Abstract
Introduction:
Postconditioning (PoCo) is an adaptive phenomenon whereby brief repetitive cycles of ischemia with intermittent reperfusion instituted immediately after prolonged ischemia at the onset of prolonged reperfusion elicit tissue protection. PoCo is noted to exert a protective effect in various organs like heart, liver, kidney and brain. Various triggers, mediators and end effectors are suggested to contribute to the protective effect of PoCo. However, the neuroprotective mechanism of PoCo is poorly understood.
Objectives:
The present study has been designed to investigate the role of nitric oxide pathway in the neuroprotective mechanism of ischemic postconditioning (iPoCo) employing a mouse model of global cerebral ischemia and reperfusion-induced injury.
Materials and Methods:
Bilateral carotid artery occlusion (BCAO) of 12 min followed by reperfusion for 24 h was employed to produce ischemia and reperfusion (I/R)-induced cerebral injury in mice. Cerebral injury was assessed in the terms of cerebral infarct, memory impairment and motor in-coordination. Brain nitrite/nitrate; acetylcholinesterase activity, thiobarbituric acid reactive species (TBARS) and glutathione level were also estimated.
Results:
BCAO followed by reperfusion produced a significant rise in cerebral infarct size, memory impairment and motor incoordination. Further a rise in acetylcholinesterase activity and TBARS level along with fall in brain nitrite/nitrate and glutathione levels was also noted. iPoCo consisting of three episodes of 10 s carotid artery occlusion and reperfusion (instituted immediately after BCAO) significantly attenuated infarct size, memory impairment, motor incoordination as well as altered biochemicals. iPoCo-induced neuroprotective effects were significantly abolished by pretreatment of L-NAME, a nonselective NOS inhibitor.
Conclusion:
It may be concluded that the nitric oxide pathway probably plays a vital role in the neuroprotective mechanism of iPoCo.
KEY WORDS: Cerebral ischemia, ischemic postconditioning, l-name, memory, neuroprotection, nitric oxide
Ischemic stroke is an acute severe manifestation of cerebrovascular disease. It is a disease characterized by rapid onset of neurological injury due to interruption of blood flow to the brain.[1] Although mortality from ischemic stroke has declined over the last decade, it still remains the second leading cause of death, since only limited therapeutic strategies exist.[1] An attempt to attenuate this injury led to the discovery of phenomenon like ischemic preconditioning and ischemic postconditioning (iPoCo).[2] Ischemic preconditioning (IPC), a well-established phenomenon, is being used since 1980s to attenuate ischemia–reperfusion (I/R)-induced injury.[3] However, inability of the preconditioning to predict the onset of ischemia in clinical settings led to the discovery of a new concept of postconditioning (PoCo), whereby brief repetitive cycles of ischemia with intermittent reperfusion immediately following prolonged ischemia elicit tissue protection.[2,4] A protective role of iPoCo has been documented in different organs like heart of several animal species,[5] brain,[4,6] and kidney.[7] iPoCo is also observed to produce cardioprotective effects in clinical settings[8] and hence iPoCo appears to hold great potential in clinical settings.
There is an impressive array of molecular mechanisms contributing to the protective effect of iPoCo which include triggers like adenosine, opioid, erythropoietin, endothelial nitric oxide, reactive oxygen species (ROS), acetylcholine, tissue factors, pro-inflammatory cytokines and bradykinin; mediators like reperfusion injury salvage kinase (RISK) pathways including phosphoinositide-3-kinase (PI3K-Akt), extra-cellular signal-regulated kinase1/2 pathway (MEK-ERK½), protein kinase G (PKG) and protein kinase C (PKC); end-effectors like mitochondrial permeability transition pore (mPTP) and mitochondrial potassium ATP channels (mKATP).[9]
Although the concept of post-conditioning is known for some time now but the mechanism of the neuroprotective effect of iPoCo is very poorly understood. Most of the studies have been carried out involving tissues other than that of brain but very little has been done on the brain.
Nitric oxide (NO) is a key biological messenger, playing a prominent role in preserving the functions of endothelium. Biochemically NO is obtained from its precursor l-Arginine with the help of enzyme endothelial nitric oxide synthase (NOS); NOS exists in three isomeric forms i.e. endothelial (eNOS), inducible (iNOS) and neuronal (nNOS) nitric oxide synthase. Recently, it has been observed that eNOS plays an important role in mediating cardio-protective effects of PoCo[10] and both eNOS and iNOS are involved in renal PoCo.[7] However, the role of NO in PoCo-mediated neuroprotection still remains to be evaluated.
Therefore, the present study has been designed to pharmacologically investigate the role of the NO pathway in the neuroprotective mechanism of iPoCo employing a mouse model of global cerebral I/R-induced injury.
Materials and Methods
Animals
Male Swiss mice weighing 20 ± 5 g, maintained on standard laboratory diet (Kisan Feeds Ltd., Mumbai, India) and having free access to tap water, were employed in the present study. They were housed in the departmental animal house and were exposed to normal light/dark cycle. All the animals used in the study were naive to Morris water maze test. The experiments were conducted in a semi-sound proof laboratory. The experimental protocol was duly approved by institutional animal ethics committee (Approval No. 2011-06). The care of the animals was carried out according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No.-107/1999/CPCSEA).
Drugs and chemicals
L-NAME (Cayman Chemicals, Ann Arbor, Mich, USA) and chloral hydrate (Riedel-deHaen, Germany) were dissolved in distilled water. All other chemicals used in the present study were of analytical quality. All drug solutions were freshly prepared before use.
Induction of global cerebral ischemia
Global cerebral ischemia was induced surgically according to methods of Himori et al., (1990)[11] and as described by Kaur et al., (2010).[6] Mice were anesthetized using chloral hydrate (400 mg/kg, ip). A midline ventral incision was made in the neck to expose right and left common carotid arteries, which were isolated from surrounding tissue and vagus nerve. A cotton thread was passed below each of the carotid arteries. Global cerebral ischemia was induced by occluding the carotid arteries. After 12 min of global cerebral ischemia, reperfusion was allowed for 24 hours. The incision was sutured back in layers. The sutured area was cleaned with 70% ethanol and was sprayed with antiseptic dusting powder. The animals were shifted individually to their home cage and were allowed to recover.
Induction of iPoCo
iPoCo was induced by introducing three cycles each consisting of 10 s ischemia followed by 10 s of reperfusion immediately after the bilateral carotid artery occlusion (BCAO) performed for 12 min i.e. before prolonged reperfusion of 24 hours.[6]
Assessment of cerebral infarct size
At the end of 24 h of reperfusion after global cerebral ischemia, the animals were subjected to behavioral assessment. Then they were sacrificed by spinal dislocation and the brains were removed and placed immediately in ice cold saline for 10 minutes. Brain samples were then sliced into uniform coronal sections of about 1 mm thickness. The slices were incubated in 1% triphenyltetrazolium chloride (TTC) at 37°C in 0.2 M tris buffer (pH 7.4) for 20 minutes.[12] The infarct size was measured by the volume method using NIH image software.[13]
Evaluation of memory using Morris water maze test
Morris water maze test was employed to assess memory of the animals.[14] MWM procedure is based on the principle where animal is placed in a large pool of water divided into four equal quadrants, as animal dislikes swimming, its tendency to escape is accomplished by finding a hidden escape platform. Each animal was subjected to four consecutive training trials (with an inter trial gap of 5 min) each day for four consecutive days in search for a hidden platform. The day 4 escape latency time (ELT) to locate the hidden platform in water maze was taken as an index of acquisition or learning. On the fifth day, the hidden platform was removed. Each animal was allowed to explore the pool for 120 seconds. Mean time spent in all the quadrants in search of hidden platform was noted. The mean time spent by the animal in the target quadrant (TSTQ) was taken as the index of retrieval or memory. After the measurement of day 4 ELT the animals were subjected to global cerebral I/R and their retrieval was tested next day i.e. on d as TSTQ.
Assessment of motor coordination
Rota-rod test
Rota rod was employed to evaluate motor coordination by testing the ability of mice to remain on revolving rod.[15] Each mouse was given five trials before the actual reading was taken. The animals staying on revolving rod for period of 5 minutes before the surgical procedure were selected and the test was again performed after 12 min of global cerebral ischemia and 24 h of reperfusion.
Inclined beam-walking test
The inclined beam-walking test was employed to evaluate fore and hind limb motor coordination.[16] The motor performance of mouse was scored on a scale ranging from 0 to 4. A grade of 0 was assigned to animal that could readily traverse the beam, grade 1 was given to animal demonstrating mild impairment, grade 2 was assigned to animal demonstrating moderate impairment, grade 3 was given to animal demonstrating severe impairment and grade 4 was assigned to animal completely unable to walk on the beam. The inclined beam-walking test was performed before global cerebral ischemia and 24 h after global cerebral I/R.
Lateral push test
A mouse was placed on a rough surface for firm grip and was evaluated for resistance to lateral push from either side of the shoulder.[16] The test was performed before global cerebral ischemia and 24 h after global cerebral I/R. Mice with increased or decreased resistance to lateral push after global ischemia were assigned + or − score, respectively.
Biochemical estimations
After behavioral tests the animals were sacrificed by cervical dislocation, brains were removed and homogenized in phosphate buffer (pH = 7.4). The homogenates were then centrifuged at 3000 rpm for 15 minutes. The supernatant of homogenates were used for biochemical estimations.
Estimation of brain acetylcholineesterase activity
The whole brain AChE activity was measured spectrophotometrically (DU 640B spectrophotometer, Beckman Coulter Inc., CA, USA) at 420 nm by the method of Ellman et al.[17] with slight modification by Voss and Sachsse (1970).
Estimation of brain total protein
The brain total protein was determined by the method of Lowry et al.[18]
Estimation of brain thiobarbituric acid reactive species
The quantitative measurement of thiobarbituric acid reactive species (TBARS), an index of lipid peroxidation in brain, was performed according to the method of Ohokawa et al.[19]
Estimation of brain reduced glutathione
The reduced glutathione (GSH) content in brain was estimated spectrophotometrically (DU 640B spectrophotometer, Beckman Coulter Inc., CA, USA) at 412 nm using method of Beutler et al.[20]
Estimation of brain nitrite/nitrate
Brain nitrite/nitrate concentration was measured spectophotometrically (DU 640B Spectrophotometer, Beckman Coulter Inc., CA, USA) at 542 nm, using method of Sastry et al.[21]
Protocol
In total seven groups were employed and each group comprised of 8 animals.
Group I (sham group): Each mouse was subjected to surgical procedure and a thread was passed below the carotid arteries but they were not occluded. After 12 min, threads were removed and the animal was sutured back and allowed to recover for 24 hours.
Group II (control group): Each mouse was subjected to 12 min global cerebral ischemia followed by reperfusion for 24 hours.
Group III (ischemic postconditioning group): Each mouse was subjected to 12 min global cerebral ischemia immediately followed by three episodes of 10 s of I/R each after which a 24 h reperfusion period was permitted.
Group IV and VII (L-NAME control group): Each mouse was administered L-NAME (1.5 mg/kg; 3 mg/kg i.p.) 30 min prior to carotid artery occlusion. The rest of the procedure was the same as described for Group II.
Group VI and VI (L-NAME ischemic postconditioning group): Each mouse was administered L-NAME (1.5 mg/kg; 3 mg/kg i.p.) 30 min prior to carotid artery occlusion. The rest of the procedure was the same as described for Group III.
Statistical analysis
The results were expressed as mean ± standard error of means (S.E.M.). Statistical analysis for all the results was done using one-way ANOVA followed by Tukey's multiple range tests as post hoc analysis. A value of P < 0.05 was considered to be statistically significant.
Results
Effect on cerebral infarct size
Global cerebral ischemia of 12 min followed by reperfusion for 24 h produced a significant (P < 0.05) increase in cerebral infarct size in the control group when compared to the sham group. iPoCo significantly (P < 0.05) attenuated I/R-induced rise in cerebral infarct size. Pretreatment of L-NAME significantly (P < 0.05) abolished iPoCo-induced decrease in infarct size. However L-NAME did not modify infarct size of ischemic control animals [Figure 1].
Figure 1.
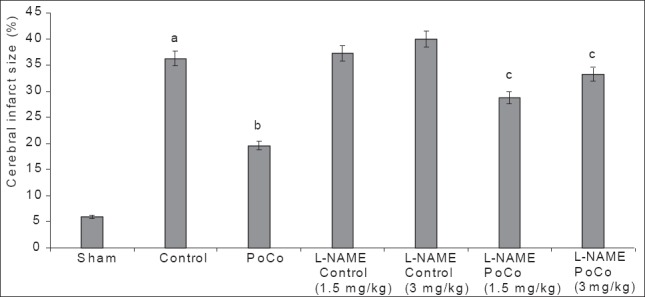
Effect of various interventions on cerebral infarct size. Each group represents mean ± S.E.M. a=P<0.05 vs sham, b=P<0.05 vs control, c=P<0.05 vs PoCo. PoCo: Postconditioning
Effect on motor performance
Effect on fall down time using Rota rod test
Global cerebral ischemia followed by reperfusion produced a significant (P < 0.05) reduction in fall down time in the control group, as measured by the rota-rod test, when compared to the sham group. iPoCo significantly attenuated I/R-induced reduction in fall down time. Further, pretreatment of L-NAME significantly abolished (P < 0.05) iPoCo-induced effect on fall down time. However, L-NAME did not modify fall down time of ischemic control animals [Figure 2].
Figure 2.
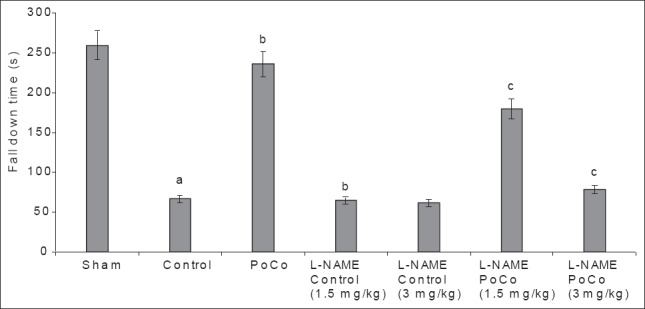
Effect of various interventions on motor performance (fall down time) using the rota rod test. Each group represents mean ± S.E.M. a=P<0.05 vs sham, b=P<0.05 vs control, c=P<0.05 vs PoCo. PoCo: Postconditioning
Effect on motor in-coordination score using inclined beam walking test
Global cerebral ischemia followed by reperfusion produced significant increase in motor incoordination score in mice noted after 24 h of reperfusion in the control group when compared to the sham group. iPoCo markedly prevented I/R-induced rise in the motor incoordination score. Further, pretreatment of L-NAME significantly (P < 0.05) abolished iPoCo-induced decrease in the motor incoordination score. However, L-NAME did not modify the motor incoordination score of ischemic control animals [Figure 3].
Figure 3.
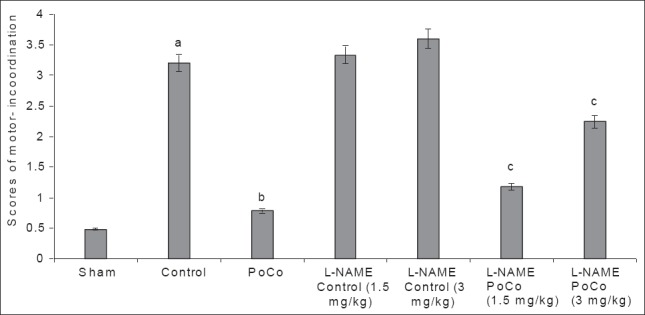
Effect of various interventions on motor performance (score) using the inclined beam-walking test. Each group represents mean ± S.E.M. a=P<0.05 vs sham, b=P<0.05 vs control, c=P<0.05 vs PoCo. PoCo: Postconditioning
Effect on lateral push response
Global cerebral ischemia followed by reperfusion produced a significant decrease in percentage resistance to lateral push noted after 24 h of reperfusion in the control group, when compared to the sham group. iPoCo significantly prevented I/R-induced decrease in percentage resistance to lateral push. Further, pretreatment of L-NAME significantly (P < 0.05) abolished iPoCo-induced effect on resistance to lateral push. However, L-NAME did not modify decrease in percentage resistance to lateral push of ischemic control animals [Figure 4].
Figure 4.
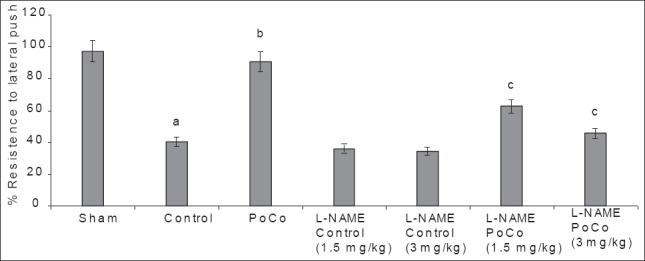
Effect of various interventions on motor performance (% resistance to lateral push) using the lateral push test. Each group represents mean ± S.E.M. a=P<0.05 vs sham, b=P<0.05 vs control, c=P<0.05 vs PoCo. PoCo: Postconditioning
Effect on memory
There was a downward trend in ELT of animals on subsequent water maze exposure during acquisition trial and a significant decrease in their day 4 ELT values was noted thereby indicating normal learning abilities [Table 1]. The sham mice when subjected to the retrieval test on day 5 spent significantly (P < 0.05) more time in the target quadrant (Q4) in search of the missing platform as compared with time spent in other quadrants (Q1, Q2 and Q3), reflecting normal memory capacity. Global cerebral ischemia followed by reperfusion markedly reduced (P < 0.05) time spent in the target quadrant (TSTQ) on day 5 when compared with the sham group mice indicating memory impairment. On the other hand, iPoCo produced significant increase (P < 0.05) in TSTQ on day 5 as compared to ischemia control group mice thus attenuating I/R-induced memory impairment. Further pretreatment of L-NAME did not modify TSTQ of ischemic control animals but it significantly abolished the effect of iPoCo on TSTQ of ischemic control animals [Figure 5]. However, this effect of L-NAME on TSTQ appears to less pronounce as compared to its effect of other above-mentioned behavioral parameters.
Table 1.
Effect of various interventions on escape latency time using Morris water maze
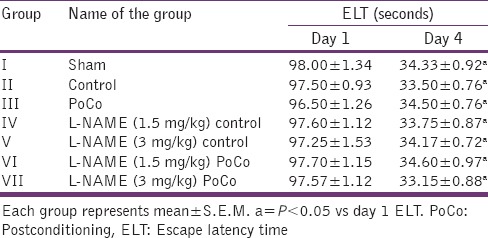
Figure 5.
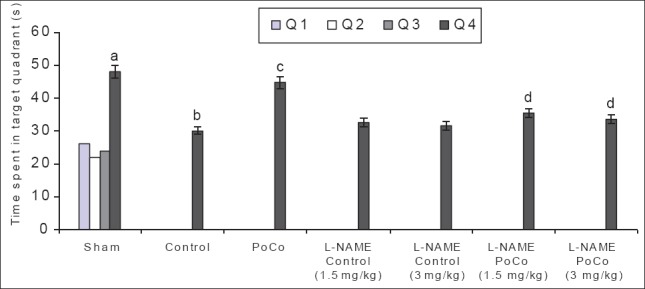
Effect of various interventions on time spent in target quadrant (TSTQ) using the Morris water maze test. Each group represents mean ± S.E.M. a=P<0.05 vs time spent in other quadrants in sham, b=P<0.05 vs TSTQ in sham, c=P<0.05 vs TSTQ in control, d=P<0.05 vs TSTQ in PoCo. PoCo: Postconditioning
Effect on various biochemical interventions
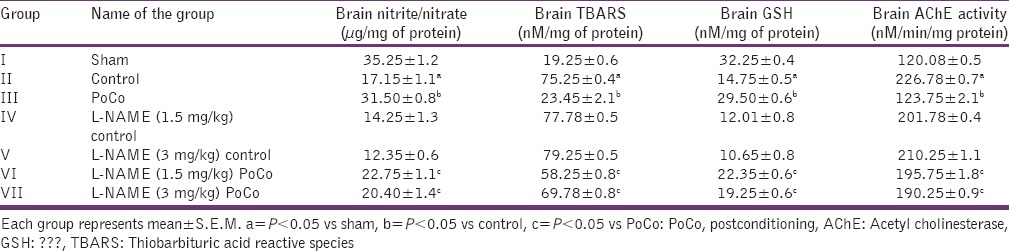
Global cerebral ischemia followed by reperfusion produced significant (P < 0.05) increase in brain AChE activity; brain TBARS; and decrease in brain GSH and brain nitrite/nitrate levels when compared to the sham group. iPoCo significantly attenuated I/R-induced changes in brain biochemicals. However, pretreatment of L-NAME significantly (P < 0.05) abolished iPoCo-induced protective effect on brain biochemicals [Table 2].
Table 2.
Effect of various interventions on brain nitrite/nitrate; oxidative stress (TBARS and GSH) levels and acetyl cholinesterase activity
Discussion
The results of present investigation indicate that iPoCo involving three episodes of 10 s carotid artery occlusion with intermittent reperfusion of 10 s produced a significant neuroprotective effect. iPoCo-mediated neuroprotective effects have been significantly abolished by pretreatment with L-NAME, a nonselective NOS inhibitor.
Male mice were used in the study because it has been reported that a high estrogen level itself exerts protection in cerebral I/R injury.[22] Global cerebral ischemia employed in this investigation is well reported to simulate the clinical situation of cerebral ischemia.[23] Cerebral ischemia has been reported to impair memory because hippocampal neurons are susceptible to the deleterious effects of I/R[24] and the hippocampus is involved in regulation of memory. Cerebral ischemia is further documented to impair sensorimotor ability as well.[25] Therefore, in the present study, we employed the Morris water maze test to assess memory and rota-rod test, lateral push test and inclined beam walk test for the evaluation of motor coordination. In addition, a battery of biochemical estimations, namely brain AChE activity (to assess brain cholinergic level), brain TBARS and glutathione levels (to assess oxidative stress) and nitrite/nitrate level (to assess brain NO levels) were also performed. Extent of I/R-induced neuronal injury, in term of cerebral infarction, was assessed by using triphenyltetrazolium chloride staining.[12] The infarct size was measured by the volume method using NIH image software.[13]
In our study, global cerebral ischemia induced by BCAO for 12 min, followed by prolonged reperfusion of 24 h produced a significant rise in infarct size along with impairment of memory as well as motor coordination. These results are in line with our earlier reports[4,16] and reports from other laboratories.[26] One of the most prominent contributors of I/R injury is oxidative stress. Cerebral I/R injury is well documented to produce oxidative stress.[27] This is further supported by observations showing enhanced oxidative stress (increased brain TBARS and decreased GSH levels) in the present study. Interestingly, in our previous finding we have noticed that enhanced brain oxidative stress is somehow related to increased brain AChE activity.[28] This contention is supported by observations showing enhanced brain AChE activity in the present study. However, the exact reason for observed enhancement of brain AChE activity remains to be determined. Cerebral I/R resulted in significant fall in brain nitrite/nitrate levels. This is also in line with the earlier studies which clearly document massive fall in NO in I/R injury.[29,30]
iPoCo, consisting of three cycles of 10 s ischemia with intermittent reperfusion of 10 s, prevented I/R-induced rise in cerebral infarct size and impairment of memory and motor coordination along with altered biochemical level. This observation complies with our earlier findings, whereby a similar protocol of iPoCo has been shown to produce neuroprotection.[4] iPoCo in earlier studies have been also been reported to alleviate oxidative stress,[31] enhanced AChE activity[28] and fall in nitrite/nitrate levels[31] induced by I/R injury.
In the present study, treatment of L-NAME (3 mg/kg, i.p.), a non-selective NOS inhibitor, per se did not modify I/R-induced rise in cerebral infarct size and associated behavioral deficits in control groups. However, it abolished the neuroprotective effects of iPoCo in a significant manner, quantitated in terms of increase in cerebral infarct size; impairment of memory; motor-incoordination and alterations in brain biochemicals. Although the effect of L-NAME on time spent in target quadrant in Morris water maze was not that much pronounced as seen in other behavioral parameters yet it was statistically quite significant. Probably this variation may be attributed to little bit small sample size.
Earlier studies have reported the implication of the NO pathway in iPoCo-mediated cardio-protection,[10] renal protection[7] and hepatic protection.[31] Therefore, it may be possible to suggest here that iPoCo-induced neuroprotection also involves the NO pathway.
This point is further supported by a recent study of Peng Le et al.(2012),[32] documenting a significant role of the eNOS and PI3K/Akt pathway in the neuroprotective mechanism of remote iPoCo. iPoCo instituted at a remote site (hind limb skeletal muscles) induced neuroprotection and memory improvement against global cerebral ischemic/reperfusion injury and an enhanced expression of eNOS was observed. The above effect of remote iPoCo was abolished by pretreatments of L-NAME (nonselective NOS inhibitor) and LY294002 (a highly selective inhibitor of PI3K). The level of NO appears to be very critical for maintenance of normal neuronal function since NO has both pro and anti-oxidant activity. In conditions like I/R probably the pro-oxidative property predominates contributing to injury. PoCo tends to provide protection by preventing this deleterious fall in NO thereby increasing NO level. However, such protection was not observed with L-NAME, a nonselective NOS inhibitor pointing that after L-NAME treatment NO levels probably falls below the critical level needed for maintenance of normal neuronal function. Further, in contrast to theoretical assumptions L-NAME at the given doses in this study did not aggravate I/R injury although there was a slight increase in infarct size but results were not statistically significant. This effect may be dose related and needed to be ascertained.
Conclusion
Therefore, with data in hand and support from the literature it is concluded that L-NAME has abolished the neuroprotective effect of iPoCo indicating that the neuroprotective mechanism of iPoCo probably involves nitric oxide-dependent pathways. Nevertheless, further in-depth studies are needed to establish the role of nitric oxide in the neuroprotective mechanism of iPoCo and substantiate these findings.
Acknowledgement
Authors are thankful to University Grants Commission, New Delhi, India, for funding this project [F.No. 39-160/2010 (SR)]
Footnotes
Source of Support: UGC, New Delhi, India
Conflict of Interest: None declared.
References
- 1.Heiss WD. The ischemic penumbra: Correlates in imaging and implications for treatment of ischemic stroke. Cerebrovasc Dis. 2011;32:307–20. doi: 10.1159/000330462. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Yellon DM. The Therapeutic Potential of Ischemic Conditioning: An Update. Nat Rev Card. 2011;8:619–29. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Rehni AK, Singh N. Role of phosphoinositide 3-kinase in ischemic postconditioning-induced attenuation of cerebral ischemia-evoked behavioral deficits in mice. Pharmacol Rep. 2007;59:192–8. [PubMed] [Google Scholar]
- 5.Zheng Z, Yang M, Zhang F, Yu J, Wang J, Ma L, et al. Gender-related difference of sevoflurane postconditioning in isolated rat hearts: Focus on phosphatidylinositol-3-kinase/Akt signaling. J Surg Res. 2011;170:e3–9. doi: 10.1016/j.jss.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Kaur H, Jaggi AS, Singh N. Modulation of neuroprotective effect of ischemic post-conditioning by dichlorobenzamil, a Na+/Ca2+ exchanger inhibitor in mice. Biol Pharma Bull. 2010;33:585–91. doi: 10.1248/bpb.33.585. [DOI] [PubMed] [Google Scholar]
- 7.Liu X, Chen H, Zhan B, Xing B, Zhou J, Zhu H, et al. Attenuation of reperfusion injury by renal ischemic postconditioning: The role of NO. Biochem Biophys Res Commun. 2007;359:628–34. doi: 10.1016/j.bbrc.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 8.Fan Q, Yang XC, Liu Y, Wang LF, Liu SH, Ge YG, et al. Postconditioning attenuates myocardial injury by reducing nitro-oxidative stress in vivo in rats and in humans. Clin Sci (Lond) 2011;120:251–61. doi: 10.1042/CS20100369. [DOI] [PubMed] [Google Scholar]
- 9.Kaur S, Jaggi AS, Singh N. Molecular aspects of ischemic postconditioning. Fund Clin Pharmacol. 2009;23:521–36. doi: 10.1111/j.1472-8206.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 10.Perrelli MG, Pagliaro P, Penna C. Ischemia/reperfusion injury and cardioprotective mechanisms: Role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200. doi: 10.4330/wjc.v3.i6.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himori N, Wantanabe H, Akaike N, Kurasawa M, ltoh J, Tanaka Y. Cerebral ischemia model with conscious mice; Involvement of NMDA receptor activation and derangement of learning and memory ability. J Pharmacol Methods. 1990;23:311–27. doi: 10.1016/0160-5402(90)90059-t. [DOI] [PubMed] [Google Scholar]
- 12.Bochelen D, Rudin M, Sauter A. Calcineurin inhibitors FK506 and SDZ ASM 981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J Pharmacol Exp Ther. 1999;288:653–9. [PubMed] [Google Scholar]
- 13.Türeyen K, Vemuganti R, Sailor KA, Dempsey RJ. Infarct volume quantification in mouse focal cerebral ischemia: A comparison of triphenyltetrazolium chloride and cresyl violet staining techniques. J Neurosci Methods. 2004;139:203–7. doi: 10.1016/j.jneumeth.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 14.Parle M, Singh N. Animal models for testing memory. Asia Pacific J Pharmacol. 2004;16:101–2. [Google Scholar]
- 15.Dunham NW, Miya TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Accoc. 1957;46:208–9. doi: 10.1002/jps.3030460322. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Singh M, Sharma A. Neuroprotective effect of antioxidants on ischaemia/reperfusion-induced cerebral injury. Pharmacol Res. 2003;48:209–15. doi: 10.1016/s1043-6618(03)00102-6. [DOI] [PubMed] [Google Scholar]
- 17.Ellman GL, Courtney KD, Andres VJ, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Far AL, Randall RJ. Protein measurement with phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Ohokawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Duron O, Kelly BM. Improved methods for determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 21.Sastry KV, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric Determination of Serum Nitrite and Nitrate by Copper–Cadmium Alloy. Anal Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 22.Zhai P, Eurell TE, Cotthaus R, Jeffery EH, Bahr JM, Gross DR. Effect of estrogen on global myocardial ischemia-reperfusion injury in female rats. Am J Physiol Heart Circ Physiol. 2000;279:H2766–75. doi: 10.1152/ajpheart.2000.279.6.H2766. [DOI] [PubMed] [Google Scholar]
- 23.Alonso de Lecinana M, Diez-Tejedor E, Cearcellar F, Roda JM. Cerebral ischemia: From animal studies to clinical practice; should the methods be reviewed? Cerebrovasc Dis. 2001;11:20–30. doi: 10.1159/000049122. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki K, Mishima K, Egashira N, Al-Khatib IH, Ishibashi D, Irie K, et al. Effect of nilvadipine on the cerebral ischemia-induced impairment of spatial memory and hippocampal apoptosis in rats. J Pharmacol Sci. 2003;93:188–96. doi: 10.1254/jphs.93.188. [DOI] [PubMed] [Google Scholar]
- 25.Dobkin BH. The rehabilitation of elderly stroke patients. Clin Geriatr Med. 1991;7:507–23. [PubMed] [Google Scholar]
- 26.Wong AM, Hodges H, Horsburgh K. Neural stem cell grafts reduce the extent of neuronal damage in a mouse model of global Ischemia. Brain Res. 2005;1063:140–50. doi: 10.1016/j.brainres.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Akhtar M, Pillai KK, Vohora D. Effect of thioperamide on oxidative stress markers in middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol. 2008;27:761–7. doi: 10.1177/0960327108094608. [DOI] [PubMed] [Google Scholar]
- 28.Gulati P, Muthuraman A, Jaggi AS, Singh N. Neuroprotective effect of gadolinium: A stretch-activatedcalcium channel blocker in mouse modelof ischemia-reperfusion injury. aNaunyn-Schmiedeberg's. Arch Pharmacol. 2013;386:255–64. doi: 10.1007/s00210-012-0819-y. [DOI] [PubMed] [Google Scholar]
- 29.Rhodena EL, Rhodenb CR, Lucasb ML, Pereira-Limaa L, Zettlerc C, Bello×-Kleind A. The role of nitric oxide pathway in the renal ischemia-reperfusion injury in rats. Transpl Immunol. 2002;10:277–84. doi: 10.1016/s0966-3274(02)00079-5. [DOI] [PubMed] [Google Scholar]
- 30.Krauss H, Sosnowski P, Biczysko M, Biczysko W, Majewski P, Jablecka A, et al. Effects of L-Arginine and NG-Nitro L-Arginine Methyl Ester (L-NAME) on Ischemia/Reperfusion Injury of Skeletal Muscle, Small and Large Intestines. Chin J Physiol. 2011;54:7–18. doi: 10.4077/cjp.2011.amk011. [DOI] [PubMed] [Google Scholar]
- 31.Guo JY, Tong Y, Sun XG, Zhou NY, Li FS, Long D, et al. Ischemic post-conditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci. 2011;18:79. doi: 10.1186/1423-0127-18-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng B, Guo QL, He ZJ, Ye Z, Yuan YJ, Wang N, et al. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by up-regulating endothelial nitric oxide synthase through PI3K/Akt pathway. Brain Res. 2012;1445:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]


