] [Google Scholar]
- 39.Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. Journal of affective disorders. 2012 May;138(3):468–72. doi: 10.1016/j.jad.2012.01.009. Epub 2012/02/24. eng. [DOI] [PubMed] [Google Scholar]
- 40.Button TM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: A twin study. Journal of abnormal psychology. 2007 Aug;116(3):554–64. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- 41.Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular psychiatry. 2007 May;12(5):432–42. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: a developmental twin study of peer-group deviance. Archives of general psychiatry. 2007 Aug;64(8):958–65. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAdams TA, Gregory AM, Eley TC. Genes of experience: explaining the heritability of putative environmental variables through their association with behavioural and emotional traits. Behavior genetics. 2013 Jul;43(4):314–28. doi: 10.1007/s10519-013-9591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker ED, Arseneault L, Brendgen M, Fontaine N, Maughan B. Joint development of bullying and victimization in adolescence: relations to delinquency and self-harm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008 Sep;47(9):1030–8. doi: 10.1097/CHI.ObO13e31817eec98. [DOI] [PubMed] [Google Scholar]
- 45.Barker ED, Salekin RT. Irritable oppositional defiance and callous unemotional traits: is the association partially explained by peer victimization? Journal of child psychology and psychiatry, and allied disciplines. 2012 Nov;53(11):1167–75. doi: 10.1111/j.1469-7610.2012.02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reijntjes A, Kamphuis JH, Prinzie P, Telch MJ. Peer victimization and internalizing problems in children: a meta-analysis of longitudinal studies. Child abuse & neglect. 2010 Apr;34(4):244–52. doi: 10.1016/j.chiabu.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in molecular medicine. 2007 Jul;13(7):269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Johannes F, Porcher E, Teixeira FK, Saliba-Colombani V, Simon M, Agier N, et al. Assessing the Impact of Transgenerational Epigenetic Variation on Complex Traits. PLoS Genet. 2009;5(6):e1000530. doi: 10.1371/journal.pgen.1000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards EJ. Inherited epigenetic variation--revisiting soft inheritance. Nature reviews Genetics. 2006 May;7(5):395–401. doi: 10.1038/nrg1834. [DOI] [PubMed] [Google Scholar]
- 50.Skeem JL, Poythress N, Edens JF, Lilienfeld SO, Cale EM. Psychopathic personality or personalities? Exploring potential variants of psychopathy and their implications for risk assessment. Aggression and Violent Behavior. 2003;8(5):513–46. [Google Scholar]
- 51.O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. The British journal of psychiatry : the journal of mental science. 2002 Jun;180:502–8. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- 52.Sadeh N, Javdani S, Jackson JJ, Reynolds EK, Potenza MN, Gelernter J, et al. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. Journal of abnormal psychology. 2010 Aug;119(3):604–9. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craig IW, Halton KE. Genetics of human aggressive behaviour. Human genetics. 2009 Jul;126(1):101–13. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.