Abstract
Aim:
A novel series of ethyl 5-(4-substituted phenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate 3a-3j, were synthesized, characterized by spectral, elemental analyses and screened for their in vitro antibacterial and Mycobacterium tuberculosis (MTB) activities.
Materials and Methods:
The in vitro antibacterial and antifungal activities were determined by agar well-diffusion and cup-plate agar diffusion methods and the anti-tuberculosis (TB) screening for test compounds were evaluated against MTB H37Rv strain by Resazurin assay.
Results:
Among the derivatives tested, most of the compounds were found to have potent activity against microbial strains. The structure-activity relationship point of view, introduced group that enhance the lipophilicity as well ester, substituted aromatic ring at thiazole quinazoline nucleus showed increasing antimicrobial and anti TB activity. The high level of activity shown by the compounds with electron withdrawing groups in the para position on the benzene ring (3 g) suggests that these compounds could serve as leads for development of novel synthetic compounds with enhanced antibacterial and anti TB activities.
Conclusion:
These results provide a further insight into the structural requirements for targeting thiazolo quinazoline carboxylate to develop potential new agents to combat TB treatment.
KEY WORDS: Anti tuberculosis, Gram-negative and fungal pathogenic strains, Gram-positive, thiazolo quinazoline carboxylates
The microbial infections pose a continuous and serious threat to human health and it's responsible for millions of human deaths and represents a global threat to human health.[1,2,3,4] Such infections most commonly affect patients with decreased immunity, neoplastic disorders and undergoing organ transplantation.[5] In order to prevent this serious medical problem, the elaboration of new types of antibacterial agents or the expansion of bioactivity of the biosensitive synthetic compounds is a very interesting research problem. On the other hand, tuberculosis (TB) is a treatable infectious disease; aggravated by the increasing number of identified multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB), as well as coincidence of TB with HIV-infection and atypical mycobacterial infections; however, its treatment fails with the emergence of MDR-TB. The global rate of TB is increasing due to the lack of treatment compliance, supply of poor quality drugs and recent direct transmission from MDR-TB patients.[6,7] The global plan millennium development goal targets halting and reversing the incidence of TB by 2015. Furthermore, the indication of Mycobacterium tuberculosis (MTB) strains resistant to first line drugs (pyrazinamide, isoniazid, rifampin, fluoroquinolone) leading to the MDR-TB and second line drugs (amikacin, kanamycin) leading to the XDR-TB. The development of new drugs with activity against MDR-TB, XDR-TB, and latent TB is a priority task. Therefore, identification of novel drugs that are essential for the bacterial growth and persistence should provide additional targets for the rational design and development of novel therapies. The main part of our research has been devoted to synthetic methods leading to new ring systems, containing the thiazolo quinazoline nucleus, as a pharmacophore moiety for potential drugs. In particular, the thiazolo quinazoline nucleus represents a very attractive scaffold to obtain new molecules endowed with many pharmacological activities. In the course of our medicinal chemistry research program on thiazolo quinazoline, we have reported the successful procedure affording novel 2-(4’-substituted benzylidine) thiazolo (2,3-b) quinazolin-3 (2H)-ones and 2-(4’-substituted benzylidine)-3-(4-nitrophenyl amino) thiazolo quinazolines. Along this line of research, we have documented its potent pharmacological activities such as HIV-1 integrase inhibition, phosphodiesterase inhibition, anticonvulsant, anti-nociceptive and anti-inflammatory.[8,9,10,11] The functional moieties urea and thiourea derivatives are exceptionally versatile building blocks for the synthesis of a diversity of heterocyclic compounds and ureas and thioureas have been studied for the systematic control of TB and anti HIV,[12] for example, isoxyl (ISO, thiocarlide), a thiourea derivative, is known by its strong anti TB activity. On the other hand, the tetra hydro imidazo benzodiazepine thiones (TIBO) derivative 9-Cl-TIBO and the phenyl ethyl thiazolyl thiourea derivatives LY73497 and trovirdine play a significant role in the inhibition of HIV reverse transcriptase [Figure 1]. As an extension of our research, in order to gain more insight into the thiazolo quinazoline series, and particularly with regard to their anti TB performance, we have designed to synthesize novel thiazolo quinazoline carboxylates derivatives. The title compounds are expected to exhibit improving biological activity. We herein report the synthesis and structure of ethyl 5-(4-substituted phenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate 3a-3j, which have been characterized by spectral data and elemental analysis.
Figure 1.
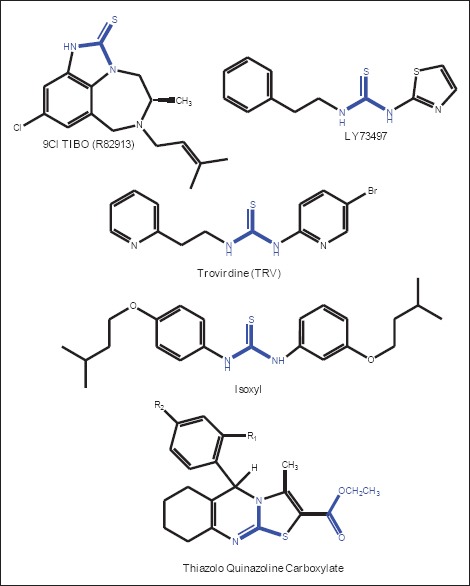
Comparison of pharmacophoric features of known anti tuberculosis compounds and synthesized compounds
Materials and Methods
Materials
Synthetic starting material, reagents, and solvents were of analytical grade or of the highest quality commercially available. The chemicals were purchased from Aldrich Chemical Co., and Merck Chemical Co., Biotium, Inc., and were dried whenever necessary.
The melting points were determined in open capillary tubes and are uncorrected. Infrared (IR) spectra were recorded with KBr pellets (ABB Bomem FT-IR spectrometer MB 104 ABB Limited, Bangaluru, India). Proton nuclear magnetic resonance (1H NMR) spectra (Bruker 400 NMR spectrometer, Mumbai, India) were recorded with tetramethylsilane as internal reference. Mass spectral data were recorded with a quadrupol mass spectrometer (Shimadzu GC MS QP 5000, Chennai, India), and microanalyses were performed using a vario EL V300 elemental analyzer (Analysensysteme GmbH, Chennai, India). The purity of the compounds was checked by TLC on precoated SiO2 gel (HF254, 200 mesh) aluminum plates (E. Merck). IR, 1H-NMR, mass spectral data, and elemental analyses were consistent with the assigned structures.
Methods
The synthetic strategy leading to the target compounds are illustrated in Scheme 1. Ethyl- 5-(4-substitutedphenyl)- 3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazolin -2-carboxylate 3a-3j prepared from equimolar quantities of cyclohexanone (0.039 mol), 2 and 4 substituted aldehyde (0.039 mol) was transferred into a 500 mL beaker. After adding a solution of sodium hydroxide to make the solution alkaline, the beaker was shaken and kept aside. The obtained solid was filtered, washed with water and recrystallized from absolute ethanol. A mixture of 2 and 4 substituted benzylidine cyclohexanone (0.039 mol), thiourea (0.03 mol) and potassium hydroxide (2.5 g) in ethanol (100 mL) was heated under reflux for 3 h. The reaction mixture was concentrated to half of its volume, diluted with water, then acidified with dilute acetic acid and kept overnight. The obtained solid was filtered, washed with water and recrystallized from ethanol to give 2- and 4-substituted phenyl 3, 4, 5, 6, 7, 8-hexahydro quinazolin-2-thione 2. To a solution of ethylacetoacetate (0.72 mmol) in 2 mL of 1,2 dichloroethane, bromine (0.72 mmol) was added slowly with constant stirring until the solution turns colorless. To this mixture compound 2 was added slowly followed by the addition of triethylamine (0.72 mmol) with constant stirring. The reaction mixture was heated to 80°C with vigorous shaking and heating was continued for 2 h. The solvent was removed under reduced pressure and the residue was filtered, and recrystallized from absolute ethanol.
Scheme 1.
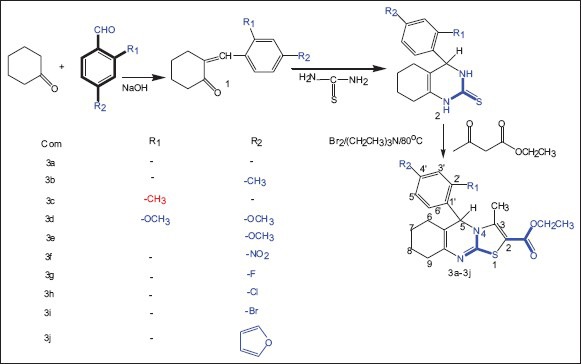
Synthetic protocols of target compounds 3a-3j
Biological activity
Antibacterial screening
Antibacterial activity was performed by using agar well diffusion method.[13] The activity of the synthesized compounds was tested against four Gram-positive bacteria (Staphylococcus aureus ATCC 9144, Staphylococcus epidermidis ATCC 155, Micrococcus luteus ATCC 4698 and Bacillus cereus ATCC 11778) and three Gram-negative bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 2853, and Klebsiella pneumoniae ATCC 11298). The compounds were dissolved in dimethyl formamide at final concentration of 50 mg/mL. The nutrient agar medium (1% typtone, 0.5% yeast extract, 1% NaCl, 0.5% agar, 1.5%, pH 7.4) were seeded with 18 h old exponentially grown culture containing 106-108 CFU/mL. The petri dishes were prepared by pouring 20 mL of seeded nutrient agar to give a depth of 2-3 mm and plate was allowed to solidify at room temperature. After solidification, wells (5 mm diameter) were created in the medium by using sterile metallic borer and it was filled with 50 μL of test compounds. The activity was determined by measuring the diameter of the inhibition zone (in mm). The plates were kept for incubation at 37°C for 24 h and then the plates were examined for the formation of inhibition zone. Growth inhibition was calculated with standard ciprofloxacin (100 μg/mL). Each inhibition zone was measured three times by caliper to get an average value. The test was performed in triplicate for each bacterium culture.
Antifungal activity
The antifungal activity of the synthesized compounds was tested against two fungal pathogenic strains (Aspergillus niger ATCC 9029 and Candida albicans ATCC 2091) by cup-plate agar diffusion method. The prepared sabouraud-dextrose agar plates were stored in to room temperature and 100 μL of fungal culture was grown in 5 mL sabouraud-dextrose broth (glucose:Peptone, 40:10). The fungal culture growth was taken 5 days to achieve 105 CFU/mL then, the grown culture was spread out uniformly on the sabouraud-dextrose agar plates and it was allowed 15 min for absorption. The prepared 5 mm wells were used to load 50 mg/mL of title compounds, and then the wells were incubated at 30°C for 3-4 days. Ketoconazole was used as standard drug. The zone of inhibition was measured in mm for each organism. Each inhibition zone was measured three times by caliper to get an average value. The test was performed in triplicate for each fungal culture.
Anti tuberculosis screening
The first and second levels of anti TB screening for test compounds were evaluated against MTB H37Rv strain (ATCC 25618) by Resazurin assay.[14] The standard drug isoniazid was used for comparison. MTB strains were grown in Middlebrook 7H9 (Difco) broth with 10% albumin-dextrose-catalase and 0.2% glycerol was thawed and diluted in broth to 105 CFU/mL dilutions. Stock solutions (2 mg/mL) of the test compounds were prepared in dimethyl sulfoxide (DMSO). The compounds were tested at 1, 10 and 100 mg/mL concentrations. Further the second level testing was carried out at concentrations 0.3125, 0.625, 1.25, 2.5, and 5 mg/mL. Control tubes had the same volumes of DMSO without any substrate. Each culture incubated at 37°C for 7 days and then 20 mL of 0.01% resazurin in water was added to each tube. Resazurin is a redox dye and its blue color in the oxidized state and turns pink color when reduced state by the growth of viable cells. Compounds, which prevented the change of color of the dye were considered to be inhibitory to MTB.
Results and Discussion
The series of heterocycles, ethyl 5-(4-substituted phenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate 3a-3j were synthesized by the reaction of 2- and 4-substituted phenyl 3, 4, 5, 6, 7, 8-hexahydro quinazolin-2-thione 2 with appropriate solution of ethylacetoacetate as presented in Scheme 1. The IR, 1H-NMR, mass spectroscopy and elemental analyses for the new compound are in accordance with the assigned structures. The IR spectrum of compounds 3a-3j showed bands of aliphatic ester group at 1735-1730/cm. In 3a-3j, alkane-CH stretching, alkane-CH2 bending and alkane-CH3 bending bands appears at 3000-2850/cm, 1375-1370/cm, 1460-1465/cm respectively. The appearance of a strong intensity band in the IR spectra of compounds 3a-3j in the range of 1300-1000/cm attributable to ester C-O bend provides a strong evidence for the condensation and also confirms the formation of the ester. The proton magnetic resonance spectra of thiazolo quinazoline carboxylate and their corresponding derivatives have been recorded in CDCl3. In this 3a-3j ester CH2 and ester CH3 signals appear at 4.00-4.59 (q, J = 7.2 Hz, 2H; ester-CH2), 1.20-1.44 (t, J = 7.2 Hz, 3H; ester-CH3) ppm, respectively. The position and presence of ester proton signals in the 1H-NMR spectra of final compounds confirms the carboxylate in thiazolo quinazoline moiety. All these observed facts clearly envisages that the thiazolo[2,3-b] quinazoline-2-carboxylate formation as indicated in Scheme 1 and confirms the proposed structure 3a-3j.
Antimicrobial screening
Antimicrobial screening results of novel ethyl 5-(2 or 4-substituted phenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate 3a-3j derivatives exhibited moderate to significant activity. Among these synthesized compounds, 3g, 3i, 3f, 3h, 3j, 3d, 3e, and 3b were found to possess significant antibacterial and antifungal activity when compared to standard drug Ciprofloxacin and Ketoconazole. Compound 3a and 3c displayed moderate antimicrobial activity whereas the remaining compounds shown good activity. The compound ethyl- 5- (4- fluorophenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline- 2- carboxylate 3g, was found to exhibit the highest antimicrobial activity against both bacterial and fungal strains. On the basis of biological data, improvement in biological activity was observed with electronic effects and positions (ortho, para) of the substituents. The influence of electronic effects on the biological activities, we introduced electron withdrawing and donating groups on the thiazolo[2,3-b] quinazoline-2-carboxylate ring. We synthesized different analogues with electron withdrawing groups, e.g. nitro, fluorine, chlorine and bromine and donating groups like methyl and methoxy with ortho, para positions on the thiazolo[2,3-b] quinazoline-2-carboxylate ring. The potent antimicrobial exhibited by the compounds may be due to the incorporation of electron withdrawing and donating groups. The interesting results we observed that the electrons withdrawing and donating groups were found to increase the antimicrobial activity, whereas ortho substituted donating group exhibited lesser degree of activity. In exceptional case, unsubstituted analog (3a) was found moderate in activity. The compound 3g was found to possess antibacterial activity almost equivalent to standard drug. This is because it has been well established that the fluorine substituted molecules have got a significant important in modern medicinal chemistry[15] and it may be explained that the esters possess preferable lipophilicity and show optimal membrane permeability. The values for the antimicrobial studies of the compounds and the standard are represented in Table 1.
Table 1.
Antimicrobial activities of the synthesized compounds 3a-3j
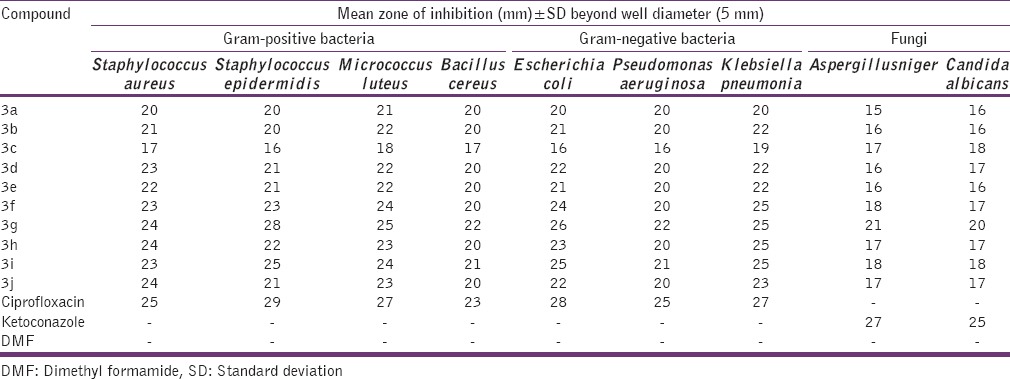
Anti tuberculosis screening
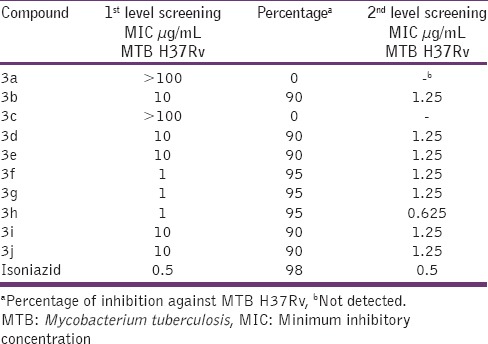
Ten designed derivatives were first measured for their first and second levels of anti TB screening at 1, 10, and 100 mg/mL and 0.3125, 0.625, 1.25, 2.5, and 5 mg/mL concentrations respectively. The data of the anti TB activity screening reveals that the compounds 3g, 3i, 3f, 3h, 3j, 3d, 3e, and 3b were active in 1 and 10 mg/mL concentrations against MTB H37Rv strain. The active compounds from the first level investigation were further subjected to 2nd level of testing [Figure 2]. The compounds (3a, 3c) which were active at 100 mg/mL concentration were not taken for further studies. In the second level testing, amongst the tested compounds, 3g, 3i, 3f, 3h, 3j, 3d, 3e, and 3b were active at 0.625 and 1.25 mg/mL concentrations. It is interesting to note that compounds 3g, 3f and 3h showed enhanced activity and this compound was considered as a most potent analog against MTB strain and was found to indicate similar anti TB potency as that of standard drug isoniazid. MTB is unique to surround by a thick and waxy cell wall, so that efficient anti TB drugs should have a reasonable lipophilicity to penetrate the cell wall. The most frequently encountered heterocycles are reported to have strong lipophilic characters, which are plays an essential role in membrane permeation in biological system.[16] In this study, we have mainly concentrated at the area 1 and 2 of the synthesized molecules [Figure 3]. Results of antimicrobial screening revealed that the halogen as well as methyl and methoxy derivatives showed good activity. In case of antimicrobial and anti-TB activity results, there was specific relationship observed between minimum inhibitory concentration profiles versus position and substitution. In majority of the cases, compounds having electron withdrawing and donating atoms with para substitutions exhibited good activity than ortho substitutions. These encouraging findings of activity, physicochemical parameters, displayed by a novel innovative structural combination system of thiazolo quinazoline makes a privileged structure to achieve more active derivatives in ongoing studies. The zone of inhibition values for the anti TB studies of the compounds (3a-3j) and the standard are represented in Table 2.
Figure 2.
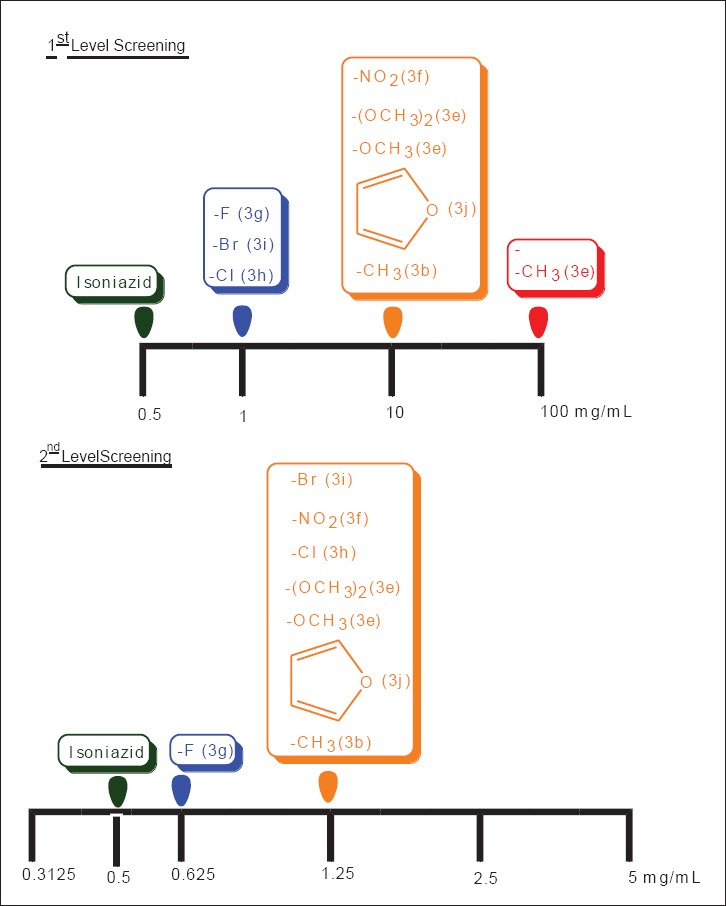
Comparison of the minimum inhibitory concentration range against Mycobacterium tuberculosis H37Rv of the various synthesized compounds 3a-3j
Figure 3.
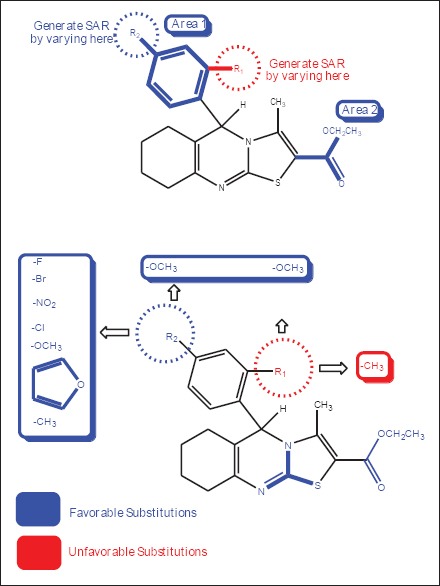
Selected area of interest for comparative analysis
Table 2.
Activity of the synthesized compounds 3a-3j against MTB H37Rv
Chemistry
Ethyl-3-methyl-5-phenyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate (3a)
The compound was obtained as a red solid; yield: 71%; m.p. 167-169°C; IR cm-1: 3112 (Ar-CH strecth), 2916 (alkane-CH strecth), 1737 (aliphatic ester), 1461 (alkane-CH2 bend), 1376 (alkane-CH3 bend), 1319 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.64-6.89 (m, 5H; Ar-H), 5.21 (s, 1H; -CH), 4.11-4.53 (q, J = 7.2 Hz, 2H; Ester-CH2), 1.81-2.49 (m, 8H; CH2, CH2, CH2, CH2), 1.76 (s, 3H; -CH3), 1.26-1.32 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M+): 354 (calcd for C20H22N2O2S; 354.5). Anal. Calcd for C20H22N2O2S; C, 67.77; H, 6.26; N, 7.90; found: C, 67.78; H, 6.22; N, 7.94.
Ethyl-3-methyl-5-(p-tolyl)-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate (3b)
The compound was obtained as a yellow solid; yield: 67%; m.p. 171-173°C; IR cm-1: 3116 (Ar-CH strecth), 2913 (alkane-CH strecth), 1739 (aliphatic ester), 1464 (alkane-CH2 bend), 1372 (Alkane-CH3 bend), 1297 (Ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.82-7.04 (m, 4H; Ar-H), 5.03 (s, 1H; -CH), 4.16-4.59 (q, J = 7.2 Hz, 2H; Ester-CH2), 3.13 (s, 3H; -CH3), 1.83-2.32 (m, 8H; CH2, CH2, CH2, CH2), 1.69 (s, 3H;-CH3), 1.22-1.35 (t, J = 7.2 Hz, 3H; Ester-CH3). EI-MS m/z (M+): 368 (calcd for C21H24N2O2S; 368.5). Anal. Calcd for C21H24N2O2S; C, 68.45; H, 6.56; N, 7.60; found: C, 68.43; H, 6.58; N, 7.58.
Ethyl-3-methyl-5-(o-tolyl)-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylate (3c)
The compound was obtained as a red solid; yield: 76%; m.p. 179-181°C; IR cm-1: 3123 (Ar-CH strecth), 2917 (alkane-CH strecth), 1731 (aliphatic ester), 1467 (alkane-CH2 bend), 1373 (alkane-CH3 bend), 1292 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.72-7.24 (m, 4H; Ar-H), 5.10 (s, 1H; -CH), 4.15-4.57 (q, J = 7.2 Hz, 2H; Ester-CH2), 3.17 (s, 3H; -CH3), 1.86-2.38 (m, 8H; CH2, CH2, CH2, CH2), 1.71 (s, 3H;-CH3), 1.24-1.37 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M+): 368 (calcd for C21H24N2O2S; 368.5). Anal. Calcd for C21H24N2O2S; C, 68.45; H, 6.56; N, 7.60; found: C, 68.44; H, 6.56; N, 7.61.
Ethyl-5-(2,4-dimethoxyphenyl)-3-methyl-6,7,8,9-tetrahydro-5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3d)
The compound was obtained as a brown solid; yield: 69%; m.p. 164-166°C; IR cm-1: 3127 (Ar-CH strecth), 2911 (alkane-CH strecth), 1734 (Aliphatic ester), 1461 (alkane-CH2 bend), 1375 (alkane-CH3 bend), 1295 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.75-7.27 (m, 3H; Ar-H), 4.96 (s, 1H; -CH), 4.24-4.42 (q, J = 7.2 Hz, 2H; ester-CH2), 3.42 (s, 6H; -OCH3), 3.12 (s, 3H;-CH3), 1.82-2.14 (m, 8H; CH2, CH2, CH2, CH2), 1.21-1.34 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M+): 414 (calcd for C22H26N2O4S; 414.5). Anal. Calcd for C22H26N2O4S; C, 63.75; H, 6.32; N, 6.76; found: C, 63.72; H, 6.35; N, 6.78.
Ethyl-5-(4-methoxyphenyl)-3-methyl-6,7,8,9-tetrahydro-5H- thiazolo[2,3-b] quinazoline- 2-carboxylate (3e)
The compound was obtained as a brick brown solid; yield: 72%; m.p. 172-174°C; IR cm-1: 3120 (Ar-CH strecth), 2915 (alkane-CH strecth), 1721 (aliphatic ester), 1462 (alkane-CH2 bend), 1374 (alkane-CH3 bend), 1293 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.71-7.23 (m, 4H; Ar-H), 4.91 (s, 1H;-CH), 4.21-4.44 (q, J = 7.2 Hz, 2H; ester-CH2), 3.38 (s, 3H; -OCH3), 3.17 (s, 3H; -CH3), 1.73-2.11 (m, 8H; CH2, CH2, CH2, CH2), 1.14-1.31 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M+): 384 (calcd for C21H24N2O3S; 384.5). Anal. Calcd for C21H24N2O3S; C, 65.60; H, 6.29; N, 7.29; found: C, 65.62; H, 6.31; N, 7.27.
Ethyl-5- (4-nitrophenyl)-3-methyl-6,7,8,9-tetrahydro-5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3f)
The compound was obtained as a dark brown solid; yield: 83%; m.p. 151-153°C; IR cm-1: 3107 (Ar-CH strecth), 2933 (alkane-CH strecth), 1712 (aliphatic ester), 1514 (Ar-NO2), 1452 (alkane-CH2 bend), 1342 (alkane-CH3 bend), 1265 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.75-7.12 (m, 4H; Ar-H), 4.97 (s, 1H; -CH), 4.27-4.46 (q, J = 7.2 Hz, 2H; ester-CH2), 3.12 (s, 3H; -CH3), 1.79-2.17 (m, 8H; CH2, CH2, CH2, CH2), 1.15-1.37 (t, J = 7.2 Hz, 3H; Ester-CH3). EI-MS m/z (M+): 399 (calcd for C20H21N3O4S; 399.5). Anal. Calcd for C20H21N3O4S; C, 60.13; H, 5.30; N, 10.52; found: C, 60.11; H, 5.32; N, 10.54.
Ethyl-5-(4-fluorophenyl)-3- methyl- 6,7,89- tetrahydro-5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3g)
The compound was obtained as a brown solid; yield: 87%; m.p. 171-173°C; IR cm-1: 3129 (Ar-CH strecth), 2917 (alkane-CH strecth), 1721 (aliphatic ester), 1464 (Alkane-CH2 bend), 1379 (alkane-CH3 bend), 1291 (ester C-O bend), 1121 (C-F). 1H NMR (CDCl3) δ (ppm): 6.71-7.19 (m, 4H; Ar-H), 4.91 (s, 1H; -CH), 4.19-4.41 (q, J = 7.2 Hz, 2H; ester-CH2), 3.16 (s, 3H; -CH3), 1.71-2.19 (m, 8H; CH2, CH2, CH2, CH2), 1.12-1.29 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M+): 372 (calcd for C20H21 FN2O2S; 372.5). Anal. Calcd for C20H21 FN2O2S; C, 64.49; H, 5.68; N, 7.52; found: C, 64.47; H, 5.69; N, 7.53.
Ethyl-5-(4- chlorophenyl)- 3- methyl- 6,7,89- tetrahydro- 5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3h)
The compound was obtained as a brown solid; yield: 77%; m.p. 176-178°C; IR cm-1: 3122 (Ar-CH strecth), 2914 (alkane-CH strecth), 1727 (aliphatic ester), 1463 (alkane-CH2 bend), 1372 (Alkane-CH3 bend), 1292 (ester C-O bend), 738 (C-Cl). 1H NMR (CDCl3) δ (ppm): 6.75-7.11 (m, 4H; Ar-H), 4.92 (s, 1H; -CH), 4.11-4.46 (q, J = 7.2 Hz, 2H; ester-CH2), 3.12 (s, 3H; -CH3), 1.68-2.20 (m, 8H; CH2, CH2, CH2, CH2), 1.14-1.39 (t, J = 7.2 Hz, 3H; ester-CH3). EI-MS m/z (M + 2): 391 (calcd for C20H21 ClN2O2S; 388.9). Anal. Calcd for C20H21 ClN2O2S; C, 61.77; H, 5.44; N, 7.20; found: C, 61.75; H, 5.43; N, 7.21.
Ethyl-5-(4-bromophenyl)-3-methyl-6,7,8,9-tetrahydro-5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3i)
The compound was obtained as a yellow solid; Yield: 92%; m.p. 181-182°C; IR cm-1: 3178 (Ar-CH strecth), 2929 (alkane-CH strecth), 1708 (aliphatic ester), 1467 (alkane-CH2 bend), 1381 (Alkane-CH3 bend), 1295 (ester C-O bend), 692 (C-Br). 1H NMR (CDCl3) δ (ppm): 6.79-7.14 (m, 4H; Ar-H), 4.96 (s, 1H; -CH), 4.15-4.47 (q, J = 7.2 Hz, 2H; Ester-CH2), 3.15 (s, 3H; -CH3), 1.63-2.24 (m, 8H; CH2, CH2, CH2, CH2), 1.12-1.41 (t, J = 7.2 Hz, 3H; Ester-CH3). EI-MS m/z (M + 2): 435 (calcd for C20H21 BrN2O2S; 433.4). Anal. Calcd for C20H21 BrN2O2S; C, 55.43; H, 4.88; N, 6.46; found: C, 55.45; H, 4.89; N, 6.48.
Ethyl-5- (furan-2-yl)-3-methyl- 6,7,8,9-tetrahydro-5H- thiazolo[2,3-b] quinazoline-2-carboxylate (3j)
The compound was obtained as a black solid; yield: 73%; m.p. 197-199°C; IR cm-1: 3124 (Ar-CH strecth), 2929 (alkane-CH strecth), 1717 (aliphatic ester), 1461 (alkane-CH2 bend), 1383 (alkane-CH3 bend), 1299 (ester C-O bend). 1H NMR (CDCl3) δ (ppm): 6.82-7.29 (m, 3H; Furan-H), 4.92 (s, 1H; -CH), 4.12-4.42 (q, J = 7.2 Hz, 2H; Ester-CH2), 3.12 (s, 3H; -CH3), 1.62-2.22 (m, 8H; CH2, CH2, CH2, CH2), 1.12-1.42 (t, J = 7.2 Hz, 3H; Ester-CH3). EI-MS m/z (M+): 334 (calcd for C18H20N2O3S; 334.4). Anal. Calcd for C18H20N2O3S; C, 62.77; H, 5.85; N, 8.13; found: C, 62.79; H, 5.87; N, 8.11.
Conclusion
The current study revealed that novel 5-(4-substituted phenyl)-3-methyl-6,7,8,9-tetrahydro-5H-thiazolo[2,3-b] quinazoline-2-carboxylates are promising candidates for their use as antimicrobial and anti TB. The possible activity achieved by modifications in the substituents on the basic quinazoline nucleus. The presence of the fluoro group on the aromatic ring has highly increased the activity of the compound compared with other substituents of the compounds. In the view of the above findings and to identify new candidates that may value in designing novelty, selectivity, less toxicity anti TB agents who might serve as novel analog, we can promise here that, our synthesized analogues will generates a very good impact to the chemists and research scholars for further investigations in this field of thiazolo quinazoline selectively being influence of electronic effects as well as change in the basic nucleus.
Acknowledgments
The authors gratefully acknowledge the Chemistry Department at PES's Rajaram and Tarabai Bandekar College of Pharmacy for all facilities provided in terms the use of the available chemicals and equipments. Furthermore, we would like to thank the VIT, Vellore, India for the wet lab facilities, for biological screening of the compounds used in this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Tenover FC, McDonald LC. Vancomycin-resistant staphylococci and enterococci: Epidemiology and control. Curr Opin Infect Dis. 2005;18:300–5. doi: 10.1097/01.qco.0000171923.62699.0c. [DOI] [PubMed] [Google Scholar]
- 2.Pfeltz RF, Wilkinson BJ. The escalating challenge of vancomycin resistance in Staphylococcus aureus. Curr Drug Targets Infect Disord. 2004;4:273–94. doi: 10.2174/1568005043340470. [DOI] [PubMed] [Google Scholar]
- 3.Roberts MC. Distribution of macrolide, lincosamide, streptogramin, ketolide and oxazolidinone (MLSKO) resistance genes in Gram-negative bacteria. Curr Drug Targets Infect Disord. 2004;4:207–15. doi: 10.2174/1568005043340678. [DOI] [PubMed] [Google Scholar]
- 4.Babu GR. Opportunities for improving public health system in India analysis of current state of affairs and pointers for future. Ann Trop Med Public Health. 2011;4:69–70. [Google Scholar]
- 5.Nathan C. Antibiotics at the crossroads. Nature. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson E, Nunn P, Uplekar M, Floyd K, Jaramillo E, Lönnroth K, et al. MDR tuberculosis - Critical steps for prevention and control. N Engl J Med. 2010;363:1050–8. doi: 10.1056/NEJMra0908076. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Multidrug and extensively drug. resistant TB (M/XDRTB): global report on surveillance and response. 2010. [Last accessed on Mar 2010]. Available from: http://www.whqlibdoc.who.int/publications/2010/9789241599191_eng,http://go.worldbank.org/K2CKM78CC0 .
- 8.Selvam TP, Kumar PV, Siva Kumar A, Arnold Emerson I. Study of inhibitory mechanism and binding mode of the thiazolo quinazoline compounds to HIV-1 integrase by docking. J Pharm Res. 2010;3:1637–47. [Google Scholar]
- 9.Selvam TP, Kumar PV. Synthesis and cAMP-dependent phosphodiesterase inhibition of novel thiazoloquinazoline derivatives. Acta Pharm. 2011;61:103–13. doi: 10.2478/v10007-011-0009-3. [DOI] [PubMed] [Google Scholar]
- 10.Selvam TP, Kumar PV, Prakash CR, Raja S. Synthesis and anticonvulsant activity of novel 6,7,8,9 tetra hydro-5h-5-(2,’-hydroxy phenyl)-2-(4’-substituted benzylidine)-3-(4-nitrophenyl amino) thiazolo quinazoline derivatives. Toxicol Environ Chem. 2001;93:643–55. [Google Scholar]
- 11.Selvam TP, Kumar PV. Synthesis of novel 6,7,8,9-tetra hydro-5h-5-hydroxy phenyl-2-benzylidine-3-substituted hydrazino thiazolo (2, 3-b) quinazoline as potent antinociceptive and anti-inflammatory agents. Bull Korean Chem Soc. 2010;31:1–7. [Google Scholar]
- 12.Sriram D, Yogeeswari P, Dinakaran M, Thirumurugan R. Antimycobacterial activity of novel 1-(5-cyclobutyl-1,3-oxazol-2-yl)-3-(sub) phenyl/pyridylthiourea compounds endowed with high activity toward multidrug-resistant Mycobacterium tuberculosis. J Antimicrob Chemother. 2007;59:1194–6. doi: 10.1093/jac/dkm085. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A, Choudhary ML, Thomsen WJ. The Netherlands: Harwood Academic Publishers; 2001. Bio-Assay Techniques for Drug Development. [Google Scholar]
- 14.Taneja NK, Tyagi JS. Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J Antimicrob Chemother. 2007;60:288–93. doi: 10.1093/jac/dkm207. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama H, Ishihara K, Akiba S, Uenishi J. Synthesis of N-[2-(2,4-Difluorophenoxy) trifluoromethyl-3-pyridyl] sulfonamides and their inhibitory activities against secretory phospholipase A2. Chem Pharm Bull (Tokyo) 2011;59:1069–72. doi: 10.1248/cpb.59.1069. [DOI] [PubMed] [Google Scholar]
- 16.Parker MA, Kurrasch DM, Nichols DE. The role of lipophilicity in determining binding affinity and functional activity for 5-HT2A receptor ligands. Bioorg Med Chem. 2008;16:4661–9. doi: 10.1016/j.bmc.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


