Key Points
Ghr is specifically expressed on HSCs within the hematopoietic system and is dynamically regulated upon HSC aging and activation.
Ghr is dispensable for HSC function.
Abstract
Growth hormone receptor (Ghr) signaling is important in a wide variety of cellular processes including aging; however, the role of Ghr signaling in hematopoietic stem cell (HSC) biology remains unexplored. Within the hematopoietic system, Ghr is expressed in a highly HSC-specific manner and is significantly upregulated during aging. Exposure of young and old HSCs to recombinant growth hormone ex vivo led to diminished short-term reconstitution and restored B-cell output from old HSCs. Hematopoietic-specific genetic deletion of Ghr neither impacted steady-state hematopoiesis nor serial transplantation potential. Repeat challenge with 5-fluorouracil showed that Ghr was dispensable for HSC activation and homeostatic recovery in vivo and, after challenge, Ghr-deficient HSCs functioned normally through serial transplantation. Although exogenous Gh induces age-dependent HSC effects, these results indicate that Ghr signaling appears largely dispensable for HSC function and aging.
Introduction
Upon aging, the hematopoietic system displays diminished regenerative potential, reduced immune competence, and a predisposition toward myelogenous disease.1 The importance of cell autonomous regulation of hematopoietic stem cell (HSC) potential throughout aging is well established, although emerging evidence suggests that HSC potential may also be regulated by environmental cues that are subject to age-related variation.2
Growth hormone (Gh) signaling has been implicated in a variety of age-related hematopoietic phenotypes,3-5 and exogenous Gh can enhance or restore young or aged hematopoietic cellularity and function, respectively.5-10 Studies have proposed that Gh mediates its hematopoietic effects indirectly through nonhematopoietic cells within the bone marrow (BM)7,11; however, as the Gh responsive cell was not identified in these studies, it remains unclear whether Gh directly targets hematopoietic stem/progenitor cells in a cell autonomous manner. Here, we have addressed the cell intrinsic impact of Gh signaling on HSCs during aging using gain-of-function and loss-of-function approaches. We found that Gh receptor (Ghr) is specifically expressed on HSCs within the hematopoietic system and ex vivo exposure of HSCs to Gh compromised the short-term reconstitution potential of young but not old HSCs and led to restored B-lymphocyte potential in old HSCs. Hematopoietic deletion of Ghr surprisingly did not impact hematopoietic steady-state homeostasis or HSC activity upon 5-fluorouracil (5-FU) challenge or serial transplantation. Although exogenous Gh exposure elicits age-dependent effects in HSCs, these results show that Ghr signaling is nonessential to HSC biology and aging.
Study design
Mice
Ghrfl/fl mice were bred with Vav1Cre/+ mice to generate Ghrfl/fl;Vav1Cre/+ experimental and Ghr+/+;Vav1Cre/+ control mice. All mice were maintained according to Boston Children’s Hospital Animal Facility protocols. Procedures were performed with consent from local ethics committees.
Ex vivo rGh administration
For transplantation experiments, fluorescence-activated cell sorter isolated HSCs were cultured in S-Clone 0.75% bovine serum albumin, 50 ng/mL each stem cell factor, thrombopoietin, and IL12+/− 100 ng/mL recombinant mouse Gh (rGh) for 6 days. Media was changed at days 2 and 4. At day 6, each well was transplanted into 3 recipient mice with 3 × 105 competitive BM.
5-FU treatment
Four doses of 5-FU at 150 mg/kg were administered every 3 weeks by intraperitoneal injection to Ghrfl/fl;Vav1Cre/+ (n = 4) and Ghr+/+;Vav1Cre/+ (n = 4) littermates. For genotyping primers, see supplemental Table 1, available on the Blood Web site.
See supplemental Methods for full details.
Results and discussion
Ghr is HSC-specific and age-regulated
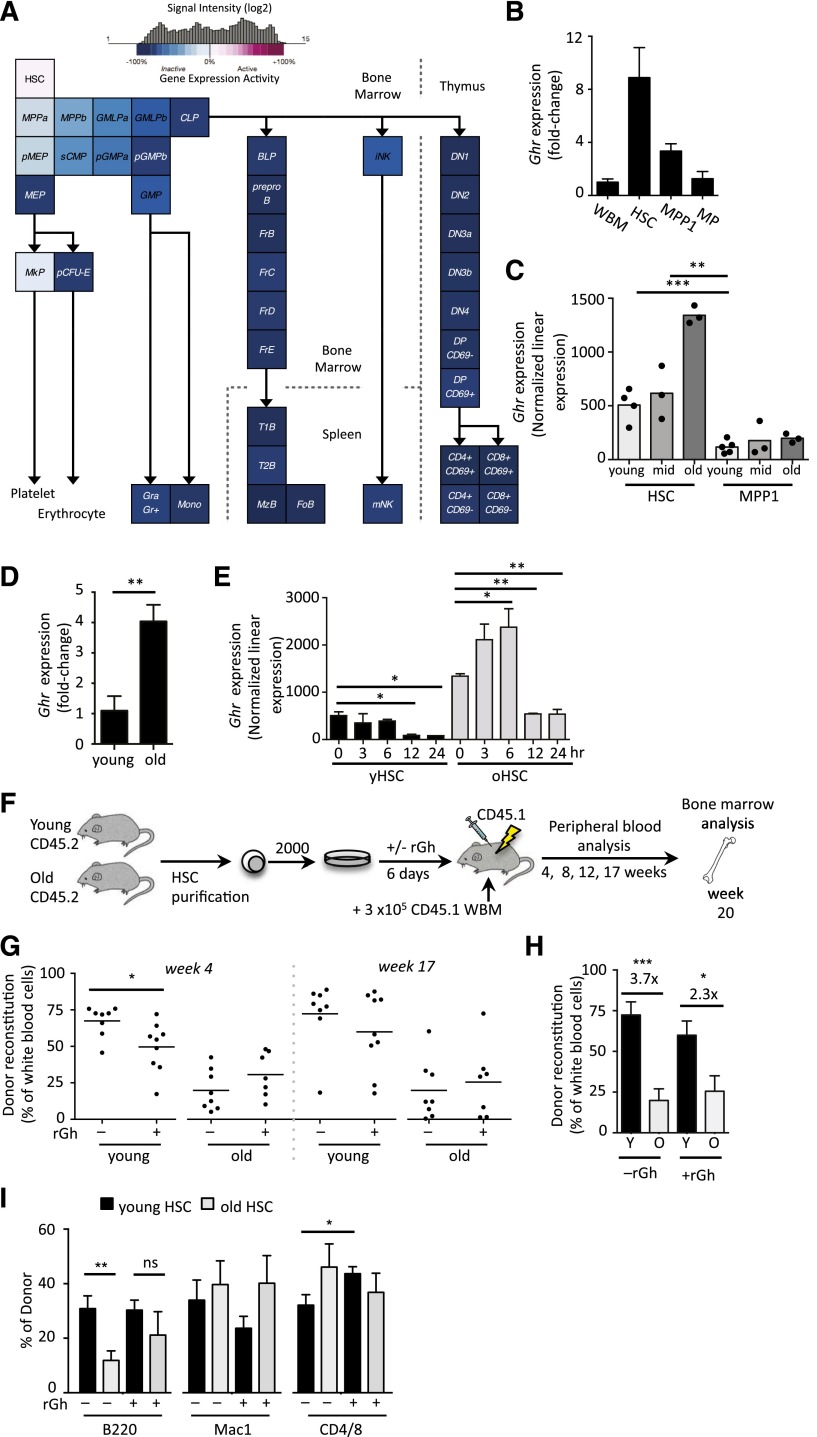
We identified Ghr as a gene whose expression was highly restricted to HSCs in comparison with their downstream progeny through analysis of a number of comprehensive expression profiling studies generated by ourselves and others12-14 (Figure 1A-B). Interestingly, Ghr was substantially upregulated (3.7-fold) in HSCs isolated from old mice, whereas no change in downstream MPP1s was observed during aging (Figure 1C-D). Protein analysis confirmed that Ghr was expressed on HSCs (supplemental Figure 1A); however, in contrast to the differential age regulation at the messenger RNA level, no difference in protein expression was observed during aging (supplemental Figure 1B). As the activity of Ghr signaling has been shown to be age-dependent,15-17 we assessed expression of Ghr signaling targets Igf118 and suppressor of cytokine signaling 2 (Socs2)19 in purified HSCs from young and old mice after exposure to recombinant Gh. The rGh exposure marginally upregulated Igf1 expression in young HSCs, whereas Socs2 was significantly upregulated in old HSCs (supplemental Figure 1C-D).
Figure 1.
Dynamic regulation of Ghr on HSCs induces age-dependent effects upon ex vivo rGH stimulation. (A) Expression of Ghr in hematopoiesis in the indicated populations as revealed by microarray analysis. (B) Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) of Ghr expression in HSCs (LSKCD34−Flk2−), multipotent progenitor-1 (MPP1; LSKCD34+Flk2−) and myeloid progenitors (MP) (Lin-Sca1−cKit+) young (4-month-old) mice. (C) Expression of Ghr in young, middle age, and old HSC (LSKFlk2−CD34−) and MPP1 (LSKCD34+Flk2–) populations. (D) The qRT-PCR of Ghr expression in young and old HSCs. (E) Ghr expression in young and old HSCs over 24 hours of ex vivo culture. (F) Experimental design of ex vivo rGh treatment of isolated young and old HSCs (LSKCD34−Flk2−CD150+) followed by in vivo functional analysis. (G-I) PB analysis after transplantation of rGh-treated or control-treated young and old HSCs showing (G) donor engraftment at 4 and 17 weeks posttransplant. (H) Fold-change in PB engraftment between by young and old untreated and rGh-treated HSCs at week 17 posttransplant. (I) Lineage reconstitution at 17 weeks posttransplant. Unpaired Student t test: *P < .05; **P < .01; ***P < .001. ns, not significant; WBM, whole BM.
To determine whether Ghr was dynamically regulated upon HSC activation, we examined its expression in young and old HSCs over 24 hours postcytokine stimulation in vitro (Figure 1E).20 Interestingly, Ghr exhibited a dynamic age-dependent response in which old, but not young, HSCs rapidly upregulated Ghr upon ex vivo stimulation peaking at 6 hours (Figure 1E); both young and old HSCs downregulated Ghr starting at 12 hours poststimulation. Together, these results show that Ghr expression is largely HSC-specific within the hematopoietic system and that Ghr signaling cascades are dynamically regulated with age upon HSC activation.
Ex vivo rGh exposure impacts HSC function in an age-dependent manner
To investigate the possibility that the differential Ghr signaling we observed may contribute to the functional changes observed during HSCs aging, we cultured purified young and old HSCs in the presence of rGh, and then we assessed their potential by competitive transplantation (Figure 1F). This allowed us to assay Gh signaling in HSCs cell autonomously without confounding effects arising from systemic treatment with Gh. Interestingly, whereas old HSCs exhibited similar reconstitution kinetics independent of rGh exposure, young HSCs exposed to rGh exhibited significantly diminished short-term reconstitution that largely recovered at later time points posttransplant (Figure 1G). Comparison of young and old cohorts showed that total reconstitution was significantly diminished with aging, independent of rGh treatment (Figure 1H). Interestingly however, exposure to rGh partially mitigated the loss in B-cell potential (Figure 1I), that is characteristic of old HSCs.21 We also observed a significant increase in T-cell potential arising from rGh-treated young HSCs, which was not observed with the old HSCs(Figure 1I).
BM analysis performed at week 20 posttransplant revealed that donor-derived chimerism and frequency of progenitors and HSCs within the BM were not impacted by exposure to rGh (supplemental Figure 2A-D). We did not observe changes in myeloid potential reported in earlier studies that used systemic rGh treatment5,7 suggesting that these previous observations reflect noncell autonomous changes in hematopoietic lineage potential. Together, our results show that exogenous rGh treatment differentially affects lineage potential of young and old HSCs, and induces a more balanced lineage output from aged HSCs.
Genetic ablation reveals that Ghr is dispensable for HSC function
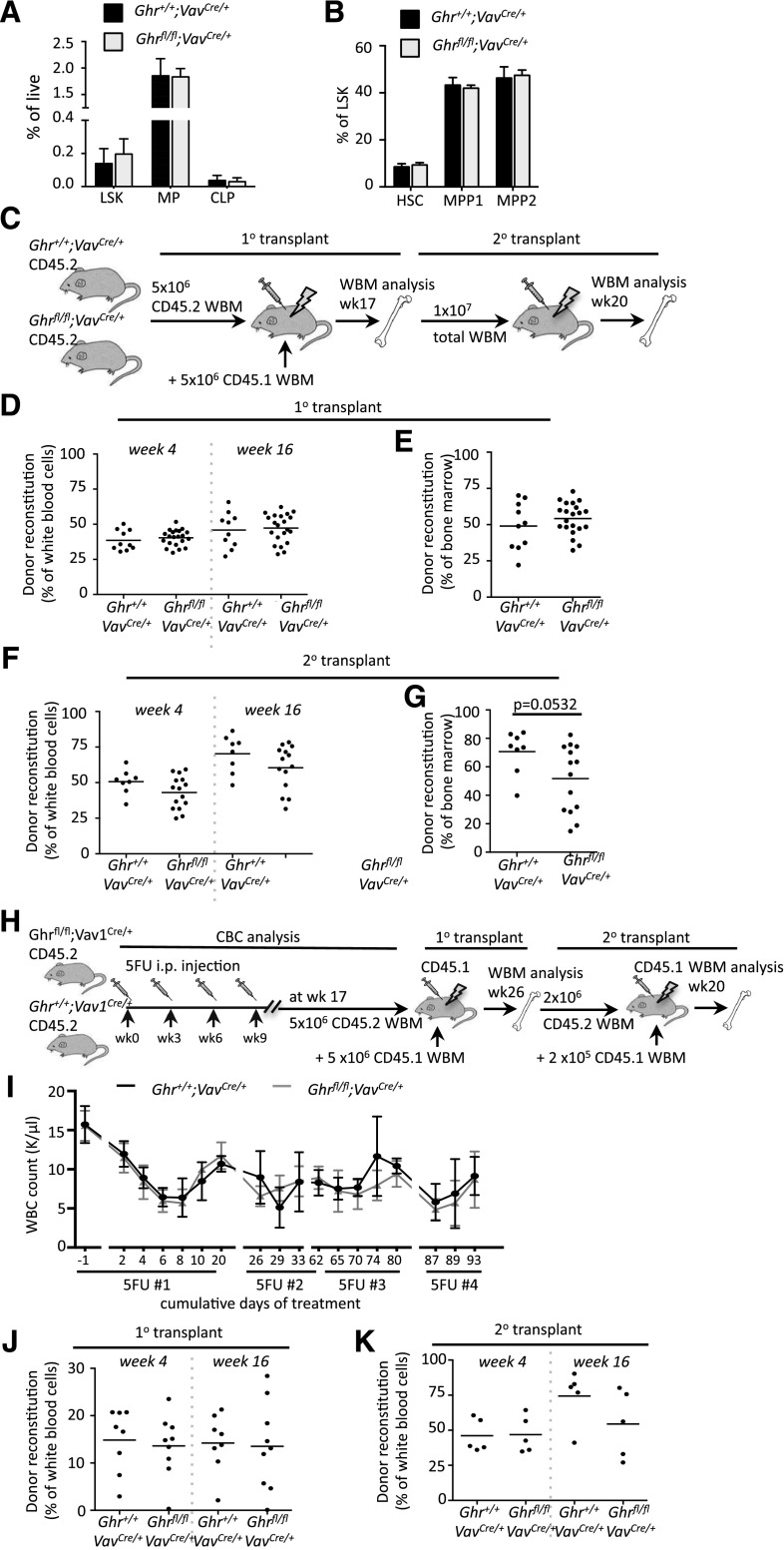
To address how loss of Ghr signaling would impact HSC potential, we generated Ghrfl/fl;Vav1Cre/+ experimental and Ghr+/+;Vav1Cre/+ control mice.22 Genomic PCR of fluorescence-activated cell sorter isolated BM cells confirmed efficient hematopoietic deletion of Ghr in the experimental mice (supplemental Figure 3A). To assess how loss of Ghr in HSCs impacted steady-state hematopoiesis, we analyzed complete blood cell counts and peripheral blood (PB) composition of age-matched experimental and control mice. A significant decrease in the number of platelets was observed in the absence of Ghr, but no other lineages were altered (supplemental Figure 3B-F). Analysis of progenitor compartments in the BM of Ghrfl/fl;Vav1Cre/+ and Ghr+/+;Vav1Cre/+ mice revealed that all were maintained at comparable frequencies regardless of Ghr status (Figure 2A-B).
Figure 2.
Ghr is dispensable for HSC function. (A-B) BM analysis of steady-state hematopoiesis in Ghrfl/fl;Vav1Cre/+ experimental and Ghr+/+;Vav1Cre/+ control mice showing BM frequency of (A) lineage marker negative, Sca1+, and c-Kit+ (LSK), MP, and common lymphoid progenitor (CLP) populations, and (B) LSK compartment including HSCs. (C) Experimental design for analysis of recipient mice competitively transplanted with Ghrfl/fl;Vav1Cre/+ and Ghr+/+;Vav1Cre/+ BM. (D-E) Primary transplant analysis showing (D) donor PB reconstitution and (E) donor BM reconstitution. (F-G) Secondary transplant analysis showing (D) donor PB reconstitution and (E) donor BM reconstitution. (H) Experimental overview of serial 5-FU exposure followed by competitive BM transplantation of Ghrfl/fl;Vav1Cre/+ experimental and Ghr+/+;Vav1Cre/+ control mice. (I) Analysis of white blood cell (WBC) counts after post-5-FU injection over the indicated time-course in Ghrfl/fl;Vav1Cre/+ (gray line) and Ghr+/+;Vav1Cre/+ (black line) mice. Arrows indicate time points of 5-FU injection. (J) The 1° transplant analysis showing donor reconstitution in PB at 4 and 16 weeks posttransplant. (K) Secondary transplant analysis showing donor reconstitution in PB at 4 and 16 weeks posttransplant. CBC, complete blood counts; i.p., intraperitoneal.
To address the possibility that deletion of Ghr from HSCs might preserve or enhance HSC potential, we competitively transplanted whole BM cells from Ghrfl/fl;Vav1Cre/+ and Ghr+/+;Vav1Cre/+ mice (Figure 2C). PB analysis over 16 weeks did not reveal any differences in the reconstitution or lineage potential (Figure 2D and supplemental Figure 4A). BM analysis at 17 weeks posttransplantation similarly revealed no difference in donor chimerism or in stem and progenitor compartments (Figure 2E and supplemental Figure 4B-C). To examine the impact of Ghr deletion on HSC self-renewal, we performed secondary transplants. PB analysis showed that loss of Ghr did not alter reconstitution or lineage potential (Figure 2F and supplemental Figure 4D). BM analysis at 20 weeks postsecondary transplant revealed diminished total chimerism, although this did not reach significance (Figure 2G). Similarly, progenitors and HSCs were not impacted by loss of Ghr (supplemental Figure 4E-F).
Due to the dynamic regulation of Ghr expression in HSCs during ex vivo activation (Figure 1E), we examined the functional response of HSCs subjected to repeated cycles of activation induced by 5-FU exposure (Figure 2H). The 5-FU exposure activates HSCs,23-25 and we have shown repeat exposure compromises HSC functional potential.26 Complete blood cell count analysis showed that Ghr deletion did not compromise the ability of HSCs and progenitors to mount an effective recovery of white blood cells, red blood cells, or platelets after each 5-FU exposure (Figure 2I, supplemental Figures 5 and 6A-B).
To assess how serial 5-FU exposure impacted HSC potential in Ghrfl/fl;Vav1Cre/+ and Ghr+/+;Vav1Cre/+ mice, competitive BM transplantation was performed (Figure 2H). Deletion of Ghr did not impact PB engraftment or lineage potential (Figure 2J; supplemental Figure 6C), and BM analysis at 26 weeks posttransplantation showed no significant difference in the level of engraftment or the frequency of BM progenitors and HSCs (supplemental Figure 6D-F). To assess HSC self-renewal, competitive secondary transplants were performed. No difference in PB engraftment or lineage reconstitution potential was observed over 16 weeks postsecondary transplant (Figure 2K; supplemental Figure 6G), and no change in BM reconstitution or in the frequency of BM progenitors or HSCs was found at 20 weeks (supplemental Figure 6H-J). The lack of differential recovery of the hematopoietic system in steady-state or after transplantation as a consequence of Ghr deletion demonstrated, surprisingly, that Ghr is dispensable for HSC activation and recovery, and further, serial activation of HSCs did not affect the engraftment or lineage potential of Ghr null HSCs.
In conclusion, although exogenous Gh can impact HSC potential in an age-dependent manner, our results suggest that Ghr signaling appears largely dispensable to HSC function and aging.
Acknowledgments
The authors thank A. Zguro for animal husbandry and technical assistance, and all the members of the D.J.R. Laboratory.
This work was supported by grants from the National Institutes of Health (National Institute of Aging; R00AG029760) (D.J.R.), (National Institute of Diabetes and Digestive and Kidney Diseases; UO1DK072473-01) (D.J.R.), the Leona M. and Harry B. Helmsley Charitable Trust (D.J.R.), the New York Stem Cell Foundation (D.J.R.), and the Harvard Stem Cell Institute (D.J.R.).
D.J.R. is a New York Stem Cell Foundation Robertson Investigator.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.H.S. performed experiments; M.H.S. and D.J.R. designed experiments and wrote the manuscript; I.B., P.G.-M., and B.G. helped with the experiments; and E.J.G. and D.L. provided Ghrfl/fl animals.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Derrick J. Rossi, 200 Longwood Ave, Warren Alpert Building, Room WAB-149e, Boston Children’s Hospital, Boston, MA 02115; e-mail: derrick.rossi@childrens.harvard.edu.
References
- 1.Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. 2010;22(4):500–506. doi: 10.1016/j.coi.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nat Rev Immunol. 2013;13(5):376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 3.Ding J, Sackmann-Sala L, Kopchick JJ. Mouse models of growth hormone action and aging: a proteomic perspective. Proteomics. 2013;13(3-4):674–685. doi: 10.1002/pmic.201200271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010;10(4):408–424. doi: 10.1016/j.coph.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French RA, Broussard SR, Meier WA, et al. Age-associated loss of bone marrow hematopoietic cells is reversed by GH and accompanies thymic reconstitution. Endocrinology. 2002;143(2):690–699. doi: 10.1210/endo.143.2.8612. [DOI] [PubMed] [Google Scholar]
- 6.Carlo-Stella C, Di Nicola M, Milani R, et al. Age- and irradiation-associated loss of bone marrow hematopoietic function in mice is reversed by recombinant human growth hormone. Exp Hematol. 2004;32(2):171–178. doi: 10.1016/j.exphem.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Chen BJ, Deoliveira D, Spasojevic I, et al. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PLoS ONE. 2010;5(6):e11056. doi: 10.1371/journal.pone.0011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pello OM, Moreno-Ortiz MC, Rodríguez-Frade JM, et al. SOCS up-regulation mobilizes autologous stem cells through CXCR4 blockade. Blood. 2006;108(12):3928–3937. doi: 10.1182/blood-2006-02-006353. [DOI] [PubMed] [Google Scholar]
- 9.Carlo-Stella C, Di Nicola M, Milani R, et al. Use of recombinant human growth hormone (rhGH) plus recombinant human granulocyte colony-stimulating factor (rhG-CSF) for the mobilization and collection of CD34+ cells in poor mobilizers. Blood. 2004;103(9):3287–3295. doi: 10.1182/blood-2003-07-2428. [DOI] [PubMed] [Google Scholar]
- 10.van der Klaauw AA, Pereira AM, Rabelink TJ, et al. Recombinant human GH replacement increases CD34+ cells and improves endothelial function in adults with GH deficiency. Eur J Endocrinol. 2008;159(2):105–111. doi: 10.1530/EJE-08-0179. [DOI] [PubMed] [Google Scholar]
- 11.Hanley MB, Napolitano LA, McCune JM. Growth hormone-induced stimulation of multilineage human hematopoiesis. Stem Cells. 2005;23(8):1170–1179. doi: 10.1634/stemcells.2004-0322. [DOI] [PubMed] [Google Scholar]
- 12.Seita J, Sahoo D, Rossi DJ, et al. Gene Expression Commons: an open platform for absolute gene expression profiling. PLoS ONE. 2012;7(7):e40321. doi: 10.1371/journal.pone.0040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazit R, Garrison BS, Rao TN, et al. Immunological Genome Project Consortium. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Reports. 2013;1(3):266–280. doi: 10.1016/j.stemcr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Bennett SA, Ingram RL, Sonntag WE. Decreases in growth hormone receptor signal transduction contribute to the decline in insulin-like growth factor I gene expression with age. Endocrinology. 1995;136(10):4551–4557. doi: 10.1210/endo.136.10.7664676. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman SA, Mitchell AM, Marcus R, Hintz RL, Hoffman AR. The insulin-like growth factor I generation test: resistance to growth hormone with aging and estrogen replacement therapy. Horm Metab Res. 1994;26(5):229–233. doi: 10.1055/s-2007-1001671. [DOI] [PubMed] [Google Scholar]
- 17.Velasco B, Cacicedo L, Melian E, Fernández-Vázquez G, Sánchez-Franco F. Sensitivity to exogenous GH and reversibility of the reduced IGF-I gene expression in aging rats. Eur J Endocrinol. 2001;145(1):73–85. doi: 10.1530/eje.0.1450073. [DOI] [PubMed] [Google Scholar]
- 18.Rotwein P. Mapping the growth hormone—Stat5b—IGF-I transcriptional circuit. Trends Endocrinol Metab. 2012;23(4):186–193. doi: 10.1016/j.tem.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vesterlund M, Zadjali F, Persson T, et al. The SOCS2 ubiquitin ligase complex regulates growth hormone receptor levels. PLoS ONE. 2011;6(9):e25358. doi: 10.1371/journal.pone.0025358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. 2014;15(1):37–50. doi: 10.1016/j.stem.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi DJ, Bryder D, Zahn JM, et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci USA. 2005;102(26):9194–9199. doi: 10.1073/pnas.0503280102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121(6):2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison DE, Lerner CP. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78(5):1237–1240. [PubMed] [Google Scholar]
- 24.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 25.Randall TD, Weissman IL. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood. 1997;89(10):3596–3606. [PubMed] [Google Scholar]
- 26.Beerman I, Bock C, Garrison BS, et al. Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell. 2013;12(4):413–425. doi: 10.1016/j.stem.2013.01.017. [DOI] [PubMed] [Google Scholar]