Abstract
There is growing evidence that lesions of the anterior thalamic nuclei cause long-lasting intrinsic changes to retrosplenial cortex, with the potential to alter its functional properties. The present study had two goals. The first was to identify the pattern of changes in eight markers, as measured by in-situ hydridisation, in the granular retrosplenial cortex (area Rgb) following anterior thalamic lesions. The second was to use retrograde trans-neuronal tracing methods to identify the potential repercussions of intrinsic changes within granular retrosplenial cortex. In Experiment 1, adult rats received unilateral lesions of the anterior thalamic nuclei and were perfused four weeks later. Of the eight markers, four (c-fos, zif268, 5ht2rc, kcnab2) showed a very similar pattern of change, with decreased levels in superficial retrosplenial cortex (lamina II) in the ipsilateral hemisphere but little or no change in deeper layers (lamina V). A fifth marker (cox6b) showed a shift in activity levels in the opposite direction to the previous four markers. Three other markers (cox6a1, CD74, ncs-1) did not appear to change activity levels after surgery. The predominant pattern of change, a decrease in superficial cortical activity, points to potential alterations in plasticity and metabolism. In Experiment 2, wheat germ agglutin (WGA) was injected into the anterior thalamic nuclei in rats given different survival times, sometimes in combination with the retrograde, fluorescent tracer, Fast Blue. Dense aggregations of retrogradely labeled cells were always found in lamina VI of granular retrosplenial cortex, but additional labeled cells in lamina II were only found: 1) in WGA cases, i.e. never after Fast Blue injections, and 2) after longer WGA survival times (three days). These layer II Rgb cells are likely to have been trans-neuronally labeled, revealing a pathway from lamina II of Rgb to those deeper retrosplenial cells that project directly to the anterior thalamic nuclei.
Keywords: Alzheimer’s disease, cingulate cortex, pathology, plasticity, rat, thalamus
Introduction
The anterior thalamic nuclei have dense, reciprocal connections with the retrosplenial cortex (Vogt et al., 1981; van Groen et al., 1993; Shibata, 1993, 1998), a part of the posterior cingulate region in the primate brain. In the rat brain, the retrosplenial cortex comprises the entire posterior cingulate region (Vogt and Peters, 1981). Both electrophysiological studies with rabbits (Gabriel et al., 1983; Gabriel, 1993) and cross-lesion disconnection studies with rats (Sutherland and Hoesing, 1993) show that retrosplenial cortex function partially depends on the integrity of the anterior thalamic nuclei (ATN). Interest in this relationship is stimulated by growing evidence that both regions are vital for memory (Maguire, 2001; Aggleton and Brown, 2006; Vann et al., 2009). One possible component of this interdependency is that anterior thalamic lesions disrupt plasticity in the retrosplenial cortex. Support comes from the recent finding that retrosplenial brain slices taken from rats with anterior thalamic lesions show a loss of long-term depression (Garden et al., 2009), though these disruptions were lamina specific as the loss of plasticity was restricted to the superficial layers of the retrosplenial cortex (Garden et al., 2009). The present study sought to examine the nature of this thalamic – cortical interdependency in two ways. First, the impact of unilateral anterior thalamic lesions upon gene activity was measured in the retrosplenial cortex using methods that permit cortical lamina to be compared. Second, trans-neuronal mapping methods were used to determine how different laminae within the retrosplenial cortex project directly and indirectly to the anterior thalamic nuclei.
It has been shown that large, unilateral thalamic lesions in rats produce a decrease in a marker of cell metabolism, cytochrome oxidase, in granular retrosplenial cortex (van Groen et al., 1993). The same thalamic lesions also reduced levels of cholinergic, serotonergic, and noradrenergic markers in the retrosplenial cortex (van Groen et al., 1993). In every case, the most pronounced changes were in superficial cortical layers. Limitations with this study include the fact that the lesions were very extensive, involving multiple thalamic nuclei, and that some of the changes in neurotransmitter markers could have reflected the degeneration of presynaptic terminals (van Groen et al., 1993). The anterior thalamic – retrosplenial relationship has recently been re-examined in rat studies that involve much more discrete ATN lesions (Jenkins et al., 2004, Poirier et al., 2008; Poirier and Aggleton, 2009). Immunohistochemical mapping of the protein products of two immediate-early genes (IEGs), c-Fos and Zif268, revealed that anterior thalamic lesions are sufficient to produce a dramatic loss of IEG activity in retrosplenial neurons. For both IEGs, these decreases were most prominent in the superficial layers of the granular retrosplenial cortex, though there was no evidence of a reduction in retrosplenial neuronal numbers (Jenkins et al., 2004; Poirier et al., 2008; Poirier and Aggleton, 2009). The most extreme IEG changes were for c-Fos, with reductions of up to 90% of all c-Fos-positive cells in lamina II and upper lamina III in granular retrosplenial cortex.
To provide a broader test of these ATN lesion effects, Experiment 1 used in situ hybridization to measure the status of eight target markers in the granular retrosplenial cortex, with particular emphasis on comparisons between superficial (lamina II) and deep (lamina V) cortical laminae. Additional interest concerned the relationship between the current findings and those from a microarray study into the effects of unilateral ATN lesions upon granular retrosplenial tissue (Poirier et al., 2008). While numerous changes in retrosplenial gene activity were recorded in that study (Poirier et al., 2008), especially for genes associated with plasticity and energy metabolism, the microarray analysis concerned tissue from across all cell layers. Consequently, it was not possible to compare cortical lamina.
Experiment 1 examined the impact of unilateral ATN lesions as the thalamic projections to the retrosplenial cortex remain ipsilateral (van Groen et al. 1993; Wang et al., 2001), making it possible to compare across hemispheres in the same animals. Cytotoxic lesions were made with the goal of minimizing white matter damage. Target genes were selected on the basis of: 1) previous evidence from other techniques (e.g. microarray) that their activity might be compromised by ATN lesions (e.g. c-fos,, cox6a1, cox6b, 5htr2c, cd74, ncs-1, kcnab2 (Poirier et al., 2008), 2) prior evidence of an involvement in excitability or plasticity [e.g. c-fos, zif268, 5htr2c (Carr et al., 2002; Boothman et al., 2006; Liu et al., 2007), cd74 (Håvik et al., 2007), ncs-1 (Génin et al., 2001), kcnab2 (Rettig et al., 1994; Salinas et al., 1997; Richardson et al., 2000)], 3) a likely involvement in retrosplenial energy metabolism [e.g. cox6a1, cox6b, (Valla et al., 2001; Liang et al., 2008; Schneider et al., 2008), and 4) evidence in Alzheimer’s disease of alterations in gene expression [cox6a1, cd74 (Kong et al., 2009)], especially those genes linked to changes within the posterior cingulate cortex [cox6a1, cox6b (Liang et al., 2008)].
Experiment 2 also sought to examine the nature of the relationship between the retrosplenial cortex and the anterior thalamic nuclei, but used anatomical tracing techniques. It is well known that there are dense projections from lamina VI of the retrosplenial cortex to the anterior thalamic nuclei (Van Groen et al., 1993; Shibata, 1998; van Groen and Wyss, 2003). The issue was whether it is possible to identify the inputs to these specific cells in lamina VI and, in particular, whether intrinsic projections from within the retrosplenial cortex might indirectly innervate the anterior thalamic nuclei. Attention was focused on lamina II cells in granular retrosplenial cortex as they principally project within the retrosplenial cortex (Sripanidkulchai and Wyss, 1987; van Groen and Wyss, 1990, 2003), i.e. they have intrinsic connections. Accordingly, wheat germ agglutin (WGA) was injected into the anterior thalamic nuclei and sufficient survival time was allowed for retrograde trans-neuronal transport (Takeuchi et al., 1985).
Experiment 1: Granular retrosplenial cortex (Rgb) changes after anterior thalamic lesions measured by in situ hybridization
Methods
Subjects
Twelve male rats (Dark Agouti strain, Harlan, Bicester,UK), weighing between 200 and 225 g at the time of surgery were all housed in pairs under a 13 hour light/11 hour dark cycle with ad libitum access to food and water. Each animal was extensively habituated to handling. All experiments were carried out in accordance with UK Animals (Scientific Procedures) Act, 1986, and associated guidelines, and all efforts were made to minimize animal suffering.
Surgery
Excitotoxic, unilateral lesions were placed in the anterior thalamic nuclei. Animals (n=12) were first anesthetized with an intraperitoneal injection of pentobarbitone sodium (Sagatal, 75mg/kg) and then placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Chloramphenicol eye ointment (Martindale Pharmaceuticals, Romford, UK) was topically applied to the eyes to protect the cornea. A craniotomy was made over both hemispheres. Excitotoxic lesions were produced by injecting 0.20 μl of N-methyl-D-aspartate (NMDA; Sigma Chemicals, Poole, UK; 0.12 M in phosphate buffered saline (PBS), pH 7.2) into two sites in the same hemisphere using a 1 μl syringe (Hamilton, Switzerland).
The stereotaxic coordinates were as follows: anterio-posterior, −0.5 from bregma; medio-lateral, 1.0 and 1.7 from the midline; dorso-ventral, −6.3 and −5.7 from the top of the dura for the medial and lateral injections, respectively. The incisor bar was set at +5.0. Antibiotic powder (Aureomycin, Fort Dodge Animal Health, Southampton, UK) was subsequently applied topically, and all rats also received a 5 ml subcutaneous injection of glucose saline. Paracetamol was dissolved in the rats’ drinking water, and they were observed daily until recovery. In five rats, the lesion was placed in the right hemisphere, and in six rats the lesion was in the left hemisphere.
Anatomical targets and nomenclature
In the rat brain, there is no posterior cingulate area 23 or area 31, so that the retrosplenial cortex (areas 29 and 30) occupies the entire posterior cingulate region. The rat retrosplenial cortex comprises two major subregions (Vogt and Peters, 1981; Anzalone et al., 2009): granular (area 29) and dysgranular (area 30). The granular retrosplenial cortex (area 29) can be subdivided (Van Groen and Wyss 1990, 1992, 2003) into a caudal area a (Rga) and a more rostral and dorsal area b (Rgb). Area Rgb was the principal region of interest in the present study for four reasons. First, Rgb receives dense inputs from the anterior thalamic nuclei (Vogt et al., 1981; Van Groen et al., 1993). Second, the most prominent retrosplenial IEG changes have been observed in this region after anterior thalamic lesions (Jenkins et al., 2004). Third, microarray analyses of retrosplenial cortex following anterior thalamic lesions have targeted tissue from this subregion (Poirier et al., 2008). Fourth, lesions of granular retrosplenial cortex in rats are sufficient to impair learning and memory (Van Groen et al., 2004; Pothuizen et al., 2010).
Grain counts focused on lamina II and lamina V of area Rgb. Lamina II was selected because previous studies have found this lamina within the granular retrosplenial cortex to be the most consistently disrupted by anterior thalamic lesions (Jenkins et al., 2004; Garden et al., 2009; Poirier and Aggleton, 2009). Cells in lamina V are also of particular interest as they are the major source of extrinsic projections from Rgb (Sripanidkulchai and Wyss, 1987; Van Groen and Wyss, 2003). The principal exception concerns the projections from Rgb to the anterior thalamic nuclei, which arise from lamina VI (Sripanidkulchai and Wyss, 1987; Van Groen and Wyss, 2003). As the anterior thalamic nuclei have been lesioned in this preparation, it is the other, extrinsic projections of Rgb that are potentially of greatest significance.
Tissue preparation
Four weeks after surgery, the rats were killed 30 min after individual exposure to a novel environment (new room and visual stimuli in a cage measuring 56 × 39 × 19 cm, with grid floor) by CO2 exposure and decapitation. Whole brains were rapidly removed, placed in a Perspex rat brain matrix (World Precision Instruments, Stevenage, UK, specific for rats weighing 175–300 g) and a coronal cut was made, separating the brain into a caudal portion containing the retrosplenial cortex and a rostral portion with the anterior thalamic nuclei and the very rostral end of the retrosplenial cortex. The retrosplenial cortex portion was immediately frozen on dry ice, then stored at −70°C until sections (14 μm) were cut on a cryostat (Leica Instruments) and thaw-mounted onto poly-L-lysine (hydrobromide; molecular mass >300,000; Sigma, Poole, UK)-coated glass slides (0.02 mg/ml DEPC-treated water). These sections were air-dried for ≥30 min, fixed in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS), pH 7.4, for 5 min, rinsed in PBS for 1 min, delipidated in 70% ethanol for 4 min, and stored in 95% ethanol at 4°C. In order to confirm the anterior thalamic nuclei lesions, the rostral portion of the brain was placed overnight in 4% paraformaldehyde in PBS for fixation and then again overnight in 25% sucrose in PBS. A series of coronal sections (40 μm) was cut with a cryostat, mounted onto gelatine-coated slides, stained with cresyl violet and coverslipped using DPX mountant (Fisher Scientific, Loughborough, UK).
In situ hybridization and silver grain counts
Hybridizations were performed essentially as described by Hall et al. (2001). cDNA antisense probes (45mers) were synthesized commercially (Sigma-Genosys, Cambridge, UK) complementary to nucleotides of the genes of interest (Table 1). These oligonucleotides were 3′-end-labelled with [α- 35S] dATP (1200 Ci/mmol; Perkin Elmer-NEN, Hounslow, UK) in a 30:1 molar ratio of radio-labelled ATP:oligonucleotide using terminal deoxynucleotidyl transferase (Promega, Southampton, UK) as described previously (Wisden and Morris, 1994). Specific activity of the 35S-labelled probe was between 2.0 ×105 and 6.0 × 105 dpm/μl probe. To define non-specific hybridization, adjacent slide-mounted sections were incubated with radiolabeled oligonucleotide in the presence of an excess (100×) concentration of unlabeled oligonucleotide probe. This method gave us an estimate of the spurious formation of grains, contributing to the “non-specific” signal. This number is then subtracted from the total signal obtained with the labelled probe, thus yielding a better estimate of the “specific signal”.
Table 1. List of oligonucleotide probes used for in situ hybridisation.
| Target | NCBI Accession No. | Nucleotides | Probe Sequence |
|---|---|---|---|
| cFos | NM 022197 | 153-197 | ggaggatgacgcctcgtagtccgcgttgaaacccgagaacatcat |
| Zif268 | NM 012551 | 3-47 | ccgttgctcagcagcatcatctcctccagtttggggtagttgtcc |
| 5ht2rc | NM 012765 | 1538-1582 | gagctccctcccagacaaagcagtggcagcaaccctaggaatctg |
| Cox6a1 | NM 012814 | 118-162 | acgaagtaggtgagggccctccaaatacgagctgaaccctcctcg |
| Cox6b | NM 025628 | 115-159 | agggggcagttttgtagttcttgattttagtcttgatgtcttcag |
| ncs-1 | NM 024366 | 71-115 | ttctggtcagctcctccacaacttcaggcttcaacttgctgttgg |
| CD74 | NM 013069 | 558-602 | ttcatccagctctcaaagaccttccagtccagaccattcatagag |
| Kcnab2 | NM 017304 | 567-611 | gcccagattcctgtaaaactggagctgtcttttgggactcccata |
After hybridization, sections were opposed to Kodak BioMax MR x-ray film (Sigma-Aldrich Company Ltd. Poole UK) for 1–2 weeks. After obtaining appropriate x-ray film exposures, sections were dipped in Ilford K5 photographic emulsion (Agar Scientific, Stansted, UK). Sections were exposed for 5–10 weeks at 4°C, before development and counterstaining with 0.01% thionin.
The silver grain counts were always obtained by an investigator blind to the treatment of each hemisphere. Grain density was assessed in laminae II (superficial) and V (deep) of Rgb, typically from three non-adjacent sections spread across the range −2.8 to −6.0 mm from bregma (Paxinos and Watson, 1997). Images of sections were captured with LeicaQWIN imaging software (Leica Microsystems, Milton Keynes, UK) using an objective (×100) with oil-immersion. Grains (total and non-specific) were counted over 8-10 cells per hemisphere for each lamina, and the mean number of silver grains per cell calculated. Silver grains were counted using the LeicaQWIN imaging software, with the exception of three genes (ncs1, zif268, kcnab2), for which the ImageJ imaging software (http://rsbweb.nih.gov/ij/) was employed. Retrosplenial counts of cox6b grains made by both methods were compared in two separate Anovas (laminae II and layer V), with the within factors of hemisphere (lesioned or intact) and count method (LeicaQWIN or ImageJ). In neither lamina (II or V) was there evidence that the count method affected the overall grain count (both p>0.1). Furthermore, the lack of any interaction (both F<1) between the scores for the two count methods and status of hemisphere (thalamic lesion or intact) showed that the two methods gave matching patterns of results that could not be distinguished.
Statistical analyses
Statistical analyses (SPSS 14.0, Chicago) on specific grain counts started with a two-way ANOVA with the within-subjects factors surgical treatment (hemisphere) and lamina. Post hoc tests were done where appropriate. As a protection for Type 1 errors after multiple comparisons, a Bonferroni adjustment was applied. Statistical significance was set at the p≤0.05 level.
Experiment 2: Trans-neuronal tracing of indirect retrosplenial projections to the anterior thalamus
Methods
Injections of WGA were made into the anterior thalamic nuclei, and by varying the survival time it was possible to look for cells that had potentially been labelled trans-neuronally. To confirm the status of such candidate cells, we injected both WGA and the retrograde fluorescent tracer, Fast Blue (Kuypers et al., 1980) in the same syringe, into exactly the same site in a number of cases. Fast Blue is known to travel trans-neuronally, and so the direct comparison with WGA labelled cells should reveal those cells that are most likely to be labelled trans-neuronally. Although WGA travels both anterogradely and retrogradely, any retrograde label is readily distinguished when visualised immunohistologically, as it is seen as intracellular grains. Anterograde label appears as an extra-cellular dusting of neuropil.
Subjects
Experiment 2 used 13 male Lister Hooded rats (Harlan, Bicester, UK) weighing 270-300g at the time of surgery. All rats were housed in pairs under a 13 hour light/11 hour dark cycle. The procedures were carried out in accordance with UK Animals (Scientific Procedures) Act, 1986, and associated guidelines, and all efforts were made to minimize animal suffering.
Surgical procedures
Both tracers were injected stereotaxically via a 0.5μl Hamilton syringe. In 7 cases, Fast Blue (Polysciences Inc, Eppelheim, Germany) was injected in combination with WGA (Vector Labs, Peterborough, UK). In an initial series of rats (n=6), only WGA was injected to help determine the time course of any potential trans-neuronal uptake. Additional experiments, involving WGA injections into the mammillary bodies (not reported here), were also used to determine the time course of any trans-neuronal transport.
Both Fast Blue and WGA were made up in sterile 0.1M PBS. For combined tracer experiments, Fast Blue was made up as a 5% solution, while WGA was made up as a 4% solution. Immediately prior to injections, Fast Blue and WGA were combined at a ratio of 2 parts Fast Blue to 1 part WGA, as this had previously resulted in equivalently sized injection sites. For WGA only experiments, WGA was made up as a 1-2% solution (comparable to the concentrations used in combined WGA/Fast Blue injections).
The surgical procedures were essentially the same as for Experiment 1, and again a craniotomy was made above the anterior thalamus. For cases with a single injection, the target was where the anterior medial and anterior ventral nuclei meet, so as to involve both nuclei. The coordinates in these cases (n=8) were: AP −0.2, ML +/− 1.4, DV −6.7 (all from bregma). For cases with two separate injections, the two targets were, respectively, the anterior medial and anterior ventral nuclei in the same hemisphere (n=5). The coordinates for the anterior medial nucleus were: AP −0.2, ML +/− 1.0, DV −6.9 (all from bregma), and for the anterior ventral nucleus were: AP −0.2, ML +/− 1.8, DV −6.2. Injection volumes of 0.01-0.04μl were made into the anterior thalamic nuclei. Following each injection, the syringe was left in place for at least 7 minutes to help limit any tracer travelling back up the syringe tract. Post-operative care matched that described for Experiment 1.
Tissue preparation
Following a postoperative period of between 6 hours to 6 days, the animals were deeply anesthetized with sodium pentobarbital (Euthatal, Merial, Harlow, UK). The rats were then perfused intracardially with 0.1M PBS at room temperature followed by 4% paraformaldehyde in 0.1M PBS at ~4°C. Brains were removed and placed in the dark for 4 hours in paraformaldehyde and then transferred to 30% sucrose solution in 0.1M PBS for 24 hours in the dark to cryoprotect the tissue before cutting. Brains were placed on a freezing platform and a 1-in-3 series of 40μm coronal sections was cut on a sledge microtome (Leica 1400). One series was either stained with cresyl violet or immunologically reacted for the presence of NeuN (Santa Cruz Biotechnology, Inc., Heidelberg, Germany). This allowed localisation of injection sites within the ATN and displayed the laminar structure of the retrosplenial cortex. The second series was viewed for fluorescence microscopy, and the third series was immunologically reacted for the presence of the WGA tracer. All sections were mounted directly onto gelatine-subbed slides, and then allowed to dry in the dark at room temperature before dehydrating and coverslipping with DPX (Raymond Lamb, Eastbourne, UK). Brightfield microscopy was undertaken with a Leica DMRB microscope equipped with an Olympus DP70 digital camera and AnalySIS image acquisition software (Soft Imaging System, Münster, Germany). For fluorescence microscopy, a Leica DM5000B microscope with a Leica DFC350FX digital camera and Leica Application Suite image acquisition software was used to capture images. Photoshop (Adobe Systems Incorporated) helped to enhance the sharpness and contrast of the photomicrographs, and also prepare figures.
Wheat germ agglutinin immunohistochemistry
An antiserum directed against WGA was used at a dilution of 1:2000 and incubated at 4°C for 48 hours (Vector Labs catalogue# AS-2024, produced in goats). The antigen-antibody complex was localised with a standard avidin-biotin process (ABC Elite Kit, Vector Labs). The chromagen diaminobenzidine (DAB) produced the visualised reaction product, which was further enhanced with a nickel solution (DAB Substrate Kit, Vector Labs). Reacted sections were then mounted onto gelatine-subbed slides and dehydrated through increasing concentrations of alcohol before being cover-slipped from xylene with DPX.
Results
Experiment 1: Granular retrosplenial cortex (Rgb) changes after anterior thalamic lesions measured by in situ hybridization
Nissl confirmation of unilateral thalamic lesions
Two rats with incomplete lesions were rejected, leaving ten rats with unilateral, anterior thalamic lesions (largest and smallest depicted in Fig. 1). The remaining thalamic lesions were essentially as intended as they always included most of the cells in the anterior dorsal and anterior ventral nuclei. Cell loss in the rostral part of the anterior thalamic nuclei (anterior dorsal, anterior medial, anterior ventral) was almost always complete. In six cases the anterior dorsal nucleus was completely absent throughout its length, while the anterior ventral nucleus was totally lost in three cases. Sparing in the latter nucleus was typically confined to its most lateral margin. The only other area to show repeated sparing was the most medial part of the anterior medial nucleus (including parts of the interoanteromedial nucleus). Cell loss continued caudally from the anterior dorsal nucleus to include the most rostral portion of the adjacent lateral dorsal nuclei in five cases. None of the lesions crossed the midline. The largest lesions extended into parts of the ventral anterior nucleus, immediately ventral to the anterior ventral thalamic nucleus, the lateral parataenial nucleus, and very restricted portions of the very rostral reticular nucleus (Fig. 1). In half of the cases there was some very restricted cell loss in the ventral blade of the septal dentate gyrus.
Figure 1.
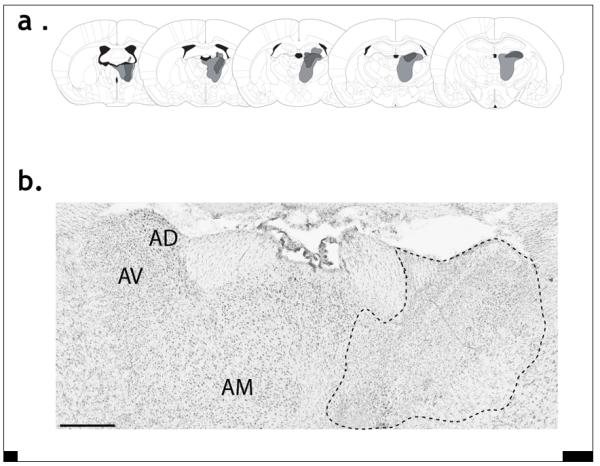
a) The smallest and largest lesions are represented in dark and light grey, respectively. The plates, from left to right, represent the distances −0.92, −1.4, −1.8, −2.12, and −2.56 mm from bregma. b) Photomicrograph of a Nissl-stained coronal section, contrasting the anterior thalamic nuclei in a normal hemisphere (left) and a lesioned hemisphere (right). The outline highlights the area of gliosis and cell loss. Note that the tissue was not perfused, hence the poor differentiation. AD, anterior dorsal thalamic nucleus; AM, anterior medial thalamic nucleus; AV anterior ventral thalamic nucleus. The scale bar represents 500 μm.
In situ findings
All analyses were based on comparisons between the retrosplenial cortex ipsilateral to the thalamic lesions and the cortex contralateral to the lesion, i.e. in an intact hemisphere.
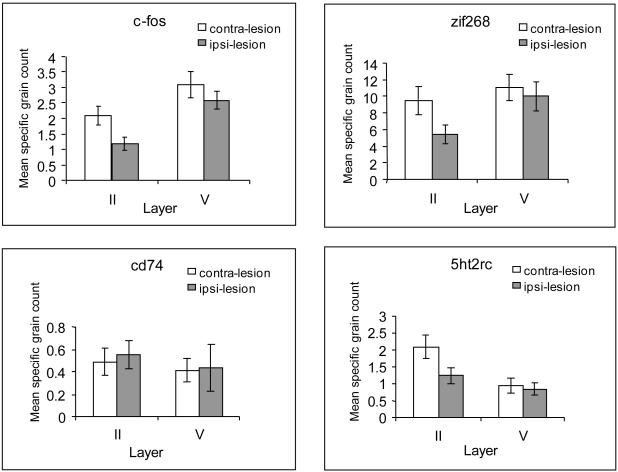
c-fos
As show in Figure 2A, unilateral anterior thalamic nuclei lesions produced a reduction of c-fos mRNA in ipsilateral retrosplenial cortex [F(1,8)=10.2, p<0.005, but no interaction of lesion and laminae, F<1]. Follow-up tests (Bonferroni adjusted for multiple comparisons) revealed that the c-fos reduction was significant in lamina II [F(1,8)=8.6, p<0.05], while it narrowly failed to achieve statistical significance for lamina V [F(1,8)=4.7, p=0.061].
Figure 2.
Graphs showing the mean silver grain count per cell in laminae II and V of granular retrosplenial cortex (Rgb). Data are presented for c-fos (A), Zif268 (B), 5ht2rc (C), and CD74 (D). In all cases, the graphs compare the grain counts in the hemisphere ipsilateral to the anterior thalamic lesion (ipsi-lesion) and contralateral to the lesion (contra-lesion), i.e. intact. Error bars represent the standard error of the mean, but note that the comparisons are within-subject.
zif268
While unilateral anterior thalamic nuclei lesions appeared to cause an overall reduction of zif268 mRNA in ipsilateral retrosplenial cortex (Fig. 2B), this change was not significant [F(1,9)=2.3, p>0.16]. Although, by inspection, the reduction was more pronounced in lamina II, the interaction of lesion and laminae failed to reach statistical significance [F(1,9)=3.7, p=0.085]. Planned comparisons in the light of previous repeated findings of a superficial laminae sensitivity of Zif268-positive cells to ATN lesions (Jenkins et al., 2004; Poirier et al., 2009) did, however, support the apparent reduction observed in lamina II [F(1,9)=3.7, p<0.05].
5htr2c
Retrosplenial cortex 5htr2c mRNA expression was reduced by the anterior thalamic nuclei lesions [main effect of lesion F(1,9)=49.0, p<0.001]. As seen in Figure 2C, the superficial lamina II appeared more sensitive to the lesions than the deep lamina V [interaction of lesion and layer, F(1,9)=11.2, p<0.01]. This pattern was confirmed by the follow-up analyses as there was a significant decrease in grain counts in lamina II [F(1,9)=34.8, p<0.001; see Fig. 3] but not in lamina V (F<1; see Fig. 3). These follow-up analyses were Bonferroni adjusted for multiple comparisons.
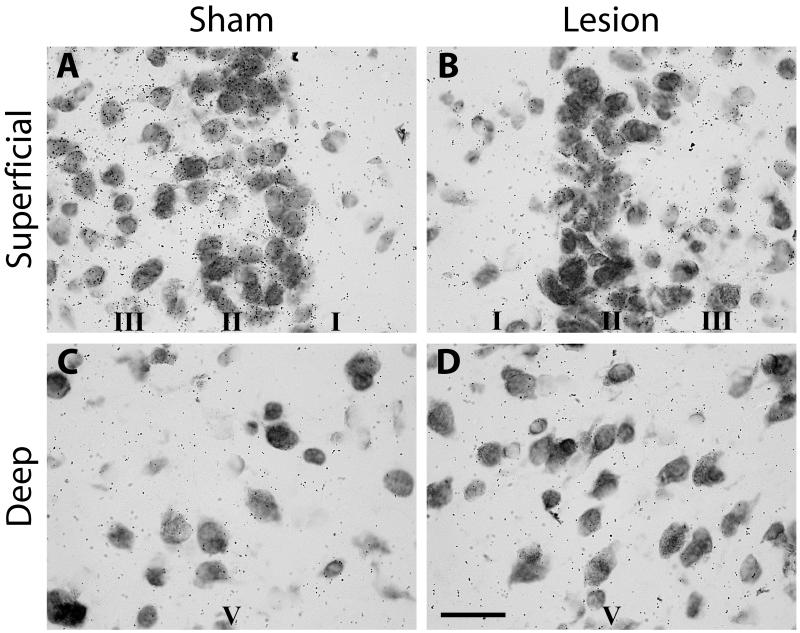
Figure 3.
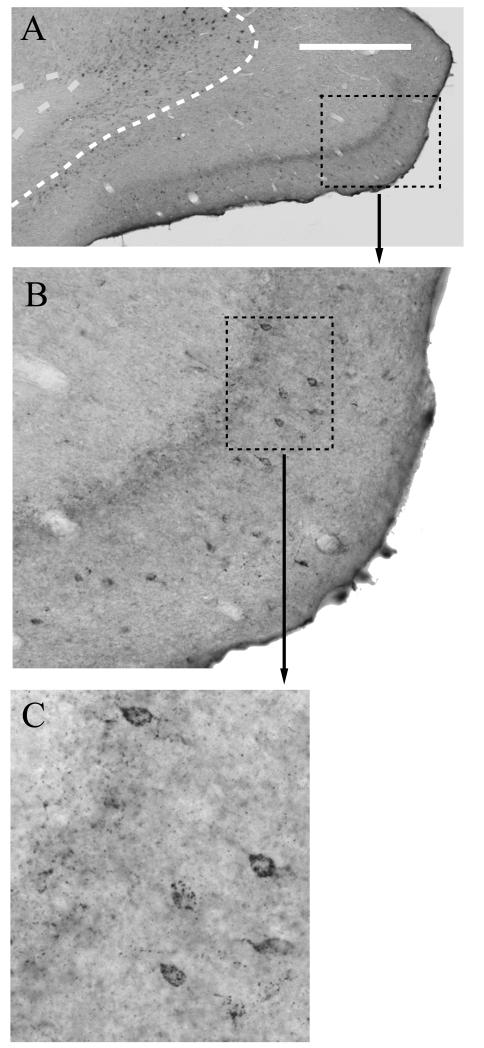
Photomicrographs of silver grains reflecting levels of 5htr2c mRNA in the granular retrosplenial cortex (Rgb) in a rat with a unilateral anterior thalamic lesion. There is a clear reduction of grain counts in the superficial laminae of Rgb in the hemisphere (Lesion) with the thalamic lesion. ‘Sham’ refers to the intact hemisphere. The upper row shows silver grain in the superficial laminae (I, II, III), while the lower row (Deep) depicts laminaV. Scale bar = 25 μm.
cd74
There was no effect of the anterior thalamic lesions on the levels of cd74 (Fig. 2D) in retrosplenial cortex laminae II and V (all comparisons, F<1).
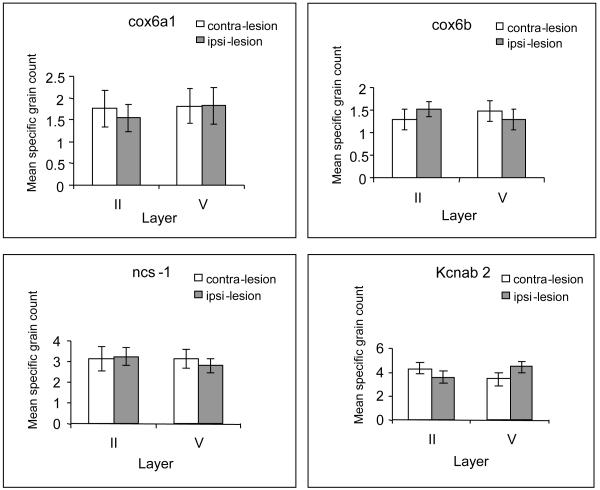
cox6a1
Counts of lamina II and lamina V cox6a1 mRNA expression (Fig. 4A) failed to reveal any effects of the anterior thalamic nuclei lesions (main effect of lesions, F<1; interaction of laminae and lesions, F<1).
Figure 4.
Graphs showing the mean silver grain count per cell in laminae II and V of granular retrosplenial cortex (Rgb). Data are presented for cox6a1 (A), cox6b (B), ncs-1 (C), and kcns2(kvβ2) (D). In all cases, the graphs compare the grain counts in the hemisphere ipsilateral to the anterior thalamic lesion (ipsi-lesion) and contralateral to the lesion (contra-lesion), i.e. intact. Error bars represent the standard error of the mean, but note that the comparisons are within-subject.
cox6b
The effect of the anterior thalamic nuclei lesions on cox6b grain counts (Fig. 4B) depended on the lamina examined [interaction of lamina and lesion, F(1,7)=6.3, p<0.05; no main effect of lesion, F<1]. Follow up analyses showed that the reduction in lamina V was close to significant [F(1,7)=4.9, p=0.062; Bonferroni adjusted for multiple comparisons], and that the interaction reflected the opposing patterns of change (Fig. 4B).
ncs-1
There was no effect of anterior thalamic nuclei lesions on grain counts of ncs-1 (Fig. 4C) in laminae II and V of retrosplenial cortex [main effect of lesions, F<1; interaction of lesions and laminae, F(1,8)=2.4, p>0.16].
kcnab2 (also known as kvb2)
There was no overall effect (F<1) on kcnab2 expression (Fig. 4D), however, the opposite pattern of effects in each laminae was confirmed by the significant lesion by lamina interaction [F(1,8)=16.2, p<0.005]. Examination of the simple effects revealed a significant reduction in lamina II [F(1,8)=8.6, p<0.05], though the increase in layer V narrowly failed to reach significance [F(1,8)=4.7, p=0.061].
Experiment 2: Trans-neuronal tracing of indirect retrosplenial projections to the anterior thalamus
Injection sites
In all 13 cases, the tracer injections were centered within the anterior medial and anterior ventral nuclei, though in some cases the tracers also involved the anterior dorsal nucleus. The single injection site in the 3-day survival was situated at the junction of the anterior medial and anterior ventral thalamic nuclei, with the tracer extending into both nuclei.
Importantly, the apparent spread of Fast Blue and WGA was very similar for those cases with combined injections of both tracers into the same site (e.g. Figs. 5G,H). The locations of the injection sites for the 1-day survival cases were very similar to those of the 3-day survival cases.
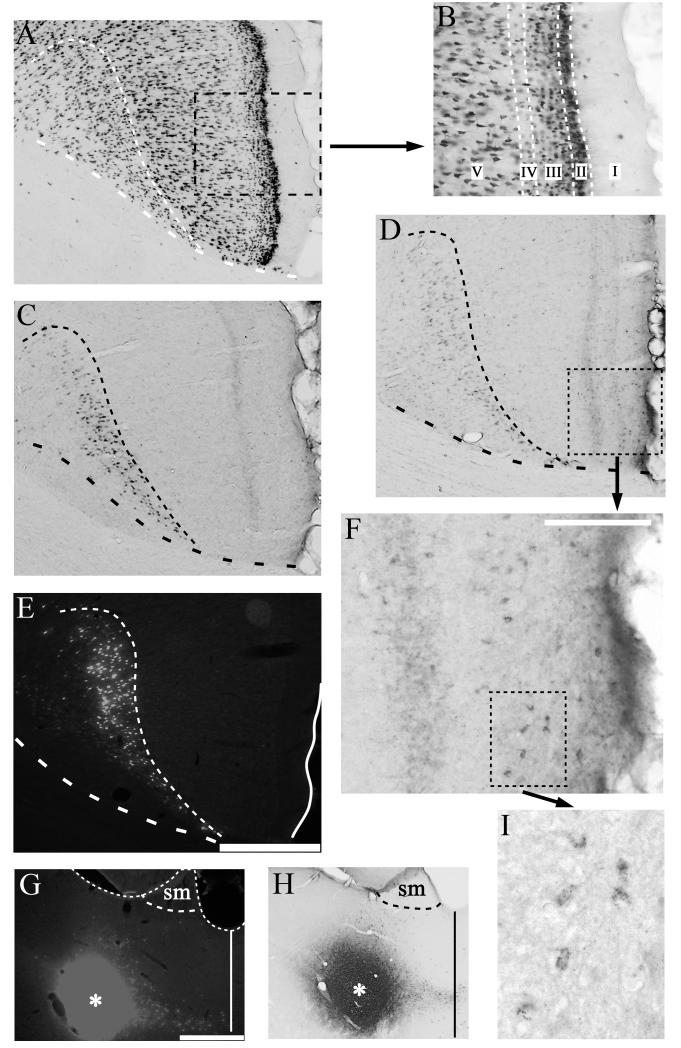
Figure 5.
Photomicrographs of retrosplenial region Rgb showing the presence of additional (trans-neuronal) retrograde label in lamina II (see D,F) following extended survival times after an injection of wheat germ agglutin (WGA) into the anterior thalamic nuclei:compare C (1 day) with D (3 days). A) Photomicrograph of retrosplenial region Rgb reacted for NeuN to show the laminar pattern of neurons. The boundary between laminae V and VI is indicated by narrow dashes. The midline is at the extreme right of the photomicrograph. B) Inset from (A) to show lamina structure of retrosplenial area Rgb. C) Photomicrograph of retrosplenial region Rgb reacted for WGA following a 24 hour survival period after a unilateral injection of WGA into the anterior thalamic nuclei. D) Photomicrograph of Rgb reacted for WGA following a 3 day survival period after a unilateral injection of combined Fast Blue/WGA into the anterior thalamic nuclei. Note the presence of additional, superficial label. E: Fluorescence photomicrograph of Fast Blue labelling in Rgb after 3 day survival. (The adjacent section is shown in D). The boundary between laminae V and VI is indicated by narrow dashes. F) High magnification photomicrograph of region indicated by a dashed box in (D), showing retrograde label in lamina II cells. G & H) Injection sites for Fast Blue (G, asterisk) and WGA (H, asterisk) in the anterior thalamic nuclei for the same 3-day survival case (also see D &E). The dashed line shows the border with the stria medullaris (sm), while the solid line marks the midline. I) Further high magnification photomicrograph of the retrograde label in the region indicated by the dashed box in F. Scale bar for A), C), D), E) displayed in E) (bar = 250μm). Scale bar in F) = 100μm. Scale bar in G), H) = 500μm. All sections are in the coronal plane.
Time course of trans-neuronal WGA labelling
Six cases with WGA injections into the anterior thalamic nuclei were examined where the survival times varied between 6 hours and 6 days. Comparisons with adjacent cresyl violet stained or NeuN reacted sections (Fig. 5A) were used to assign WGA labelling to the associated lamina of the Rgb. With increasing survival times (6–24 hours), the retrogradely labelled cells within lamina VI of region Rgb increased both in their number and in the intensity of their labelling. This lamina (VI) is regarded as the sole source of the direct projections to the anterior thalamic nuclei (Shibata, 1998; Van Groen and Wyss, 2003). In those cases with survival times of up to 24 hours, no other labelled cells were present in more superficial layers of the Rgb (Fig. 5C).
When post injection survival times were increased to three days, numerous intensely labelled cells were again located within lamina VI (Fig. 5D), but now there was also a scattered layer of lightly labelled cells in lamina II of ipsilateral Rgb. Despite the low intensity of the labelling, the characteristic granular nature of the DAB reaction product was clearly identifiable (Figs. 5F,I). This light, superficial label extended along the length of the granular retrosplenial cortex, so that it was also found in Rga along with caudal Rgb (Fig. 6A). While the large majority of this additional retrograde label was found in lamina II, occasional cells in lamina III were also labelled (Fig. 6B). At the longest survival time (6 days), labelling was still present in lamina VI, but the intensity of labelling was considerably reduced in comparison to shorter survival times of 1-3 days. In addition, the light labelling previously noted within lamina II was now absent.
Figure 6.
Photomicrograph of caudal retrosplenial cortex showing the presence of additional (trans-neuronal) retrograde label in lamina II (see B,C) following extended survival times after a bilateral injection of wheat germ agglutin (WGA) into the anterior thalamic nuclei. A) Photomicrograph of caudal retrosplenial cortex (Rga, Rgb) reacted for WGA following a 3 day survival period. The dashed line marks the boundary between lamina V and VI. Scale bar = 500μm. B) Higher magnification photomicrograph of region indicated by a dashed box in (A), showing both anterograde label (laminae I-IV) and retrograde label concentrated in lamina II cells. C) Further high magnification photomicrograph of the retrograde label in the region indicated by the dashed box in B. Note, the occasional cell in lamina III/IV. All sections are in the coronal plane.
In all WGA cases with survival times of 24 hours or more, bands of anterograde label were also visible in the retrosplenial cortex (Figs. 5C,D). Within Rgb, this anterograde label was most prominent in laminae I and IV (Figs. 5D,F).
Combined Fast Blue and WGA injections cases
By combining injections of Fast Blue and WGA within the anterior thalamic nuclei in the same case, and then processing adjacent sections for the presence of each tracer, it is possible to determine whether populations of labelled cells within Rgb project directly or trans-neuronally to the thalamus. Following on from the results of the previous WGA time course experiments, a case with a three day survival period is described in detail.
In this case, the spread of Fast Blue and WGA appeared very similar (Figs. 5G,H). Numerous retrogradely labelled Fast Blue cells were located within lamina VI (Fig. 5E). These cells appeared pyramidal in shape and no Fast Blue labelled cells were detected in the more superficial Rgb layers. In contrast, the immediately adjacent WGA-processed sections (Fig. 5D) not only contained many labelled pyramidal cells with a high intensity of label in lamina VI but also had a band of lightly labelled cells scattered ipsilaterally in lamina II of Rgb (Figs. 5D,F,H). No corresponding Fast Blue label occurred in this region. This pattern of WGA label in the superficial laminae, but no Fast Blue label, after three day survival periods was confirmed in other cases (Fig. 6).
Discussion
Pathology involving the anterior thalamic nuclei can cause extensive changes in retrosplenial cortex activity (Gabriel, 1993; van Groen et al., 1993; Jenkins et al., 2004, Poirier et al., 2008), including a loss of cortical plasticity (Garden et al., 2009). The interlinked goals of the present study were to characterize these cortical changes further and to determine whether previous findings, which indicate that these retrosplenial changes are especially pronounced in the superficial lamina (Jenkins et al., 2004; Garden et al., 2009), are representative of a wider range of neurochemical changes. Using in situ hybridization, Experiment 1 found evidence that unilateral anterior thalamic lesions changed the relative expression of c-fos, zif268, 5ht2rc, cox6b, and kcnab2 across the hemispheres in the granular retrosplenial cortex (area Rgb). For four of these five genes, the changes reflected a pronounced decrease in lamina II expression in the retrosplenial cortex ipsilateral to the anterior thalamic lesion. To this list can be added gabrd, which also shows a reduction in superficial granular retrosplenial cortex after anterior thalamic lesions (Poirier et al., 2008). Of these genes, the exception was cox6b, which tended to be lower in lamina V yet relatively higher in lamina II, resulting in a significant interaction. For the three other genes, that were examined (cox6a1, CD74, ncs-1) there was no clear evidence of an anterior thalamic lesion effect within the granular retrosplenial cortex layers examined. It should be added that previous studies have repeatedly shown that anterior thalamic lesions do not result in a loss of neurons in the retrosplenial cortex (van Groen et al., 1993; Jenkins et al., 2004; Poirier et al., 2008; Poirier and Aggleton, 2009), so excluding a potential confound.
This bias towards superficial hypoactivity in retrosplenial cortex after anterior thalamic damage has also been seen in immunohistochemical studies of the expression of the two immediate-early genes, c-Fos and Zif268 (Jenkins et al., 2004). A potential clue to this relationship comes from a consideration of the interconnections between the anterior thalamic nuclei and the retrosplenial cortex. Projections from all three major anterior thalamic nuclei (anterior medial, anterior ventral, and anterior dorsal) terminate in the retrosplenial region under investigation (Rgb). While the three major anterior thalamic nuclei have slightly different patterns of lamina inputs within Rgb (Shibata, 1993), they all preferentially target the superficial lamina of the cortex, with lamina I often receiving particularly dense termination (Vogt et al., 1981: Shibata, 1993; van Groen and Wyss, 1995, 2003; see also present study). The neurons in lamina II have tight bundles of dendrites in lamina I (van Groen and Wyss, 1993) that are thought to have a particularly close relationship with the anterior thalamic inputs (Ichinohe and Rockland, 2002), so potentially explaining the sensitivity of these particular cells to thalamic disconnection. At the same time, the deeper retrosplenial cells also have apical dendrites that could interact with the anterior thalamic inputs to the more superficial granular retrosplenial cortex (Vogt and Peters, 1981; Shibata, 1998), showing how thalamic deafferentation could have more widespread lamina effects.
The target genes were selected with a view to: i) helping to understand better the nature of any cortical dysfunction, and ii) cross-validating with other studies. The results for c-fos, zif268, kcnab2, and 5hrt2c showed good agreement in comparison with previous anterior thalamic lesion studies that had used other methodologies (Jenkins et al., 2004; Poirier et al., 2008). The cross-validated IEG depletions are potentially important as both c-fos and zif268 have repeatedly been linked to learning mechanisms in a wide range of structures, including the retrosplenial cortex (Beck and Fibiger, 1995; Vann et al., 2000; Maviel et al., 2004; Pothuizen et al., 2009). In the cases of c-fos and zif268, immunohistological studies have shown marked reductions of Fos and Zif268 protein in the retrosplenial cortex after ATN lesions (Jenkins et al., 2004; Poirier and Aggleton, 2009). The c-fos findings are especially intriguing as the activity of this IEG is thought to be regulated by negative feedback (Sassone-Corsi et al., 1988), such that high levels of c-Fos protein should normally restrict c-fos RNA production. For this reason, the discovery in this study that c-fos RNA and Fos protein levels parallel one another might not have been anticipated. Given that the activity of this IEG is often well correlated with neuronal activity (Herdegen and Leah, 1998; Lawrence et al., 2004; Herry et al., 2007; Yamazaki et al., 2008), the decrease in c-fos activity may reflect a long-term compensatory change in Rgb responsiveness to stimuli after remote anterior thalamic nuclei lesions. Alternatively, rather than a compensatory change, c-fos mRNA could be de-sensitized as a result of chromatin remodelling, for example via deacetylation (e.g. Renthal et al., 2008). Such a possibility may be supported by reduced expression of a member of histone acetyltransferase complex (ruvbl1) in Rgb after thalamic lesions (Poirier et al., 2008). Meanwhile, the zif268 hypoactivity may have relevance for memory consolidation, as retrosplenial changes in zif268 activity are correlated with the reorganisation of spatial memories over time (Maviel et al., 2004).
The gene 5htr2c, showed a selective depletion of RNA levels in lamina II of the retrosplenial cortex (Fig. 3). An earlier study found that extensive thalamic lesions, which included the anterior thalamic nuclei, reduced levels of binding to serotonin1B (5-hydroxytryptamine, 5-HT1B) throughout laminae I-III in Rgb cortex (van Groen et al., 1993). It was supposed that this change largely reflected the loss of presynaptic binding, though in the present study the changes in 5hrt2c RNA potentially reflected changes to retrosplenial neurons, as this receptor can be found post-synaptically (Bockaerts et al., 2006). The finding that cutting the mammillothalamic tract is sufficient to induce very pronounced decreases in c-fos activity within the retrosplenial cortex (Vann and Albasser, 2009) reveals how changes seen in the retrosplenial cortex can be via a loss of afferent signalling rather than the degeneration of axon terminals.
Two closely related genes, cox6a1 and cox6b, which code for cytochrome oxidase, were examined. Cytochrome oxidase has been used as an index of long-term metabolic activity (Wong-Riley, 1989), and learning can be associated with levels of cytochrome oxidase (Poremba et al., 1998; Callaway et al., 2002). Further interest in this enzyme comes from findings that extensive unilateral thalamic lesions will reduce cytochrome oxidase in the rat retrosplenial cortex (van Groen et al., 1993), along with the discovery that patients with Alzheimer’s disease have unusually low levels of cytochrome oxidase within the posterior cingulate/retrosplenial cortex region (Valla et al., 2001; Liang et al., 2008). Of the two genes measured in the present study, only cox6b showed evidence of a lesion-induced change, with a modest increase in lamina II and decrease in lamina V, resulting in a significant interaction.
Levels of kcnab2 levels were affected by the anterior thalamic lesion in a layer-dependent fashion, with a reduction in lamina II accompanied by a modest increase in lamina V. Although little is known about the contribution of this delayed-rectifier potassium-gated channel subunit, its activation may play a role in the inhibition of cell firing (Richardson et al., 2000). As such, its reduced presence in lamina II could be involved in the long-term depression deficit observed in that specific layer after anterior thalamic lesions (Garden et al., 2009). While this remains speculation, kcnab2 does again provide clear evidence of a qualitative difference in lamina sensitivity.
One of the first demonstrations of functional differences between retrosplenial laminae came from a series of pioneering electrophysiological studies (Gabriel et al., 1983; Gabriel, 1993) in which rabbits learnt an auditory avoidance discrimination between two tones. Recording studies during acquisition plotted the emergence of training-induced activity in the superficial and deep laminae of the retrosplenial cortex (Gabriel et al., 1983; Gabriel, 1993). While activity in lamina V and VI appeared to discriminate between the tones in early/intermediate stages of training, comparable changes in the superficial lamina were not seen until later training. Such findings highlight a recurrent theme of the present study, the presence of qualitative differences between the superficial and deep layers of the retrosplenial cortex. Furthermore, the finding that recordings in the anterior thalamic nuclei (anterior ventral nucleus) show a similar pattern of late discrimination as that seen in superficial retrosplenial cortex (Gabriel, 1993) once again links these particular subregions. Consistent with this specific relationship, lesions of the anterior thalamic nuclei only disrupted late aspects of performance (Gabriel et al., 1989), although electrophysiological studies failed to confirm a selective disruption of just superficial retrosplenial cortex following anterior thalamic lesions in rabbits. This latter finding might reflect long-term compensatory effects following surgery. Evidence for such long-term changes comes from the finding that, following anterior thalamic lesions in rats, the IEG changes seem largely confined to the superficial lamina in the first few weeks after surgery, but by one year post surgery, these IEG changes become much more pervasive, with decreases across both superficial and deep lamina (Jenkins et al., 2004; Poirier and Aggleton, 2009).
In a complementary anatomical study, we examined whether cells in lamina II of the retrosplenial cortex (those most sensitive to disconnection) project indirectly back to the anterior thalamus. Strong support came from the WGA survival series (1-6 days) and from direct comparisons with Fast Blue label. Although cells in lamina II of Rgb were labelled trans-neuronally (as well as occasional cells in lamina III), we cannot definitively specify the route by which this occurred. It is, however, most likely that the route was via those cells labelled directly in the deeper layers of the retrosplenial cortex. It is known that the principal efferent target of the lamina II Rgb cells is to other sites within the retrosplenial cortex, both ipsilaterally and contralaterally, i.e. lamina II has mainly intrinsic projections (Sripanidkulchai and Wyss, 1987; van Groen and Wyss, 1990, 2003). As a consequence, it very probable that the labelled cells in lamina II project ipsilaterally to retrosplenial lamina VI cells that, in turn, project to the anterior thalamic nuclei. It can be added that the cases with unilateral thalamic injections of WGA injections showed no evidence of transcallosal label in lamina II.
As the importance of the retrosplenial cortex for learning and memory emerges (Maguire, 2001; Vann et al., 2009), it becomes clear that retrosplenial cortex dysfunction could contribute to a wide range of cognitive disorders (Reed et al., 2005). It is for this reason that it is valuable to identify potential causes of retrosplenial dysfunction. There is, in fact, a growing list of disorders that exhibit both memory impairments and evidence of retrosplenial cortex dysfunction (Vann et al., 2009). In the majority of these cases, the retrosplenial cortex is hypoactive, as determined by reductions in glucose utilization, regional cerebral blood flow or blood-oxygen-level-dependent activity (Minoshima et al., 1997; Reed et al., 1999; Aupée et al., 2001; Archer et al., 2003; Nestor et al., 2003a; Reed et al., 2003; Martinez-Bisbal et al., 2004; Laurens et al., 2005). Disorders associated with retrosplenial hypoactivity include Alzheimer’s disease (e.g. Minoshima et al., 1997; Nestor et al., 2003b), mild cognitive impairment (Nestor et al., 2003a), vascular dementia (Martinez-Bisbal et al., 2004), diencephalic amnesia (Aupée et al., 2001; Reed et al., 2003), and temporal lobe amnesia (Reed et al., 1999, 2005; Aupée et al., 2001).
In a number of these conditions (e.g. Alzheimer’s disease), the origin of this hypoactivity could be intrinsic, i.e. due to pathology within the retrosplenial cortex (e.g. Pengas et al., 2010), or extrinsic, i.e. due to distal changes that then influence the retrosplenial cortex (Aggleton, 2008; Vann et al., 2009; Chetelat at al., 2010), or both. The present study explored the impact of extrinsic changes by showing how unilateral anterior thalamic lesions disrupt retrosplenial cortex activity. Widespread abnormalities in retrosplenial cortex were found, which were especially concentrated in the superficial laminae. Other manipulations that preferentially target the superficial lamina of Rgb include the deposition of amyloid in transgenic mouse models of features of Alzheimer’s disease (van Groen et al., 2003). The potential significance of this thalamic – retrosplenial relationship is highlighted by the frequent presence of anterior thalamic pathology in conditions that disrupt memory (Masliah et al., 1989; Braak and Braak, 1991a, 1991b; Aggleton & Brown, 1999; Harding et al., 2000; Van der Werf et al., 2003). Future studies might seek to contrast the impact of selective lesions of the three major anterior thalamic nuclei, as they each have unique patterns of limbic connectivity (Vogt et al., 1981; Shibata, 1993, 1998; van Groen and Wyss, 1995; Aggleton et al., 2010; Wright et al., 2010). A related task will be to determine whether the molecular retrosplenial changes that follow anterior thalamic damage are sufficient to stop this cortical area from conducting its normal functions. In view of the repeated finding that anterior thalamic lesions do not alter the appearance of the retrosplenial cortex when measured by Nissl stains (van Groen et al., 1983; Jenkins et al., 2004; Poirier and Aggleton, 2009), such findings would point to the presence of ‘covert’ pathology within this cortical region (Garden et al., 2009).
List of abbreviations used in manuscript
- AD
anterior dorsal thalamic nucleus
- AM
anterior medial thalamic nucleus
- ATN
anterior thalamic nuclei
- AV
anterior ventral thalamic nucleus
- DAB
diaminobenzidine
- DEPC
diethylpyrocarbonate
- IEG
immediate-early gene
- NeuN
neuronal nuclei antibody
- NMDA
N-methyl-D-aspartic acid
- PBS
phosphate buffered saline
- SM
stria medullaris
- WGA
wheat germ agglutin
REFERENCES
- Aggleton JP. Understanding anterograde amnesia: disconnections and hidden lesions. Q J Exp Psych. 2008;61:1441–1471. doi: 10.1080/17470210802215335. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. Understanding retrosplenial amnesia: Insights from animal studies. Neuropsychologia. 2010 doi: 10.1016/j.neuropsychologia.2009.09.030. in press. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Interleaving brain systems for episodic and recognition memory. Trends Cog Sci. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. Hippocampal - anterior thalamic pathways for memory: Uncovering a network of direct and indirect actions. Eur J Neurosci. 2010 doi: 10.1111/j.1460-9568.2010.07251.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone S, Roland J, Vogt B, Savage S. Acetylcholine efflux from retrosplenial areas and hippocampal sectors during maze exploration. Behav Brain Res. 2009;201:272–278. doi: 10.1016/j.bbr.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer JS, Abbott DF, Waites AB, Jackson GD. fMRI “deactivation” of the posterior cingulate during generalized spike and wave. NeuroImage. 2003;20:1915–1922. doi: 10.1016/s1053-8119(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Aupée AM, Desgranges B, Eustache F, Lalevée C, de la Sayette V, Viader F, Baron JC. Voxel-based mapping of brain hypometabolism in permanent amnesia with PET. NeuroImage. 2001;13:1164–1173. doi: 10.1006/nimg.2001.0762. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. 2006;149:861–9. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Alzheimer’s disease affects limbic nuclei of the thalamus. Acta Neuropathologica. 1991a;81:261–268. doi: 10.1007/BF00305867. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991b;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Callaway NL, Riha PD, Wrubel KM, McCollum D, Gonzalez-Lima F. Methylene blue restores spatial learning memory retention impaired by an inhibitor of cytochrome oxidase in rats. Neurosc Lett. 2002;332:83–86. doi: 10.1016/s0304-3940(02)00827-3. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci. 2002;22:6846–55. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat G, Villain N, Baron J-C. Posterior cingulate hypometabolism in early Alzheimer’s disease: what is the contribution of local atrophy versus disconnection? Brain. 2010;132:1–2. e133. doi: 10.1093/brain/awp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. A discriminative avoidance learning system. In: Vogt BA, Gabriel M, editors. Neurobiology of the cingulate cortex and limbic thalamus: a comprehensive handbook. Birkhauser; Boston: 1993. pp. 478–523. [Google Scholar]
- Gabriel M, Lambert RW, Foster K, Orona E, Sparenborg S, Maiorca RR. Anterior thalamic lesions and neuronal activity in the cingulate and retrosplenial cortices during discriminative avoidance behavior in rabbits. Behav Neurosci. 1983;97:675–696. doi: 10.1037//0735-7044.97.5.675. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg S, Kubota Y. Anterior and medial thalamic lesions, discriminative avoidance learning, and cingulate cortical neuronal learning in rabbits. Exp Brain Res. 1989;76:441–457. doi: 10.1007/BF00247901. [DOI] [PubMed] [Google Scholar]
- Garden D, Massey PV, Caruana DA, Johnson B, Warburton EC, Aggleton JP, Bashir ZI. Anterior thalamic lesions stop synaptic plasticity in retrosplenial cortex slices: expanding the pathology of diencephalic amnesia. Brain. 2009;132:1847–1857. doi: 10.1093/brain/awp090. [DOI] [PubMed] [Google Scholar]
- Genin A, Davis S, Meziane H, Doyere V, Jeromin A, Roder J, Mallet J, Laroche S. Regulated expression of the neuronal calcium sensor-1 gene during long-term potentiation in the dentate gyrus in vivo. Neuroscience. 2001;106:571–577. doi: 10.1016/s0306-4522(01)00301-3. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123:141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Håvik B, Røkke H, Dagyte G, Stavrum AK, Bramham CR, Steen VM. Synaptic activity-induced global gene expression patterns in the dentate gyrus of adult behaving rats: induction of immunity-linked genes. Neuroscience. 2007;148:925–36. doi: 10.1016/j.neuroscience.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, Di Salle F, Perrig WJ, Scheffler K, Lüthi A, Seifritz E. Processing of temporal unpredictability in human and animal amygdala. J Neuro. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N, Rockland KS. Parvalbumin positive dendrites co-localize with apical dendritic bundles in rat retrosplenial cortex. NeuroReport. 2002;13:757–761. doi: 10.1097/00001756-200205070-00005. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Vann SD, Amin E, Aggleton JP. Anterior thalamic lesions stop immediate early gene activation in selective laminae of the retrosplenial cortex: evidence of covert pathology in rats? Eur J Neurosci. 2004;19:3291–3304. doi: 10.1111/j.0953-816X.2004.03421.x. [DOI] [PubMed] [Google Scholar]
- Kong W, Mou X, Liu Q, Chen Z, Vanderburg CR, Rogers JT, Huang X. Independent component analysis of Alzheimer’s DNA microarray gene expression data. Mol Neurodegener. 2009;4(1):5. doi: 10.1186/1750-1326-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers HGJM, Bentivoglio M, Catsman-Berrevoets CE, Bharos AT. Double retrograde neuronal labeling through divergent axon collaterals, using two fluorescent tracers with the same excitation wavelength which label different features of the cell. Exp Brain Res. 1980;40:383–392. doi: 10.1007/BF00236147. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ETC, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schiz Res. 2005;75:159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lawrence J, Stroman PW, Bascaramurty S, Jordan LM, Malisza KL. Correlation of functional activation in the rat spinal cord with neuronal activation detected with immunocytochemistry. Neuroimage. 2004;22:1802–1807. doi: 10.1016/j.neuroimage.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Liang WS, Reiman EM, Valla J, Dunckley T, Beach TG, Grover A, Niedzielko TL, Librizzi L, Regondi MC, Pastori C, Frigerio S, Frassoni C, de Curtis M. Expression of adhesion factors induced by epileptiform activity in the endothelium of the isolated guinea pig brain in vitro. Epilepsia. 2007;48:743–751. doi: 10.1111/j.1528-1167.2007.01047.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin2C receptor localization in GABA neurons of the rat medial prefrontal cortex: implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1677–88. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E. The retrosplenial contribution to human navigation: A review of lesion and neuroimaging findings. Scand J Psych. 2001;42:225–238. doi: 10.1111/1467-9450.00233. [DOI] [PubMed] [Google Scholar]
- Martinez-Bisbal MC, Arana E, Marti-Bonmati L, Molla E, Celda B. Cognitive impairment: classification by 1H magnetic resonance spectroscopy. European J Neurol. 2004;11:187–193. doi: 10.1046/j.1468-1331.2003.00746.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Terry R, Buzsaki G. Thalamic nuclei in Alzheimer disease: evidence against the cholinergic hypothesis of plaque formation. Brain Res. 1989;493:240–246. doi: 10.1016/0006-8993(89)91159-1. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- Minoshima SG, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) Eur J Neurosci. 2003a;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Smielewski P, Hodges JR. Limbic hypometabolism in Alzheimer’s disease and mild cognitive impairment. Annals Neurol. 2003b;54:343–351. doi: 10.1002/ana.10669. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Third edition Academic Press; San Diego: 1997. [Google Scholar]
- Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer’s disease. Neurobiol Aging. 2010;31:25–33. doi: 10.1016/j.neurobiolaging.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Poirier GL, Shires KL, Sugden D, Amin E, Thomas KL, Carter DA, Aggleton JP. Anterior thalamic lesions produce chronic and profuse transcriptional de-regulation in retrosplenial cortex: A model of retrosplenial hypoactivity and covert pathology. Thalamus and Related Systems. 2008;4:59–77. doi: 10.1017/S1472928808000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier GL, Aggleton JP. Post-surgical interval and lesion location within the limbic thalamus determine extent of retrosplenial cortex hypoactivity. Neuroscience. 2009;160:452–469. doi: 10.1016/j.neuroscience.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Poremba A, Jones D, Gonzalex-Lima F. Classical conditioning modifies cytochrome oxidase in the auditory system. Eur J Neurosci. 1998;10:3035–3043. doi: 10.1046/j.1460-9568.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Pothuizen HJ, Davies M, Aggleton JP, Vann SD. Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behav Brain Res. 2010;208:566–575. doi: 10.1016/j.bbr.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Pothuizen HJ, Davies M, Albasse MM, Aggleton JP, Vann SD. Granular and dysgranular retrosplenial cortices provide qualitatively different contributions to spatial working memory: evidence from immediate-early gene imaging in rats. Eur J Neurosci. 2009;30:877–888. doi: 10.1111/j.1460-9568.2009.06881.x. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Brigt P, Stanhope N,E, Kopelman MD. Correlations of regional cerebral metabolism with memory performance and executive function in patients with herpes encephalitis or frontal lobe lesions. Neuropsychology. 2005;19:555–565. doi: 10.1037/0894-4105.19.5.555. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Marsden P, Lasserson D, Sheldon N, Lewis P, Stanhope N, Guinan E, Kopelman MD. FDG-PET analysis and findings in amnesia resulting from hypoxia. Memory. 1999;7:599–612. doi: 10.1080/096582199387779. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Lasserson D, Marsden P, Stanhope N, Stevens T, Bello F, Kingsley D, Colchester A, Kopelman MD. FDG-PET findings in the Wernicke-Korsakoff syndrome. Cortex. 2003;39:1027–1045. doi: 10.1016/s0010-9452(08)70876-1. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, 3rd, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–9. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Richardson FC, Kaczmarek LK. Modification of delayed rectifier potassium currents by the Kv9.1 potassium channel subunit. Hearing Res. 2000;147:21–30. doi: 10.1016/s0378-5955(00)00117-9. [DOI] [PubMed] [Google Scholar]
- Salinas M, Duprat F, Heurteaux C, Hugnot JP, Lazdunski M. New modulatory a subunits for mammalian Shab K1 channels. J Biol Chem. 1997;272:24371–24379. doi: 10.1074/jbc.272.39.24371. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P, Sissson JC, Verma IM. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988;334:314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schneider LE, Mastroeni D, Caselli R, Kukull W, Morris JC, Hulette CM, Schmechel D, Rogers J, Stephan DA. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc Natl Acad Sci U S A. 2008;105:4441–4446. doi: 10.1073/pnas.0709259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. J Comp Neurol. 1993;330:533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Shibata H. Organization of projections of rat retrosplenial cortex to the anterior thalamic nuclei. Eur J Neurosci. 1998;10:3210–3219. doi: 10.1046/j.1460-9568.1998.00328.x. [DOI] [PubMed] [Google Scholar]
- Sripanidkulchai K, Wyss JM. The laminar organization of efferent neuronal cell bodies in the retrosplenial granular cortex. Brain Res. 1987;406:255–269. doi: 10.1016/0006-8993(87)90790-6. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Hoesing JM. Posterior cingulate cortex and spatial memory: A microlimnology analysis. In: Vogt BA, Gabriel M, editors. Neurobiology of the cingulate cortex and limbic thalamus: a comprehensive handbook. Birkhauser; Boston: 1993. pp. 461–477. [Google Scholar]
- Takeuchi Y, Allen GV, Hopkins DA. Transnuclear transport and axon collateral projections of the mamillary nuclei in the rat. Brain Res Bull. 1985;14:453–468. doi: 10.1016/0361-9230(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer’s patients: superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–4930. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154:483–491. doi: 10.1016/j.bbr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- van Groen T, Liu L, Ikonen S, Kadish I. Diffuse amyloid deposition, but not plaque number is reduced in amyloid precursor protein/presenilin 1 doubel-transgenic mice by pathway lesions. Neuroscience. 2003;119:1185–1197. doi: 10.1016/s0306-4522(03)00215-x. [DOI] [PubMed] [Google Scholar]
- van Groen T, Vogt BA, Wyss JM. Interconnections between the thalamus and retrosplenial cortex in the rodent brain. In: Vogt BA, Gabriel M, editors. Neurobiology of the cingulate cortex and limbic thalamus: a comprehensive handbook. Birkhauser; Boston: 1993. pp. 123–150. [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol. 1990;300:593–606. doi: 10.1002/cne.903000412. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol. 1992;315:200–216. doi: 10.1002/cne.903150207. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 2003;463:249–263. doi: 10.1002/cne.10757. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. Projections from the anterodorsal and anteroventral nucleus of the thalamus to the limbic cortex in the rat. J Comp Neurol. 1995;358:584–604. doi: 10.1002/cne.903580411. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802. doi: 10.1038/nrn2733. 2009. [DOI] [PubMed] [Google Scholar]
- Vann SD, Albasser MM. Hippocampal, retrosplenial, and prefrontal hypoactivity in a model of diencephalic amnesia: evidence towards an interdependent subcortical-cortical memory network. Hippocampus. 2009;19:1090–1102. doi: 10.1002/hipo.20574. [DOI] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Aggleton JP. Fos expression in the rostral thalamic nuclei and associated cortical regions in response to different spatial memory tests. Neuroscience. 2000;101:983–991. doi: 10.1016/s0306-4522(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: Areas 32, 24, and 29. J Comp Neurol. 1981;195:603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Rosene DL, Peters A. Synaptic termination of thalamic and callosal afferents in cingulate cortex of the rat. J Comp Neurol. 1981;201:265–283. doi: 10.1002/cne.902010210. [DOI] [PubMed] [Google Scholar]
- Wang B, Gonzalo-Ruiz A, Sanz JM, Campbell G, Lieberman AR. Glutamatergic components of the retrosplenial granular cortex in the rat. J Neurocytology. 2001;30:427–441. doi: 10.1023/a:1015069727171. [DOI] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ hybridization protocols for the brain. Academic Press; London: 1994. pp. 9–34. [Google Scholar]
- Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101. doi: 10.1016/0166-2236(89)90165-3. [DOI] [PubMed] [Google Scholar]
- Wright N, Erichsen JT, Vann SD, O’Mara S, Aggleton JP. Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. J Comp Neurol. 2010;518:2334–2354. doi: 10.1002/cne.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Ren K, Shimada M, Iwata K. Modulation of paratrigeminal nociceptive neurons following temporomandibular joint inflammation in rats. Exp Neurol. 2008;214:209–218. doi: 10.1016/j.expneurol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]