ABSTRACT
Sister chromatid bi-orientation on the mitotic spindle is essential for proper chromosome segregation. Defects in bi-orientation are sensed and corrected to prevent chromosome mis-segregation and aneuploidy. This response depends on the adaptor protein Sgo1, which associates with pericentromeric chromatin in mitosis. The mechanisms underlying Sgo1 function and regulation are unclear. Here, we show that Sgo1 is an anaphase-promoting complex/cyclosome (APC/C) substrate in budding yeast (Saccharomyces cerevisiae), and that its mitotic destruction depends on an unusual D-box-related sequence motif near its C-terminus. We find that the removal of Sgo1 from chromosomes before anaphase is not dependent on its destruction, but rather on other mechanisms responsive to tension between sister chromatids. Additionally, we find that Sgo1 recruits the protein phosphatase 2A (PP2A) isoform containing Rts1 to the pericentromeric region prior to bi-orientation, and that artificial recruitment of Rts1 to this region of a single chromosome is sufficient to perform the function of Sgo1 on that chromosome. We conclude that in early mitosis, Sgo1 associates transiently with pericentromeric chromatin to promote bi-orientation, in large part by recruiting the Rts1 isoform of PP2A.
KEY WORDS: Sgo1, APC/C, PP2A, Rts1, Chromosome segregation, Bi-orientation
INTRODUCTION
Accurate duplication and segregation of chromosomes during cell division is essential for the faithful propagation of the genome, and errors in these processes can lead to cancer, genetic disorders or infertility. To ensure accurate chromosome segregation, sister chromatid pairs are held together by cohesin and are bi-oriented on the mitotic spindle, with each chromatid attached to the opposite spindle pole. Following bi-orientation, sister chromatid separation and segregation are triggered by the anaphase-promoting complex/cyclosome (APC/C), a ubiquitin-protein ligase or E3 that initiates anaphase by promoting the proteasomal destruction of securin, cyclins and other proteins (Foley and Kapoor, 2013; Morgan, 2007; Sullivan and Morgan, 2007).
The shugoshins are a conserved family of centromeric proteins that are necessary for proper bi-orientation and ultimately for accurate chromosome segregation. Most eukaryotes express two shugoshin family members, Sgo1 and Sgo2 (also known as SGOL1 and SGOL2, respectively, in mammals), whereas the budding yeast Saccharomyces cerevisiae has a single shugoshin protein, Sgo1 (Kitajima et al., 2004; Rabitsch et al., 2004). These proteins have functions in the control of both sister chromatid cohesion and bi-orientation. Shugoshin was first identified on the basis of its function during anaphase I of meiosis, when it is required to protect cohesin from cleavage by separase, thereby maintaining sister chromatid cohesion when the homologs are segregated (Katis et al., 2004; Kerrebrock et al., 1992; Kitajima et al., 2004; Marston et al., 2004). In metazoans, shugoshin also protects centromeric cohesin during mitosis from separase-independent removal by the ‘prophase pathway,’ which allows sister chromatid arms to condense and resolve while maintaining cohesion between the centromeric regions (Liu et al., 2013). Thus, depletion of shugoshin in metazoans results in premature sister chromatid separation and an arrest in metaphase that results from activation of the spindle assembly checkpoint (Kitajima et al., 2005; McGuinness et al., 2005; Salic et al., 2004; Tang et al., 2004).
Shugoshins protect centromeric cohesin by recruiting protein phosphatase 2A (PP2A), which removes phosphates from cohesin and thereby inhibits its cleavage (Brar et al., 2006; Ishiguro et al., 2010; Katis et al., 2010; Kitajima et al., 2006; Riedel et al., 2006; Tang et al., 2006; Tanno et al., 2010). Similarly, during prophase, shugoshins recruit PP2A to dephosphorylate sororin, allowing it to block cohesin removal by Wapl (Liu et al., 2013; Nishiyama et al., 2010). PP2A is normally composed of three subunits: the scaffold (A), the regulatory subunit (B), and the catalytic subunit (C). Cells contain multiple distinct B subunits, and shugoshin has thus far been found associated with PP2A complexes containing the B′ subunit (B56 in humans, Rts1 in budding yeast). Structural analysis indicates that human Sgo1 interacts with PP2A through contacts between the Sgo1 N-terminal coiled-coil domain and both the B′ and C subunits of PP2A (Xu et al., 2009).
Shugoshins are also an important part of the kinetochore-based machinery that detects and corrects erroneous microtubule attachments, ensuring that sister chromatids achieve bi-orientation before the onset of anaphase (Huang et al., 2007; Indjeian et al., 2005; Kawashima et al., 2007; Vanoosthuyse et al., 2007). Deletion of Sgo1 in budding yeast renders cells unable to respond to a lack of tension between sister chromatids, a hallmark of mis-oriented chromatids (Indjeian et al., 2005; Kitajima et al., 2004; Rabitsch et al., 2004). In yeast and humans, shugoshin recruits the chromosomal passenger complex (CPC) containing the protein kinase Aurora B (Peplowska et al., 2014; Tsukahara et al., 2010; Vanoosthuyse et al., 2007; Verzijlbergen et al., 2014; Yamagishi et al., 2010). In the absence of kinetochore tension, Aurora B phosphorylates multiple substrates at the kinetochore, thereby destabilizing microtubule attachments, and signaling to the spindle assembly checkpoint to delay anaphase until the attachments have been corrected (Lampson and Cheeseman, 2011; Musacchio and Salmon, 2007). Aurora B is silenced, and attachments are thereby stabilized, when sister kinetochores are properly attached to microtubules from opposite spindle poles. The ability of shugoshin to recruit the CPC provides a logical explanation for its function in bi-orientation, but it is unclear whether budding yeast Sgo1 works through direct recruitment of the CPC in mitosis (Katis et al., 2004; Kerrebrock et al., 1992; Kitajima et al., 2004; Marston et al., 2004; Storchová et al., 2011; Verzijlbergen et al., 2014) or whether the bi-orientation function of Sgo1 depends in part on its association with PP2A. There is evidence both for and against a role for the isoform of PP2A containing Rts1 (PP2A-Rts1) in budding yeast bi-orientation, and thus the importance of this phosphatase in bi-orientation remains unresolved (Nerusheva et al., 2014; Peplowska et al., 2014; Verzijlbergen et al., 2014).
Shugoshin function is regulated in part by its recruitment to pericentromeric regions, which is governed by kinetochore kinases. Phosphorylation of the outer kinetochore protein Spc105 by Mps1 recruits Bub1 to kinetochores, which in turn phosphorylates local histone H2A to create a binding site for shugoshin (Fernius and Hardwick, 2007; Kawashima et al., 2010; Liu et al., 2013; London et al., 2012; Tang et al., 2004). In fission yeast and humans, shugoshin also binds pericentromeric heterochromatin proteins to further refine its localization (Brar et al., 2006; Ishiguro et al., 2010; Katis et al., 2010; Kitajima et al., 2006; Riedel et al., 2006; Tang et al., 2006; Yamagishi et al., 2008). Together, these mechanisms focus shugoshin at the pericentromeric region of chromatin.
Shugoshin disappears from the centromere in mitosis and its total levels drop sharply, suggesting that its destruction might be important for its inactivation (Huang et al., 2007; Indjeian et al., 2005; Katis et al., 2004; Kawashima et al., 2007; Kitajima et al., 2004; Lianga et al., 2013; Marston et al., 2004; Vanoosthuyse et al., 2007). In human cells, Sgo1 has been shown to be an APC/C substrate, but its destruction is not necessary for anaphase progression, indicating that there might be other mechanisms for its inactivation (Karamysheva et al., 2009; Liu et al., 2013; Nishiyama et al., 2010). Recent work in budding yeast suggests that the recruitment of PP2A by Sgo1 could be contributing to its own release from centromeres by reversing phosphorylation of histones or other substrates in response to tension between sister chromatids (Nerusheva et al., 2014; Xu et al., 2009).
In this study, we examined the regulation and function of Sgo1 in the budding yeast Saccharomyces cerevisiae. We found that Sgo1 is an APC/C substrate in budding yeast, but that an additional mechanism ensures that Sgo1 is removed from centromeres once sister chromatids have properly attached to the spindle. We also found that Sgo1 recruits Rts1 to centromeres, and that recruitment of Rts1 is sufficient for bi-orientation, supporting a function for PP2A-Rts1 in sensing and responding to the tension between sister chromatids.
RESULTS
Sgo1 is an APC/C substrate in budding yeast
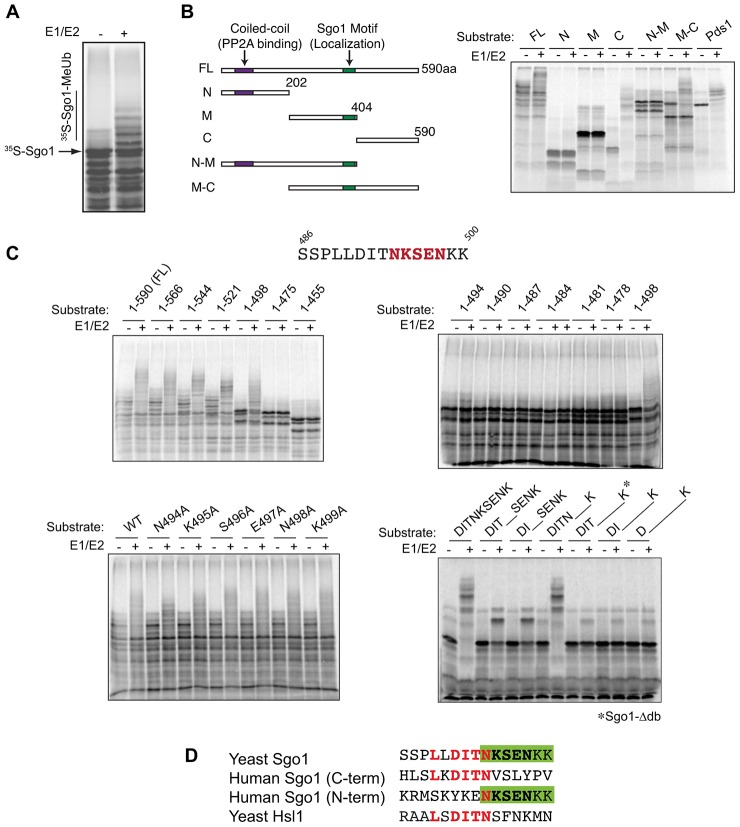
Sgo1 levels oscillate during the budding yeast cell cycle, accumulating at metaphase and declining near anaphase onset (Huang et al., 2007; Indjeian et al., 2005; Katis et al., 2004; Kawashima et al., 2007; Kitajima et al., 2004; Lianga et al., 2013; Marston et al., 2004; Vanoosthuyse et al., 2007). This pattern of protein levels is similar to that of known substrates of the APC/C, and vertebrate shugoshin is known to be a substrate for the APC/C (He et al., 2013; Karamysheva et al., 2009; Salic et al., 2004). Deletion of the APC/C in budding yeast results in Sgo1 stabilization (Lianga et al., 2013), suggesting that Sgo1 could also be a target of the APC/C in yeast. To test this possibility directly, we translated radiolabeled budding yeast Sgo1 in vitro and incubated it with purified APC/C components and ubiquitin. Sgo1 was extensively modified, suggesting that Sgo1 is ubiquitylated by the APC/C (Fig. 1A).
Fig. 1.
Budding yeast Sgo1 is an APC/C substrate, and its destruction depends on a non-canonical D-box. (A) Radiolabeled Sgo1 was translated in rabbit reticulocyte lysates and incubated with purified APC/C, ubiquitin and Cdh1, an APC/C activator. Purified E1 and E2 enzymes were added to promote ubiquitylation as indicated. Reaction products were analyzed by SDS-PAGE and autoradiography. Methylated ubiquitin was used to prevent polyubiquitin chain synthesis, which facilitates the clear detection of reaction products. The number of products generated in these reactions reflects the number of lysine residues that have been modified on the substrate, and modification of large numbers of lysine residues can lead to band heterogeneity and smearing. (B) Fragments of Sgo1 were used in APC/C ubiquitylation assays to identify the Sgo1 D-box. Radiolabeled fragments were translated in vitro and assayed as in A using a higher percentage polyacrylamide gel to resolve smaller fragments. Yeast securin (Pds1) was used as a positive control in the reactions at far right. FL, full-length Sgo1. (C) Various Sgo1 fragments and point mutants were used in APC/C assays, as in A, to further define the D-box. In the top two panels, fragments containing the indicated residues were translated from truncated PCR products. In the bottom two panels, reactions were carried out with translated full-length Sgo1 carrying the indicated point mutations or short deletions. An asterisk indicates the Sgo1-Δdb mutant. (D) Comparison of the budding yeast Sgo1 D-box to the Hsl1 D-box and the two APC/C degrons of human Sgo1 (Burton and Solomon, 2001; He et al., 2013; Karamysheva et al., 2009). Red text highlights similarities between all four sequences, and the green box highlights the similarities between C-terminal residues of the yeast Sgo1 D-box and the N-terminal degron of human Sgo1.
To assess the importance of Sgo1 ubiquitylation at anaphase, we sought a mutant that would be resistant to ubiquitylation by the APC/C. Ubiquitylation of APC/C substrates typically depends on short sequence motifs or degrons, such as the ‘D-box’ or ‘KEN box’, which interact with specific binding sites on the APC/C (Primorac and Musacchio, 2013). APC/C assays with a series of Sgo1 fragments suggested that Sgo1 ubiquitylation required amino acids 495–498 (Fig. 1B,C). Single point mutations in this region had little effect on Sgo1 ubiquitylation, but short deletions of multiple amino acids revealed that removal of amino acids 494–498 (NKSEN) greatly reduced its ubiquitylation by the APC/C in vitro (Fig. 1C). These and surrounding residues are reminiscent of the human Sgo1 degrons and the high-affinity D-box of yeast Hsl1 (Burton and Solomon, 2001; Karamysheva et al., 2009) (Fig. 1D). Hereafter, we refer to the Sgo1 mutant lacking the NKSEN sequence as the Sgo1-Δdb mutant.
The studies in Fig. 1 were performed using Cdh1 as the APC/C activator, as this activator is more stable and reliable in vitro. In further studies, we also found that Sgo1 can be ubiquitylated in vitro, in a destruction-box-dependent manner, when Cdc20 is used as the activator (supplementary material Fig. S1).
We constructed a yeast strain expressing Sgo1-Δdb at the endogenous locus, and measured Sgo1 levels by western blotting of lysates of cells released from an α-factor-mediated G1 arrest. Whereas wild-type Sgo1 levels rose after G1 and fell at anaphase, Sgo1-Δdb levels remained high throughout the cell cycle, indicating that it is resistant to the APC/C in vivo (Fig. 2A). The sgo1-Δdb strain did not display detectable defects in proliferation rate or sensitivity to the spindle poison benomyl, suggesting that Sgo1 destruction during mitosis is not required for normal cell function (Fig. 2B).
Fig. 2.
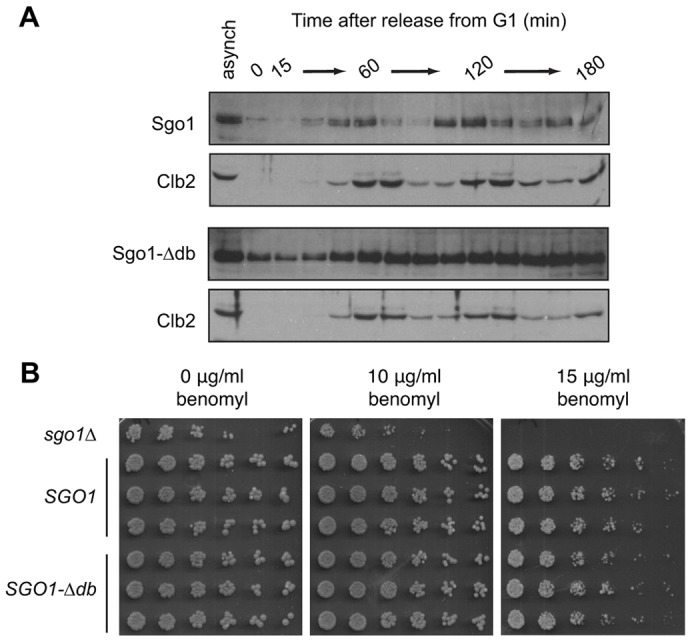
Deletion of the budding yeast Sgo1 D-box stabilizes Sgo1. (A) Wild-type (top panels) or SGO1-Δdb cells (bottom panels) were arrested in G1 with α-factor for 3 h and then released from the arrest. Samples were taken every 15 min, and lysates were analyzed by western blotting with antibodies against Sgo1 or Clb2. Lysates of asynchronous cultures were also analyzed (asynch). (B) The indicated strains (sgo1Δ, or three isolates each of SGO1 or SGO1-Δdb) were plated as serial dilutions (left to right) on YPD or YPD containing the indicated concentrations of benomyl.
Wild-type Sgo1 levels declined earlier than those of the mitotic cyclin Clb2 (Fig. 2A), consistent with previous studies showing that Sgo1 levels decline at the same time as those of securin (Indjeian et al., 2005; Lianga et al., 2013). The timing of Sgo1 disappearance, together with evidence that APC/CCdc20 (the form of APC/C containing Cdc20) ubiquitylates Sgo1 in vitro (supplementary material Fig. S1), suggests that Sgo1 is a target of the Cdc20-activated form of the APC/C.
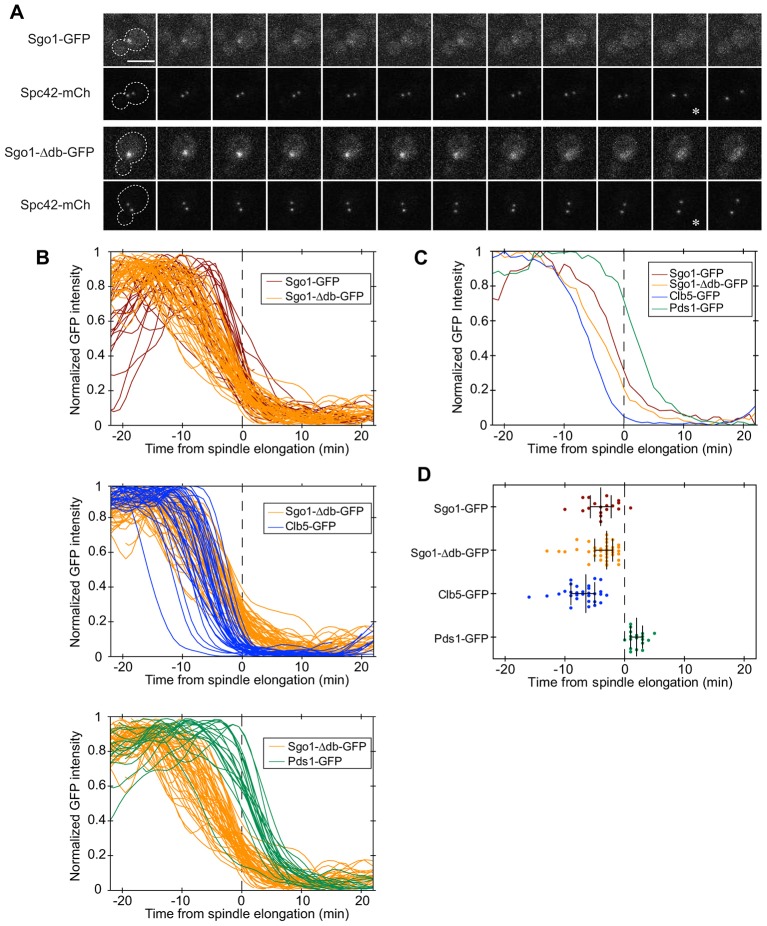
To assess the effects of APC/C-dependent destruction on Sgo1 localization dynamics, we tagged Sgo1 with GFP at the endogenous SGO1 locus and analyzed its localization and levels by spinning-disk confocal fluorescence microscopy. We also tagged Spc42, a spindle pole body (SPB) component, with mCherry to mark the mitotic spindle poles. The initial separation of duplicated SPBs provides a useful indication of mitotic entry, whereas the initiation of spindle elongation marks the onset of anaphase (Lu et al., 2014; Pearson et al., 2001; Straight et al., 1997; Yaakov et al., 2012). Wild-type Sgo1–GFP appeared as a diffuse single or bi-lobed dot, first appearing at about the same time that the duplicated SPBs separated at the beginning of mitosis. Sgo1 remained localized within the region of the mitotic spindle, and then disappeared just before the spindle began to elongate at the onset of anaphase (Fig. 3A).
Fig. 3.
Sgo1 and Sgo1-Δdb disappear from the pericentromere with similar timing. (A) Sgo1 or Sgo1-Δdb was tagged with GFP and imaged at 1-min intervals using spinning disk confocal microscopy. Spindle pole bodies were marked by fusing Spc42 to mCherry. An asterisk denotes the onset of spindle elongation. Scale bar: 5 µm. (B) Quantification of GFP intensities relative to the onset of spindle elongation (Lu et al., 2014). The intensity of the brightest 5×5 pixel square in strains containing Sgo1–GFP (n = 28), Sgo1-Δdb–GFP (n = 53), Pds1–GFP (n = 20) or Clb5–GFP (n = 36) was measured as a function of time, smoothed, normalized to the maximum intensity, and plotted relative to the onset of spindle elongation (dashed line). Data before normalization are shown in supplementary material Fig. S2. (C) Averaged traces of results shown in B, where unsmoothed traces from all cells were first aligned to the same time reference point, averaged at each time point, and then normalized to maximum intensity (Lu et al., 2014). (D) To quantify and compare the timing of fluorescence changes for each protein, we determined the time point when 50% of the GFP intensity remained in each cell. This analysis was carried out with the subset of cells in which a clear fluorescence plateau was present before and after the decline. Each dot represents a single cell, the middle bar represents the median of each strain dataset, and the error bars represent the 25th and 75th percentiles.
The fluorescence intensity of Sgo1-Δdb–GFP was significantly higher than that of wild-type Sgo1–GFP, presumably owing to higher protein levels (Fig. 2A; Fig. 3A). Surprisingly, however, the stabilized Sgo1 mutant accumulated with normal timing as a diffuse focus between the spindle poles and then disappeared from the spindle at about the same time as the wild-type protein, while remaining concentrated in the nucleus. Thus, destruction through the APC/C is not required for the removal of Sgo1 from centromeres, indicating that other mechanisms promote Sgo1 delocalization.
Pericentromeric Sgo1 is removed when the spindle assembly checkpoint is satisfied
We carried out a more detailed analysis of the timing of Sgo1 disappearance, using spinning-disk confocal video microscopy and quantification of the intensity of the Sgo1–GFP focus in single cells. We compared the timing and rate of the Sgo1–GFP dot disappearance with the timing of destruction of GFP-tagged Clb5 and securin (Pds1), two major APC/C substrates that are destroyed in sequence during mitosis (Lu et al., 2014). We measured the intensity of the brightest 25-pixel (5×5) square in each of the strains, representing the highest concentration of GFP-tagged protein.
In the Sgo1–GFP and Sgo1-Δdb–GFP strains, fluorescence was concentrated primarily in the pericentromeric focus, and measurement of the brightest 25-pixel square enabled us to follow pericentromeric Sgo1 levels over time. We found that both Sgo1–GFP and Sgo1-Δdb–GFP began to disappear from the centromere 8–10 min before the onset of spindle elongation (Fig. 3B–D). These results further indicate that destruction of Sgo1 through the APC/C does not make a significant contribution to the timing of Sgo1 delocalization from centromeres prior to anaphase onset.
As expected, the fluorescence intensity of Sgo1-Δdb–GFP was higher than that of the wild-type protein, and declined to a higher baseline in anaphase (supplementary material Fig. S2). The intensity of the wild-type protein declined to very low levels as Sgo1 dissociated from centromeres. These results, together with measurements of total Sgo1 levels by western blotting (Fig. 2A), suggest that APC/C-mediated Sgo1 destruction occurs at about the same time or soon after its dissociation from centromeres.
Further insights were gained by comparing the timing of Sgo1–GFP dot disappearance with the timing of Clb5–GFP and securin–GFP destruction (Fig. 3B–D). For these proteins, fluorescence intensity is distributed throughout the nucleus, and measurement of the brightest 25-pixel square provides the rate and timing of Clb5 or securin destruction (Lu et al., 2014). Our recent work indicates that Clb5 is destroyed immediately following spindle assembly checkpoint inactivation, whereas securin destruction begins about 6 min later, just before the onset of spindle elongation and anaphase (Lu et al., 2014). Both Sgo1–GFP and Sgo1-Δdb–GFP began to disappear from the centromere at about the same time as the beginning of Clb5 destruction; dot intensities then declined at a slower rate than the rate of Clb5 destruction (Fig. 3B,C).
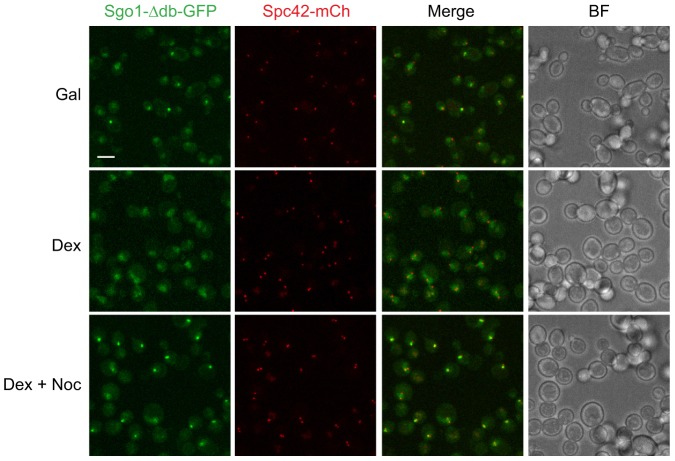
The onset of Clb5 destruction is determined by the spindle assembly checkpoint: defects in the checkpoint result in premature Clb5 destruction but have no effect on the timing of securin destruction (Lu et al., 2014). The coincidence of Sgo1 delocalization and Clb5 destruction therefore suggested that Sgo1 delocalization occurs when sister chromatids achieve bi-orientation and the spindle assembly checkpoint is satisfied. To address this possibility further, we arrested cells in metaphase by shutting off expression of CDC20, which encodes the mitotic activator of the APC/C (Fig. 4). In this arrest, sister chromatids are bi-oriented and the spindle checkpoint is satisfied. We observed very few Sgo1-Δdb–GFP foci in this arrest, indicating that Sgo1 is not localized at centromeres when sister chromatids have bi-oriented. However, when we treated the arrested cells with nocodazole to promote spindle disassembly, Sgo1-Δdb–GFP formed chromosomal foci in most cells. Thus, as suggested by other recent studies, our results argue that Sgo1 is localized to pericentromeres specifically when sister chromatids are improperly attached to the spindle (Nerusheva et al., 2014).
Fig. 4.
Sgo1 is removed when the spindle assembly checkpoint is satisfied. Cells contained Sgo1-Δdb–GFP and Spc42–mCherry as in Fig. 3, as well as CDC20 under the control of the GAL promoter. In the presence of galactose (Gal, top panels), cells at all stages of the cell cycle were present. After shifting the cells into medium containing dextrose for 3 h, Cdc20 synthesis was shut off and cells arrested in mitosis (Dex, middle panels). Nocodazole (20 µg/ml) was then added to the arrested cells (Dex + Noc, bottom panels) to promote spindle disassembly. In two independent experiments with over 50 cells each, 6.7% (±1.4%; ±s.d.) of cells displayed Sgo1-Δdb–GFP foci in the presence of dextrose, and 93% (±2.2%; ±s.d.) of cells displayed foci following addition of nocodazole. BF, brightfield image. Scale bar: 5 µm.
Rts1 localization requires Sgo1
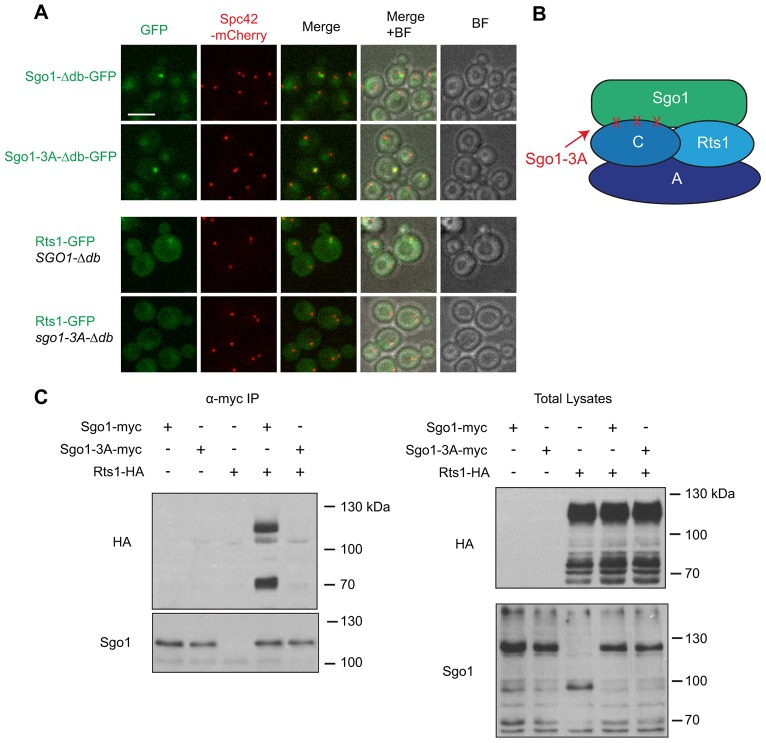
Budding yeast Sgo1 is known to interact with PP2A-Rts1 in meiotic cells (Riedel et al., 2006). In mitotic cells, Rts1 is found in foci that colocalize with kinetochores, raising the possibility that Sgo1 also recruits PP2A-Rts1 to pericentromeric regions in mitotic cells (Gentry and Hallberg, 2002; Peplowska et al., 2014). To test this possibility, we used fluorescence microscopy to determine whether Sgo1 is required for Rts1 localization in mitosis. We tagged Rts1 with GFP at the endogenous locus in a strain containing either wild-type or the stabilized SGO1-Δdb mutant. Interestingly, the fluorescence intensity of Rts1–GFP was increased in the SGO1-Δdb mutant. Microscopy of this strain revealed that localization of Rts1–GFP is similar to that of Sgo1-Δdb–GFP: it appeared as a diffuse dot in the vicinity of the mitotic spindle during early mitosis, and was absent in anaphase cells (Fig. 5A) (Gentry and Hallberg, 2002).
Fig. 5.
Sgo1 recruits Rts1 to the mitotic spindle. (A) The sgo1-3A mutant fails to recruit Rts1 to foci. Rts1–GFP was visualized in cells containing Sgo1-Δdb or Sgo1-3A-Δdb. Rts1–GFP foci were present in 29% of cells containing Sgo1-Δdb and in 1.6% of cells containing Sgo1-3A-Δdb (n = 110 and 126, respectively). Sgo1-Δdb–GFP and Sgo1-3A-Δdb–GFP both localized normally. BF, brightfield image. Scale bar: 5 µm. Rts1–GFP fluorescence intensity was weak and difficult to quantify in a wild-type SGO1 strain (not shown). (B) Diagram showing locations of the mutations in the sgo1-3A mutant relative to the structure of PP2A. The 3A mutations disrupt the interaction between Sgo1 and the catalytic subunit. (C) Sgo1 and Rts1 physically interact. Sgo1–9myc or Sgo1-3A–9myc were immunoprecipitated from lysates of strains containing Rts1–6HA, and western blots of the immunoprecipitation (IP) and of total lysates were probed using anti-HA and anti-Sgo1 antibodies. Rts1–6HA was partly proteolyzed in the cell lysates, generating ∼70-kDa fragments.
Structural analysis has identified residues in the human Sgo1 coiled-coil domain that interact with the catalytic and regulatory subunits of PP2A-B56 (Xu et al., 2009). Mutations in residues of human Sgo1 that contact the catalytic subunit of PP2A abolish the interaction between Sgo1 and PP2A-B56. Mutating the analogous residues in budding yeast Sgo1 (sgo1-3A, Fig. 5B) causes defects both in meiotic cohesin protection and mitotic spindle assembly checkpoint activation (Xu et al., 2009). We found that the sgo1-3A mutant was also defective in recruiting Rts1 to the centromere in mitosis (Fig. 5A), consistent with a role for Sgo1 in recruitment of Rts1 to the centromere.
We next tested whether Sgo1 and Rts1 physically interact. We tagged Rts1 with an HA epitope in strains containing either Sgo1–9myc or Sgo1-3A–9myc. We immunoprecipitated the Myc-tagged Sgo1 proteins and western blotted the precipitates with an anti-HA antibody. We found that Sgo1–9myc co-immunoprecipitated with Rts1–6HA, and that this interaction was abolished in the sgo1-3A mutant (Fig. 5C). Together with the fluorescence microscopy data, these results suggest that Sgo1 directly recruits Rts1 to the centromere, consistent with recent studies (Peplowska et al., 2014; Verzijlbergen et al., 2014).
The Sgo1–PP2A interaction is required for bi-orientation
Sgo1 is known to be required for bi-orientation in cells released from a spindle assembly checkpoint arrest, but the mechanism underlying this function is unclear (Indjeian et al., 2005; Storchová et al., 2011). The sgo1-3A mutant is defective in both its response to spindle damage and in recruitment of Rts1, and so we tested whether Rts1 is required for Sgo1 to promote bi-orientation. To measure bi-orientation, we used an established assay with a strain in which the centromere of chromosome V is marked with a fluorescent dot by integration of a LacO array and expression of GFP-tagged LacI (Biggins et al., 1999; Indjeian et al., 2005; Riedel et al., 2006; Storchová et al., 2011; Straight et al., 1997). We treated cells with benomyl to promote spindle disassembly and trigger a spindle assembly checkpoint arrest in mitosis. We then released the cells from benomyl and analyzed the fidelity of chromosome segregation 60 min after release (Fig. 6). Under these conditions, sgo1Δ cells cannot detect attachment errors that are made when the spindle reforms, and therefore the sister chromatids mis-orient and segregate randomly, resulting in co-segregation of the GFP dots in ∼50% of the cells (Fig. 6A). We found that sgo1-3A cells also co-segregated the GFP dots ∼50% of the time, suggesting that Sgo1 promotes bi-orientation through its interaction with PP2A-Rts1. Similar results have recently been reported by others (Peplowska et al., 2014). Interestingly, deletion of Rts1 resulted in only a minor segregation defect, suggesting that Rts1 is not essential for bi-orientation. However, given that Sgo1 is known to interact directly with the PP2A catalytic subunit through the residues mutated in the Sgo1-3A mutant (Fig. 5B) (Xu et al., 2009), we suspect that the recruitment of PP2A, with or without the Rts1 subunit, promotes bi-orientation.
Fig. 6.
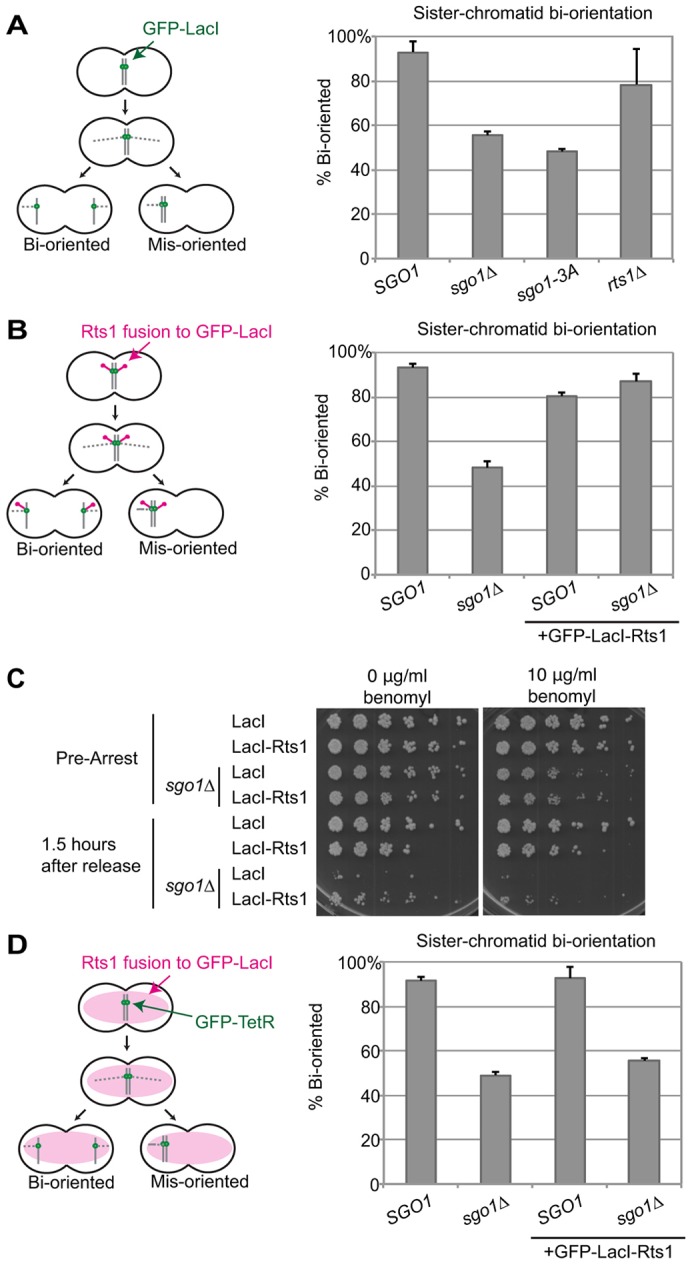
Recruitment of Rts1 is sufficient for bi-orientation. Bi-orientation of sister chromatids after release from benomyl. (A) sgo1Δ or sgo1-3A mutants fail to properly bi-orient after release from benomyl. Cells containing a GFP–LacI/LacO dot near the centromere of chromosome V were arrested in medium containing benomyl to promote spindle disassembly. Cells were then released into medium without benomyl to allow the spindle to reform. After 60 min, cells were fixed and stained with DAPI to visualize DNA. Only cells that had fully segregated their DNA were scored for localization of chromosome V GFP dots (n≥48 cells). Results are the mean of two experiments, with error bars representing the standard deviation. (B) A GFP–LacI–Rts1 fusion protein rescues the bi-orientation defect of sgo1Δ cells. The same bi-orientation assay as in A was performed using a GFP–LacI–Rts1 fusion in place of GFP–LacI. (C) GFP–LacI–Rts1 does not restore normal proliferation to cells lacking SGO1. Cells were arrested in benomyl and then released into medium without benomyl. After 90 min, cells were plated in serial dilutions on media with or without benomyl as indicated. (D) The GFP–LacI–Rts1 fusion protein restores bi-orientation to sgo1Δ cells only when localized to the centromere. A GFP–LacI–Rts1 fusion protein was expressed as in B, but in this case there were no LacO arrays on the chromosome. Chromosome segregation was instead monitored with a TetR–GFP/TetO dot on chromosome V.
Recruitment of Rts1 is sufficient for bi-orientation
To further address the importance of PP2A-Rts1 in Sgo1 function in bi-orientation, we determined whether Rts1 recruitment to pericentromeres is sufficient to promote bi-orientation in the absence of Sgo1. We fused Rts1 to the C-terminus of GFP–LacI (GFP–LacI–Rts1) in the strain carrying a LacO array near the centromere of chromosome V (Fig. 6B). Wild-type cells expressing GFP–LacI–Rts1 properly bi-oriented chromosome V after release from benomyl. Most importantly, GFP–LacI–Rts1 rescued the bi-orientation defect of sgo1Δ cells, indicating that recruitment of Rts1 to the centromere is sufficient to restore Sgo1 function.
We also plated cells 90 min after benomyl release to assess their viability (Fig. 6C). As expected, sgo1Δ cells were unable to recover from the arrest, presumably due to massive chromosome mis-segregation. The growth defect of sgo1Δ cells was only slightly rescued by expressing GFP–LacI–Rts1, suggesting that the effect of recruiting Rts1 to the centromere is specific to chromosome V, and that Rts1 promotes bi-orientation primarily on the chromosome to which it is recruited. These results also indicate that constitutive recruitment of Rts1 to the pericentromere, even after Sgo1 would normally be removed, does not result in significant growth defects.
As a control, we expressed GFP–LacI–Rts1 in a strain with chromosome V marked with a TetR–GFP/TetO system, which does not recruit GFP–LacI–Rts1 (Fig. 6D). In this case, GFP–LacI–Rts1 did not rescue the bi-orientation defect of the sgo1Δ strain. Our results, like similar results published recently (Peplowska et al., 2014), indicate that the primary direct function of Sgo1 in bi-orientation is to recruit PP2A to the centromere.
DISCUSSION
Shugoshin proteins function by two distinct mechanisms to promote normal chromosome segregation: they protect centromeric cohesin from premature removal and they participate in sensing and correcting kinetochore attachment defects. The shugoshins carry out these functions in large part by serving as adaptor proteins that recruit two enzyme complexes to the centromere: PP2A-Rts1 and the CPC. Most eukaryotes have two homologs of shugoshin to complete these tasks. In the case of fission yeast, the two homologs are functionally distinct: Schizosaccharomyces pombe (sp)Sgo1 recruits PP2A and spSgo2 recruits the CPC. Budding yeast has a single homolog, Sgo1, which recruits both PP2A and the CPC and fulfills both the cohesin protection and bi-orientation functions. Although it is clear that Sgo1 recruits PP2A-Rts1 in meiosis to protect centromeric cohesin, there is no clear indication that Sgo1 serves a cohesin protection function in mitotic yeast cells, which do not employ a ‘prophase pathway’ to remove cohesin prior to metaphase and are therefore not expected to need centromeric cohesin protection. Instead, our work and other recent studies suggest that, in budding yeast mitosis, Sgo1 recruits PP2A-Rts1 to promote bi-orientation, probably in collaboration with the CPC (Peplowska et al., 2014; Verzijlbergen et al., 2014). We found that PP2A-Rts1 is recruited by Sgo1 to the pericentromere during mitosis, and that defects in PP2A recruitment result in bi-orientation defects when cells recover from severe spindle damage.
We also found that recruitment of Rts1 to a single centromere is sufficient to allow bi-orientation at that centromere in the absence of Sgo1. To understand how Sgo1 and PP2A contribute to bi-orientation, it will be important to identify the key substrate(s) of PP2A. Studies in human cells have shown that PP2A-B56 (Rts1) is important for stabilizing kinetochore–microtubule attachments and ensuring proper chromosome alignment on the mitotic spindle (Foley et al., 2011). Interestingly, phosphorylation of Dsn1 and Knl1 (both substrates of Aurora B) and BubR1 (a substrate of Plk1) increases in cells depleted of the B56 subunit, suggesting that these could be direct targets of PP2A (Foley et al., 2011; Suijkerbuijk et al., 2012). Another possibility is that PP2A-Rts1 regulates localization of Aurora B and the CPC to promote bi-orientation, as recent studies in budding yeast suggest that Sgo1 and Rts1 are required for maintenance of Aurora B (Ipl1) at the centromere (Peplowska et al., 2014; Verzijlbergen et al., 2014). Interestingly, a screen for Rts1 substrates identified two components of the CPC, Sli15 and Bir1, as being hyperphosphorylated in rts1Δ cells (Zapata et al., 2014).
In budding yeast, deletions of SGO1 or BUB1 also cause aberrations in mitotic chromosome architecture when cells are challenged with spindle poisons (Haase et al., 2012), suggesting that Sgo1 could be modulating the spatial conformation of sister chromatid attachments to promote bi-orientation. Sgo1 can recruit condensin to chromosomes and has the capability to dephosphorylate cohesin, both of which contribute to chromosome structure and organization (Peplowska et al., 2014; Stephens et al., 2011; Verzijlbergen et al., 2014).
Interestingly, Rts1 itself is not strictly required for bi-orientation, as seen in our work and another recent study (Verzijlbergen et al., 2014). We suggest that Sgo1 is still able to recruit the catalytic subunit of PP2A even in the absence of Rts1. Binding studies with PP2A subunits in vitro reveal that mouse (m)Sgo2 can associate with the C subunit of PP2A even in the absence of a B subunit (Xu et al., 2009). Thus, the B subunit might not be required for interaction of budding yeast Sgo1 with the C subunit. Additionally, mSgo2 can associate with three structurally distinct versions of human B subunits: B, B′ and B″ (Xu et al., 2009). Further experiments will be required to determine whether Sgo1 can recruit active PP2A with the alternative regulatory subunit Cdc55 or without any regulatory subunit at all.
Sgo1 and its associated PP2A-Rts1 dissociate from pericentromeres following bi-orientation, suggesting that their function is no longer required after satisfaction of the spindle assembly checkpoint. Interestingly, we found that constitutive recruitment of Rts1 to one pericentromere throughout mitosis did not cause significant mitotic defects, suggesting that removal of phosphatase activity is not essential after bi-orientation has been achieved.
We also find that Sgo1 is an APC/C substrate in budding yeast, but that it is removed from centromeres independently of its destruction once sister chromatid bi-orientation has been achieved. Consistent with this finding, studies in human cells demonstrate that localization of the PP2A-B56 subunit is also dependent on microtubule attachment (Foley et al., 2011). Mps1 and Bub1 kinase activities are required for Sgo1 localization to the centromere, and phosphatases such as PP1 have been shown to counteract these kinases (London et al., 2012). It is likely that phosphatase activation following bi-orientation is required to silence the spindle checkpoint, and that displacement of Sgo1 is an important outcome of this silencing mechanism. Recent work suggests that Rts1 itself might contribute to release of Sgo1 following bi-orientation, though this is not the sole mechanism (Nerusheva et al., 2014). Taken together, these results demonstrate that Sgo1 recruits PP2A to centromeres in response to errors in sister chromatid attachment, and that centromeric PP2A promotes bi-orientation to ensure accurate chromosome segregation.
MATERIALS AND METHODS
Yeast strains and plasmids
Strains used in this study are listed in supplementary material Table S1. All yeast strains were derivatives of W303. C-terminal tagging of Sgo1, Rts1 and Spc42, and introduction of a GAL promoter upstream of CDC20, were performed using standard methods at the endogenous loci (Janke et al., 2004; Longtine et al., 1998). The SGO1 gene was subcloned from the budding yeast genome into pBS, pRS306, or pRS316 containing a 9×Myc tag and the HIS3 marker. The 3A or Δdb mutations, as well as point mutants for ubiquitylation assays, were made in these vectors using Quikchange site-directed mutagenesis (Agilent Technologies, La Jolla, CA). Lac or Tet operator arrays were integrated 1 kb from the centromere of chromosome V using a two-step method (Rohner et al., 2008). pCUP1-GFP-LacI, pCUP1-GFP-LacI-RTS1, or pCUP1-GFP-TetR were cloned into pRS303 or single-copy integrating vectors and integrated at the corresponding locus (Chau et al., 2012; Yaakov et al., 2012; Zalatan et al., 2012).
Cells were grown in YPD, except where noted. To arrest cells in G1, cells were treated with 1 µg/ml α-factor for 3 h. To arrest cells in mitosis, cells were treated for 4 h at 30°C with 60 µg/ml benomyl or 2% dextrose (in strains containing CDC20 under control of the GAL promoter). Cells in a GAL-CDC20 arrest were treated with 20 µg/ml nocodazole to promote spindle disassembly.
APC/C ubiquitylation assays
E1 (Uba1), E2 (Ubc4) and APC/C (from a cdh1Δ strain) were purified and used in ubiquitylation assays as previously described (Carroll and Morgan, 2002; Rodrigo-Brenni and Morgan, 2007; Lu et al., 2014). Cdh1 was translated using the TnT quick coupled transcription/translation systems (Promega, Madison, WI) and purified using a ZZ-tag and magnetic Dynabeads (Life Technologies, Carlsbad, CA) coupled to IgG. Cdh1 was cleaved from the beads using the TEV protease. PCR products of full-length Sgo1 or truncations containing a T7 promoter were translated in vitro in the presence of [35S]methionine and subsequently treated with NEM to inhibit activities of the E1 and E2 enzymes in the reticulocyte lysates. E1 and E2 enzymes were charged with methylated ubiquitin (Boston Biochem, Cambridge, MA) and added to reactions containing the purified Cdh1, APC/C and Sgo1 translations to initiate ubiquitylation of Sgo1. After 1 h, reaction products were separated by SDS-PAGE and visualized using a Typhoon Phosphorimager (GE Healthcare).
Sgo1 immunoprecipitations
50 ml cell cultures were grown to an absorbance at 600 nm (A600) of ∼1.0 and frozen as pellets in liquid nitrogen. Cell pellets were lysed by bead beating in IP lysis buffer (50 mM Hepes pH 8.0, 150 mM NaCl, 1% NP40, 50 mM β-glycerophosphate, 50 mM NaF, 1 mM DTT, 1 µg/ml leupeptin, 1 µg/ml pepstatin, 1 µg/ml aprotinin, 1 mM PMSF, 10% glycerol, 0.63 mg/ml benzamidine, 1 mM MgCl2, 50 U/ml DNase I) and lysates were incubated with Protein G dynabeads (Life Technologies, Carlsbad, CA) pre-incubated with an anti-Myc antibody (9E10, Covance, Princeton, NJ). Beads were washed with lysis buffer and eluted from the beads in sample buffer. Elution products were separated by SDS-PAGE and analyzed by western blotting. Sgo1–9myc was detected using an antibody against Sgo1 (a gift of Adam Rudner, University of Ottawa, Canada), Rts1–6HA was detected using an antibody against HA (12CA5, Roche), and Clb2 was detected using the sc-9071 antibody from Santa Cruz Biotechnology.
Fluorescence microscopy and data processing
Cells were grown in synthetic complete medium containing 2% dextrose or galactose and raffinose to minimize background fluorescence. Cells were plated onto coverslips coated with concanavalin A (ConA) and imaged at the UCSF Nikon Imaging Center using a spinning disk confocal microscope (Nikon Ti-E inverted microscope with a Yokogawa CSU-22 scanner unit and a Photometrics Evolve EMCCD camera), 491 nm and 561 nm lasers, and Chroma ET525/50 m and ET610/60 m emission filters. Between 13 and 17 z-slices (0.5 µm each) were taken for each image using a 60× 1.4 NA oil objective with a final pixel size of ∼0.15 µm/pixel. Images for movies were acquired every minute for ∼50 min. To obtain traces of GFP intensity relative to spindle elongation, images were processed as described previously (Lu et al., 2014).
Bi-orientation assays
Cells were arrested in YPD containing benomyl for 4 h and released into YPD without benomyl. After 60 min, cells were collected and fixed using 3.7% formaldehyde. After washing with PBS, cells were mixed with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing 1 µg/ml DAPI on microscope slides, and imaged using spinning disk confocal fluorescence microscopy.
Supplementary Material
Acknowledgments
We thank members of the Morgan laboratory for discussions; Dan Lu (UCSF) for reagents and assistance with image data processing; Scott Foster (UCSF) for assistance with ubiquitylation assays; Norman Davey (UCSF) for assistance with D-box sequence analysis; Matilde Galli, Drew Thacker and Dan Lu (UCSF) for comments on the manuscript; Kurt Thorn and the UCSF Nikon Imaging Center for assistance with microscopy; and Gilad Yaakov (UCSF), Adam Rudner (University of Ottawa, Canada) and Susan Gasser (Friedrich Mieser Institute, Basel, Switzerland) for reagents.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
H.D.E. performed all experiments and wrote the manuscript, with guidance from D.O.M.
Funding
This work was supported by funding from the National Institute of General Medical Sciences [grant numbers R01-GM094173, R37-GM053270]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.161273/-/DC1
References
- Biggins S., Severin F. F., Bhalla N., Sassoon I., Hyman A. A., Murray A. W. (1999). The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 13, 532–544 10.1101/gad.13.5.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar G. A., Kiburz B. M., Zhang Y., Kim J-E., White F., Amon A. (2006). Rec8 phosphorylation and recombination promote the step-wise loss of cohesins in meiosis. Nature 441, 532–536 10.1038/nature04794 [DOI] [PubMed] [Google Scholar]
- Burton J. L., Solomon M. J. (2001). D box and KEN box motifs in budding yeast Hsl1p are required for APC-mediated degradation and direct binding to Cdc20p and Cdh1p. Genes Dev. 15, 2381–2395 10.1101/gad.917901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C. W., Morgan D. O. (2002). The Doc1 subunit is a processivity factor for the anaphase-promoting complex. Nat. Cell Biol. 4, 880–887 10.1038/ncb871 [DOI] [PubMed] [Google Scholar]
- Chau A. H., Walter J. M., Gerardin J., Tang C., Lim W. A. (2012). Designing synthetic regulatory networks capable of self-organizing cell polarization. Cell 151, 320–332 10.1016/j.cell.2012.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernius J., Hardwick K. G. (2007). Bub1 kinase targets Sgo1 to ensure efficient chromosome biorientation in budding yeast mitosis. PLoS Genet. 3, e213 10.1371/journal.pgen.0030213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E. A., Kapoor T. M. (2013). Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 14, 25–37 10.1038/nrm3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E. A., Maldonado M., Kapoor T. M. (2011). Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat. Cell Biol. 13, 1265–1271 10.1038/ncb2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. S., Hallberg R. L. (2002). Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol. Biol. Cell 13, 3477–3492 10.1091/mbc.02-05-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Stephens A., Verdaasdonk J., Yeh E., Bloom K. (2012). Bub1 kinase and Sgo1 modulate pericentric chromatin in response to altered microtubule dynamics. Curr. Biol. 22, 471–481 10.1016/j.cub.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Chao W. C. H., Zhang Z., Yang J., Cronin N., Barford D. (2013). Insights into degron recognition by APC/C coactivators from the structure of an Acm1-Cdh1 complex. Mol. Cell 50, 649–660 10.1016/j.molcel.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Feng J., Famulski J., Rattner J. B., Liu S. T., Kao G. D., Muschel R., Chan G. K. T., Yen T. J. (2007). Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J. Cell Biol. 177, 413–424 10.1083/jcb.200701122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indjeian V. B., Stern B. M., Murray A. W. (2005). The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307, 130–133 10.1126/science.1101366 [DOI] [PubMed] [Google Scholar]
- Ishiguro T., Tanaka K., Sakuno T., Watanabe Y. (2010). Shugoshin-PP2A counteracts casein-kinase-1-dependent cleavage of Rec8 by separase. Nat. Cell Biol. 12, 500–506 10.1038/ncb2052 [DOI] [PubMed] [Google Scholar]
- Janke C., Magiera M. M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E. et al. (2004). A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 10.1002/yea.1142 [DOI] [PubMed] [Google Scholar]
- Karamysheva Z., Díaz-Martinez L. A., Crow S. E., Li B., Yu H. (2009). Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J. Biol. Chem. 284, 1772–1780 10.1074/jbc.M807083200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katis V. L., Gálová M., Rabitsch K. P., Gregan J., Nasmyth K. (2004). Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr. Biol. 14, 560–572 10.1016/j.cub.2004.03.001 [DOI] [PubMed] [Google Scholar]
- Katis V. L., Lipp J. J., Imre R., Bogdanova A., Okaz E., Habermann B., Mechtler K., Nasmyth K., Zachariae W. (2010). Rec8 phosphorylation by casein kinase 1 and Cdc7-Dbf4 kinase regulates cohesin cleavage by separase during meiosis. Dev. Cell 18, 397–409 10.1016/j.devcel.2010.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S. A., Tsukahara T., Langegger M., Hauf S., Kitajima T. S., Watanabe Y. (2007). Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes Dev. 21, 420–435 10.1101/gad.1497307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima S. A., Yamagishi Y., Honda T., Ishiguro K., Watanabe Y. (2010). Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327, 172–177 10.1126/science.1180189 [DOI] [PubMed] [Google Scholar]
- Kerrebrock A. W., Miyazaki W. Y., Birnby D., Orr-Weaver T. L. (1992). The Drosophila mei-S332 gene promotes sister-chromatid cohesion in meiosis following kinetochore differentiation. Genetics 130, 827–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima T. S., Kawashima S. A., Watanabe Y. (2004). The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427, 510–517 10.1038/nature02312 [DOI] [PubMed] [Google Scholar]
- Kitajima T. S., Hauf S., Ohsugi M., Yamamoto T., Watanabe Y. (2005). Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 15, 353–359 10.1016/j.cub.2004.12.044 [DOI] [PubMed] [Google Scholar]
- Kitajima T. S., Sakuno T., Ishiguro K., Iemura S., Natsume T., Kawashima S. A., Watanabe Y. (2006). Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441, 46–52 10.1038/nature04663 [DOI] [PubMed] [Google Scholar]
- Lampson M. A., Cheeseman I. M. (2011). Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 21, 133–140 10.1016/j.tcb.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianga N., Williams E. C., Kennedy E. K., Doré C., Pilon S., Girard S. L., Deneault J-S., Rudner A. D. (2013). A Wee1 checkpoint inhibits anaphase onset. J. Cell Biol. 201, 843–862 10.1083/jcb.201212038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Rankin S., Yu H. (2013). Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 15, 40–49 10.1038/ncb2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N., Ceto S., Ranish J. A., Biggins S. (2012). Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 22, 900–906 10.1016/j.cub.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., McKenzie A., III, Demarini D. J., Shah N. G., Wach A., Brachat A., Philippsen P., Pringle J. R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 [DOI] [PubMed] [Google Scholar]
- Lu D., Hsiao J. Y., Davey N. E., Van Voorhis V. A., Foster S. A., Tang C., Morgan D. O. (2014). Multiple mechanisms determine the order of APC/C substrate degradation in mitosis. J. Cell Biol (in press). 10.1083/jcb.201402041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A. L., Tham W. H., Shah H., Amon A. (2004). A genome-wide screen identifies genes required for centromeric cohesion. Science 303, 1367–1370 10.1126/science.1094220 [DOI] [PubMed] [Google Scholar]
- McGuinness B. E., Hirota T., Kudo N. R., Peters J-M., Nasmyth K. (2005). Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 3, e86 10.1371/journal.pbio.0030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. O. (2007). The Cell Cycle: Principles of Control London: New Science Press. [Google Scholar]
- Musacchio A., Salmon E. D. (2007). The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Nerusheva O. O., Galander S., Fernius J., Kelly D., Marston A. L. (2014). Tension-dependent removal of pericentromeric shugoshin is an indicator of sister chromosome biorientation. Genes Dev. 28, 1291–1309 10.1101/gad.240291.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T., Ladurner R., Schmitz J., Kreidl E., Schleiffer A., Bhaskara V., Bando M., Shirahige K., Hyman A. A., Mechtler K. et al. (2010). Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143, 737–749 10.1016/j.cell.2010.10.031 [DOI] [PubMed] [Google Scholar]
- Pearson C. G., Maddox P. S., Salmon E. D., Bloom K. (2001). Budding yeast chromosome structure and dynamics during mitosis. J. Cell Biol. 152, 1255–1266 10.1083/jcb.152.6.1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peplowska K., Wallek A. U., Storchová Z. (2014). Sgo1 regulates both condensin and Ipl1/Aurora B to promote chromosome biorientation. PLoS Genet. 10, e1004411 10.1371/journal.pgen.1004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primorac I., Musacchio A. (2013). Panta rhei: the APC/C at steady state. J. Cell Biol. 201, 177–189 10.1083/jcb.201301130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabitsch K. P., Gregan J., Schleiffer A., Javerzat J-P., Eisenhaber F., Nasmyth K. (2004). Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr. Biol. 14, 287–301 10.1016/j.cub.2004.01.051 [DOI] [PubMed] [Google Scholar]
- Riedel C. G., Katis V. L., Katou Y., Mori S., Itoh T., Helmhart W., Gálová M., Petronczki M., Gregan J., Cetin B. et al. (2006). Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441, 53–61 10.1038/nature04664 [DOI] [PubMed] [Google Scholar]
- Rodrigo-Brenni M. C., Morgan D. O. (2007). Sequential E2s drive polyubiquitin chain assembly on APC targets. Cell 130, 127–139 10.1016/j.cell.2007.05.027 [DOI] [PubMed] [Google Scholar]
- Rohner S., Gasser S. M., Meister P. (2008). Modules for cloning-free chromatin tagging in Saccharomyces cerevisae. Yeast 25, 235–239 10.1002/yea.1580 [DOI] [PubMed] [Google Scholar]
- Salic A., Waters J. C., Mitchison T. J. (2004). Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118, 567–578 10.1016/j.cell.2004.08.016 [DOI] [PubMed] [Google Scholar]
- Stephens A. D., Haase J., Vicci L., Taylor R. M., II and Bloom K. (2011). Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J. Cell Biol. 193, 1167–1180 10.1083/jcb.201103138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchová Z., Becker J. S., Talarek N., Kögelsberger S., Pellman D. (2011). Bub1, Sgo1, and Mps1 mediate a distinct pathway for chromosome biorientation in budding yeast. Mol. Biol. Cell 22, 1473–1485 10.1091/mbc.E10-08-0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight A. F., Marshall W. F., Sedat J. W., Murray A. W. (1997). Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277, 574–578 10.1126/science.277.5325.574 [DOI] [PubMed] [Google Scholar]
- Suijkerbuijk S. J., Vleugel M., Teixeira A., Kops G. J. (2012). Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev. Cell 23, 745–755 10.1016/j.devcel.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Sullivan M., Morgan D. O. (2007). Finishing mitosis, one step at a time. Nat. Rev. Mol. Cell Biol. 8, 894–903 10.1038/nrm2276 [DOI] [PubMed] [Google Scholar]
- Tang Z., Sun Y., Harley S. E., Zou H., Yu H. (2004). Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis. Proc. Natl. Acad. Sci. USA 101, 18012–18017 10.1073/pnas.0408600102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Shu H., Qi W., Mahmood N. A., Mumby M. C., Yu H. (2006). PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell 10, 575–585 10.1016/j.devcel.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Tanno Y., Kitajima T. S., Honda T., Ando Y., Ishiguro K., Watanabe Y. (2010). Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 24, 2169–2179 10.1101/gad.1945310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T., Tanno Y., Watanabe Y. (2010). Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467, 719–723 10.1038/nature09390 [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse V., Prykhozhij S., Hardwick K. G. (2007). Shugoshin 2 regulates localization of the chromosomal passenger proteins in fission yeast mitosis. Mol. Biol. Cell 18, 1657–1669 10.1091/mbc.E06-10-0890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijlbergen K. F., Nerusheva O. O., Kelly D., Kerr A., Clift D., de Lima Alves F., Rappsilber J., Marston A. L. (2014). Shugoshin biases chromosomes for biorientation through condensin recruitment to the pericentromere. eLife 3, e01374 10.7554/eLife.01374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Cetin B., Anger M., Cho U. S., Helmhart W., Nasmyth K., Xu W. (2009). Structure and function of the PP2A-shugoshin interaction. Mol. Cell 35, 426–441 10.1016/j.molcel.2009.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaakov G., Thorn K., Morgan D. O. (2012). Separase biosensor reveals that cohesin cleavage timing depends on phosphatase PP2A(Cdc55) regulation. Dev. Cell 23, 124–136 10.1016/j.devcel.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y., Sakuno T., Shimura M., Watanabe Y. (2008). Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455, 251–255 10.1038/nature07217 [DOI] [PubMed] [Google Scholar]
- Yamagishi Y., Honda T., Tanno Y., Watanabe Y. (2010). Two histone marks establish the inner centromere and chromosome bi-orientation. Science 330, 239–243 10.1126/science.1194498 [DOI] [PubMed] [Google Scholar]
- Zalatan J. G., Coyle S. M., Rajan S., Sidhu S. S., Lim W. A. (2012). Conformational control of the Ste5 scaffold protein insulates against MAP kinase misactivation. Science 337, 1218–1222 10.1126/science.1220683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata J., Dephoure N., Macdonough T., Yu Y., Parnell E. J., Mooring M., Gygi S. P., Stillman D. J., Kellogg D. R. (2014). PP2ARts1 is a master regulator of pathways that control cell size. J. Cell Biol. 204, 359–376 10.1083/jcb.201309119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.