Abstract
Glucagon-like peptide 1 (GLP-1), a peptide secreted from the intestine in response to nutrient ingestion, is perhaps best known for its effect on glucose-stimulated insulin secretion. GLP-1 is also secreted from neurons in the caudal brainstem, and it is well-established that, in rodents, central administration of GLP-1 potently reduces food intake. Over the past decade, GLP-1 has emerged not only as an essential component of the system that regulates blood glucose levels but also as a viable therapeutic target for the treatment of type 2 diabetes mellitus. However, although GLP-1 receptor agonists are known to produce modest but statistically significant weight loss in patients with diabetes mellitus, our knowledge of how endogenous GLP-1 regulates food intake and body weight remains limited. The purpose of this Review is to discuss the evolution of our understanding of how endogenous GLP-1 modulates energy balance. Specifically, we consider contributions of both central and peripheral GLP-1 and propose an integrated model of short-term and long-term control of energy balance. Finally, we discuss this model with respect to current GLP-1-based therapies and suggest ongoing research in order to maximize the effectiveness of GLP-1-based treatment of obesity.
Introduction
Successful regulation of energy balance requires accurate matching of energy intake and expenditure so that the metabolic demands of the body are met and appropriate fuel stores are maintained. Whereas terminal starvation represents one extreme of energy imbalance that is imminently fatal, obesity represents the other extreme. Although not immediately life-threatening, obesity disrupts normal functioning and kills insidiously through comorbid conditions, such as diabetes mellitus, dyslipidemia and hypertension. To prevent these pathological states, a complex neuroendocrine network has evolved that enables peripheral tissues to convey the status of energy stores to the central nervous system (CNS), which in turn modulates caloric intake and expenditure to maintain appropriate energy balance.
Energy balance is regulated by both short-term and long-term negative feedback. In the short term, meal size is determined by means of various ‘satiation signals’, such as the secretion of the peptide cholecystokinin (CCK) from duodenal I cells in response to nutrient ingestion. Through local action on intestinal vagal afferent nerves and subsequent modulation of neurotransmission in the hindbrain and hypothalamus, CCK limits individual meal size, which ensures that nutrient intake is restricted to what can be assimilated at a given time.1 In the long term, the amount of adiphose tissue, the body’s principle energy store, is determined by various ‘adiposity signals’, the most important of which involves the peptide leptin. The adipokine leptin, which is secreted into the circulation from white adipose tissue in direct proportion to total body adiposity, directly conveys the size of peripheral adipose tissue stores to leptin-sensitive neurons in the CNS, whose coordinated actions alter food intake and energy expenditure to maintain an appropriate body weight and level of adiposity.2
Glucagon-like peptide 1 (GLP-1), a product of the proglucagon (GCG) gene, is secreted into the hepatic portal circulation from intestinal L cells in response to nutrient ingestion.3,4 Numerous factors have been implicated in the complex regulation of GLP-1 secretion, including nutrients and dietary fiber, proximal-to-distal intestinal neural signaling, neurotransmitters and other gastrointestinal hormones, G-protein coupled receptors activated by free fatty acids, as well as L-cell taste receptors. A detailed review of GLP-1 secretion can be found elsewhere.5 GLP-1 is perhaps best known for its effect to augment glucose-stimulated insulin secretion from pancreatic β cells—an action termed the incretin effect.3,4 This ‘insulinotropic’ effect has prompted the successful development of GLP-1-based therapies for type 2 diabetes mellitus,6 and increased GLP-1 signaling is thought to be one mechanism by which type 2 diabetes mellitus rapidly improves following gastric bypass surgery.7 Nonetheless, the anatomy of the GLP-1 system suggests additional roles, including regulation of energy balance. In addition to intestinal L cells, GLP-1 is produced in the caudal nucleus of the solitary tract and in the ventro lateral medulla,8 and GLP-1 receptors (GLP1R) are expressed in the hindbrain and in hypothalamic nuclei that are known to regulate energy balance.9
On the basis of the initial finding that centrally administered GLP-1 potently reduces short-term food intake in rats,10,11 traditional models proposed that peripheral GLP-1 regulates glucose homeostasis, whereas central GLP-1 regulates food intake. However, evidence has accumulated to support roles for both central and peripheral GLP-1 in the regulation of energy balance, in the short and in the long term. The purpose of this Review is to address our current understanding of how central and peripheral GLP-1 action regulates food intake and body weight. Additionally, we propose an integrated model of short-term and long-term control of energy balance and discuss this model with respect to current GLP-1-based therapeutics.
Central GLP-1 and energy balance
Evidence of a role for GLP-1 in the regulation of energy balance was first reported in 1996, as central administration of GLP-1 caused robust inhibition of food intake in rats.10,11 This effect was short-lived (1–2 h), dose-dependent and sensitive to blockade by the GLP1R antagonist exendin (9–39). Moreover, the anorectic effect of GLP-1 seemed to be mediated primarily via central GLP1R, as peripheral administration of GLP-1 exerted little to no effect on food intake.12 On the basis of these data, central GLP-1 was thought to act as a physiological satiation signal. However, this hypothesis was quickly challenged by the work of Rinaman and others, who proposed that central GLP-1 regulates illness-induced and stress-induced anorexia rather than satiety.13,14 Specifically, rat hindbrain neurons that express GCG were activated by noxious stimuli such as lithium chloride (LiCl), lipopolysaccharide (LPS) and experimental gastric distension but not by ingestion of a large meal.13,14 Moreover, the anorectic effects of both LiCl and LPS, as well as the effect of LiCl to elicit a conditioned taste aversion in rats, could be blocked by centrally administered exendin 9.15–17 These data, therefore, shifted the hypothesized role of central GLP-1 from satiation signal to mediator of visceral illness, of which reduced food intake is just one manifestation.
What has since become clear is that central GLP1R activity mediates a range of diverse effects. With respect to food intake inhibition, studies in rats employing site-specific infusion of GLP-1 or exendin 4 have implicated several hypothalamic nuclei, most notably the paraventricular nucleus, as well as the hindbrain.18–22 However, centrally administered GLP-1 also engages the hypothalamic–pituitary–adrenal (HPA) axis in rats,23 and GLP1R activity in the paraventricular nucleus and central nucleus of the amygdala is necessary to mediate neuroendocrine and behavioral responses to interoceptive and psychogenic stressors.24,25 In turn, physiological stressors as well as glucocorticoids themselves modulate GCG transcription in the hindbrain.26 Furthermore, centrally administered exendin 4 engages the sympathetic nervous system in rats, including medullary catecholamine neurons and the adrenal medulla.27 In rodents, sympathetic nervous system activity is necessary for several central GLP1R-mediated effects, including inhibition of gastric emptying, adipogenesis inhibition and exendin 4-induced hyperglycemia.28–30 More over, centrally administered GLP-1 engages the parasympathetic nervous system in rats, stimulating pancreatic vagal efferent neurons ex vivo and accelerating colonic transit in vivo.31–33 Finally, central GLP1R activity regulates glucose homeostasis, specifically by sensing enteric glucose, stimulating insulin secretion, modulating hepatic and muscle glucose metabolism and possibly by redirecting peripheral blood flow.34–37
The above examples illustrate that activation of GLP1R in the CNS is sufficient to elicit a wide variety of effects. However, what is not so easy to reconcile is how these putative effects can all be mediated by such a small collection of neurons confined to the caudal brain stem. Although GLP1R expression within the rodent CNS is widespread,9,38 the only characterized projections of hindbrain GCG neurons have been to the paraventricular nucleus and the dorsal medial nucleus of the hypothalamus (DMH).39 Fortunately, this problem may easily be remedied with conventional neuronal tracing methods, as well as transgenic mouse models that express stable fluorescent proteins under control of the GCG promoter—approaches that are now being used to address the projections of GCG-expressing neurons. Additionally, more detailed analysis of GCG neuronal activation has already revealed that discrete subsets of GCG neurons appear to be activated by noxious versus nutritional stimuli, which implies some degree of parallel processing with respect to these roles.40 The numerous effects of central GLP1R activation may potentially reflect postprandial physiological adaptations mediated by central GLP-1. Furthermore, central GLP-1 may elicit differential effects under various physiological conditions (that is, hyperglycemia versus euglycemia).
For some of the putative effects of central GLP-1, most notably visceral illness and glucose homeostasis, loss-of-function studies strongly support a role for the endogenous peptide. However, with respect to energy balance, the data are less clear. Initial studies in rats using acute and repeated central GLP1R blockade with intracerebroventricular exendin 9 revealed hyperphagia and increased weight gain,11,41 which supports a necessary role for central GLP1R activity in the regulation of energy balance. However, this conclusion was challenged by the fact that these effects were not observed in initial studies of GLP1R−/− mice, which lack GLP1R expression, when maintained on either a standard chow or high-fat diet.42,43 Subsequent studies of these animals have further complicated the picture, as they report a hyperphagic, hypermetabolic phenotype with no overall weight gain in GLP1R−/− mice maintained on a high-fat diet.44 Interestingly, a similar phenotype has been observed in wild-type mice treated with chronic intra cerebroventricular exendin 9 and fed a high-fat, low-carbohydrate diet.45
The above discrepancies complicate our current understanding of the role of central GLP-1 in the regulation of energy balance; however, they may be explained by experimental factors. First and foremost is the possibility of bona fide species differences between rats and mice. In support of this argument, evidence for species differences already exists with respect to the role of GLP-1 in mediating visceral illness.46 Second, it is well-known that genetic mouse models are subject to developmental compensations, thus limiting interpretations of negative data as true absence of a functional role for the deleted product. Third, strain differences may complicate our interpretations of mouse data, as GLP1R−/− mice on a CD1 background exhibited no differences in food intake or body weight,42,43 whereas GLP1R−/− mice on a C57BL/6J background seem to exhibit a hyperphagic, hypermetabolic phenotype on a high-fat diet.44
An awareness of these potential confounders supports the use of alternative experimental models. The use of Cre–loxP technology perhaps holds the most promise, as it enables site-specific as well as conditional knockout of GLP-1 and its receptor and thereby confers selective and thorough loss of function while circumventing develop mental compensations. In addition, a greater awareness and understanding of species differences in GLP-1 physiology is critical. In support of a physiological role for central GLP-1 in the regulation of energy balance in rats, our group has found that downregulation of GCG expression in the caudal nucleus of the solitary tract through viral-mediated expression of RNA interference leads to hyperphagia, as well as increased fat accumulation in response to a high-fat diet.47
Moreover, in contrast to the mouse data of Knauf and colleagues,45 but similar to other mouse studies,29 our group found that chronic intracerebroventricular injection of exendin 9 results in hyperphagia and weight gain in rats fed either standard chow or a high-fat diet.47 Combined with earlier studies using exendin 9, these data suggest that, at least in rats, central GLP-1 is necessary for normal energy balance. Furthermore, the weight gain that occurs in response to sustained central GLP1R blockade suggests a role in the regulation of long-term energy balance, which is discussed in more detail below.
Peripheral GLP-1 and energy balance
The putative role of central GLP-1 as a satiation signal was based largely on initial reports of robust anorexia following central administration versus little to no anorexia following peripheral administration of GLP-1.10–12 This hypothesis was supported by the knowledge that GCG and GLP1R are expressed in key areas of the CNS known to regulate food intake.8,48,49 In addition, the fact that circulating GLP-1 is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4) argued against activation of GLP1R in the CNS by peripheral GLP-1.50 Nonetheless, reports of marked anorexia in rats following peripheral administration of exendin 4 raised the possibility that peripheral GLP-1 may reduce food intake in part via activation of peripheral GLP1R.51,52 However, these findings were easily explained by the enhanced stability of exendin 4 in the circulation, as well as the reported ability of exendin 4 to cross the mouse blood–brain barrier.53,54 Thus, it remained possible that peripheral exendin 4 reduced food intake via direct activation of GLP1R in the CNS.
Baggio and colleagues reported that peripheral administration of albumin-conjugated GLP-1 and exendin 4, a modification that prevents these peptides from crossing the blood–brain barrier, significantly reduces food intake in mice,55,56 which suggests that these effects are not dependent upon direct activation of GLP1R in the CNS. However, these effects could be mediated via GLP1R located in brain circumventricular organs, such as the area postrema and subfornical organ.8,9 One study raises doubts about this explanation, as the investigators observed an intact anorectic response to intraperitoneal injection of exendin 4 in rats with lesions of either the area postrema, subfornical organ or both.57 Nonetheless, this finding should not be considered definitive, as the dose of intraperitoneal exendin 4 used in these studies may have been sufficiently high to activate central GLP1R and elicit enough anorexia to overshadow the putative contributions of GLP1R activation in circumventricular organs. At present, further studies are needed to clarify the role of circumventricular organs in mediating peripheral GLP-1-induced anorexia, for example, by site-specific infusion of GLP-1 and exendin 9.
Work by Williams and colleagues on rats treated with exendin 9 provides substantial evidence in favor of both a peripheral mechanism for GLP-1-induced anorexia as well as a role for endogenous peripheral GLP-1 in the regulation of food intake. Specifically, acute intraperitoneal administration of exendin 9 increases light-phase food intake, and pretreatment with intraperitoneal exendin 9 blocks anorexia in response to a nutrient preload, which indicates that nutrient-induced satiation is at least in part GLP-1-mediated.58 Moreover, blockade of central GLP1R with exendin 9 fails to attenuate peripheral GLP-1-induced anorexia,58 which suggests that this effect is indeed mediated by peripheral GLP1R. Finally, intraperitoneal injection of exendin 4 reduces meal size and duration and increases the interval between meals,58 and similar satiating effects in response to exogenous GLP1R agonism have been observed in nonhuman primates and humans.59,60 Taken together, these data support the hypothesis that peripheral GLP-1 acts as a satiation signal in a manner similar to that of CCK.
One aspect that warrants further consideration is the effect of intraperitoneal exendin 9 to increase food intake during the light phase. After all, given that nutrient intake in rats is modest during this period, nutrient-induced release of endogenous GLP-1 should be minimal as well. Even low rates of L-cell secretion may be sufficient to maintain satiety during anticipated long intervals between meals. Also possible, but less probable, are un expected effects of exendin 9, such as inverse agonism,61 an action demonstrated in cultured cells but not in vivo. The temporal influence of GLP-1 on food intake is undoubtedly complex, as exemplified by a report that rats habituated to restricted food access exhibit a preprandial increase in GLP-1 secretion.62 This preprandial GLP-1 spike is thought to represent an anticipatory response that facilitates preparation for a nutrient load. In support of this hypothesis, food intake is paradoxically decreased in these rats when given a GLP1R antagonist peripherally prior to the GLP-1 spike.62 Again, this finding underscores the notion that the effects of GLP-1 vary under different physiological conditions. Moreover, experiments in humans could be designed to correlate peripheral GLP-1 secretion to various meal patterns to test the hypothesis that dysregulated GLP-1 secretion contributes to the metabolic derangements observed in shift workers.
Like CCK, peripheral GLP-1 appears to reduce food intake via activation of vagal afferent neurons. In rats, GLP1R mRNA is expressed in cell bodies of the nodose ganglion,63,64 and intravenous infusion of high physiological concentrations of GLP-1 increases activity of the ventral gastric branch of the vagus nerve.65 In addition, bilateral subdiaphragmatic truncal vagotomy in rats and capsaicin pretreatment in mice significantly blunt the anorectic effects of peripherally administered GLP-1 and exendin 4, respectively.66,67 Interestingly, GLP-1 reduces meal size in rats similarly when administered into the hepatic portal vein, the inferior vena cava or the peritoneum, but only the effect of intraperitoneal GLP-1 was blunted by subdiaphragmatic vagal deafferentation.68 The investigators attribute this effect to inhibition of local intestinal vagal neurotransmission, and several lines of evidence indirectly support this hypothesis. First, intravascular infusions of GLP1R antagonists, either into the hepatic portal or jugular veins, have uniformly failed to increase food intake in rats,69,70 whereas intraperitoneal administration of exendin 9 significantly increases light-phase food intake and blocks nutrient-induced satiation. 58 Second, intraperitoneal exendin 4 activates both submucosal and myenteric neurons of the rat duodenum but, interestingly, not of the jejunum or ileum.71 Further experiments employing selective intestinal GLP1R blockade or elimination are warranted to pinpoint the critical GLP1R populations necessary for this effect. Nonetheless, the above findings reinforce the notion that a highly complex intraintestinal neural circuitry coordinates both the secretion and effects of peripheral GLP-1.
Overall, the majority of evidence strongly supports the hypothesis that peripheral GLP-1 acts as a physiologically relevant satiation signal via peripheral GLP1R activation and vagal neurotransmission. Moreover, the lack of effect of central GLP1R blockade on intra peritoneal GLP-1-induced anorexia suggests that this role takes place independent of central GLP1R activation by circulating ligands.58 Nonetheless, work by Hayes and colleagues provides evidence that central GLP-1 may indeed be implicated in meal-induced satiation. Specifically, blockade of hindbrain GLP1R in rats attenuates satiation induced by nutrient preload consumption or artificial gastric distension but not intraduodenal nutrient delivery.72 These data suggest a mechanism whereby nutrient consumption induces satiation via release of both peripheral and central GLP-1, both of which then act on local GLP1R to influence neurotransmission.
Despite the evidence in support of a role for both central and peripheral GLP1R in mediating meal-induced satiation, the precise roles of these receptor populations and their relationship to each other remain unknown. One possibility is a serial model whereby nutrient consumption triggers secretion of peripheral GLP-1, activation of intestinal GLP1R and induction of vagal afferent neurotransmission, which in turn triggers release of central GLP-1, activation of hindbrain GLP1R and alteration of efferent neurotransmission to elicit coordinated changes leading to decreased food intake. A second possibility is a parallel model whereby nutrients and gastric distension trigger peripheral and central GLP-1 release, respectively, both of which then activate local GLP1R, alter discrete neural circuitry and ultimately elicit specific changes (that is, decreased appetite and delayed gastric emptying) leading to decreased food intake.
Whether hindbrain GCG neurons are activated by peripheral GLP-1 must be clarified, as the only evidence to date that this occurs is an anecdotal report by Yamamoto and colleagues for which no explicit data has been provided. 73 Assuming this phenomenon is indeed real, it may occur via indirect versus direct stimulation, given the rapid inactivation of circulating GLP-1 by DPP-4 and the previously reported lack of GLP1R expression in mouse hindbrain GCG neurons.74 Gaykema and colleagues have provided evidence that noxious and nutritional stimuli activate distinct subsets of rat hindbrain GCG neurons,40 and a similar comparative approach could be applied to intraintestinal nutrients versus gastric distension. Finally, correlation of behavioral findings with emerging data from neuronal tracing studies has the potential to elucidate specific hind brain targets and downstream effectors of intestinal versus gastric vagal afferent nerves.
Short-term versus long-term control
Historically, GLP-1 has been hypothesized to regulate food intake only on a short-term meal-to-meal basis. Although this role was initially attributed to central GLP-1, evidence which demonstrates that peripheral GLP-1 acts as a gut-derived satiation signal similar to CCK ultimately supports a role for peripheral GLP-1 in the short-term regulation of energy balance. Nonetheless, peripheral GLP-1 may also interact with known regulators of long-term energy balance such as leptin, implicating it in both rapid and long-term regulation of energy balance.
One proposed mechanism by which leptin reduces food intake is by increasing sensitivity to peripheral satiation signals. In support of this hypothesis, intra peritoneal coinfusion of subthreshold doses of leptin and CCK— meaning doses that do not significantly reduce food intake when given individually—in mice synergistically reduces short-term food intake, and this effect is blocked by either capsaicin or the CCK-A receptor antagonist devazepide.75 A similar anorectic synergism has been observed with coinfusion of intra cerebroventricular leptin and intraperitoneal CCK in rats,76 and further studies have revealed leptin receptors in both the hypothalamic arcuate nucleus and the hindbrain dorsal vagal complex to be critical mediators of leptin-induced CCK sensitization in rats.77,78 Finally, chronic subcutaneous leptin replacement restores sensitivity to intraperitoneal CCK that is lost in fasted rats,79 which provides further evidence that the satiating effect of CCK is modulated by circulating leptin levels.
Williams and colleagues demonstrate a similar relation ship between leptin and peripheral GLP-1.80 First, intra peritoneal administration of set doses of GLP-1 reduces short-term food intake in wild-type but not obese, leptin-receptor-deficient Koletsky rats. Second, co infusion of subthreshold doses of intraperitoneal or intra cerebroventricular leptin with either intra peritoneal GLP-1 or exendin 4 synergize to reduce food intake and body weight. Third, the anorectic effects of both intraperitoneal GLP-1 and exendin 4 are significantly blunted by fasting, yet restored by chronic subcutaneous leptin replacement. Together, these data not only underscore the similarities between CCK and GLP-1 as peripheral satiaion signals but also indicate that peripheral GLP-1 indirectly contributes to long-term energy balance regulation via potentiation of its satiating effect by leptin.
Whereas reports on the above relationship between peripheral GLP-1 and leptin are relatively new, evidence of a relationship between central GLP-1 and leptin dates back to 1997, when Goldstone and colleagues reported the presence of leptin receptors on mouse hindbrain GCG neurons, as well as blockade of intracerebroventricular-leptin-induced anorexia in rats by intra cerebroventricular exendin 9.81 Shortly thereafter, the same group reported that intracerebroventricular injection of leptin prevents fasting-induced decreases in hindbrain GCG expression and hypothalamic GLP-1 content in rats.82 Taken together, these data suggest that leptin positively regulates central GLP-1 activity and that central GLP-1, among other neuropeptides, is a critical downstream mediator of leptin action. Further support for this hypothesis has come from the findings that rat hindbrain GCG neurons are activated by leptin. Moreover, leptin directly de polarizes mouse hindbrain GCG neurons.74,83
Nevertheless, although blockade of intra cerebroventricular leptin action with intra cerebroventricular exendin 9 has been replicated in rats,84 GLP1R−/− mice were found to have a normal anorectic response to exogenous leptin,85 which either argues against a critical role for central GLP-1 as a downstream mediator of leptin action or suggests developmental compensation. Further more, discrepancies between leptin effects on hindbrain GCG neurons in mice versus rats have been reported. Specifically, intraperitoneal leptin induces STAT3 phosphorylation in mouse but not rat hindbrain GCG neurons.86 In addition, intraperitoneal leptin prevents the fasting-induced decrease in hindbrain GCG expression in mice but not rats.86 Although these discrepancies raise the possibility of species differences in the relationship between leptin and central GLP-1, it is important to note that mice and rats were fasted for the same amount of time and sacrificed at the same time interval after intra peritoneal leptin administration in these studies. Thus, the observed species differences may in fact be secondary to differences in the relative impact of a 24 h fast and/or the time course of leptin action in mice versus rats.
Interestingly, the discrepancy in the effect of fasting on hindbrain GCG expression in mice versus rats suggests an alternative interpretation of the data. Because a 24 h fast probably results in more substantial weight loss in mice versus rats, perhaps hindbrain GCG expression is associated more with whole-body energy status than short-term fasting or feeding status. In fact, this hypothesis is supported by Vrang and colleagues,87 who have reported significantly increased hindbrain GCG expression in obese versus lean Zucker rats. Moreover, although not reported as a significant correlation, a positive association was noted between hindbrain GCG mRNA levels and body weight in lean Zucker rats who were either food-restricted, ad libitum fed or overfed.87 Finally, Primeaux and colleagues88 have reported a strikingly similar association between intestinal GCG expression and body weight in obesity-resistant S5B/Pl rats and in obesity-prone Osborne–Mendel rats maintained on either a low-fat or high-fat diet. Together, these data suggest that both hindbrain and intestinal GCG expression vary directly with body weight.
On the other hand, GCG expression may not so much be associated with body weight, but rather with body adiposity. Knauf and colleagues have reported increased hindbrain GCG expression in mice fed a high-fat, low-carbohydrate diet. Interestingly, these animals did not differ significantly in body weight from chow-fed controls. 45 However, body composition data was not reported; hence, consumption of a high-fat, low-carbohydrate diet may have resulted in greater body adipose tissue stores despite a lack of overall weight gain. Along the same lines, a significant positive correlation between hindbrain GCG expression and body weight in rats fed a high-fat diet has been observed, which becomes even stronger when comparing hindbrain GCG expression and body adiposity.47 Collectively, these data support the hypo thesis that expression of both hindbrain and intestinal GCG varies directly with the size of peripheral adipose tissue stores; interestingly, this relationship may exist in dependent of leptin.
GLP-1 in obesity and weight loss
The above evidence that expression of both central and peripheral GCG varies in direct proportion to stores of body adipose tissue raises the possibility that GLP-1 physiology is altered in obese states. However, little is known about the nature of these changes, as one can not interpret GCG gene expression levels as a surrogate for GLP-1 system activity. GLP1R−/− mice were first reported to exhibit no differences in either baseline food intake and body weight or hyperphagia and weight gain induced by high-fat diet compared with wild-type mice.42,43 How ever, these animals were subsequently reported to be hypermetabolic, exhibiting increased baseline food intake, energy expenditure and activity but again a largely normal response to a high-fat diet.44 This hyper metabolic phenotype has since been reproduced by Knauf and colleagues in a mouse model of chronic central GLP1R blockade using exendin 9, although in this experiment neither exendin 9 nor a high-fat diet resulted in increased body weight.45
Whereas current findings from mouse models collectively indicate little to no overall effect of GLP-1 loss of function on body weight or sensitivity to diet-induced obesity, rat models paint a very different picture. Meeran and colleagues41 reported sustained hyperphagia and weight gain in response to repeated daily injections of intracerebroventricular exendin 9, which suggests that central GLP1R activity tonically inhibits food intake and weight gain. These findings have been corroborated and extended by our group; hyperphagia, as well as increased weight gain and adiposity, was shown in response to chronic intracerebroventricular infusion of exendin 9.47 This anabolic response was not significantly different in rats fed standard chow or a high-fat diet, which suggests that central GLP1R activity is not altered by obesity or high-fat intake. However, Vrang and colleagues reported increased sensitivity to the hyperphagic effect of intracerebroventricular exendin 9 in obese versus lean Zucker rats,87 which suggests that obesity is associated with increased central GLP1R activity. A third possibility is that obesity is associated with ‘CNS GLP-1 resistance’, a hypothesis raised by the work of Al-Barazanji and colleagues, which suggests that the anorectic effect of centrally administered exendin 4 is diminished in obese versus lean Zucker rats.89 Finally, and perhaps most convincingly, obesity may be associated with impaired GLP-1 release, which has been demonstrated with respect to L cells in mice and is supported by several studies that report decreased plasma GLP-1 levels and GLP-1 secretion in humans with obesity.90–95
Speculation of a role for GLP-1 in the treatment of obesity has been ongoing since the initial discovery that central administration of GLP-1 reduces food intake in rats.10,11 Although initial studies employing chronic or repeated intracerebroventricular administration of GLP-1 in rats yielded conflicting results with respect to ongoing anorexia and weight loss,41,96 chronic studies using the long-acting GLP1R agonist exendin 4 have been quite promising. These studies have indicated not only potent and dose-dependent anorexia and weight loss across several animal models of obesity but also a reliable means to stimulate key GLP1R involved in energy balance regulation via the peripheral route.51,97,98 Similar properties have been demonstrated for the long-acting acylated GLP-1 molecule liraglutide in rats,99 yet by contrast, DPP-4 inhibitors have thus far elicited conflicting effects on food intake and body weight in animal studies.98,99
With increased knowledge of the changes in GLP-1 physiology in response to bariatric surgery as well as ongoing loss-of-function studies in humans using exendin 9, direct evidence of a role for endogenous GLP-1 in the regulation of energy balance in humans may not be lacking for much longer. Gain-of-function studies have generally indicated that intravenous infusion of GLP-1 reduces food intake and promotes satiety.100–106 A meta-analysis of these studies revealed that these effects were dose-dependent, not significantly different in lean individuals versus patients with obesity and not associated with adverse scores of well-being or food palatability.107 Since then, clinical trials of exenatide and liraglutide have revealed that these compounds produce small but significant and dose-dependent reductions in body weight over time.108–112 The most prominent adverse effects were nausea and vomiting, which were dose-dependent, generally transient and, interestingly, not related to the degree of weight loss. DPP-4 inhibitors, on the other hand, have thus far not been shown to cause weight change in clinical trials.113–117
Modeling GLP-1 physiology
Collectively, the current data support a model (Figure 1) whereby peripheral and central GLP-1 combine to regulate both short-term and long-term energy balance. Within a given meal, intraluminal nutrients stimulate intestinal L cells to secrete GLP-1, which acts locally via GLP1R expressed on intestinal vagal afferent nerve terminals to initiate feedback to key hindbrain nuclei such as the caudal nucleus of the solitary tract. These hindbrain nuclei integrate a variety of meal-related signals, including those from peripheral GLP-1 and gastric distension and, in turn, modulate efferent neurotransmission to promote satiety. Hindbrain GCG neurons and GLP1R participate in this feedback loop, perhaps specifically as mediators of gastric distension-induced satiety, yet they may also integrate signals initiated by peripheral GLP-1. In addition, leptin augments the satiating effects of both peripheral and central GLP-1, perhaps via both direct stimulation of GLP-1 release and indirect modulation of GLP-1-responsive neurons.
Figure 1.
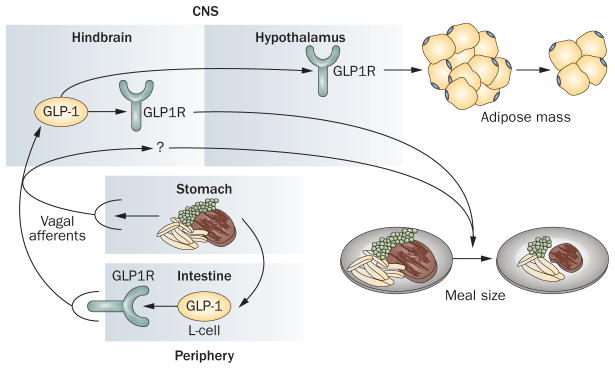
Model of GLP-1 physiology. Nutrient ingestion stimulates intestinal L-cell secretion of glucagon-like peptide 1 (GLP-1), which activates local GLP-1 receptors (GLP1R) to initiate afferent vagal neurotransmission to the hindbrain. These vagal afferent signals, combined with those initiated by gastric distension, may activate hindbrain GLP-1-producing neurons, which in turn activate hindbrain GLP1R, as well as other hindbrain neurons to produce efferent signals leading to decreased meal size. In addition, signaling from hindbrain GLP-1-producing neurons to hypothalamic targets promotes decreased fat mass.
Whereas both peripheral and central GLP-1 regulate short-term energy balance via a gut–hindbrain feedback loop, central GLP-1 also regulates long-term energy balance, as evidenced by loss-of-function studies of GCG-expressing neurons in the caudal nucleus of the solitary tract and central GLP1R activity. Although a neuroanatomical model for such regulation is admittedly speculative, we propose that it involves ascending projections of hindbrain GLP-1 neurons to key hypothalamic nuclei such as the paraventricular nucleus. In addition, central GLP-1 serves as a downstream mediator of central leptin action, which is potentially one mechanism by which central GLP-1 regulates total body adiposity. Finally, we propose that central GLP-1 activity is altered in obesity and is characterized by impaired GLP-1 release. This hypothesis is consistent not only with data that demonstrate decreased circulating GLP-1 levels in humans with obesity but also with the fact that GLP1R agonists retain their efficacy in these individuals.
The above model provides an intriguing framework for consideration of the differential effects of GLP1R agonists and DPP-4 inhibitors on weight. Specifically, the ability of the GLP1R agonists exenatide and liraglutide to cause weight loss in humans may be underscored by their enhanced ability to engage the central GLP-1 system. Such engagement may involve direct central GLP1R activation, which is possible with exenatide given that it has been shown to cross the blood–brain barrier in mice.54 In addition, these compounds may indirectly engage the central GLP-1 system via sustained activation of intestinal vagal afferent neurons that express GLP1R. By contrast, DPP-4 inhibitors may fail to cause weight loss because their effects are largely limited to potentiation of peripheral GLP-1 signaling. Whereas DPP-4 inhibition may augment the satiating effect of peripheral GLP-1, it may also lead to compensatory changes in meal patterns that limit its ability to elicit weight loss, as has been shown with repeated administration of CCK. In addition, DPP-4 inhibition alters activity of a variety of gut hormones, including PYY3–36,118 and these combined effects may ultimately neutralize each other.
In order to better understand the mechanisms that underlie GLP1R agonist-induced weight loss in humans, as well as maximize the therapeutic potential of these compounds, several lines of investigation are warranted. First, direct comparisons of the anorectic effects of exenatide and liraglutide are necessary to establish relative dose–response curves. Based on the results of the LEAD-6 study, it appears that liraglutide 1.8 mg once daily and exenatide 10 μg twice daily produce comparable weight loss in patients.119 One ongoing controversy, however, is the extent to which the increased potency of exenatide over GLP-1 is secondary to greater plasma stability versus differential GLP1R activation.120 By use of animal models, comparison of peripheral administration of these compounds has the potential to address the possibility that the increased potency of exenatide is secondary to greater plasma stability, whereas comparison of central administration of these compounds may clarify whether they activate GLP1R in different ways. Moreover, direct comparison of neuronal activation in response to peripheral exenatide and liraglutide may clarify the extent to which each compound engages central GLP-1-dependent neural circuitry.
Finally, site-specific elimination of GLP-1 and/or its receptor using either Cre-loxP or viral-mediated RNA interference technology has the most potential to elucidate which components of the GLP-1 system are truly critical to mediating weight loss, particularly in response to bariatric surgery. In fact, one could predict that increased direct nutrient stimulation of intestinal GLP-1-producing neurons in patients with Roux-en-Y gastric bypass leads to increased GLP-1 secretion. This process, in turn, leads to increased satiety either via higher circulating GLP-1 levels or enhancement of the gut–brain feedback loop initiated by GLP1R on vagal afferent neurons, if these connections remain intact after surgery. In addition, if the function of central GLP-1 is indeed impaired in obesity, it is possible that weight loss due to caloric restriction in patients who have undergone bariatric surgery improves central GLP-1 activity, further contributing to a decreased set point for adipose tissue mass. On the other hand, if central GLP-1 activity is promoted by leptin, the levels of which could fall in response to bariatric surgery, central GLP-1 activity may decrease, which would suggest that it is not a critical mediator of bariatric surgery-induced weight loss.
Conclusions
Our knowledge of the physiology of GLP-1 as well as its therapeutic relevance has grown rapidly over the past decade. First and foremost, this statement applies to our knowledge of the role of GLP-1 in regulating peripheral glucose homeostasis, which has ultimately led to the FDA approval of several GLP-1-based therapies for type 2 diabetes mellitus, as well as intriguing hypotheses regarding the role of GLP in diabetes improvement following bariatric surgery. Perhaps equally impressive, however, is the evolution of our understanding of the role of GLP-1 in the regulation of energy balance, which has taken us well beyond its initially proposed role as a ‘central satiety factor’. In light of the well-established, albeit modest, beneficial effects of exenatide and liraglutide on weight loss in humans, it is critical that we continue to seek a better understanding of GLP-1 physiology as it relates to food intake and body weight. Moreover, it will be interesting to observe the results of loss-of-function studies designed to test the hypothesis that enhanced GLP-1 activity in part mediates weight loss following bariatric surgery. Such information, combined with detailed pharmacological analyses and comparisons of the weight-reducing effects of GLP-1 analogues in both animals and humans, has the potential to maximize our ability to apply GLP-1-based therapies to the treatment of obesity.
Key points.
Animal studies suggest that endogenous, central glucagon-like peptide 1 (GLP-1) regulates both short-term and long-term energy balance, potentially via activation of hindbrain and hypothalamic GLP-1 receptors (GLP1R), respectively
Endogenous peripheral GLP-1 may limit meal size by activation of GLP1R on local vagal afferent nerves and stimulation of a gut–brain feedback loop
Increasing GLP1R activity by administering GLP-1 receptor agonists reduces food intake and promotes weight loss in humans, but the extent to which endogenous GLP-1 regulates energy balance in humans remains unknown
Although diabetes mellitus and obesity appear to be associated with altered GLP-1 system activity, GLP1R agonists retain their efficacy in these contexts, making them a viable therapeutic tool
A better understanding of how endogenous central and peripheral GLP-1 regulate energy balance has the potential to maximize our application of GLP-1-based therapies for the treatment of obesity
Review criteria.
Articles cited in this Review were found either by searching PubMed or referring to the reference lists of selected articles. The most commonly used search terms, used both alone and in conjunction with various modifiers, were “GLP-1”, “glucagon-like peptide-1” and “exendin”. No restrictions were placed on year of publication or language; however, all cited articles and their corresponding abstracts were in English. In most cases, full-text online or PDF versions of articles were available for viewing or download.
Footnotes
Competing interests
D. A. Sandoval declares an association with the following company: Ethicon. D. A. D’Alessio declares an association with the following companies: Amylin, Johnson and Johnson, MannKind, Merck, Novo Nordisk, Sanofi-Aventis, Takeda. R. J. Seeley declares an association with the following companies: Alkermes, Amylin, Eli Lilly, Johnson and Johnson, MannKind, Merck, Novo Nordisk, Roche, Zafgen. See the article online for details of the relationships. J. G. Barrera declares no competing interests.
Author contributions
All authors researched the data for the article, provided a substantial contribution to discussions of the content, contributed equally to writing the article and reviewed and/or edited the manuscript before submission.
References
- 1.Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- 2.Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog Horm Res. 2004;59:267–285. doi: 10.1210/rp.59.1.267. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 4.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 5.Parker HE, Reimann F, Gribble FM. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert Rev Mol Med. 2010;12:e1. doi: 10.1017/S146239940900132X. [DOI] [PubMed] [Google Scholar]
- 6.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med. 2010;123:S28–S37. doi: 10.1016/j.amjmed.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19:217–229. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–2300. doi: 10.1111/j.1460-9568.1995.tb00650.x. [DOI] [PubMed] [Google Scholar]
- 10.Tang-Christensen M, et al. Central administration of GLP-1-(7--36) amide inhibits food and water intake in rats. Am J Physiol. 1996;271:R848–R856. doi: 10.1152/ajpregu.1996.271.4.R848. [DOI] [PubMed] [Google Scholar]
- 11.Turton MD, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 12.Navarro M, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem. 1996;67:1982–1991. doi: 10.1046/j.1471-4159.1996.67051982.x. [DOI] [PubMed] [Google Scholar]
- 13.Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–R590. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- 14.Vrang N, Phifer CB, Corkern MM, Berthoud HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol. 2003;285:R470–R478. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 15.Rinaman L, Comer J. Antagonism of central glucagon-like peptide-1 receptors enhances lipopolysaccharide-induced fever. Auton Neurosci. 2000;85:98–101. doi: 10.1016/S1566-0702(00)00227-7. [DOI] [PubMed] [Google Scholar]
- 16.Seeley RJ, et al. The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–1621. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grill HJ, Carmody JS, Amanda Sadacca L, Williams DL, Kaplan JM. Attenuation of lipopolysaccharide anorexia by antagonism of caudal brain stem but not forebrain GLP-1-R. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1190–R1193. doi: 10.1152/ajpregu.00163.2004. [DOI] [PubMed] [Google Scholar]
- 18.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–R29. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 19.McMahon LR, Wellman PJ. Decreased intake of a liquid diet in nonfood-deprived rats following intra-PVN injections of GLP-1 (7–36) amide. Pharmacol Biochem Behav. 1997;58:673–677. doi: 10.1016/s0091-3057(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 20.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 21.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–4455. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- 24.Kinzig KP, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–6170. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–10476. doi: 10.1523/JNEUROSCI.22-23-10470.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA. 2009;106:5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto H, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakade Y, Tsukamoto K, Pappas TN, Takahashi T. Central glucagon like peptide-1 delays solid gastric emptying via central CRF and peripheral sympathetic pathway in rats. Brain Res. 2006;1111:117–121. doi: 10.1016/j.brainres.2006.06.090. [DOI] [PubMed] [Google Scholar]
- 29.Nogueiras R, et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. J Neurosci. 2009;29:5916–5925. doi: 10.1523/JNEUROSCI.5977-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pérez-Tilve D, et al. Exendin-4 increases blood glucose levels acutely in rats by activation of the sympathetic nervous system. Am J Physiol Endocrinol Metab. 2010;298:E1088–E1096. doi: 10.1152/ajpendo.00464.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Wan S, Browning KN, Travagli RA. Glucagon-like peptide-1 modulates synaptic transmission to identified pancreas-projecting vagal motoneurons. Peptides. 2007;28:2184–2191. doi: 10.1016/j.peptides.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Wan S, Coleman FH, Travagli RA. Glucagon-like peptide-1 excites pancreas-projecting preganglionic vagal motoneurons. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1474–G1482. doi: 10.1152/ajpgi.00562.2006. [DOI] [PubMed] [Google Scholar]
- 34.Knauf C, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–3563. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knauf C, et al. Role of central nervous system glucagon-like Peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57:2603–2612. doi: 10.2337/db07-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cabou C, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes. 2008;57:2577–2587. doi: 10.2337/db08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 39.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007;1149:118–126. doi: 10.1016/j.brainres.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 40.Gaykema RP, et al. Immune challenge and satiety-related activation of both distinct and overlapping neuronal populations in the brainstem indicate parallel pathways for viscerosensory signaling. Brain Res. 2009;1294:61–79. doi: 10.1016/j.brainres.2009.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeran K, et al. Repeated intracerebroventricular administration of glucagon-like peptide-1-(7–36) amide or exendin-(9–39) alters body weight in the rat. Endocrinology. 1999;140:244–250. doi: 10.1210/endo.140.1.6421. [DOI] [PubMed] [Google Scholar]
- 42.Scrocchi LA, et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med. 1996;2:1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 43.Scrocchi LA, Drucker DJ. Effects of aging and a high fat diet on body weight and glucose tolerance in glucagon-like peptide-1 receptor −/− mice. Endocrinology. 1998;139:3127–3132. doi: 10.1210/endo.139.7.6092. [DOI] [PubMed] [Google Scholar]
- 44.Hansotia T, et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007;117:143–152. doi: 10.1172/JCI25483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Knauf C, et al. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology. 2008;149:4768–4777. doi: 10.1210/en.2008-0180. [DOI] [PubMed] [Google Scholar]
- 46.Lachey JL, et al. The role of central glucagon-like peptide-1 in mediating the effects of visceral illness: differential effects in rats and mice. Endocrinology. 2005;146:458–462. doi: 10.1210/en.2004-0419. [DOI] [PubMed] [Google Scholar]
- 47.Barrera JG, et al. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like Peptide-1 loss of function. J Neurosci. 2011;31:3904–3913. doi: 10.1523/JNEUROSCI.2212-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shughrue PJ, Lane MV, Merchenthaler I. Glucagon-like peptide-1 receptor (GLP1-R) mRNA in the rat hypothalamus. Endocrinology. 1996;137:5159–5162. doi: 10.1210/endo.137.11.8895391. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu I, Hirota M, Ohboshi C, Shima K. Identification and localization of glucagon-like peptide-1 and its receptor in the brain. Endocrinology. 1987;121:1076–1082. doi: 10.1210/endo-121-3-1076. [DOI] [PubMed] [Google Scholar]
- 50.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36) amide, petide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 51.Rodriquez de Fonseca F, et al. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism. 2000;49:709–717. doi: 10.1053/meta.2000.6251. [DOI] [PubMed] [Google Scholar]
- 52.Szayna M, et al. Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology. 2000;141:1936–1941. doi: 10.1210/endo.141.6.7490. [DOI] [PubMed] [Google Scholar]
- 53.Thum A, et al. Endoproteolysis by isolated membrane peptidases reveal metabolic stability of glucagon-like peptide-1 analogs, exendins-3 and -4. Exp Clin Endocrinol Diabetes. 2002;110:113–118. doi: 10.1055/s-2002-29087. [DOI] [PubMed] [Google Scholar]
- 54.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 55.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–2500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 56.Baggio LL, Huang Q, Cao X, Drucker DJ. An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology. 2008;134:1137–1147. doi: 10.1053/j.gastro.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Baraboi ED, Smith P, Ferguson AV, Richard D. Lesions of area postrema and subfornical organ alter exendin-4-induced brain activation without preventing the hypophagic effect of the GLP-1 receptor agonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1098–R1110. doi: 10.1152/ajpregu.00326.2009. [DOI] [PubMed] [Google Scholar]
- 58.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology. 2009;150:1680–1687. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293:R983–R987. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 60.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Serre V, et al. Exendin-(9–39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′, 5′-monophosphate levels and β-cell glucose competence. Endocrinology. 1998;139:4448–4454. doi: 10.1210/endo.139.11.6295. [DOI] [PubMed] [Google Scholar]
- 62.Vahl TP, Drazen DL, Seeley RJ, D’Alessio DA, Woods SC. Meal-anticipatory glucagon-like peptide-1 secretion in rats. Endocrinology. 2009;151:569–575. doi: 10.1210/en.2009-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakagawa A, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 64.Vahl TP, et al. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology. 2007;148:4965–4973. doi: 10.1210/en.2006-0153. [DOI] [PubMed] [Google Scholar]
- 65.Bucinskaite V, et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil. 2009;21:978–e78. doi: 10.1111/j.1365-2982.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- 66.Abbott CR, et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology. 2005;146:3748–3756. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 68.Rüttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150:1174–1181. doi: 10.1210/en.2008-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim DH, D’Alessio DA, Woods SC, Seeley RJ. The effects of GLP-1 infusion in the hepatic portal region on food intake. Regul Pept. 2009;155:110–114. doi: 10.1016/j.regpep.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rüttimann EB, Arnold M, Geary N, Langhans W. GLP-1 antagonism with exendin (9–39) fails to increase spontaneous meal size in rats. Physiol Behav. 2009;100:291–296. doi: 10.1016/j.physbeh.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 71.Washington MC, Raboin SJ, Thompson W, Larsen CJ, Sayegh AI. Exenatide reduces food intake and activates the enteric nervous system of the gastrointestinal tract and the dorsal vagal complex of the hindbrain in the rat by a GLP-1 receptor. Brain Res. 2010;1344:124–133. doi: 10.1016/j.brainres.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 72.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 2009;150:2654–2659. doi: 10.1210/en.2008-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yamamoto H, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–2946. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like peptide 1 neurons. Diabetes. 2010;59:1890–1898. doi: 10.2337/db10-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol. 1999;276 (Pt 2):R1545–R1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 77.Morton GJ, et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest. 2005;115:703–710. doi: 10.1172/JCI200522081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams DL, Baskin DG, Schwartz MW. Hindbrain leptin receptor stimulation enhances the anorexic response to cholecystokinin. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1238–R1246. doi: 10.1152/ajpregu.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology. 2000;141:4442–4448. doi: 10.1210/endo.141.12.7815. [DOI] [PubMed] [Google Scholar]
- 80.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 81.Goldstone AP, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett. 1997;415:134–138. doi: 10.1016/s0014-5793(97)01103-4. [DOI] [PubMed] [Google Scholar]
- 82.Goldstone AP, et al. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-proglucagon mRNA. Biochem Biophys Res Commun. 2000;269:331–335. doi: 10.1006/bbrc.2000.2288. [DOI] [PubMed] [Google Scholar]
- 83.Elias CF, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 84.Nowak A, Bojanowska E. Effects of peripheral or central GLP-1 receptor blockade on leptin-induced suppression of appetite. J Physiol Pharmacol. 2008;59:501–510. [PubMed] [Google Scholar]
- 85.Scrocchi LA, Brown TJ, Drucker DJ. Leptin sensitivity in nonobese glucagon-like peptide I receptor −/− mice. Diabetes. 1997;46:2029–2034. doi: 10.2337/diab.46.12.2029. [DOI] [PubMed] [Google Scholar]
- 86.Huo L, Gamber KM, Grill HJ, Bjørbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology. 2008;149:492–497. doi: 10.1210/en.2007-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vrang N, et al. Upregulation of the brainstem preproglucagon system in the obese Zucker rat. Brain Res. 2008;1187:116–124. doi: 10.1016/j.brainres.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 88.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes (Lond) 2010;34:1427–1433. doi: 10.1038/ijo.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Barazanji KA, Arch JR, Buckingham RE, Tadayyon M. Central exendin-4 infusion reduces body weight without altering plasma leptin in (fa/fa) Zucker rats. Obes Res. 2000;8:317–323. doi: 10.1038/oby.2000.38. [DOI] [PubMed] [Google Scholar]
- 90.Ranganath LR, et al. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. 1996;38:916–919. doi: 10.1136/gut.38.6.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613. doi: 10.2337/diabetes.50.3.609. [DOI] [PubMed] [Google Scholar]
- 92.Vilsbøll T, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706–2713. doi: 10.1210/jc.2002-021873. [DOI] [PubMed] [Google Scholar]
- 93.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 94.Muscelli E, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–1348. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 95.Carr RD, et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 96.Donahey JC, van Dijk G, Woods SC, Seeley RJ. Intraventricular GLP-1 reduces short-but not long-term food intake or body weight in lean and obese rats. Brain Res. 1998;779:75–83. doi: 10.1016/s0006-8993(97)01057-3. [DOI] [PubMed] [Google Scholar]
- 97.Mack CM, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–1340. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 98.Lamont BJ, Drucker DJ. Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes. 2008;57:190–198. doi: 10.2337/db07-1202. [DOI] [PubMed] [Google Scholar]
- 99.Raun K, et al. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 100.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Näslund E, Gutniak M, Skogar S, Rössner S, Hellström PM. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 102.Näslund E, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 103.Long SJ, et al. No effect of glucagon-like peptide-1 on short-term satiety and energy intake in man. Br J Nutr. 1999;81:273–279. [PubMed] [Google Scholar]
- 104.Gutzwiller JP, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. doi: 10.1136/gut.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gutzwiller JP, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276 (Pt 2):R1541–R1544. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 106.Flint A, Raben A, Ersbøll AK, Holst JJ, Astrup A. The effect of physiological levels of glucagon-like peptide-1 on appetite, gastric emptying, energy and substrate metabolism in obesity. Int J Obes Relat Metab Disord. 2001;25:781–792. doi: 10.1038/sj.ijo.0801627. [DOI] [PubMed] [Google Scholar]
- 107.Verdich C, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 108.Buse JB, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 109.Kendall DM, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 110.DeFronzo RA, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 111.Vilsboll T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 112.Astrup A, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 113.Raz I, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 114.Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428. doi: 10.1055/s-2006-944546. [DOI] [PubMed] [Google Scholar]
- 115.Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–386. doi: 10.1111/j.1463-1326.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 116.DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315–2317. doi: 10.2337/dc08-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taskinen MR, et al. Safety and efficacy of linagliptin as add-on therapy to metformin in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab. 2011;13:65–74. doi: 10.1111/j.1463-1326.2010.01326.x. [DOI] [PubMed] [Google Scholar]
- 118.Aaboe K, et al. Twelve weeks treatment with the DPP-4 inhibitor, sitagliptin, prevents degradation of peptide YY and improves glucose and non-glucose induced insulin secretion in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2010;12:323–333. doi: 10.1111/j.1463-1326.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 119.Buse JB, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 120.Barrera JG, D’Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes. 2009;58:2820–2827. doi: 10.2337/db09-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]


