Abstract
Aim of the Study:
The synthesis, characterization and application of biologically synthesized nanomaterials are an important aspect in nanotechnology.
Materials and Methods:
The present study deals with the synthesis of silver nanoparticles (Ag-NPs) using the coconut water (C. nucifera) as the reducing agent. The formation of Ag-NPs was characterized by UV-Visible Spectroscopy, Scanning Electron Microscopy (SEM), EDX, X-ray Diffraction (XRD) and FTIR spectroscopy.
Results:
The synthesized Ag-NPs were predominately polydispersed. Crystalline nature of the nanoparticle in the face centered cubic (fcc) structure are confirmed by the peaks in the XRD pattern corresponding to (111), (200), (220) and (311) planes. Fourier Transform Infra-Red (FT-IR) spectroscopy analysis showed that the synthesized nanoparicles was capped with bimolecular compounds which are responsible for the reduction of silver ions.
Conclusion:
The approach of green synthesis appears to be cost efficient, ecofriendly and easy alternative to conventional methods of silver nanoparticle synthesis.
KEY WORDS: Coconut water, ecofriendly, green synthesis, silver nanoparticle
Nanotechnology is the principally attractive area of research related with production of nanoparticles of variable sizes, shapes, chemical compositions, controlled dispersity and their possible application for human being benefits. Creation, manipulation and utilization of metallic nanoparticles because of the reduction of materials dimensions, affect the physical properties and results in displaying extraordinary chemical, physical, thermal, optical and electronic properties of nanomaterials.[1] Metallic nanoparticles are presently applied in different fields such as electronics, biotechnology, DNA labeling, drug delivery, cosmetics, coatings and packaging.[2,3] Among the metal-nanoparticle, silver nanoparticles (Ag-NPs) exhibit tremendous applications in spectrally selective coatings for solar energy absorption, optical receptors, bio-labeling, intercalation materials for electrical batteries, filters, antimicrobial agents and sensors.[4,5,6,7,8] Silver nanoparticle-embedded antimicrobial paint[9] is a promising area of environmentally friendly applications. Hence, a variety of techniques including physical and chemical methods have been developed to synthesize Ag-NPs. The physical methods[10] are highly expensive[11] and chemical methods are harmful to the environment.[12] Therefore, there is a growing need to develop the environmentally benign nanoparticle synthesis processes that do not use toxic chemicals in the synthesis protocols.
Green processes with the use of economic, efficient and ecofriendly catalysis are gaining much importance due to the benefits associated with their use. In the recent years, ‘green synthesis’ of the NPs has paid much more attention in the rapidly growing area of nanoscience and nanotechnology.[13,14,15,16] Utilization of cheap nontoxic chemicals, eco-friendly solvents and renewable materials are some of the pivotal issues that merit important concern in a green synthesis strategy for nanomaterials. Earlier reports cite the use of microorganism such as Bacillus koreensis, B. subtilis, etc., and fungi such as A. fumigates, F.oxysporum, etc., for the biosynthesis of Ag NPs.[17,18,19] Recently, several plant extracts such as M. oleifera,[20] E. hirta,[21] C. roseus,[22] S. tumbuggaia,[23] and Diopyros kaki[24] etc.
Coconut (Cocos nucifera linn): It belongs to the family of Arecaceae. Indigenous people of tropical countries use young coconut juice in the treatment of stomach upsets, diarrhea and dysentery. Coconut water contains sugar, fiber, proteins, antioxidants, vitamins and minerals[25] and provides an isotonic electrolyte balance, making it a nutritious food source. Coconut water is used in place of dextrose\glucose in medical emergencies. During the World War II, young coconut water was used as an emergency room glucose supply in the absence of sterile glucose; it is also used as an antidote for poisons.[26] It is used to expel intestinal parasites like tapeworms and Helicobacter pylori.[27] Coconut water is traditionally used as a growth supplement in plant tissue culture/micropropagation.[28] In this study, we explored for the first time the potential of the C.nucifera to enlarge the scope of non-toxic biological systems for the biogenic synthesis of metallic nanomaterials.
Materials and Methods
Collection of coconut water
The fresh fruit of coconut was collected from local market. The fruits were washed thoroughly to remove adhered material. Then make a hole to that fruits and collect the coconut water into a clean and conical flask. Then that water was filtered with the help of filtration set and filtrate was collected.[29] The filtered coconut water was used for further process.
Biogenic synthesis of silver nanoparticles
Typically, 10 mL of coconut water was added to 90 mL of 1 mM aqueous AgNO3 solution for the reduction of Ag+ ions. The mixture were heated to 80°C for 15 to 20 minutes on a steam bath until the color of solutions changes from watery to reddish color was observed. The synthesized NPs were extracted from the solution by centrifugation at 18,000 rpm for 20 minutes by using a REMI ultracentrifuge.
UV-VIS Spectra analysis
The reduction of pure Ag + ions was monitored by measuring the UV-Vis spectrum of the reaction medium at 2 h after diluting a small aliquot of the sample into distilled water. UV-Vis spectral analysis was done by using UV-VIS spectrophotometer UV-2450 (Shimadzu).
SEM analysis of silver nanoparticles
Scanning electron microscopic (SEM) analysis was done using Hitachi S-4500 SEM machine. Thin films of the sample were prepared on a carbon coated copper grid by just dropping a very small amount of the sample on the grid, extra solution was removed using a blotting paper and then the film on the SEM grid were allowed to dry by putting it under a mercury lamp for 5 minutes.
EDAX measurements
In order to carry out EDAX analysis, the coconut water reduced Ag-NPs were dried and drop coated on to carbon film and performed on Hitachi S-3400 N SEM instrument equipped with a Thermo EDAX attachments.
XRD measurement
The X-ray diffraction (XRD) measurements of the coconut water -reduced Ag NPs were carried out on drop-coated films of the respective solutions onto glass substrates using a Phillips PW 1830 instrument operating at a voltage of 40 kV with Cu Kα radiation.
Fourier transforms infrared spectroscopy (FTIR) measurements
To identify the possible biomolecules responsible for the reduction of the Ag ions and capping of the bioreduced Ag-NPs synthesized by coconut water, FTIR spectroscopy measurements were carried out. Ag-NPs powder sample was prepared by centrifuging the synthesized Ag-NPs solution at 18,000 rpm for 20 min. The pellet which contains Ag NPs was redispersed with sterile deionized water three times to get rid of the unattached biological impurities and remove the free proteins/enzymes that are not capping ligands for the silver nanoparticles. The samples were dried in an oven overnight at 60°C and grinded with KBr pellets and analyzed on a Shimadzu FTIR 8000 model in the diffuse reflectance mode operating at a resolution of 4 cm-1.
Results and Discussion
UV-VIS Spectra analysis
The formation of the metal NPs through the reduction of the aqueous metal ions during exposure to the coconut water can easily be followed by UV-Vis spectroscopy. During the process of the metal NPs reduction, it is well-known that Ag NPs exhibit a watery-reddish color in the reaction mixture, which arises due to the excitation of surface plasmon vibrations in the metal NPs.[30,31] As the coconut water was mixed in the aqueous solution of the Ag ion complex, it started to change the color from watery to reddish brown due to reduction of silver ion; which indicated formation of Ag-NPs [Figure 1].
Figure 1.

Color change of coconut water (C. nucifera) containing silver before and after the synthesis of Ag-NPs (a) Before, (b) After
The result obtained in this investigation is very interesting in terms of identification of potential coconut water for synthesizing the Ag-NPs. UV-Vis spectrograph of the colloidal solution of Ag-NPs has been recorded as a function of time viz. 10,20,30….120. Absorption spectra of Ag-NPs formed in the reaction media at 10 min has absorbance peak at 423 nm and the broadening of peak indicated that the particles are polydispersed [Figure 2].
Figure 2.
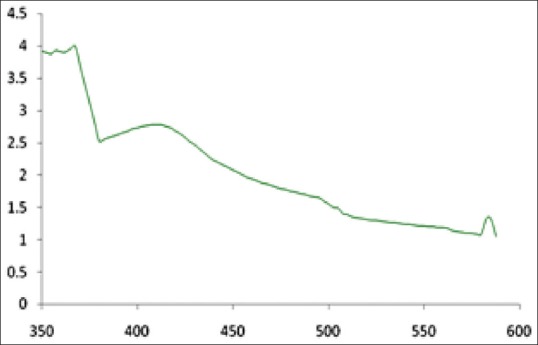
UV-VIS absorption spectra of Ag-NPs synthesized from the coconut water (C. nucifera) at 1 mM silver nitrate
SEM and EDAX studies
Figure 3a shows representative SEM images recorded from the drop-coated films of the Ag-NPs synthesized by treating AgNO3 solution with coconut water. The Ag-NPs formed were predominantly polydispersed shape. It is known that the shape of the metal nanoparticles considerably change their optical and electronic properties.[32] The SEM image showed relatively polydispersed shape nanoparticle formed with diameter range 70-80 nm. Similar phenomenon was reported by Chandran et al.[33]
Figure 3.
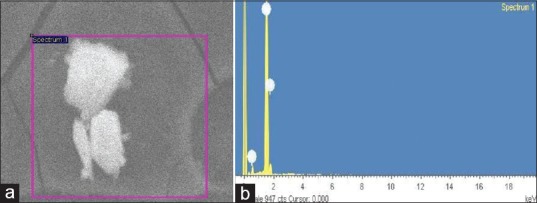
(a) SEM and (b) EDAX image of Ag-NPs formed by coconut water (C. nucifera)
Analysis through Energy Dispersive X-ray (EDX) spectrometers confirmed the presence of elemental silver signal of the Ag-NPs [Figure 3b]. The vertical axis displays the number of X-ray counts whilst the horizontal axis displays the energy in KeV. Identification lines for the major emission energies for silver (Ag) are displayed and these correspond with peaks in the spectrum, thus giving confidence that silver has been correctly identified.
XRD analysis
The XRD result shows four distinct diffraction peaks at 38.12°, 44.27°, 64.27° and 76.23°, which are indexed for the planes (111), (200), (220) and (311) respectively of the face centered cubic Ag [Figure 4]. This data was matched with the database of joint committee on powder diffraction standards (JCPDS) for silver. Thus, the formation of Ag is confirmed. The additional peak obtained at 30° may be due to the bio-inorganic compounds and protein matters present in the coconut water (as evidenced from the intensity of the Bragg reflections of strong X-ray scattering points in the crystalline phase arise from proteins in the NPs synthesis).[31] Other minor peaks are due to noise only. The average grain size of the Ag-NPs synthesized by the greener method was calculated using Scherr's formula [d = (0.89λ×180°)/βcosθπ] and observed as 48 nm. Hence, the synthesized Ag was in nano size only.
Figure 4.
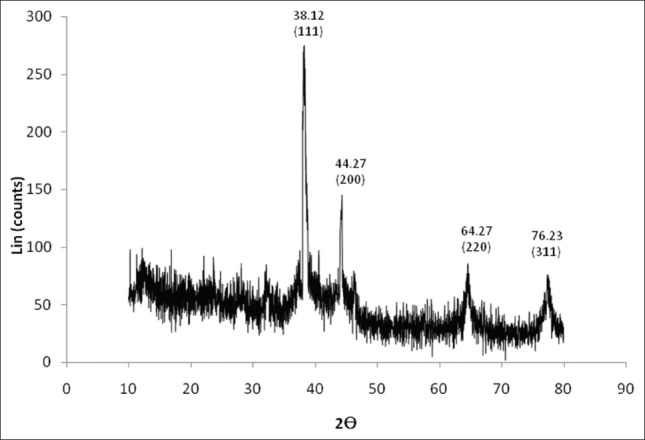
XRD patterens of capped Ag-NPs synthesised using the coconut water (C. nucifera)
FTIR spectroscopy
FTIR measurement was carried out to study the interaction of the nanoparticles and to identify the possible biomolecules in coconut water responsible for capping leading to efficient stabilization of the NPs. The intense IR bands [Figure 5] are observed at 3398, 1613, 1368, 1213 and 1069 cm-1. The bands observed at 3398 and 1613cm-1 are assigned to the stretching vibrations of the C-H and C-C bonds, respectively. The broad band at 1213 cm-1 is due to C-O and C-H stretching modes. The absorption bands located at 1368 may be attributed to C-H, NO3 and C-N stretching modes,[34] respectively. The bonds or functional group such indicates that's. Therefore, it may be assumed that water soluble compounds such as flavonoids, silver nanoparticles synthesized using the coconut water surrounded by some proteins and metabolites such as terpenoids, flavonoides which are capping ligands of the nanoparticles.
Figure 5.
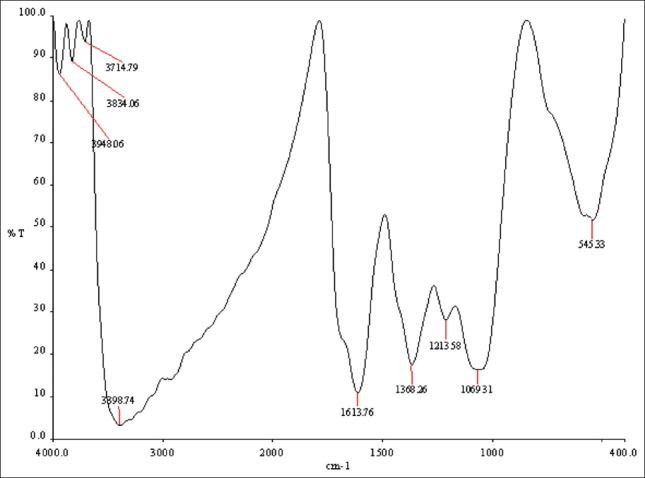
FTIR spectrum recorded by making KBr with synthesized Ag-NPs using the coconut water (C. nucifera)
Conclusion
The bio-reduction of aqueous Ag+ ions by coconut water (C.nucifera) has been demonstrated. This green chemistry approach towards the synthesis of Ag-NPs has many advantages such as ease with which the process can be scaled up, economic viability, etc., This environmentally friendly method of biological Ag-NPs can potentially be applied in various products that directly come in contact with the human body, such as cosmetics, foods and besides medical applications.
Footnotes
Source of Support: The authors grateful thanks to Department of Collegiate Education, Government of Tamilnadu, India for financial assistance
Conflict of Interest: No.
References
- 1.Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: Technological concepts and future applications. J Nanopart Res. 2008;10:507–17. [Google Scholar]
- 2.Kohler JM, Csaki A, Reichert R, Straube W, Fritzche W. Selective labeling of oligonuckleotide monolayers by metallic nanobeads for fast optical readout of DNA chips Sensors and Actuators B: Chemical. 2001;76:166–72. [Google Scholar]
- 3.Schatz GC, Lazarides AA, Kelly KL, Jensen TR. Optical properties of metal nanoparticles aggregates important in biosensors. J Mol Structure (Theochem) 2000;529:59–63. [Google Scholar]
- 4.Smitha SL, Nissamudeen KM, Philip D, Gopchandran KG. Studies on surface plasmon resonance and photoluminescence of silver nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 2008;71:186–90. doi: 10.1016/j.saa.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65:150–3. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Mukherjee P, Roy M, Mandal BP, Dey GK, Mukherjee PK, Khatak J, et al. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology. 2008;19:075103. doi: 10.1088/0957-4484/19/7/075103. [DOI] [PubMed] [Google Scholar]
- 7.Shahverdi AR, Minacian S, Shahverdi HR, Jamalifar H, Nohi AA. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Process Biochem. 2007;42:919–23. [Google Scholar]
- 8.Sangi R, Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol. 2009;100:501–4. doi: 10.1016/j.biortech.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Vemula PK, Ajayan PM, John G. Silver nanoparticles embedded anti-microbial paints based on vegetable oil. Nat Mater. 2008;7:236–41. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 10.Raffi M, Rumaiz AK, Hasan MM, Shah SI. J. Structural effects of silver doping on titanium dioxide nanoparticles. Mater Res. 2007;22:3378–84. [Google Scholar]
- 11.Parikh RY, Singh S, Prasad BL, Patole MS, Sastry M, Shouche YS. Extracellular synthesis of crystalline silver nanoparticles and molecular evidence of silver resistance from Morganella sp.: Towards understanding biochemical synthesis mechanism. Chembiochem. 2008;9:1415–22. doi: 10.1002/cbic.200700592. [DOI] [PubMed] [Google Scholar]
- 12.Lee KJ, Jun BH, Choi J, Lee Y, Joung J, Oh YS. Environmentally friendly synthesis of organic-soluble silver nanoparticles for printed electronics. Nanotechnology. 2007;18:335601. [Google Scholar]
- 13.Korbekandi H, Iravani S, Abbasi S. Production of nanoparticles using organisms. Crit Rev Biotech. 2009;29:279–306. doi: 10.3109/07388550903062462. [DOI] [PubMed] [Google Scholar]
- 14.Basu N, Bhattacharya R, Mukherjee P. Protein-mediated autoreduction of gold salts to gold nanoparticles. Nanotechnology. 2008;3:034105. doi: 10.1088/1748-6041/3/3/034105. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Rheem Y, Yoo B, Chong Y, Bozhilov KN, Kim D, et al. Peptide-mediated shape- and size-tunable synthesis of gold nanostructures. Acta Biomater. 2010;7:2681. doi: 10.1016/j.actbio.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine (Lond) 2010;5:33–40. doi: 10.2217/nnm.09.77. [DOI] [PubMed] [Google Scholar]
- 17.Sharma S, Ahmad N, Prakash A, Singh VN, Ghosh KA, Mehta BR. Synthesis of crystalline Ag nanoparticles (AgNPs) from microorganisms. Mater Sci Appl. 2010;1:1–7. [Google Scholar]
- 18.Bhainsa KC, D’souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B. 2006;47:160–4. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadian A, Shojaosadati SA, Rezaee MH. Fusarium oxysporum Mediates Photogeneration of silver. Sci Iran. 2007;14:323–32. [Google Scholar]
- 20.Prasad T, Elumalai E. Biofabrication of Ag nanoparticles using Moringa oleifera leaf extract and their antimicrobial activity. Asian Pac J Trop Biomed. 2011;1:439–42. doi: 10.1016/S2221-1691(11)60096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elumalai E, Prasad T, Hemachandran J, Viviyan T, Thirumalai T, David E. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res. 2010;2:549–54. [Google Scholar]
- 22.Mukunthan K, Elumalai E, Patel TN, Murty VR. Catharanthus roseus: A natural source for the synthesis of silver nanoparticles. Asian Pac J Trop Biomed. 2011;1:270–4. doi: 10.1016/S2221-1691(11)60041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkateswarlu P, Ankanna S, Prasad T, Elumalai EK, Nagajyothi PC, Savithramma N. Green synthesis of Silver Nanoparticle Using Shorea tumbuggaia Stem Bark. Int J Drug Dev Res. 2010;2:720–3. [Google Scholar]
- 24.Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract. Korean J Chem Eng. 2008;25:808–11. [Google Scholar]
- 25.Obidoa Onyechi J, Parker E, Nkechi J. Phytochemical Analysis of Cocos nucifera L. J Pharm Res. 2010;3:280–6. [Google Scholar]
- 26.Chan E, Elevitch CR. Species profiles for pacific island, Agroforestry. [Last accessed on 2008 Jul 26]. pp. 1–27. Available from: http://www.traditionaltree.org .
- 27.Fife B. Piccadilly Books Ltd. USA: Healthwise publications, Colorado Springs, Co; 2000. The Healing Miracles of Coconut Oil; pp. 1–46. [Google Scholar]
- 28.Charles W, Fetroo Juan R. Copy Right. 9th ed. 2004. Avila The Complete Guide To Herbal Medicines”.7. Bertram G “Basic and clinical pharmacology” Katzung; pp. 158–60. [Google Scholar]
- 29.Pattigadapa HS, Ramesh M, Praneeth Sagar CH, Bhaskar Rao U, Lakshman G, Ankaiah M, Balu Naik J. Cardiotonic activity of coconut water (Cocos nucifera) Recent Res Sci Technol. 2011;3:155–7. [Google Scholar]
- 30.Kalishwaralal K, Deepak V, Ram Kumar Pandian S, Kottaisamy M, BarathmaniKanth S, Kartikeyan B, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B. 2010;77:257–62. doi: 10.1016/j.colsurfb.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Shankar SS, Rai A, Ankamwar A, Singh Ahmad A, Sastry M. Biological synthesis of triangular gold nanoprisms. Nat Mater. 2004;3:482–8. doi: 10.1038/nmat1152. [DOI] [PubMed] [Google Scholar]
- 32.Xu H, Kall M, J. Morphology effects on the optical properties of silver nanoparticles. Nanosci Nanotechnol. 2002;4:254. doi: 10.1166/jnn.2004.034. [DOI] [PubMed] [Google Scholar]
- 33.Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22:577–83. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- 34.Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett. 2008;62:4588–90. [Google Scholar]


