ABSTRACT
The Wnt pathway plays important roles in multiple physiological and pathophysiological processes. Here, we report a novel mechanism that regulates the Wnt pathway through heterodimerization of the Wnt co-receptor low-density lipoprotein-receptor-related protein 6 (LRP6) and very low-density lipoprotein receptor (VLDLR); the latter belongs to the same protein family as LRP6 and was originally known as a receptor for lipoproteins. Knockdown of Vldlr expression elevated LRP6 protein levels and activated Wnt/β-catenin signaling, whereas overexpression of Vldlr suppressed Wnt signaling. Moreover, we demonstrate that the VLDLR ectodomain is essential and sufficient for inhibition of Wnt signaling. The VLDLR ectodomain accelerated internalization and degradation of LRP6 through heterodimerization with the LRP6 extracellular domain. Monoclonal antibodies specific for the VLDLR ectodomain blocked VLDLR–LRP6 heterodimerization, resulting in enhanced Wnt/β-catenin signaling in vitro and in vivo. Taken together, these findings suggest that heterodimerization of receptors in the membrane accelerates the turnover of LRP6, and represent a new mechanism for the regulation of Wnt/β-catenin signaling.
KEY WORDS: Wnt signaling, Lipoprotein receptor-related protein, LRP, VLDL
INTRODUCTION
Wnt ligands, a group of secreted cysteine-rich glycoproteins, bind to a co-receptor complex consisting of members of the G protein-coupled receptor family Frizzled (Fzd) and lipoprotein receptor-related proteins 5 and 6 (LRP5 and LRP6, respectively) and, subsequently, activate an intracellular signaling pathway (Logan and Nusse, 2004). In the absence of Wnt ligands, the transcription factor β-catenin is continuously phosphorylated by GSK-3β and then degraded. Upon binding of Wnt ligands to the receptors, LRP6 becomes phosphorylated. Subsequently, the GSK-3β–axin complex is sequestered to the plasma membrane, preventing β-catenin phosphorylation and degradation (MacDonald et al., 2009). A more recent study indicates that Wnt induces sequestration of GSK-3β from the cytosol into multivesicular bodies, so that cytosolic substrates are separated from this enzyme (Taelman et al., 2010). As a result, stabilized β-catenin accumulates in the cytosol and translocates to the nucleus. It then associates with T cell factor (TCF) to activate transcription of Wnt target genes including cyclin D1 (Ccnd1), Myc proto-oncogene (Myc), vascular endothelial growth factor (VEGF), and Axin2 (Klaus and Birchmeier, 2008).
Wnt/β-catenin signaling plays central roles in the regulation of multiple physiological processes (Clevers, 2006). Dysregulation of Wnt signaling results in aberrant regulation of proliferation, migration, apoptosis and differentiation, and are associated with developmental defects, neoplasia and neovascular disorders (Clevers, 2006; Wodarz and Nusse, 1998; Zhang and Ma, 2010). Owing to the broad biological functions of Wnt/β-catenin signaling, regulation of Wnt signaling is of great significance for understanding biological processes and for the development of clinical applications (Rey and Ellies, 2010). Modulation of Wnt/β-catenin signaling is known to occur at multiple levels through conserved cellular mechanisms (MacDonald et al., 2009). Of numerous regulators, those targeting the Wnt co-receptor LRP6 are of particular importance, as LRP6 plays a significant role in ligand reception and signal amplification. LRP6 contains numerous Wnt-ligand-binding sites in its extracellular domain as well as five repeats of the PPSPxS motif in the intracellular domain of LRP6, which are sufficient to transmit signals from Wnt ligands to the intracellular cascade when phosphorylated (MacDonald et al., 2008; Zeng et al., 2008).
LRP6 is a member of the low-density lipoprotein receptor (LDLR) family (Hussain et al., 1999). Several structural and functional features are conserved within the LDLR family, including a large ectodomain, a single transmembrane domain, and an intracellular domain. The ectodomains of the LDLR family proteins share some structural similarities, including domains with distinct functions, such as an LDLR type-A domain for lipoprotein interaction and an LDLR type-B domain with EGF-precursor homology domains composed of YWTD β-propeller structures for Wnt interaction (Ettenberg et al., 2010; Krieger and Herz, 1994). Moreover, the YWTD–EGF repeats have been shown to mediate LRP6 homodimer formation (Liu et al., 2003).
Very low-density lipoprotein receptor (VLDLR) is another member of the LDLR family and is known to mediate lipid metabolism (Goudriaan et al., 2001). Vldlr−/− mice have been shown to develop abnormal angiogenesis in the retina, and their phenotypes recapitulate those of human diseases that involve intra- and sub-retinal neovascularization, including wet age-related macular degeneration, choroidal anastomosis, retinal angiomatous proliferation and macular telangiectasia (Chen et al., 2007; Heckenlively et al., 2003; Hu et al., 2008; Li et al., 2007). We have previously shown that neovascularization in the retinas of Vldlr−/− mice occurs through activation of Wnt/β-catenin signaling, suggesting that VLDLR has an inhibitory role in Wnt/β-catenin signaling (Chen et al., 2007). However, the mechanism for VLDLR regulation of Wnt/β-catenin signaling was not understood, and it was unclear whether VLDLR interacts directly with Wnt/β-catenin signaling. In the present study, we have investigated the interactions of VLDLR with LRP6, and elucidated the mechanism by which VLDLR regulates Wnt signaling through physical interaction with LRP6.
RESULTS
Knockdown of Vldlr expression upregulates Wnt/β-catenin signaling by increasing LRP6 levels
We speculated that the retinal pigment epithelium (RPE) contributes to neovascularization by secreting pro-angiogenic factors as the neovasculature grows towards the RPE and accumulates in the sub-retinal space in Vldlr−/− mice. Thus, we used cultured human RPE cells (hTERT-RPE-1) to investigate the direct impact of VLDLR deficiency on the activation of Wnt signaling, which contributes to neovascularization. siRNA knockdown of Vldlr significantly increased the activity of TCF/β-catenin in the absence and presence of the Wnt ligand Wnt3A, as indicated by increased TOPFLASH activity (Fig. 1A). Consistently, secretion of VEGF, encoded by β-catenin target genes, was upregulated by 2.5-fold following Vldlr knockdown in the absence of Wnt3A and 5.5-fold in the presence of Wnt3A, as measured in the culture medium using ELISA (Fig. 1B). To determine whether Vldlr deficiency activates Wnt/β-catenin signaling through the canonical Wnt pathway, we measured LRP6 phosphorylation and β-catenin stabilization. The Vldlr siRNA induced a substantial increase of phosphorylated LRP6 (pLRP6) as indicated by mobility shift in the presence of Wnt3A-conditioned medium (see Materials and Methods for further information on how conditioned media were obtained). Western blot analysis with an antibody specific for non-phosphorylated Ser33/Ser37/Thr41 residues of β-catenin (non-p-β-catenin) demonstrated that Vldlr knockdown increased levels of non-p-β-catenin in cells treated with Wnt3A-conditioned medium (Fig. 1C). Taken together, these results indicated that VLDLR deficiency potentiated Wnt signaling in response to the Wnt ligand, Wnt3A.
Fig. 1.
Knockdown of Vldlr upregulated canonical Wnt signaling. RPE cells were transfected with siRNA (20 nmol/l) for Vldlr or control siRNA and, when needed, co-transfected with siRNA and TOPFLASH (0.2 µg) and control pRL-TK (0.04 µg) reporter plasmids. Cells were subsequently treated with 20% Wnt3A-conditioned medium (Wnt3A CM) and with L-cell-conditioned medium (LCM) as control. (A) TOPFLASH activity was measured by luciferase assay after a 16 h treatment with Wnt3A CM. Values are expressed as the firefly:Renilla ratio normalized to the control LCM group (n = 5, *P<0.05). Knockdown efficiency was confirmed by western blot analysis for VLDLR in the same cells. (B) Quantification of VEGF levels secreted into the culture medium by ELISA after an 18 h exposure to Wnt3A CM. Values are the levels of VEGF relative to those of cells transfected with the control siRNA and treated with LCM (n = 3, †P<0.001). (C) pLRP6 (Ser1490; P, phosphorylated species, U, unphosphorylated species), total LRP6, VLDLR and non-p-β-catenin (Ser33/Ser37/Thr41) in cells after a 2 h exposure to Wnt3A CM were measured by western blot analysis and semi-quantified by densitometry (n = 3; **P<0.01).
The ectodomain of VLDLR is essential and sufficient for suppression of Wnt/β-catenin signaling
The expression vector for the full-length (FL) LRP6 was co-transfected with a vector expressing VLDLR or its deletion mutants into HEK-293T cells. Overexpression of LRP6 alone induced TCF/β-catenin activity, whereas co-expression of FL VLDLR diminished this induction. To determine which domain in VLDLR is essential for inhibition of Wnt/β-catenin signaling, we generated deletion mutants of VLDLR, including a C-terminus deletion mutant (VLDLRΔC), in which the entire intracellular domain was deleted, the N-terminus deletion mutant (VLDLRΔN), in which the entire extracellular domain was deleted, and a soluble peptide of the VLDLR ectodomain (sVLDLR-N). A TOPFLASH assay showed that the FL VLDLR and VLDLRΔC mutant, but not the VLDLRΔN mutant, inhibited TCF/β-catenin activity, suggesting that the ectodomain of VLDLR (VLDLR-N) is required for the inhibition of Wnt/β-catenin signaling (Fig. 2A). In accordance with TOPFLASH activity assay, the expression of an endogenous Wnt target gene, Axin2, was downregulated by FL VLDLR and VLDLRΔC, but not by VLDLRΔN (Fig. 2A). Furthermore, our results indicated that FL VLDLR and VLDLRΔC, but not VLDLRΔN, suppressed Wnt3A-mediated Wnt/β-catenin signaling, suggesting that the extracellular domain of VLDLR is essential for inhibition of Wnt signaling (Fig. 2B).
Fig. 2.
sVLDLR-N suppressed Wnt/β-catenin signaling. (A,B) HEK-293T cells were co-transfected with the TOPFLASH vector and plasmids expressing LRP6 (LRP6), full-length VLDLR (FL), VLDLRΔC (ΔC) and VLDLRΔN (ΔN). (A) TOPFLASH activity was measured 48 h following the transfection and expressed as the firefly/Renilla ratio normalized to control cells transfected with an empty plasmid (mock; mk) (n = 4, **P<0.01, †P<0.001). Axin2 mRNA levels were measured 48 h following the transfection and normalized to the levels of 18S (n = 4, **P<0.01, †P<0.001) (B) TOPFLASH activity and Axin2 mRNA levels in the cells 48 h post-transfection, and further treated with Wnt3A-conditioned medium (Wnt3A CM) for another 16 h (n = 3, †P<0.001). (C) RPE cells were transfected with the TOPFLASH vector followed by a 16 h treatment with sLDLR-conditioned medium (LN) or sVLDLR-N-conditioned medium (VLN). TOPFLASH activity was measured and normalized (n = 3, **P<0.01). (D) The RPE cells were pre-treated for 2 h with increasing volumes of sVLDLR-N-conditioned medium, and then exposed to Wnt3A CM for another 2 h. pLRP6 and total LRP6 were measured using western blot analysis. Added sVLDLR-N was detected in the medium by using an anti-His-tag antibody. (E) RPE cells were infected with adenovirus expressing VLDLR-N or control virus expressing GFP for 48 h, followed by membrane/nuclear extraction. Nuclear β-catenin levels were measured. The membrane and nuclear fractions were validated using a nuclear marker Fibrillarin and a membrane marker Na+/K+-ATPase. (F) RPE cells were transfected with the plasmid expressing VLDLR-N or empty plasmid (Mock) for 48 h, and further treated with Wnt3A CM for another 16 h. Axin2 mRNA levels were normalized to the 18S levels (n = 3, †P<0.001). (G) Complex formation of Fzd8-CRD–Wnt3A–LRP6N and its inhibition by sVLDLR-N. Conditioned media from HEK-293T cells expressing Fzd8-CRD, LRP6N or VLDLR-N were incubated with Wnt3A CM for 2 h. The resulting mix of conditioned media was then incubated with protein A/G-agarose to precipitate the Fzd8-CRD–Wnt3A–LRP6N complex; precipitates were then immunoblotted with the anti-Myc antibody to detect Myc-tagged LRP6N. (H) The VLDLR FL expression plasmid was co-transfected with the TOPFLASH vector into RPE cells. The effect of VLDLR on TCF/β-catenin transcriptional activity by Wnt3A CM (20%) or LiCl (30 mmol/l) was measured by TOPFLASH assay (n = 4, **P<0.01; †P<0.001; N.S, not statistically significant). (I) TOPFLASH activity assay in HEK-293T cells co-transfected with the TOPFLASH vector and plasmids expressing the full-length LRP6 (LRP6FL) and LRP6ΔN with the treatment of sVLDLR-N-conditioned medium for 16 h (n = 3, †P<0.001, N.S; not statistically significant). Expression levels of LRP6FL and LRP6ΔN were confirmed using different anti-LRP6 antibodies as indicated.
Next, we determined whether the sVLDLR-N peptide alone is sufficient to elicit the inhibitory effect of VLDLR on Wnt/β-catenin signaling. Conditioned medium containing secreted sVLDLR-N was collected from HEK-293T cells stably transfected with an expression plasmid of sVLDLR-N. As a negative control, conditioned medium containing the soluble ectodomain of low-density lipoprotein receptor (sLDLR-N; sLDLR-N-conditioned medium) was used because LDLR deficiency does not upregulate Wnt/β-catenin signaling (Ishibashi et al., 1993; Rudolf et al., 2005). In cultured RPE cells, which express relatively high levels of endogenous LRP6, sVLDLR-N suppressed TCF/β-catenin activity in the presence and absence of Wnt3A-conditioned medium in a concentration-dependent manner (Fig. 2C). Interestingly, an excess amount of sVLDLR-N suppressed basal activity of Wnt/β-catenin signaling in the absence of Wnt ligands (Fig. 2C). The results showed that 4 h exposure of RPE cells to sVLDLR-N decreased Wnt3A-induced LRP6 phosphorylation in a concentration-dependent manner (Fig. 2D), which might be why sVLDLR-N suppresses TCF/β-catenin activity. In accordance with the suppressed LRP6 phosphorylation, the level of nuclear β-catenin was reduced by VLDLR-N (Fig. 2E). Moreover, Wnt3A-induced overexpression of Axin2 mRNA was decreased by sVLDLR-N (Fig. 2F). Next, we sought to determine whether sVLDLR-N interferes with Wnt-induced formation of the complex between Fzd and LRP6, an initiation step of Wnt/β-catenin signaling. Co-immunoprecipitation assays indicated that the Fzd8 cystein-rich domain (Fzd-CRD) co-precipitated with LRP6N in the presence of Wnt3A, suggesting the Wnt ligand-induced dimerization of LRP6 and Fzd. sVLDLR-N decreased dimerization of LRP6 and Fzd-CRD, as indicated by co-precipitation assay, suggesting that sVLDLR-N blocks formation of the Fzd–LRP6 receptor complex (Fig. 2G).
LiCl inhibits GSK-3β activity and, consequently, stabilizes β-catenin, thus inducing TCF/β-catenin activity independently of Wnt ligand–receptor interaction (Klein and Melton, 1996). In RPE cells, overexpression of FL VLDLR inhibited Wnt3A-mediated Wnt/β-catenin signaling, but not signaling activated by LiCl, suggesting that VLDLR suppresses Wnt/β-catenin signaling at the level of the Wnt receptor (Fig. 2H). To determine whether sVLDLR-N inhibits Wnt signaling through interactions with the ectodomain of LRP6, we activated Wnt/β-catenin signaling by using the extracellular domain deletion mutant of LRP6 (LRP6ΔN), which is known as a constitutively active mutant that triggers the Wnt pathway in the absence of a Wnt ligand (Liu et al., 2003). As shown by a TOPFLASH assay, sVLDLR-N abolished Wnt/β-catenin signaling activated by full-length LRP6 but not Wnt/β-catenin signaling activated by LRP6ΔN, suggesting that the LRP6 extracellular domain is essential for the suppression of Wnt/β-catenin signaling mediated through VLDLR (Fig. 2I).
sVLDLR-N decreases total and cell surface LRP6 levels by accelerating internalization of LRP6
We next sought to determine whether total LRP6 levels and membrane localization of LRP6 are affected by VLDLR. We first determined whether the Vldlr siRNA affects total LRP6 levels. Interestingly, VLDLR knockdown increased total LRP6 protein levels 2.5 fold in RPE cells (Fig. 3A). A previous study has shown that Lrp6 mRNA levels were increased in the eyecup of the Vldlr−/− mice, compared with those of wild-type mice (Chen et al., 2007). However, LRP6 mRNA levels were not significantly altered following Vldlr knockdown in cultured RPE cells (Fig. 3B), suggesting that resultant LRP6 upregulation occurs at the protein level in RPE cells. The discrepancy between knockout mice and cultured RPE cells could be attributed to the context-specific alteration. Therefore, we investigated whether addition of sVLDLR-N decreased total LRP6 levels. Although a short incubation (4 h) with sVLDLR-N decreased pLRP6 levels but not total LRP6 levels in the RPE cells (Fig. 2D), prolonged incubation (16 h) with sVLDLR-N decreased levels of total LRP6 in a concentration-dependent manner (Fig. 3C).
Fig. 3.
sVLDLR-N downregulated total and cell surface LRP6 levels by accelerating degradation of LRP6. (A) Western blot and densitometry analysis of total LRP6 and VLDLR in the RPE cells 36 h after transfection of the siRNAs as indicated. The LRP6/β-actin ratio was expressed as fold of that in cells transfected with the control siRNA (n = 3, *P<0.05). (B) The Vldlr and Lrp6 mRNA levels were measured by real-time RT-PCR analysis 36 h after the transfection and normalized to 18S RNA levels (n = 3, †P<0.001, N.S; not statistically significant). (C) Western blot analysis of LRP6 in RPE cells exposed to the conditioned medium containing Myc-tagged LDLR-N or increasing concentrations of His-tagged sVLDLR-N for 16 h. sVLDLR-N and sLDLR-N in conditioned medium were measured using antibodies for the His and Myc tags (n = 3, *P<0.05, †P<0.001). (D) RPE cells were transfected with the Vldlr siRNA or control siRNA for 36 h, and then treated with LDLR-N-conditioned medium (LN) or VLDLR-N-conditioned medium (VLN) for an additional 16 h. Cell surface proteins were biotinylated, and LRP6 was precipitated with an anti-LRP6 antibody (specific for carboxyl terminus). The biotinylated LRP6 in the immunoprecipitated proteins was detected using streptavidin-HRP. Precipitation efficiency was evaluated by measurement of precipitated total LRP6 (specific for N-terminus). Biotinylated species of total cell lysates prior to immunoprecipitation were immunoblotted using an anti-Biotin antibody. (E) Measurement of LRP6 stability: The RPE cells were transfected with the Vldlr siRNA or control siRNA for 36 h and then treated with sLDLR-N- or sVLDLR-N-conditioned medium supplemented with cycloheximide (CHX; 50 µg/ml) for the indicated durations. Total LRP6 levels were measured by western blot analysis and densitometry. p53 was used as a reference of protein half-life measurement. The results are expressed as LRP6/β-actin ratio relative to that with siRNA transfection at 0 h of CHX treatment (n = 3, *P<0.05, **P<0.01, †P<0.001).
To elucidate the mechanism by which VLDLR modulates total LRP6 levels, we measured the impact of VLDLR on LRP6 internalization and degradation. We biotinylated cell surface LRP6, immunoprecipitated total LRP6 and measured the biotinylated cell surface LRP6 by using streptavidin. The results showed that VLDLR knockdown increased biotinylated LRP6, suggesting elevated levels of cell surface LRP6 (Fig. 3D). This effect was reversed by treatment with sVLDLR-N-conditioned medium (Fig. 3D). To confirm this effect of VLDLR, we compared the rate of LRP6 degradation in the presence and absence of sVLDLR-N in order to determine whether destabilization of LRP6 is responsible for the reduced level of LRP6. To elucidate the effect of sVLDLR-N on LRP6, we first depleted endogenous VLDLR by pre-incubation with the Vldlr siRNA, with scrambled siRNA as control. LRP6 showed an apparent half-life of ∼5–6 h under normal conditions. Knockdown of Vldlr slowed the degradation of LRP6 and prolonged its half-life (>8 h) (Fig. 3E). The LRP6 half-life prolongation by the Vldlr siRNA was reversed by incubation with sVLDLR-N-conditioned medium (∼6 h), but not by LDLR-N-conditioned medium (Fig. 3E). Taken together, these results suggest that sVLDLR-N inhibits Wnt/β-catenin signaling by accelerating LRP6 degradation, which is consistent with the enhanced LRP6 internalization. Moreover, the rapid turn-over of LRP6 by sVLDLR-N might be attributed to the reduction in recycling of internalized LRP6 back to the plasma membrane, with a concomitant increase in targeting to the lysosome.
VLDLR and LRP6 form a heterodimer through interaction of their ectodomains
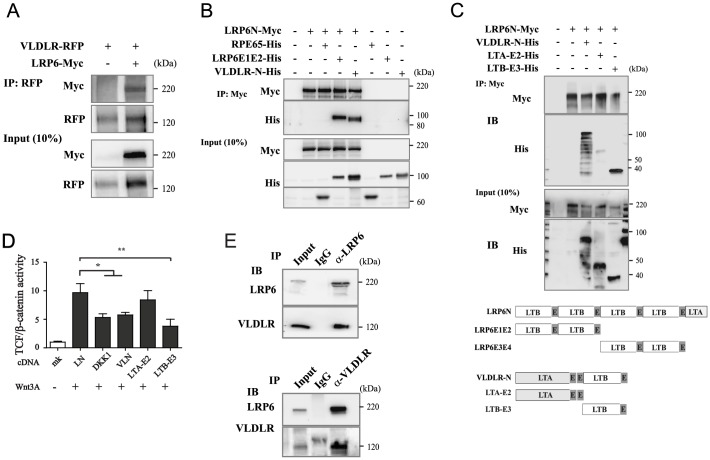
To further understand how VLDLR accelerates LRP6 degradation, we investigated the possible physical interactions between these two membrane receptors of the same gene family. VLDLR and LRP6 share a similar structure of their extracellular domains including LDLR type-A and type-B and EGF-like repeats; their intracellular domains, however, share few similarities. A previous study that focused on the molecular mechanism of LRP6 activation showed that LRP6 naturally forms a homodimer through LDLR type-B and EGF-like repeats in the ectodomain (Liu et al., 2003). Based on this observation, we investigated whether VLDLR forms a heterodimer with LRP6. As shown by co-immunoprecipitation assay, VLDLR co-precipitated with LRP6, suggesting a direct physical interaction between VLDLR and LRP6 (Fig. 4A). To determine whether the ectodomain is sufficient for this interaction, LRP6N-Myc-conditioned medium was incubated separately with either purified His-tagged LRP6E1E2 (the first two propeller domains of LRP6N) or conditioned medium containing sVLDLR-N; His-tagged RPE65 was used as negative control. When LRP6N-Myc was precipitated with an anti-Myc antibody, LRP6E1E2 and sVLDLR-N, but not RPE65, co-precipitated with LRP6N-Myc, indicating that the physical association of VLDLR and LRP6 occurs through their ectodomain (Fig. 4B). To determine whether this relationship was through a direct interaction, we further characterized the LRP6-interacting domain in VLDLR-N using a deletion mutant that includes the amino-terminus (LDLR type A with EGF-like repeat 1 and 2; LTA-E2) as well as a carboxy-terminus region (LDLR type B with EGF-like repeat 3; LTB-E3) of the VLDLR. sVLDLR-N and LTB-E3, but not LTA-E2 co-precipitated with LRP6N, suggesting that LTB-E3 mediates the physical interaction between VLDLR and LRP6 (Fig. 4C). To further examine whether the LTB-E3-specific interaction with LRP6 results in the inhibition of Wnt/β-catenin signaling, we measured TCF/β-catenin activity upon forced expression of LTB-E3. LTB-E3, but not LTA-E2, significantly suppressed Wnt3A-induced TCF/β-catenin activity, supporting a domain-specific and interaction-mediated inhibitory mechanism of VLDLR on Wnt signaling (Fig. 4D).
Fig. 4.
VLDLR formed a heterodimer with LRP6 through its ectodomain. (A) Co-immunoprecipitation of VLDLR-RFP with LRP6-Myc. HEK-293T cells were co-transfected with expression vectors for VLDLR-RFP and LRP6-Myc. At 48 h post-transfection, the membrane in total cell lysates was solubilized. VLDLR-RFP was immunoprecipitated with an anti-RFP antibody, and the precipitates were immunoblotted with an anti-Myc antibody. (B) Immunoprecipitation of LRP6N with sVLDLR-N. HEK-293T cells were transfected separately with vectors expressing LRP6N-Myc or sVLDLR-N-His. Each of the obtained conditioned medium was mixed and incubated with anti-Myc agarose. The E1E2 domain of LRP6 with a His-tag (LRP6E1E2-His) was incubated with LRP6N-conditioned medium as a positive control, whereas RPE65-His-conditioned medium was used as a negative control. LRP6N-Myc was immunoprecipitated, and the precipitates were immunoblotted with anti-His-tag antibody. (C) Top panel: Immunoprecipitation of Myc-tagged LRP6N with LDLR type-B (LTB) repeats in the VLDLR ectodomain. LRP6N-Myc was immunoprecipitated using anti-Myc agarose in conditioned medium from HEK-293 cells transfected with indicated plasmids. Precipitates were immunoblotted separately with antibodies against the His and Myc tags. Bottom panel: schematic of the constructs used. (D) TOPFLASH assay in RPE cells transfected with different deletion mutants of the VLDLR ectodomain for 48 h, followed by incubation with 20% Wnt3A-conditioned medium for 16 h (n = 3, *P<0.05 **P<0.01). (E) Co-immunoprecipitation of endogenous VLDLR and LRP6 in RPE cells. VLDLR and LRP6 in the solubilized membrane fractions were immunoprecipitated separately using antibodies against their respective C-termini. The precipitates were immunoblotted separately with antibodies against LRP6 and VLDLR. The solubilized membrane fraction prior to precipitation (10%) was used as input. Non-specific IgG was used as negative control in immunoprecipitation.
We then verified that endogenous VLDLR forms a heterodimer with LRP6 in RPE cells. Immunoprecipitation of LRP6 in the solubilized membrane fraction using an anti-LRP6 antibody specific for its C-terminus pulled down endogenous VLDLR, suggesting that endogenous VLDLR and LRP6 naturally interact in the membrane. This conclusion was supported by reverse immunoprecipitation, showing that LRP6 co-precipitated with VLDLR (Fig. 4E). Taken together, these results demonstrate that VLDLR and LRP6 heterodimerize through their extracellular domains.
Blocking the ectodomain of VLDLR with monoclonal antibodies abolishes its heterodimerization with LRP6 and results in activation of Wnt/β-catenin signaling
To further establish VLDLR–LRP6 heterodimerization as an inhibitory module of Wnt/β-catenin signaling, we developed monoclonal antibodies specifically recognizing the VLDLR ectodomain in order to block ectodomain-mediated heterodimerization of VLDLR and LRP6. A number of the monoclonal antibodies were highly specific to VLDLR, as shown in western blot analysis of the RPE cell lysates (Fig. 5A). To determine whether these monoclonal antibodies simulate the effect of Vldlr knockdown, RPE cells were treated with different clones of anti-VLDLR-N antibodies, with non-specific mouse IgG as control. A TOPFLASH assay indicated that these antibodies enhanced basal TCF/β-catenin activity in comparison to the non-specific IgG (Fig. 5B). Similar to Vldlr knockdown, these antibodies increased levels of both pLRP6 and total LRP6 (Fig. 5C). In accordance with the increase in pLRP6, non-p-β-catenin levels were elevated by the antibodies, whereas levels of VLDLR were unchanged (Fig. 5C).
Fig. 5.
Blockade of the VLDLR ectodomain abolished LRP6 heterodimerization and activated Wnt/β-catenin signaling. (A) Specificity of an anti-VLDLR-N antibodies (3D10C9, 4G4G4, and 4G4F4). The RPE cells were transfected with the Vldlr siRNA, and cell lysates were subjected to western blot analysis with anti-VLDLR-N antibodies. (B) A TOPFLASH assay after incubation with the anti-VLDLR-N antibodies. RPE cells transfected with the TOPFLASH vector were incubated with three independent clones of anti-VLDLR antibodies (20 µg/ml) for 16 h. TOPFLASH activity was measured and expressed as the firefly/Renilla ratio relative to that of control IgG incubation (n = 3, †P<0.001). (C) The RPE cells were treated with the indicated antibodies (20 µg/ml) for 6 h. pLRP6, LRP6 and non-p-β-catenin were measured using western blot analysis and densitometry, normalized to β-actin and expressed as ratio to that in un-treated cells (n = 3, †P<0.001). (D) Immunoprecipitation of endogenous LRP6-VLDLR heterodimer in the RPE cells treated with an anti-VLDLR-N antibody (3D10C9). VLDLR in the indicated membrane fraction was immunoprecipitated with an anti-VLDLR antibody (specific for the carboxyl terminus), and the precipitates were blotted with an anti-LRP6 antibody (specific for the C-terminus). Asterisk indicates a non-specific band. Molecular mass of the LRP6 band is marked by an arrow. Levels of LRP6 co-precipitated with VLDLR were normalized to input LRP6 levels and expressed as a ratio to that of control IgG treatment and averaged in three independent assays (n = 3, *P<0.05). IP, immunoprecipitation with anti-VLDLR; IB, immunoblotting with anti-LRP6 and anti-VLDLR. (E) Conditioned medium of sVLDLR-N alone, or conditioned medium of sVLDLR-N pre-incubated with either non-specific IgG or 3D10C9 for 1 h were added to the RPE cells for 16 h in the presence of Wnt3A-conditioned medium. Fzd8-CRD was used as a positive control.
We next determined whether antibody-induced stimulation of basal Wnt/β-catenin signaling was due to decreased heterodimerization of VLDLR and LRP6. As indicated by co-immunoprecipitation assay, endogenous LRP6 co-precipitated with VLDLR was significantly decreased after the incubation with a clone of the anti-VLDLR antibodies compared to that incubated with the non-specific IgG, suggesting that the antibodies, indeed, blocked the heterodimerization of VLDLR and LRP6 (Fig. 5D).
Last, when the RPE cells were incubated with sVLDLR-N-conditioned medium together with the monoclonal anti-VLDLR antibody 3D10C9, the inhibitory effect of sVLDLR-N on Wnt3A-induced TCF/β-catenin transcriptional activity was abolished as indicated by the TOPFLASH assay (Fig. 5E). This suggests that the antibody neutralizes the inhibitory activity of sVLDLR-N on Wnt signaling (Fig. 5E). Taken together, these results suggest that VLDLR-LRP6 heterodimerization is an essential inhibitory mechanism for LRP6 activation.
Wnt3A lacks physical interaction with VLDLR-N
We next sought to determine whether VLDLR serves as a trap for Wnt ligands and competes with LRP6 for ligand binding. The VLDLR extracellular domain comprises three repetitive LDLR-like domains with EGF-like repeats, which could serve as Wnt ligand-binding sites. To overcome the difficulty that VLDLR does not have defined downstream signaling cascades for quantitative measurement, we generated a chimeric mutant containing the VLDLR extracellular and LRP6 intracellular domains (VLN6C). A TOPFLASH assay showed that the chimeric receptor did not mediate Wnt signaling activated by canonical or noncanonical Wnt ligands (Wnt3A and Wnt5A) (Fig. 6A). Furthermore, Wnt3A increased phosphorylation of LRP6 but not VLN6C. Additionally, Wnt5A decreased phosphorylation of LRP6 but not VLN6C (Fig. 6B). These observations suggest that the VLDLR extracellular domain does not respond to Wnt ligands.
Fig. 6.
VLDLR was not bound by Wnt ligands. (A) HEK-293T cells were co-transfected with the TOPFLASH vector and plasmids as indicated. The cells were treated with 20% L-cell-conditioned medium (LCM, control-conditioned medium) and Wnt3A- or Wnt5A-conditioned media (Wnt3A or Wnt5A, respectively) for 16 h, and TOPFLASH activity measured. Values are relative to that in cells transfected with empty vector (pcDNA6) and LCM treatment (n = 3, †P<0.001, N.S; not statistically significant). (B) HEK-293T cells were transfected with Myc-tagged VLDLR FL (VLDLR), VLN6C and LRP6 FL (LRP6). The cells were treated with LCM, Wnt3A- and Wnt5A-conditioned medium for 2 h, followed by western blot analysis using anti-pLRP6 and anti-Myc antibodies. (C) Interaction of Wnt3A with the E3E4 domain of LRP6 (LRP6E3E4), but not sVLDLR-N. Conditioned media from HEK-293T cells that had been transfected as indicated were immunoprecipitated with anti-HA agarose, and the precipitates were blotted with an anti-His tag antibody. Interaction of Wnt3A-HA with LRP6E3E4 was used as a positive control of immunoprecipitation assay. (D) Co-immunoprecipitation of Wnt3A, LRP6 and VLDLR. HEK-293T cells were co-transfected with expression vectors for LRP6-Myc, VLDLR-N-His and Wnt3A-HA. At 48 h post-transfection, total cell lysates were subjected to immunoprecipitation of Wnt3A-HA using anti-HA agarose. Co-precipitation of VLDLR-N-His and LRP6-Myc was evaluated by western blotting. β-actin was used as a loading control.
To further confirm the lack of binding of Wnt ligands to VLDLR, we tested whether there is a direct interaction between Wnt3A and VLDLR or LRP6 exists. Immunoprecipitation of Wnt3A in LRP6E3E4- or sVLDLR-N-conditioned medium demonstrated that LRP6E3E4, but not sVLDLR-N, co-precipitated with Wnt3A, suggesting a physical interaction of Wnt3A with LRP6E3E4 but not with sVLDLR-N (Fig. 6C). However, immunoprecipitation showed that LRP6 and VLDLR-N co-precipitated with Wnt3A, suggesting the formation of a complex between the Wnt ligand and VLDLR-N/LRP6 (Fig. 6D). Taken together, these results indicate that VLDLR does not serve as an independent Wnt receptor.
Blockade of VLDLR increases levels of total LRP6 and activates Wnt signaling in vivo
To determine whether VLDLR-LRP6 heterodimerization inhibits Wnt/β-catenin signaling in the retina, we injected the purified anti-VLDLR antibody 3D10C9 into the vitreous cavity of C57BL/6J mice and injected a non-specific IgG into the contralateral eye. Retinal LRP6 and non-p-β-catenin were measured by western blotting one week following the injection. Consistent with the results in cultured RPE cells, the retinas from 3D10C9-injected eyes showed significantly increased levels of LRP6 and non-p-β-catenin, compared to those injected with the control IgG (Fig. 7A,B). Moreover, quantitative real-time PCR showed that expression levels of Wnt target genes including Axin2, Ccnd1, Myc and Vegf were significantly increased in 3D10C9-injected retinas, suggesting that blockade of the VLDLR ectodomain alone is sufficient to activate Wnt/β-catenin signaling in the retina (Fig. 7C).
Fig. 7.
Blockade of VLDLR using the anti-VLDLR antibody induced activation of Wnt signaling. (A,B) Anti-VLDLR antibody (3D10C9) was injected into the vitreous cavity of C57BL/6J mice, with the same amount of non-specific IgG as control. One week after the injection, LRP6 and non-p-β-catenin were measured in the retina, semi-quantified by densitometry and expressed as fold of that in IgG control (n = 6, *P<0.05, †P<0.001). α-tubulin was used as a loading control. (C) mRNA levels of Axin2 (Axin 2), Ccnd1 (Cyclin D1), Myc (C-myc), and Vegf in retinas from the eyes injected with 3D10C9 were measured by real-time RT-PCR, normalized to 18S RNA levels and expressed as fold expression of that in control IgG-injected retinas (n = 8–10, **P<0.01, †P<0.001). (D–G) Enhanced TCF/β-catenin-mediated in vivo reporter activity after the injection of 3D10C9. The anti-VLDLR antibody 3D10C9 (E) was injected into the vitreous cavity of BAT-gal mice (5 µg/eye), with the same amount of non-specific IgG as control (D). One week after injection, eyes were enucleated and processed for X-Gal staining. Stained eyes were sectioned and images were taken. Asterisk indicates enhanced X-Gal staining by 3D10C9 in inner retina. (F,G) Eyes enucleated from BAT-gal mice crossbred with Vldlr−/− mice (VKO-Bat-gal) were processed for X-Gal staining with the control wild-type BAT-gal mice (WT-Bat-gal). Asterisk indicates enhanced X-Gal staining in the inner retina that is equivalent to the staining in the retina of the mice injected with 3D10C9. (H,I) TCF/Lef:H2B-GFP mice received an intravitreal injection of 5 µg/eye of 3D10C9 (I), with the same amount non-specific IgG as control (H). One week after injection, the retina was sectioned and the GFP signal examined under fluorescence microscope. GCL, ganglion cell layer; RPE, retinal pigment epithelium. (J,K) Retina from TCF/Lef:H2B-GFP mice cross-bred with Vldlr−/− mice (VKO-TCF/Lef:H2B-GFP) was sectioned and the GFP signal was examined. Note that the enhanced GFP signal in the inner retina is equivalent to the one in 3D10C9-injected cells. (L-O) TCF/Lef:H2B-GFP mice received a daily intraperitoneal injection of 3D10C9 (1 mg/mouse) for 5 days. The kidney and the small intestine were dissected, sectioned and used for analysis of GFP reporter expression. Enhanced GFP intensity in glomerular epithelial cells (G) and renal tubular cells (T) after injection with 3D10C9 (M), compared to the control IgG injection (L). Enhanced GFP reporter expression in cells located in intestinal crypt by 3D10C9 injection (O), compared to control IgG injection (N). V; villi, C; crypt. Scale bars: 50 µm.
We next evaluated the effect of the anti-VLDLR antibody on Wnt signaling in vivo by using BAT-gal transgenic mice expressing the β-galactosidase reporter under the control of a promoter containing TCF-binding sites. We injected 3D10C9 into the vitreous cavity, and subjected the eye to X-Gal staining to evaluate the effect of the antibody upon Wnt signaling. Injection of the anti-VLDLR antibody increased β-galactosidase reporter expression in the inner retina, compared to that of control IgG (Fig. 7D,E). To determine whether blockade of VLDLR through the anti-VLDLR antibody enhances Wnt signaling in the inner retina, we crossbred Vldlr−/− mice with BAT-gal transgenic mice (VKO-BAT-gal). 3D10C9 injection increased Wnt signaling in the inner retina, suggesting that blockade of VLDLR upregulates Wnt signaling similar to that in Vldlr−/− mouse retina (Fig. 7F,G). We next verified the effect of 3D10C9 on Wnt signaling in vivo by using another Wnt reporter mouse (TCF/Lef:H2B-GFP), that expresses the H2B−EGFP fusion protein under the control of a promoter containing six copies of TCF/LEF1 response elements. In the retina, intravitreal injection of 3D10C9 induced a more-intense GFP signal in the inner retina, compared with that following injection with control IgG (Fig. 7H,I). In addition, the GFP signal induced by 3D10C9 injection was also observed in the inner retina of Vldlr−/− mice crossbred with TCF/Lef:H2B-GFP (VKO-TCF/Lef:H2B-GFP) (Fig. 7J,K). Daily intraperitoneal injections of 3D10C9 for 5 days resulted in enhanced expression of GFP in the kidney and small intestine. In the kidney, activated Wnt signaling by 3D10C9 was clearly observed in the renal tubular epithelial cells and the glomerular epithelial cells (Fig. 7L,M). In the small intestine, 3D10C9 enhanced GFP expression in cells located in intestinal crypts and longitudinal and/or circular network cells in the submucosa (Fig. 7N,O). Taken together, these in vivo results demonstrate that blocking the ectodomain of VLDLR enhances Wnt/β-catenin signaling, providing in vivo evidence of the biological relevance of the VLDLR–LRP6 heterodimerization in modulation of Wnt/β-catenin signaling.
DISCUSSION
The Wnt/β-catenin signaling pathway plays important roles in multiple physiological and pathophysiological processes (Clevers, 2006). However, the regulation of Wnt signaling is not completely understood. Here, we report a novel mechanism for the modulation of Wnt/β-catenin signaling through receptor heterodimerization in the plasma membrane. Our results demonstrate that VLDLR, a membrane receptor originally known for lipid metabolism and unrelated to the Wnt pathway, inhibits Wnt/β-catenin signaling through the formation of a heterodimer with LRP6. The heterodimerization blocks the Wnt ligand-induced formation of the Fzd–LRP6 receptor complex, an essential step in the activation of Wnt signaling. Furthermore, the heterodimerization also reduces LRP6 levels on the cell surface by accelerating internalization and turnover of LRP6. Our results further showed that the extracellular domain of VLDLR is essential and sufficient for dimerization with LRP6. However, blockade of VLDLR–LRP6 heterodimerization by anti-VLDLR antibodies enhances Wnt/β-catenin signaling in vitro and in vivo. These results suggest that dynamic interactions between members of the LDLR family are endogenous mechanisms that modulate Wnt/β-catenin signaling, and that VLDLR is a novel endogenous modulator of Wnt/β-catenin signaling.
Our previous study showed over-activation of the canonical Wnt pathway in the retinas of Vldlr−/− mice, suggesting that VLDLR has an inhibitory effect on Wnt signaling (Chen et al., 2007). So far, the mechanism underlying Wnt activation induced through Vldlr knockout has been unclear. This study, using VLDLR overexpression and knockdown in cultured cells, confirmed a direct inhibitory effect of VLDLR on Wnt signaling. VLDLR inhibits Wnt signaling induced by a Wnt ligand but not that induced by LiCl or a constitutively active mutant of LRP6, suggesting that the target of VLDLR is on the cell surface, probably at the receptor level. Our results demonstrate that short incubation with the sVLDLR-N peptide inhibited phosphorylation of LRP6, an early and essential step in Wnt pathway activation, whereas prolonged treatment with sVLDLR-N not only blocked phosphorylation of LRP6, but also decreased total LRP6 protein levels, suggesting that LRP6 downregulation induced by sVLDLR-N is responsible for the inhibitory effect of VLDLR on Wnt signaling.
Our co-immunoprecipitation analysis demonstrated that VLDLR specifically dimerizes with LRP6. Further, VLDLR–LRP6 heterodimerization prevents the Wnt-ligand-induced formation of the LRP6–Fzd complex. Because formation of the receptor complex is an essential step in the activation of the canonical Wnt pathway, blockade of Wnt-ligand-induced formation of the LRP6–Fzd complex might serve as a mechanism by which VLDLR inhibits Wnt signaling – similar to that of DKK1, a secreted specific inhibitor of the Wnt pathway (Semënov et al., 2001).
LRP6 has been shown to form a homodimer, which prevents its internalization and degradation (Liu et al., 2003). Recently, it has been reported that interaction between Fzd and LRP6 in the absence of Wnt3A results in the formation of an inactive complex. Upon Wnt3A binding, the Fzd–LRP6 complex leads to conformational changes of the receptors and the formation of the hexamer Wnt3A–Fzd–LRP6–LRP6–Fzd–Wnt3A that contains the LRP6 homodimer (Chen et al., 2014). Whereas LRP6 dimerizes through the LDLR type-A domain, our data indicate that VLDLR interacts with LRP6 through the LDLR type-B domain. This suggests that the mechanism for LRP6 internalization and degradation induced by VLDLR is mediated through an interacting domain that differs from the one that mediates LRP6 homodimer formation. Therefore, another possible impact of VLDLR–LRP6 heterodimerization is to diminish the formation of the LRP6 homodimer, leading to LRP6 destabilization. Our findings indicate that VLDLR increases internalization of LRP6 and decreases its stability, which supports this mechanism. Studies in which deletion mutants of VLDLR have been used showed that deletion of the ectodomain of VLDLR abolished its inhibitory effect upon Wnt signaling, whereas sVLDLR-N was sufficient to dimerize with LRP6 and inhibit Wnt signaling. Moreover, monoclonal antibodies against the ectodomain of VLDLR abolished VLDLR–LRP6 heterodimerization and eliminated the Wnt-inhibitory activity of VLDLR. These results suggest that the structure responsible for LRP6 dimerization resides in the ectodomain of VLDLR.
Regarding the mechanism of sVLDLR-N suppressing Wnt/β-catenin signaling, another possible mechanism is that VLDLR competes with LRP6 for binding to the Wnt ligand. However, our immunoprecipitation assays indicated that the Wnt ligand does not bind to VLDLR, excluding the possibility that VLDLR functions as a Wnt ligand trap. Furthermore, our results showed that sVLDLR-N reduced total LRP6 protein levels, but did not affect its mRNA levels. In addition, surface-biotinylation and LRP6-stability assays suggest that sVDLLR-N accelerates endocytosis-mediated degradation of LRP6. However, sVLDLR-N does not retain the intracellular endocytosis signal NPxY motif. Thus, the mechanism for VLDLR-mediated enhancement of LRP6 endocytosis is unclear.
LDLR is another member of the low-density lipoprotein receptor family, and shares high structural and sequence similarities with VLDLR. LDLR and VLDLR both have similar functions in lipid metabolism (Hussain et al., 1999). However, Ldlr−/− mice do not develop retinal neovascularization or over-activation of Wnt signaling in the retina, suggesting that, despite their structural similarities, VLDLR has a different function than LDLR (Rudolf et al., 2005). Our findings demonstrate that LDLR neither inhibits Wnt signaling nor heterodimerizes with LRP6, which is consistent with the phenotype of Ldlr−/− mice. Comparison of LDLR and VLDLR ectodomains raises some intriguing questions about the specificity of interaction and the inhibitory function in Wnt/β-catenin signaling of VLDLR. LDLR has seven type-A domains, whereas VLDLR has eight type-A domains. Our present data suggest that the LTB-E3 domain of VLDLR is responsible for its interaction with LRP6. However, LDLR also has a LTB-E3 domain. Therefore, that LDLR does not bind LRP6 is probably due to more subtle differences in the amino acid sequences of VLDLR and LDLR. The structural basis for the differential Wnt signaling modulations warrants further investigation. Potential interactions between LRP6 and other LDLR receptor family members are currently unknown, and remain a topic of intense investigation.
Conditional ablation of LRP1 in adipocytes exhibits reduced levels of nuclear β-catenin through modulation of GSK-3β activity during adipogenesis (Terrand et al., 2009). LRP4-mutant mice exhibited reduced bone integrity, because LRP4 serves as a receptor for DKK1 and for sclerostin in osteoblasts that regulates bone maturation and turnover (Choi et al., 2009). Although interactions between members of the LDLR family on a transcriptional level are not fully understood, current work with mutant mice suggests a functional integration of LDLR family members with LRP6-mediated signaling cascades in multiple physiological processes (Jeong et al., 2010; Li et al., 2010; Simon-Chazottes et al., 2006; Terrand et al., 2009).
One of the striking phenotypes observed in Vldlr−/− mice is hair loss (patchy fur) at weaning age from which they have recovered at the age of 6 weeks. Precise control of Wnt/β-catenin signaling is crucial not only for hair follicle development and morphogenesis, but also for postnatal hair regeneration. Tight regulation of Wnt/β-catenin signaling is also required to maintain proper hair regeneration in the dermal papilla (Andl et al., 2002; Millar et al., 1999). Thus, the patchy-fur phenotype of Vldlr−/− mice supports findings of dysregulation of Wnt/β-catenin signaling.
The biological function of Wnt/β-catenin signaling in tumorigenesis is a topic of intense investigation because aberrant activation of the Wnt pathway plays a significant role in neoplasia. Most tumors depend on Wnt/β-catenin signaling for tumor outgrowth and survival, and this signaling axis plays a particularly well-recognized role in colon cancer (Reya and Clevers, 2005). However melanoma proliferation inversely correlates with the activation of Wnt/β-catenin signaling, and stable transfection of Wnt3A suppresses melanoma growth (Chien et al., 2009). Because VLDLR is expressed in melanoma, it may play a role in melanoma outgrowth. Thus, monoclonal antibodies that block VLDLR–LRP6 heterodimerization have significant therapeutic potential for the treatment of melanoma.
In conclusion, our findings describe a novel regulatory mechanism of Wnt signaling, whereby the interaction of two membrane receptors can potently suppress the activation of Wnt/β-catenin signaling. Further, our study describes a novel function of the receptor heterodimerization (VLDLR–LRP6) as a previously unrecognized mechanism that modulates Wnt/β-catenin signaling. Although destabilization of the Wnt co-receptor LRP6 is known to have a role in the inhibition of Wnt/β-catenin signaling, we observed for the first time that VLDLR functions as an antagonizing partner of LRP6. This expands the repertoire of regulatory mechanisms of Wnt signaling and also reveals a so far unknown function of VLDLR. Our study also provides a strong rationale for investigating the clinical application of sVLDLR-N in diseases associated with Wnt/β-catenin signaling, depending upon cellular context.
MATERIALS AND METHODS
Mice and injection of antibodies
Female C57BL/6J mice (8–10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME); care, use, and treatment of all of the animals in this study were in strict agreement with the Statement for the Use of Animals in Ophthalmic and Vision Research, ARVO. Anti-VLDLR antibody 3D10C9 was injected into the vitreous cavity of anesthetized animals through sclerotomy; the same amount of non-specific IgG was injected into the contralateral eye as a control. BAT-gal transgenic mice (Maretto et al., 2003) and TCF/Lef:H2B-GFP transgenic mice (Ferrer-Vaquer et al., 2010), Vldlr−/− (Chen et al., 2007) were maintained and used according to protocols approved by the Institutional Animal Care and Use Committee, IACUC. At the age of 2 months, female BAT-gal and TCF/Lef:H2B-GFP transgenic littermates received an intravitreal injection of 3D10C9, the same amount of non-specific IgG was injected into the contralateral eye for control. To evaluate the effect of the anti-VLDLR antibody on Wnt signaling in other tissues, TCF/Lef:H2B-GFP transgenic mice received daily intraperitoneal injections of 3D10C9 (1 mg/mice) or control IgG (1 mg/mice) for 5 days. The mice were euthanized and perfused with PBS containing 4% paraformaldehyde. The eyes, kidneys, and small intestine were isolated and processed for frozen tissue sections (20 µm). Sections were mounted and examined under a fluorescence microscope (Axio imager 2, Carl Zeiss, Germany).
Expression constructs and siRNA
Plasmids that express LRP6, a truncated form of LRP6 lacking the extracellular domain (LRP6ΔN), the extracellular domain of LRP6 (LRP6N), the soluble extracellular domain of LDLR (LDLR-N) or the Frizzled 8 cysteine-rich domain (Fzd-CRD) were from Xi He (Harvard University). The full-length (FL) VLDLR was cloned into the pcDNA6 (Invitrogen, Carlsbad, CA) with a Myc tag at the C-terminus. The C-terminus deletion mutant (VLDLRΔC) – in which the entire intracellular domain was deleted, and the N-terminus deletion mutant (VLDLRΔN) – in which the entire extracellular domain except for the signal peptide was deleted, were generated and cloned into pcDNA6. A chimeric mutant composed of VLDLR ectodomain and LRP6 intracellular domain (VLN6C) was cloned into pcDNA6 with an 8×Myc tag at the C-terminus. The soluble extracellular domain of VLDLR (sVLDLR-N) comprising a histidine hexamer at the C-terminus was cloned into pcDNA6 and fused with a secretion sequence at the N-terminus. A HEK-293T cell line stably expressing sVLDLR-N was generated by transfection of a linearized expression plasmid, followed by multiple selections in medium that contained 20 µg/ml blasticidine. Small interfering RNAs (siRNA) specific for Vldlr and control siRNA with a scrambled sequence were synthesized by Life Technology Inc. (Carlsbad, CA).
Cell culture, transfection, preparation of conditioned medium, and TOPFLASH assay
HEK-293T, hTERT-RPE-1, mouse fibroblast (L) cells, L cells stably expressing Wnt3A (ATCC, Manassas, VA) were maintained in Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum. Expression plasmids (0.5 µg/well) and siRNA (50 nM) in six-well plates were transfected into hTERT-RPE-1 cells using lipofectamine 2000 (Invitrogen). Briefly, plasmids or siRNAs were mixed with opti-MEM (Cellgro, Manassas, VA) with lipofectamine 2000 following manufacturer's instruction. After 6 h of transfection, medium was changed to fresh culture medium. For co-IP or collection of VLDLR-N- or LDLR-N-conditioned media, HEK-293T cells, in 10-cm dishes were transfected with 10 µg of VLDLR-N- or LDLR-N-expressing plasmids, supplemented with control empty plasmid to achieve same amount of plasmids transfected using lipofectamine 2000. Wnt3A-, Wnt5A- or control-conditioned media were obtained from L cells stably expressing human origin Wnt3A, Wnt5A or neither of the two, respectively, (ATCC). For the measurement of TCF/β-catenin activity, TOPFLASH vector (0.2 µg) and Renilla luciferase pRL-TK (0.04 µg) reporter vector were co-transfected into hTERT-RPE-1 cells. TCF/β-catenin activity was measured using the dual luciferase reporter system (Promega, Madison, WI) and normalized to Renilla luciferase activity.
Total RNA isolation and real-time reverse-transcription-PCR
Total RNA was isolated from RPE cells and the retina of injected mice using TRIzol (Invitrogen) according to the manufacturer's instructions. cDNAs were synthesized by reverse-transcription (RT) using TaqMan Kit (Applied Biosystems, Carlsbad, CA). The cDNA generated was used for real-time PCR with specific primers and iQ SYBR Green Super mix (Bio-Rad, Hercules, CA). Specific gene amplification was detected using the MyiQ single-color real-time PCR system. The transcript level was determined using the comparative Ct method with the housekeeping gene 18S or Gapdh as the reference. Data from at least three independent cell culture dishes or five independent retinas were averaged. Primers for Vldlr and Lrp6 were designed spanning one intron junction with amplicon size between 150 and 200 base pairs. Specific primers for Vldlr (F: 5′-CAAGTGTGAATGTAGTCGTG-3′, R: 5′-ATCTTCCTGATGTCTCTTCG-3′) and Lrp6 (F: 5′-CGGGCTATTGTGTTAGATCC-3′, R: 5′- CTTGTCTGTTTTGGCATCTC-3′) were used. Primers for amplifying mouse origins of replication of Myc, Ccnd1 and Axin2 were previously described (Griffin et al., 2011). Primers for amplifying human AXIN2 (F: 5′-TTATGCTTTGCACTACGTCCCTCCA-3′, R: 5′-CGCAACATGGTCAACCCTCAG-3′) were used.
Surface biotinylation, immunoprecipitation and western blot analysis
After three washes in phosphate buffered saline (PBS), hTERT-RPE-1 cells were incubated with 0.5 mg/ml sulfo-NHS-LC-biotin (Pierce, Rockford, IL) at 4°C for 30 min and then quenched with 100 mM glycine. Cells were lysed in IP buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.2 mM PMSF, 1% Triton X-100, 0.5% NP 40), containing protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO; p2714). LRP6 was immunoprecipitated with an anti-LRP6 antibody specific for the C-terminus (C-10; Santa Cruz Biotechnology, Santa Cruz, CA); immunoprecipitates were analyzed by western blot analysis with Streptavidine-HRP (N100; Pierce) to measure surface LRP6 levels. Total LRP6 levels were measured using an antibody specific for the E1E2 domain of LRP6.
To determine endogenous interactions in immunoprecipitation assays, the membrane fraction was prepared. RPE cells in ice-cold PBS supplemented with proteinase inhibitor cocktail were disrupted in a Dounce homogenizer 30 times. Homogenates were lysed in IP buffer, centrifuged once for 30 min (16,000 g at 4°C), followed by a second centrifugation for 4 h (200,000 g at 4°C). Soluble membrane fractions were incubated with antibody against LRP6 (C-terminus; C5C7, Cell Signaling) or anti-VLDLR (C-terminus; 6A6; Santa Cruz) overnight at 4°C, followed by precipitation of immune complexes with Protein A/G Plus Agarose (Santa Cruz). Immunoprecipitates were analyzed by western blot analysis with antibodies against VLDLR (3D10C9) and LRP6 (Mab2F1) recognizing the extracellular domains. The anti-RFP antibody (ab62341; Abcam, Cambridge, MA), anti-Myc-agarose, anti-HA-agarose (Sigma-Aldrich) and protein A/G beads were used for immunoprecipitation of cell lysates and conditioned medium, and then the prey was detected with respective antibodies.
Nuclear and/or cytosolic fractions were isolated using the fractionation kit from BioVision following manufacturer's instructions (Milpitas, CA). For western blot analysis, antibodies specific for phosphorylated LRP6 (pLRP6; phosphorylated at residue Ser1490), LRP6 (C5C7) and non-phospho-β-catenin (Ser33/Ser37/Thr41) were purchased from Cell Signaling (Danvers, MA). Antibodies for LRP6 (C-10), VLDLR (6A6), Myc-tag (9E10) and human IgG were purchased from Santa Cruz (Santa Cruz). Anti-β-actin and anti-poly-histidine, anti-Fibrillarin and anti-RFP antibodies were purchased from Abcam (Cambridge, MA). Antibodies specific for the VLDLR extracellular domain and Mab2F1 specific for the LRP6E1E2 domain were generated via a contracted service by Proteintech Group (Chicago, IL) using purified recombinant proteins or peptides. Western blotting images were captured by a Chemi Genius Image Station (SynGene, Frederick, MD). Individual protein band was semi-quantified by densitometry using the GENETOOLS program (SynGene).
β-galactosidase staining and visualization of H2B-GFP transgene expression
BAT-gal transgenic mice were sacrificed and perfused with PBS containing 4% paraformaldehyde. For β-galactosidase staining, the eyes from mice of each group at post-injection day 7 were enucleated, and incubated in a β-galactosidase solution [5 mM K3FE(CN)6, 5 mM K4Fe(CN)6 ˙3H2O, 2 mM MgCl2, 0.02% NP-40, 0.1% sodium deoxycholate, 1 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-galactoside] for 12 h in a humidified chamber and then processed for frozen tissue sections (20 µm).
LRP6 stability assay
hTERT-RPE-1 cells transfected with control siRNA or with siRNA specifically targeting Vldlr were treated with control-conditioned medium, or LDLR-N- or VLDLR-N-conditioned medium. Then, the cells were incubated with cycloheximide (50 µg/ml) for 0, 2, 4, 6 and 8 h. Total cell lysates were analyzed by western blot analysis of LRP6 and quantified in densitometry assays.
Statistical analysis
Experiments were performed at least three times and representative data were shown. All of values were expressed as mean±s.d. Differences between the groups were tested for statistical significance using Student's t-test. Statistical significance was set at P<0.05.
Acknowledgments
We thank Xi He (Harvard University) for kindly providing the expression plasmids for LRP6 and its deletion mutants, and Olga Nikolaeva (The University of Oklahoma Health Sciences Center) for providing purified RPE65.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
K.L., K.P., Y.H. and J.-x.M. participated in the experimental design. K.L., K.P., Y.H., Y.S., R.C., J.M., X.H. and Y.T. performed the experiments. K.L. and J.-x.M. wrote the paper.
Funding
This study was supported by National Institutes of Health grants [grant numbers EY018659, EY012231, EY019309 and P20GM104934]; an Oklahoma Center for the Advancement of Science and Technology grant [grant number HR-13-076]; and a grant from the Juvenile Diabetes Research Foundation [grant number 2-SRA-2014-147-Q-R]. Deposited in PMC for release after 12 months.
References
- Andl T., Reddy S. T., Gaddapara T., Millar S. E. (2002). WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 10.1016/S1534-5807(02)00167-3 [DOI] [PubMed] [Google Scholar]
- Chen Y., Hu Y., Lu K., Flannery J. G., Ma J. X. (2007). Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J. Biol. Chem. 282, 34420–34428 10.1074/jbc.M611289200 [DOI] [PubMed] [Google Scholar]
- Chen J., Yan H., Ren D. N., Yin Y., Li Z., He Q., Wo D., Ho M. S., Chen Y., Liu Z. et al. (2014). LRP6 dimerization through its LDLR domain is required for robust canonical Wnt pathway activation. Cell. Signal. 26, 1068–1074 10.1016/j.cellsig.2013.12.020 [DOI] [PubMed] [Google Scholar]
- Chien A. J., Moore E. C., Lonsdorf A. S., Kulikauskas R. M., Rothberg B. G., Berger A. J., Major M. B., Hwang S. T., Rimm D. L., Moon R. T. (2009). Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc. Natl. Acad. Sci. USA 106, 1193–1198 10.1073/pnas.0811902106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. Y., Dieckmann M., Herz J., Niemeier A. (2009). Lrp4, a novel receptor for Dickkopf 1 and sclerostin, is expressed by osteoblasts and regulates bone growth and turnover in vivo. PLoS ONE 4, e7930 10.1371/journal.pone.0007930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469–480 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Ettenberg S. A., Charlat O., Daley M. P., Liu S., Vincent K. J., Stuart D. D., Schuller A. G., Yuan J., Ospina B., Green J. et al. (2010). Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc. Natl. Acad. Sci. USA 107, 15473–15478 10.1073/pnas.1007428107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Vaquer A., Piliszek A., Tian G., Aho R. J., Dufort D., Hadjantonakis A. K. (2010). A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse. BMC Dev. Biol. 10, 121 10.1186/1471-213X-10-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan J. R., Tacken P. J., Dahlmans V. E., Gijbels M. J., van Dijk K. W., Havekes L. M., Jong M. C. (2001). Protection from obesity in mice lacking the VLDL receptor. Arterioscler. Thromb. Vasc. Biol. 21, 1488–1493 10.1161/hq0901.095147 [DOI] [PubMed] [Google Scholar]
- Griffin C. T., Curtis C. D., Davis R. B., Muthukumar V., Magnuson T. (2011). The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc. Natl. Acad. Sci. USA 108, 2282–2287 10.1073/pnas.1013751108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckenlively J. R., Hawes N. L., Friedlander M., Nusinowitz S., Hurd R., Davisson M., Chang B. (2003). Mouse model of subretinal neovascularization with choroidal anastomosis. Retina 23, 518–522 10.1097/00006982-200308000-00012 [DOI] [PubMed] [Google Scholar]
- Hu W., Jiang A., Liang J., Meng H., Chang B., Gao H., Qiao X. (2008). Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest. Ophthalmol. Vis. Sci. 49, 407–415 10.1167/iovs.07-0870 [DOI] [PubMed] [Google Scholar]
- Hussain M. M., Strickland D. K., Bakillah A. (1999). The mammalian low-density lipoprotein receptor family. Annu. Rev. Nutr. 19, 141–172 10.1146/annurev.nutr.19.1.141 [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. (1993). Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92, 883–893 10.1172/JCI116663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y. H., Sekiya M., Hirata M., Ye M., Yamagishi A., Lee S. M., Kang M. J., Hosoda A., Fukumura T., Kim D. H. et al. (2010). The low-density lipoprotein receptor-related protein 10 is a negative regulator of the canonical Wnt/beta-catenin signaling pathway. Biochem. Biophys. Res. Commun. 392, 495–499 10.1016/j.bbrc.2010.01.049 [DOI] [PubMed] [Google Scholar]
- Klaus A., Birchmeier W. (2008). Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387–398 10.1038/nrc2389 [DOI] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455–8459 10.1073/pnas.93.16.8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Herz J. (1994). Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu. Rev. Biochem. 63, 601–637 10.1146/annurev.bi.63.070194.003125 [DOI] [PubMed] [Google Scholar]
- Li C., Huang Z., Kingsley R., Zhou X., Li F., Parke D. W., II and Cao W. (2007). Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: an animal model of retinal angiomatous proliferation. Arch. Ophthalmol. 125, 795–803 10.1001/archopht.125.6.795 [DOI] [PubMed] [Google Scholar]
- Li Y., Pawlik B., Elcioglu N., Aglan M., Kayserili H., Yigit G., Percin F., Goodman F., Nürnberg G., Cenani A. et al. (2010). LRP4 mutations alter Wnt/beta-catenin signaling and cause limb and kidney malformations in Cenani-Lenz syndrome. Am. J. Hum. Genet. 86, 696–706 10.1016/j.ajhg.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Bafico A., Harris V. K., Aaronson S. A. (2003). A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol. 23, 5825–5835 10.1128/MCB.23.16.5825-5835.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Nusse R. (2004). The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 20, 781–810 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., Yokota C., Tamai K., Zeng X., He X. (2008). Wnt signal amplification via activity, cooperativity, and regulation of multiple intracellular PPPSP motifs in the Wnt co-receptor LRP6. J. Biol. Chem. 283, 16115–16123 10.1074/jbc.M800327200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K., He X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar S. E., Willert K., Salinas P. C., Roelink H., Nusse R., Sussman D. J., Barsh G. S. (1999). WNT signaling in the control of hair growth and structure. Dev. Biol. 207, 133–149 10.1006/dbio.1998.9140 [DOI] [PubMed] [Google Scholar]
- Rey J. P., Ellies D. L. (2010). Wnt modulators in the biotech pipeline. Dev. Dyn. 239, 1034 10.1002/dvdy.22249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Clevers H. (2005). Wnt signalling in stem cells and cancer. Nature 434, 843–850 10.1038/nature03319 [DOI] [PubMed] [Google Scholar]
- Rudolf M., Winkler B., Aherrahou Z., Doehring L. C., Kaczmarek P., Schmidt-Erfurth U. (2005). Increased expression of vascular endothelial growth factor associated with accumulation of lipids in Bruch's membrane of LDL receptor knockout mice. Br. J. Ophthalmol. 89, 1627–1630 10.1136/bjo.2005.071183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semënov M. V., Tamai K., Brott B. K., Kühl M., Sokol S., He X. (2001). Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11, 951–961 10.1016/S0960-9822(01)00290-1 [DOI] [PubMed] [Google Scholar]
- Simon-Chazottes D., Tutois S., Kuehn M., Evans M., Bourgade F., Cook S., Davisson M. T., Guénet J. L. (2006). Mutations in the gene encoding the low-density lipoprotein receptor LRP4 cause abnormal limb development in the mouse. Genomics 87, 673–677 10.1016/j.ygeno.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Taelman V. F., Dobrowolski R., Plouhinec J. L., Fuentealba L. C., Vorwald P. P., Gumper I., Sabatini D. D., De Robertis E. M. (2010). Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143, 1136–1148 10.1016/j.cell.2010.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrand J., Bruban V., Zhou L., Gong W., El Asmar Z., May P., Zurhove K., Haffner P., Philippe C., Woldt E. et al. (2009). LRP1 controls intracellular cholesterol storage and fatty acid synthesis through modulation of Wnt signaling. J. Biol. Chem. 284, 381–388 10.1074/jbc.M806538200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A., Nusse R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88 10.1146/annurev.cellbio.14.1.59 [DOI] [PubMed] [Google Scholar]
- Zeng X., Huang H., Tamai K., Zhang X., Harada Y., Yokota C., Almeida K., Wang J., Doble B., Woodgett J. et al. (2008). Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135, 367–375 10.1242/dev.013540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Ma J. X. (2010). Wnt pathway antagonists and angiogenesis. Protein Cell 1, 898–906 10.1007/s13238-010-0112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]