Abstract
The aim of this work was to synthesize methotrexate (MTX)-polyamidoamine (PAMAM) dendritic nanoconjugates and to study their effect on cell viability in uterine sarcoma cells. The amide-bonded PAMAM dendrimer-MTX conjugates were prepared by conjugation between the amine-terminated G5 dendrimer and the carboxylic groups of the MTX using a dicyclohexylcarbodiimide coupling reaction. The formation of conjugates was evaluated by ultraviolet (UV) and 1H nuclear magnetic resonance (1H NMR) spectroscopy studies. The cell survival of MES-SA cells, a uterine sarcoma cell line, was evaluated in the presence of the dendrimer-MTX nanoconjugate, using appropriate controls. The UV and 1H NMR study confirmed the formation of covalent bonds between the drug and the dendrimer. The cell viability study indicated that the nanoconjugates had significantly improved cell killing compared to the free MTX.
KEY WORDS: Dendrimer, drug delivery, methotrexate, polyamidoamine
The impact of nanocarriers in increasing efficacy and reducing toxicity of a variety of drugs has been the subject of numerous research studies over the past three decades. One of the challenges in the delivery of drugs using nanoconjugates continues to be the fabrication of well-defined particulates with reproducible size and shape. Polyamidoamine (PAMAM) dendrimers are highly branched, circular, “tree-like” structures that consist of a carbon core that is attached to symmetrical layers (generations) of branches. Dendrimers were first described by Buhleier et al.[1] in 1978 and later studied extensively by Tomalia et al. in the context of utilizing PAMAM dendrimers in drug delivery.[2,3] A detailed review by Samad et al.[4] illustrates different types of dendrimers and their characteristic differences from hyperbranched and linear polymers, micelles and globular proteins. Dendrimers offer several structural advantages, such as (1) precise molecular weight, required for reproducible pharmacokinetic data, (2) size monodispersity, (3) compact globular shape, (4) high density of structural functionalities, potentially leading to a higher drug payload and (5) a well-defined structure that allows for better control over toxicity and release properties, which make them excellent candidates for drug delivery vehicles.[5,6,7] A drug or a ligand can be attached to the perfectly branched monodisperse macromolecules using relatively simple synthetic processes. As illustrated, the use of dendrimers in drug delivery has great potential; however, it has not been fully explored.[8] PAMAM dendrimers have been studied as carriers in gene transfection,[9,10,11] a number of drug delivery applications[12,13,14] and as carriers for a variety of therapeutic and imaging agents,[10,15,16,17,18] but the translation of dendritic nanocarriers from bench to bedside, i.e. in clinical applications, remains unrealized. A recent review has explored the application of dendrimers for a variety of drug delivery applications.[19]
Despite several advantages of PAMAM dendrimers for passive drug targeting, reports have shown that there is a possibility that lower generation anionic and polar terminal surfaced dendrimers are relatively less biocompatible compared to high generation cationic and non-polar dendrimers.[20,21] However, in a study using KB and Rat2 cells, Hong et al. showed that fairly high concentrations (>500 nM) of cationic dendrimers were required to induce toxicity in the cell lines.[22] Since most PAMAM dendrimers are small molecules, water soluble, non-immunogenic and rapidly cleared from blood,[23] it is not required to be biodegradable to prevent bioaccumulation, thus reducing the potential of carrier related toxicity.[24] The common approaches of drug delivery using dendrimers include either encapsulation of the drug into the core of the dendrimer or chemically attaching the drugs to the surface of dendrimers. Depending on the generation number of the dendrimers, the PAMAM dendrimers could have terminal acid or amine groups ending in –COOH or –NH2 and anticancer drugs such as 5-fluorouracil and methotrexate (MTX) could be successfully conjugated to dendrimers.[25,26]
In a large clinical study, the superiority of using cisplatin with MTX and other anticancer drugs in carcinoma of the uterine cervix was demonstrated.[27] MTX is an antineoplastic agent whose mechanism of action is to competitively bind to dihydrofolate reductase with greater affinity than folic acid. Thus, it inhibits the conversion of dihydrofolate to tetrahydrofolate that is responsible for the synthesis of purine nucleotides and thymidylate, which subsequently serve as substrates for deoxyribonucleic acid (DNA) synthesis necessary for cell proliferation. Therefore, MTX successfully inhibits tumor cell proliferation in the S-phase of the cell cycle. However, it interferes with normal cell proliferation as well, leading to its extensive and serious adverse effects, including cardiovascular, dermatologic, hematologic and gastrointestinal toxicities, to name a few. A number of studies indicate that conjugation of anticancer drugs, such as doxorubicin or MTX to nanostructured polymers, significantly reduces their systemic toxicity and provides an improved therapeutic outcome.[28] In addition, the principle of enhanced permeation and retention (EPR) enriches drug accumulation in the tumor tissues[24,29,30] by virtue of accumulating the nano-object in the rapid angiogenesis-prone “leaky” tumor vasculature compared to normal tissues, thus enhancing the therapeutic index of the carrier.[31] It was also postulated that the amino groups of PAMAM dendrimers could be protonated at the low pH of the tumor cells, which could facilitate endosomal osmotic swelling followed by the rupture of endosome and release of the loaded drug.[32] Therefore, the potential exists for reduction of adverse effects of MTX, along with the potential for improved tumor targeting through selective EPR by using dendrimer-MTX complexes in the treatment of carcinoma of the uterine cervix.
The objective of the present work was to synthesize MTX-PAMAM dendrimer nanoconjugates and evaluate their effect on cell viability in the uterine cancer cell line, MES-SA. Since the potential toxicity of PAMAM dendrimers has been questioned[21,33] appropriate controls were used in the cell viability experiment to study the same.
Materials and Methods
MTX, USP was purchased from Spectrum Chemical, Gardena, CA, USA. PAMAM dendrimers (Dendrimer PAMAM (NH2)128, generation-5, amino surface groups, 1,12-diaminododecane core, molecular weight: 28965.08) in methanol (10% w/v) were purchased from Sigma Aldrich, Inc., St. Louis, MO, USA. Dicyclohexylcarbodiimide (DCC) was obtained from VWR, West Chester, PA, USA. Dimethyl sulfoxide (DMSO) was received from Alfa Aesar, Ward Hill, MA, USA. Trypsin ethylenediaminetetraacetic acid (EDTA), 1 × (0.25% trypsin/2.21 mM EDTA in Hanks balanced salt solution without sodium bicarbonate, calcium and magnesium) and penicillin-streptomycin solution (5000 IU/ml penicillin, 5000 μg/ml streptomycin) were purchased from Mediatech Inc., Herndon, VA, USA. Fetal bovine serum was purchased from Atlanta Biological, Lawrenceville, GA, USA. Triple distilled Nanopure® water prepared in the laboratory was used for all applications.
Synthesis and purification of MTX-dendrimer conjugates
All lab ware was cleaned carefully to avoid the introduction of foreign particulates in the formulations and formulation processing involving potentially harmful organic solvents were limited to a certified chemical hood. MTX (1 mg), PAMAM-G5-NH2 dendrimer (10 μl, 10% w/v in methanol, 0.791 g/ml at 25°C) were dissolved in 1 ml DMSO followed by addition of DCC (5 mg). The mixture was stirred continuously for three days at room temperature in complete darkness. The MTX-dendrimer conjugate is formed by a reaction between –NH2 end groups of dendrimer and the –COOH group of MTX (especially the γ-COOH group of MTX) [Figure 1]. The reaction mixture was purified by dialyzing against DMSO for 24 hour to remove free MTX and DCC. Although the size distribution of the dendrimer-MTX conjugate was expected to be in the nanometer range, the product was filtered through a sterile membrane filter to ensure preparatory aseptic processing for cell culture studies, as well as the removal of any residual particulate dicyclohexylurea (DCU) that formed as a byproduct and may have precipitated during the conjugation process. An earlier report indicated the conjugation ratio of MTX to hydrazide-terminated dendrimers was 4.7.[26]
Figure 1.
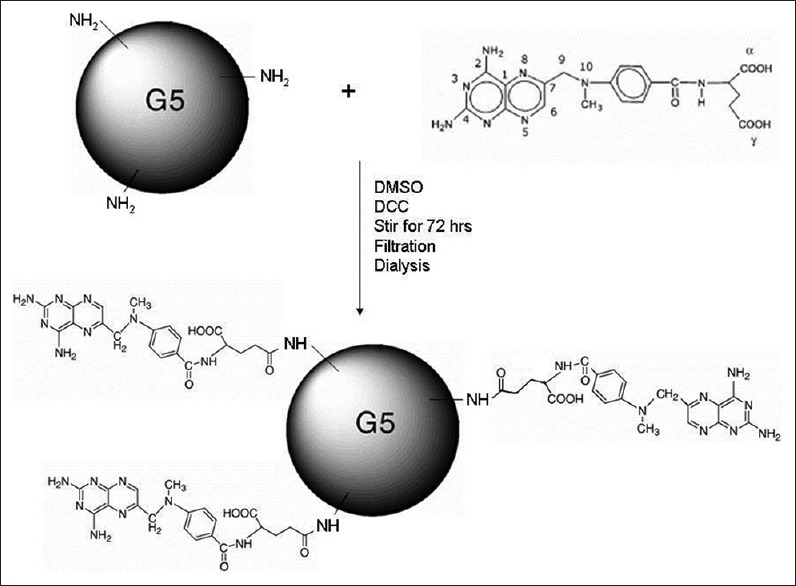
Scheme of synthesis of polyamidoamine-G5-NH2-methotrexate conjugate
The purification of dendrimer conjugates was carried out by dialysis in a 3000 Da MWCO Slide-A-Lyzer dialysis cassette (Pierce, Rockford, IL, USA) against phosphate buffered saline (PBS, pH 7.4). Approximately 3 ml samples were collected for analysis from the external phase at intervals of 15 min, for 2 hour. The volume of dialysis media removed was immediately replaced with fresh PBS to maintain sink conditions. The samples were analyzed by UV spectroscopy at 289 nm (Perkin-Elmer UV/VIS Spectrometer, Waltham, MA, USA).
NMR experiments were performed using a Bruker AVANCE 250 MHz NMR spectrometer (Richland, WA, USA) to access the formation of the MTX-dendrimer conjugate through the evaluation of its chemical structure. NMR spectra were obtained for MTX, dendrimer, DCC and the MTX-dendrimer conjugate, for comparative analysis.
Cell culture
The MES-SA cells (ATCC® CRL-1976™, human uterine sarcoma cells) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in McCoy's 5A modified medium with l-glutamine media (ATCC, Manassas, VA, USA) with antibiotics and 10% fetal bovine serum (Atlanta Biological, GA, USA). A humidified 5% CO2 environment at 37°C was used to grow the cells, which were passaged every 3-4 days.
MES-SA cell growth characteristics
In order to determine the cell seeding density and drug treatment intervals for the cells being studied, it was important to study its short-term (~72 h) growth characteristics. A growth analysis was performed to assess the rate and extent of proliferation of the MES-SA cells. Approximately 10,000 cells (by volume, based on count) were plated in each well of a 6-well cell culture plate. The cells were detached using trypsin at various time intervals (0, 22, 27, 45, 50, 69 and 73 h), centrifuged and re-suspended in a fixed volume of media, following which the number of cells was determined through counting on a hemocytometer (Hausser Scientific, Horsham, PA, USA). A growth curve was plotted using the average of three readings each from three experiments (n = 9).
MES-SA uterine sarcoma cell viability in vitro
The effect of the dendrimer-MTX conjugate on the cell survival of MES-SA cells was evaluated using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. Briefly, after the MES-SA cells grew to 80-90% confluency, the cells were trypsinized and 40,000 cells were plated in each well of three 96-well plates. Following a 24 h incubation period, standardized dilutions of MTX (125-1000 ng/ml; control) in media, MTX-dendrimer complex (equivalent to 125-1000 ng/ml MTX) in media, dendrimer (concentrations equivalent to the corresponding MTX-dendrimer treatment group; control) and blank media (control of cell viability) were equitably distributed among the 96-well plates and incubated for 48 h for cell viability study. At the end of the experimental period, 10 μl of MTT reagent was added to each well and incubated at 37°C for 4 h to allow for the cleavage of the MTT reagent which leads to the formation of the purple formazan crystals. In order to solubilize the formazan product, 100 μl of isopropanol/HCl was added to each well and mixed thoroughly by pipetting to yield a homogenous purple solution. Since MTT assay is not considered an end-point assay, fixed time protocols were followed to ensure that each treatment followed identical exposure time. Plates were read using a microplate reader (AD 340 Beckman Coulter™, Beckman Coulter, Inc., Fullerton, CA, USA) at a test wavelength of 570 nm and a reference wavelength of 630 nm.
Statistical analysis
Statistical analysis of data was carried out using Origin™ (OriginLab Corporation, MA) and the significance level was considered at P < 0.05. Standard error of mean (±) was included in every plot.
Results and Discussion
Synthesis and purification of MTX-dendrimer conjugates
Previously, attempts have been made to conjugate MTX to several natural and synthetic carriers through amide or ester bond formation using a carbodiimide -mediated reaction.[14] In this study, we chose to synthesize and study conjugates of MTX with a 5th generation cationic dendrimer (PAMAM-G5-NH2) because of the in-vivo tumor targeting potential offered by the carrier and the feasibility of amide bond formation between this particular drug and the dendrimer. The formation of the conjugate was evaluated through UV and NMR spectroscopy. Free MTX was separated from the conjugate using dialysis, followed by evaluation and quantitation by UV spectroscopy. Negligible amounts of free MTX (<0.1%) were found in the dialysate, which indicated that the bulk of the reactants culminated in the formation of conjugates between the MTX and the dendrimer. However, it is also possible that free MTX was sequestered within the DCU mass that precipitated out and was removed by filtration prior to dialysis. A symmetric UV peak indicated homogeneous attachment of MTX to the G5 dendrimer.
1H NMR studies were carried out to further confirm the conjugate formation between the MTX and the dendrimer. The 1H NMR spectra of MTX, the G5 PAMAM dendrimer with NH2 end groups, DCC and the MTX-dendrimer conjugate are shown in Figure 2. The 1H NMR data indicated that a conjugate formed between the drug and the dendrimer. The peaks in the aromatic region of 6.5-8.5 ppm are shown in the 1H NMR spectra of MTX [Figure 2a] which correlate to the aromatic rings of MTX. The peak at 3.4 ppm corresponding to the CH3 on the N and peaks corresponding to aliphatic region were also observed in the 1H NMR spectra. The spectra showed a peak at 4.9 ppm, which correlated to the CH next to the NH of the amide or the CH2 next to the NCH3. The peak at 2.5 ppm was a DMSO solvent peak. MTX has COOH groups; however, there was no peak observed in the COOH region. This could be due to a peak shift, rapid exchange of hydrogen due to hydrogen bonding, or the sample being too diluted. The sharp peak at 3.2 ppm observed in the 1H NMR spectra of the dendrimer [Figure 2b] corresponds to the free amino end groups present on the surface of the dendrimer. The spectrum of DCC was similar to its NMR spectra described in the literature. The 1H NMR spectra of DCC showed peaks in the CH2 region of 1-2 ppm and the peaks at 3.2 ppm represent CH2 groups in its cyclohexane ring and the CH-N bond, respectively [Figure 2c]. The 1H NMR spectra of the MTX-dendrimer conjugate showed a peak at 3.4 ppm which likely represents the dendrimer (CH2 attached to N of dendrimer) with a shifted peak because it was conjugated to the MTX. The peak observed at 2.6 ppm is likely a DMSO solvent peak, otherwise it may be due to the free amino groups on the dendrimer, since it was such an intense peak. There was no peak observed for DCC in the spectra for the conjugate because it was either filtered out or removed by dialysis prior to analysis. These NMR spectroscopy results indicated that a drug-dendrimer conjugate was formed, but there was no conclusive evidence of the formation of covalent bonds.
Figure 2.
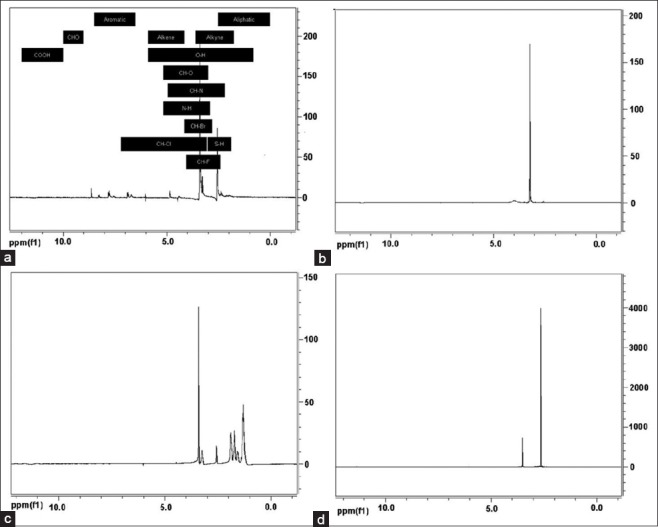
1H nuclear magnetic resonance spectra of. (a) Methotrexate (MTX). (b) G5 polyamidoamine dendrimer with NH2 end groups, (c) Dicyclohexylcarbodiimide and. (d) MTX-dendrimer conjugate
MES-SA cell growth characteristics
Knowledge of the time frames required for the doubling of cells and reproducible results regarding their healthy proliferation ensures that the results obtained from the cell survival studies (MTT assay) are reliable. The short-term growth rate of the MES-SA cells was determined to be linear with a correlation coefficient of >0.90 (R2 = 0.9082). The growth curve is shown in Figure 3. The results obtained validated the treatment schedule, i.e. the test formulations were being applied to the MES-SA cells in appropriate intervals.
Figure 3.
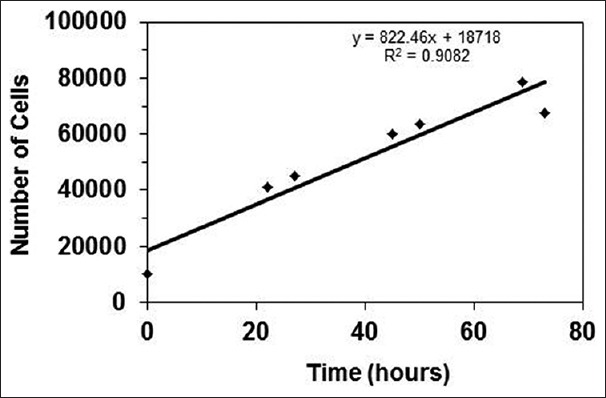
MES-SA growth curve
MES-SA uterine sarcoma cell viability in vitro
It was shown by Quintana et al. that free MTX was released upon internalization of conjugates due to the hydrolysis of the ester bonds in the acidic endosomal environment.[34] Therefore, the free dendrimer liberated will be effectively eliminated by the kidney due to its very small size of about 5.5 nm.[35] A previous report examined the effect of PAMAM dendrimer-MTX conjugates in human acute lymphoblastoid leukemia and Chinese hamster ovary cell lines.[14] Their results showed that their conjugate had 8-24 times the sensitivity as compared to the drug alone. A closer examination of MTX's mechanism of action explains why it may be beneficial to conjugate the drug to a drug delivery system, such as a dendrimer. As stated previously, MTX inhibits nucleotide synthesis, eventually halting DNA replication. MTX entry into human cells is limited due to the negative charge on the carboxylic acid groups at physiologic pH. Therefore, MTX is taken up into human cells through carrier mediated transport; however, since this is an active transport process, the capacity of the transport is limited. The cationic nature of dendrimer conjugate interacts electrostatically with negatively charged epithelial cells and enters the cells through fluid phase pinocytosis.[36] Therefore, dendrimer conjugates can increase drug payload in the diseased tissue, while limiting toxic effects to healthy tissues because of the EPR effect. Consequently, in vivo therapeutic efficacy is likely to improve, while overall toxicity may be decreased further due to a lesser amount of drug being needed per unit dose.
The MTT assay is a colorimetric assay that measures the reduction of MTT reagent by mitochondrial succinate dehydrogenase. The reduction rate and extent of the MTT reagent depends on the number of viable cells present in the well. The absorbance values obtained in the MTT assay are proportional to the viable cell number. The absorbance of this colored product is measured using a microplate reader and is directly proportional to the number of the viable cells. The extent of cell survival was calculated based on the number of viable cells present after treatment with the drug, dendrimers or conjugates, considering the cell survival upon treatment with media as 100% (control). The cell survival after treatment with blank media, MTX, dendrimer and MTX-dendrimer conjugate is shown in Figure 4. When comparing equal amounts of MTX-dendrimer conjugate and MTX, the conjugate showed a significantly greater cell kill than the drug alone. It was also noted that the percentage of decrease in cell viability was proportional to the concentration of MTX-dendrimer conjugate from 125 to 750 ng/ml while a similar trend was not followed at 1000 ng/ml of equivalent MTX. Interestingly, the percentage of decrease in cell viability for MTX from 125 to 750 ng/ml was not significantly different. This is an important observation that indicates that the increase in concentration of the drug does not affect the cell viability unless it is conjugated with dendrimer that facilitates the cellular uptake. The conjugate also killed a greater number of cells at nearly all of the concentrations. This supports the argument that the dendrimer and MTX bond is not unnecessarily strong and successfully liberates MTX in the cellular environment.[37] The cell study indicated that the conjugate is the most successful, overall, at killing the MES-SA cells.
Figure 4.
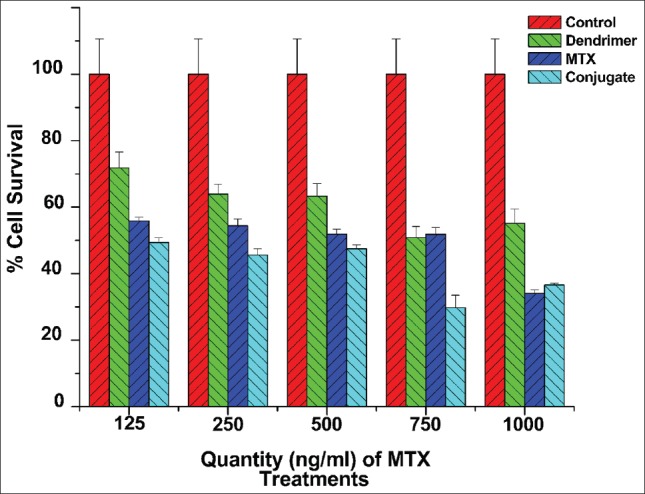
Percent cell survival of blank media, methotrexate (MTX), dendrimer and MTX-dendrimer conjugate (n = 3, mean ± standard error of mean)
It is to be noted that the dendrimer itself had an effect on the cell viability compared to the media alone. This was an observation similar to reported by Kuo et al. that cationic dendrimers affect cell viability in a dose-dependent manner.[38] Based on a recent study by Choi et al.[39] and previous reports,[22] it could be postulated that dendrimers cause changes in membrane permeability (or formation of holes) whereby the conjugated drug is internalized and released for therapeutic action. However, the toxicity of cationic dendrimers in cell culture system could be quite different from in vivo toxicity. In their study, Baker et al. have reported that in in vivo studies the experimental mice showed no gross acute or chronic toxicity for 99 days.[40] This could be attributed to rapid clearance of the dendrimer as well as its conjugates from the blood.
Conclusions
In this study, we synthesized G5 dendrimer-MTX conjugates by attaching MTX to a NH2 terminated dendrimer. The conjugation was confirmed by 1H NMR spectroscopy and UV spectroscopy. The in vitro cytotoxicity study using MES-SA cells showed significantly superior cytotoxicity of conjugates compared with free MTX. The cellular uptake of MTX dendrimer conjugates into MES-SA uterine sarcoma cells was successful. The MTX-dendrimer nanoconjugates can be used for targeting cervical cancer where it has the potential of significantly reducing the cytotoxicity to normal cells, when administered intravenously or through local tumor injection.
Acknowledgments
This project was supported through a research grant provided by the American Foundation of Pharmaceutical Education. The authors would like to thank Dr. John Esteb, Butler University Department of Chemistry, for his help with the NMR experiments.
Footnotes
Source of Support: This project was supported through a research grant provided by the American Foundation of Pharmaceutical Education
Conflict of Interest: None declared.
References
- 1.Buhleier E, Wehner W, Vögtle F. Cascade and nonskid-chain-like synthesis of molecular cavity topologies. Synthesis. 1978;2:155–8. [Google Scholar]
- 2.Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. Dendritic macromolecules: Synthesis of starburst dendrimers. Macromolecules. 1986;19:2466–8. [Google Scholar]
- 3.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–85. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Samad A, Alam MI, Saxena K. Dendrimers: A class of polymers in the nanotechnology for the delivery of active pharmaceuticals. Curr Pharm Des. 2009;15:2958–69. doi: 10.2174/138161209789058200. [DOI] [PubMed] [Google Scholar]
- 5.Ambade AV, Savariar EN, Thayumanavan S. Dendrimeric micelles for controlled drug release and targeted delivery. Mol Pharm. 2005;2:264–72. doi: 10.1021/mp050020d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majoros IJ, Thomas TP, Mehta CB, Baker JR., Jr Poly (amidoamine) dendrimer-based multifunctional engineered nanodevice for cancer therapy. J Med Chem. 2005;48:5892–9. doi: 10.1021/jm0401863. [DOI] [PubMed] [Google Scholar]
- 7.Khandare J, Kolhe P, Pillai O, Kannan S, Lieh-Lai M, Kannan RM. Synthesis, cellular transport, and activity of polyamidoamine dendrimer-methylprednisolone conjugates. Bioconjug Chem. 2005;16:330–7. doi: 10.1021/bc0498018. [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Fréchet JM. Designing dendrimers for drug delivery. Pharm Sci Technolo Today. 1999;2:393–401. doi: 10.1016/s1461-5347(99)00203-5. [DOI] [PubMed] [Google Scholar]
- 9.An M, Parkin SR, DeRouchey JE. Intermolecular forces between low generation PAMAM dendrimer condensed DNA helices: Role of cation architecture. Soft Mater. 2014;10:590–9. doi: 10.1039/c3sm52096j. [DOI] [PubMed] [Google Scholar]
- 10.Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR., Jr Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci U S A. 1996;93:4897–902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arima H, Motoyama K, Higashi T. Polyamidoamine dendrimer conjugates with cyclodextrins as novel carriers for DNA, shRNA and siRNA. Pharmaceutics. 2012;4:130–48. doi: 10.3390/pharmaceutics4010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esfand R, Tomalia DA. Poly (amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6:427–36. doi: 10.1016/s1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 13.Beezer AE, King AS, Martin IK, Mitchel JC, Twyman LJ, Wain CF. Dendrimers as potential drug carriers; encapsulation of acidic hydrophobes within water soluble PAMAM derivatives. Tetrahedron. 2003;59:3873–80. [Google Scholar]
- 14.Gurdag S, Khandare J, Stapels S, Matherly LH, Kannan RM. Activity of dendrimer-methotrexate conjugates on methotrexate-sensitive and -resistant cell lines. Bioconjug Chem. 2006;17:275–83. doi: 10.1021/bc0501855. [DOI] [PubMed] [Google Scholar]
- 15.Tomalia DA. Twenty-First Century Polymer Science After Staudinger: The Emergence of Dendrimers/Dendritic Polymers as a Fourth Major Architecture and Window to a New Nano-periodic System. Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize I. Adv Polym Sci. 2013;261:321–89. [Google Scholar]
- 16.Bielinska A, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR., Jr Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res. 1996;24:2176–82. doi: 10.1093/nar/24.11.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malik N, Evagorou EG, Duncan R. Dendrimer-platinate: A novel approach to cancer chemotherapy. Anticancer Drugs. 1999;10:767–76. [PubMed] [Google Scholar]
- 18.Wiener EC, Konda S, Shadron A, Brechbiel M, Gansow O. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest Radiol. 1997;32:748–54. doi: 10.1097/00004424-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39:268–307. [Google Scholar]
- 20.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multi-purpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–7. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 21.Jain K, Kesharwani P, Gupta U, Jain NK. Dendrimer toxicity: Let's meet the challenge. Int J Pharm. 2010;394:122–42. doi: 10.1016/j.ijpharm.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Hong S, Bielinska AU, Mecke A, Keszler B, Beals JL, Shi X, et al. Interaction of poly (amidoamine) dendrimers with supported lipid bilayers and cells: Hole formation and the relation to transport. Bioconjug Chem. 2004;15:774–82. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 23.Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 24.Lee CC, MacKay JA, Fréchet JM, Szoka FC. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23:1517–26. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 25.Zhuo RX, Du B, Lu ZR. In vitro release of 5-fluorouracil with cyclic core dendritic polymer. J Control Release. 1999;57:249–57. doi: 10.1016/s0168-3659(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 26.Kono K, Liu M, Fréchet JM. Design of dendritic macromolecules containing folate or methotrexate residues. Bioconjug Chem. 1999;10:1115–21. doi: 10.1021/bc990082k. [DOI] [PubMed] [Google Scholar]
- 27.Long HJ, 3rd, Monk BJ, Huang HQ, Grendys EC, Jr, McMeekin DS, Sorosky J, et al. Clinical results and quality of life analysis for the MVAC combination (methotrexate, vinblastine, doxorubicin, and cisplatin) in carcinoma of the uterine cervix: A Gynecologic Oncology Group study. Gynecol Oncol. 2006;100:537–43. doi: 10.1016/j.ygyno.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, Thomas TP, et al. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005;65:5317–24. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 29.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92. [PubMed] [Google Scholar]
- 30.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J Control Release. 2000;65:271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 31.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 32.Haensler J, Szoka FC., Jr Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993;4:372–9. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 33.Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–37. doi: 10.1016/j.addr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, Baker JR., Jr Design and function of a dendrimer. based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm Res. 2002;19:1310–6. doi: 10.1023/a:1020398624602. [DOI] [PubMed] [Google Scholar]
- 35.Svenson S, Tomalia DA. Dendrimers in biomedical applications – Reflections on the field. Adv Dru g Deliv Rev. 2012;64:102–15. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Najlah M, D’Emanuele A. Crossing cellular barriers using dendrimer nanotechnologies. Curr Opin Pharmacol. 2006;6:522–7. doi: 10.1016/j.coph.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Patri AK, Kukowska-Latallo JF, Baker JR., Jr Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv Rev. 2005;57:2203–14. doi: 10.1016/j.addr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Kuo JH, Jan MS, Chiu HW. Mechanism of cell death induced by cationic dendrimers in RAW 264.7 murine macrophage-like cells. J Pharm Pharmacol. 2005;57:489–95. doi: 10.1211/0022357055803. [DOI] [PubMed] [Google Scholar]
- 39.Choi SK, Thomas TP, Li MH, Desai A, Kotlyar A, Baker JR., Jr Photochemical release of methotrexate from folate receptor-targeting PAMAM dendrimer nanoconjugate. Photochem Photobiol Sci. 2012;11:653–60. doi: 10.1039/c2pp05355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JR., Jr Dendrimer-based nanoparticles for cancer therapy. Hematology Am Soc Hematol Educ Program 2009. 2009:708–19. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]


