Abstract
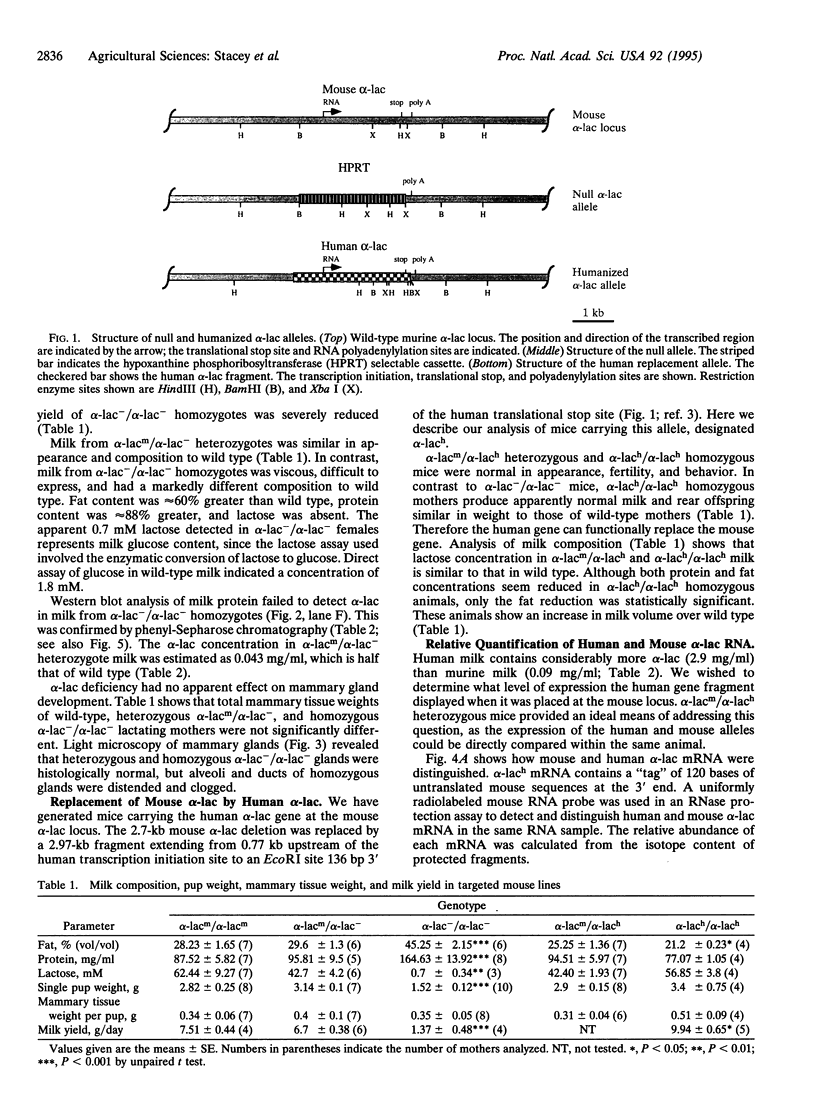
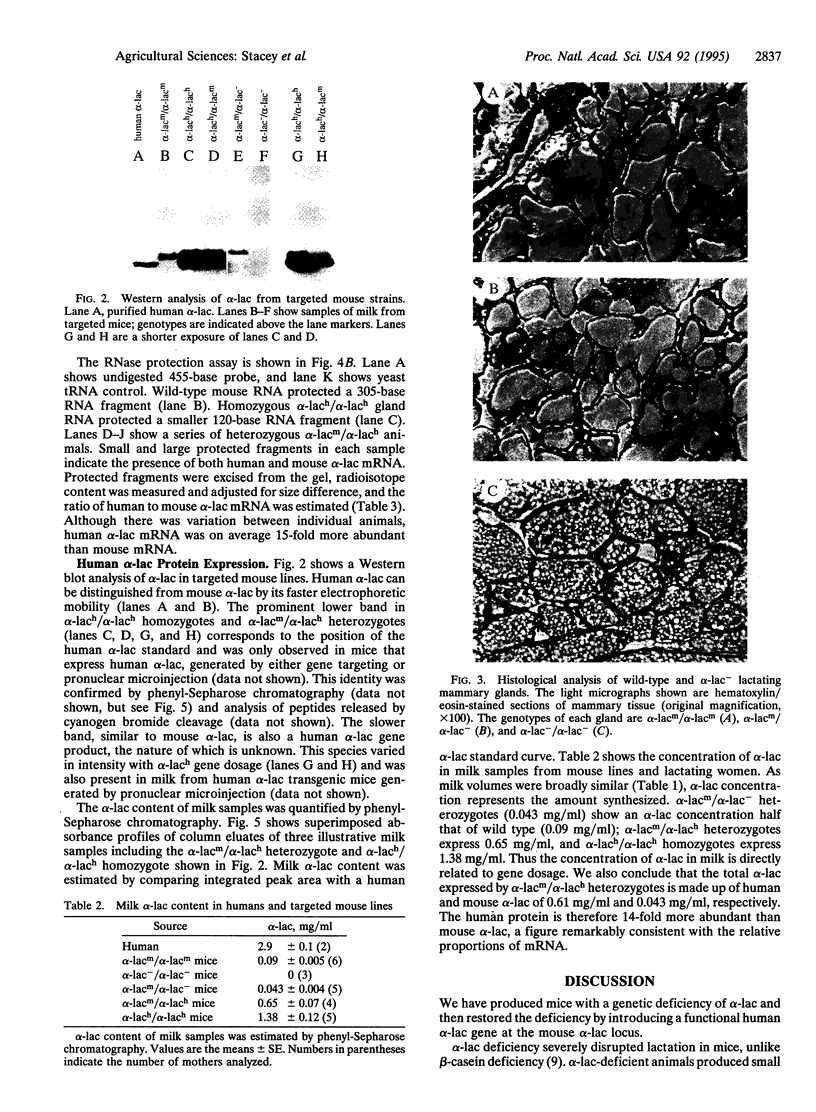
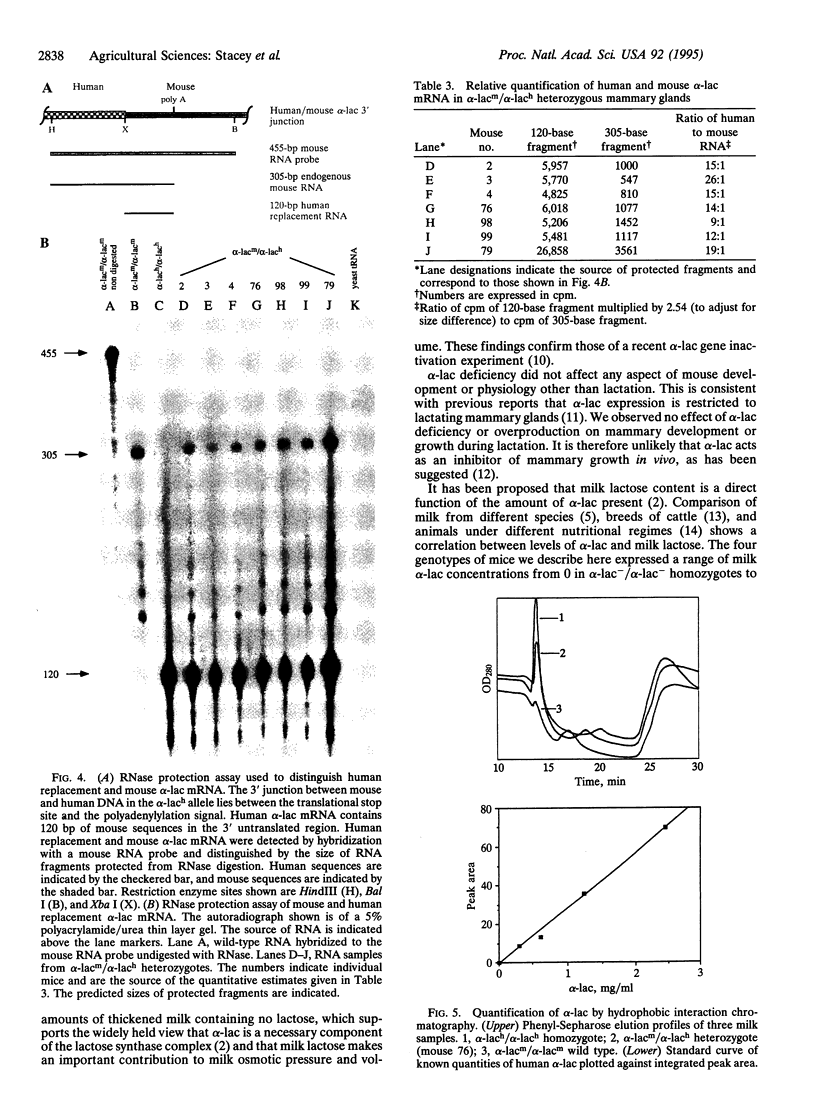
Mice carrying either a deletion of the murine alpha-lactalbumin (alpha-lac) gene (null allele) or its replacement by the human alpha-lac gene (humanized allele) have been generated by gene targeting. Homozygous null females are alpha-lac-deficient, produce reduced amounts of thickened milk containing little or no lactose, and cannot sustain their offspring. This provides definitive evidence that alpha-lac is required for lactose synthesis and that lactose is important for milk production. Females homozygous for the humanized allele lactate normally, indicating that human alpha-lac can replace murine alpha-lac. Mouse and human alpha-lac expression was compared in mice heterozygous for the humanized allele. The human gene expressed approximately 15-fold greater mRNA and approximately 14-fold greater protein than the mouse, indicating that the major determinants of human alpha-lac expression are close to, or within, the human gene and that the mouse locus does not exert a negative influence on alpha-lac expression. Variations in alpha-lac expression levels in nondeficient mice did not affect milk lactose concentration, but the volume of milk increased slightly in mice homozygous for the humanized allele. These variations demonstrated that alpha-lac expression in mice is gene dosage dependent.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. K., Brodbeck U., Kiyosawa I., Mawal R., Colvin B., Ebner K. E. Alpha-lactalbumin and the lactose synthetase reaction. J Biol Chem. 1970 Apr 25;245(8):2103–2108. [PubMed] [Google Scholar]
- Grimble R. F., Mansaray Y. K. Effects in rats of dietary protein inadequacy on lactose production, milk volume and components of the lactose synthetase complex (EC 2.4.1.22). Ann Nutr Metab. 1987;31(3):179–184. doi: 10.1159/000177266. [DOI] [PubMed] [Google Scholar]
- Khatra B. S., Herries D. G., Brew K. Some kinetic properties of human-milk galactosyl transferase. Eur J Biochem. 1974 May 15;44(2):537–560. doi: 10.1111/j.1432-1033.1974.tb03513.x. [DOI] [PubMed] [Google Scholar]
- Knight C. H., Maltz E., Docherty A. H. Milk yield and composition in mice: effects of litter size and lactation number. Comp Biochem Physiol A Comp Physiol. 1986;84(1):127–133. doi: 10.1016/0300-9629(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., White A. The role of nucleoside diphosphatase in a uridine nucleotide cycle associated with lactose synthesis in rat mammary-gland Golgi apparatus. Biochem J. 1977 Dec 15;168(3):423–433. doi: 10.1042/bj1680423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Clarke A. R., Hooper M. L., Horne D. S., Law A. J., Leaver J., Springbett A., Stevenson E., Simons J. P. Milk composition and lactation of beta-casein-deficient mice. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6138–6142. doi: 10.1073/pnas.91.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Vogel H. J. Metal-ion-dependent hydrophobic-interaction chromatography of alpha-lactalbumins. Anal Biochem. 1984 Aug 1;140(2):394–402. doi: 10.1016/0003-2697(84)90184-2. [DOI] [PubMed] [Google Scholar]
- McFadden T. B., Akers R. M., Beal W. E. Influence of breed and hormones on production of milk proteins by mammary explants from prepubertal heifers. J Dairy Sci. 1989 Jul;72(7):1754–1763. doi: 10.3168/jds.S0022-0302(89)79292-4. [DOI] [PubMed] [Google Scholar]
- Perry R. E., Blatchford G. J., Christensen M. A., Thorson A. G., Attwood S. E. Manometric diagnosis of anal sphincter injuries. Am J Surg. 1990 Jan;159(1):112–117. doi: 10.1016/s0002-9610(05)80615-4. [DOI] [PubMed] [Google Scholar]
- Powell J. T., Brew K. Glycosyltransferases in the Golgi membranes of onion stem. Biochem J. 1974 Aug;142(2):203–209. doi: 10.1042/bj1420203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulier S., Vilotte J. L., Stinnakre M. G., Mercier J. C. Expression analysis of ruminant alpha-lactalbumin in transgenic mice: developmental regulation and general location of important cis-regulatory elements. FEBS Lett. 1992 Feb 3;297(1-2):13–18. doi: 10.1016/0014-5793(92)80317-a. [DOI] [PubMed] [Google Scholar]
- Stacey A., Schnieke A., McWhir J., Cooper J., Colman A., Melton D. W. Use of double-replacement gene targeting to replace the murine alpha-lactalbumin gene with its human counterpart in embryonic stem cells and mice. Mol Cell Biol. 1994 Feb;14(2):1009–1016. doi: 10.1128/mcb.14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre M. G., Vilotte J. L., Soulier S., Mercier J. C. Creation and phenotypic analysis of alpha-lactalbumin-deficient mice. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6544–6548. doi: 10.1073/pnas.91.14.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilotte J. L., Soulier S. Isolation and characterization of the mouse alpha-lactalbumin-encoding gene: interspecies comparison, tissue- and stage-specific expression. Gene. 1992 Oct 1;119(2):287–292. doi: 10.1016/0378-1119(92)90285-w. [DOI] [PubMed] [Google Scholar]