Abstract
The intense arousal and excitement shown by adult male chimpanzees, Pan troglodytes, during territorial attacks on other chimpanzees and predation upon monkeys suggest that similar psychological mechanisms may be involved. Specifically, it has been proposed that hunting behaviour in chimpanzees evolved from intraspecies aggression. Over 32 years, chimpanzees at Gombe National Park, Tanzania were significantly more likely to engage in a territorial border patrol on days when they hunted red colobus monkeys (Procolobus spp.), and vice versa, even after statistically controlling for male chimpanzee party size. We test the hypothesis that this correlation arises because hunting and patrolling are components of a specieslevel aggressive behavioural syndrome; specifically that predation arose as a by-product of territorial aggression in this species. However, hunting was equally likely to occur after a patrol and/or an intergroup interaction as it was before, and the occurrence of an intergroup interaction in which the chimpanzees approached strangers did not increase subsequent hunting probability. We also reject the hypothesis that hunting and patrolling reflect an individual-level behavioural syndrome. We identified two ‘impact hunters’ whose presence increased hunting probability. Similarly, there were also three ‘impact patrollers’, who increased the likelihood that a visit to the periphery of the community range resulted in a patrol. While this discovery has important implications for our understanding of the proximate causes of cooperation, it does not explain the temporal correlation between patrolling and hunting, since no males had such an impact in both contexts. Instead, the data suggest that the correlation arose because patrols typically involved males travelling long distances, which increased the probability of encountering prey. Additionally, parties that travelled to the periphery were more likely to encounter colobus in woodland, where hunts are more likely to occur and to succeed. Therefore, we conclude that ecological, rather than psychological, factors promote the co-occurrence of hunting and territorial aggression in this species.
Keywords: behavioural syndrome, chimpanzee, collective action, cooperation, hunting, impact males, lethal aggression, Pan troglodytes, territoriality
Male chimpanzees, Pan troglodytes, collectively defend group territories by conducting boundary patrols, advertising territory ownership with vocalizations and aggressively repelling members of other groups, sometimes injuring or killing them (Wrangham 1999; Wilson & Wrangham 2003; Watts et al. 2006; Boesch et al. 2008). Male chimpanzees also engage in group hunts of monkeys, particularly red colobus monkeys, Procolobus spp. (reviewed by Gilby 2012), which involve many of the same behavioural elements as aggression against conspecifics. The intense arousal and excitement shown during attacks on both chimpanzees and monkeys has prompted the suggestion that similar physiological and psychological mechanisms may be involved in predation and intergroup aggression (Goodall et al. 1979; van Hooff 1990; Wrangham & Peterson 1996; Wrangham 1999; Watts & Mitani 2001). Specifically, predation by chimpanzees may have evolved as a by-product of selection for intraspecific territorial aggression (Kortlandt 1972; Eibl-Eibesfeldt 1975; Wrangham 1999). This idea contrasts with the finding that distinct mechanisms underlie predation and intraspecific aggression in other taxa (e.g. rodents: Parmigiani & Palanza 1991; Wersinger et al. 2007; but see Siegel & Victoroff 2009).
We used long-term data on wild chimpanzees in the Kasekela community in Gombe National Park, Tanzania to test the hypothesis that predation and territoriality are components of an ‘aggressive’ behavioural syndrome in this species. A behavioural syndrome is a suite of similar traits that evolved in concert due to shared genetic or epigenetic mechanisms (Sih et al. 2004a, b). In addition to explaining interindividual behavioural variation (personality), a behavioural syndrome may also account for species-level differences (Sih & Bell 2008). For example, Thierry et al. (2008) found that several traits associated with conflict resolution existed as ‘an integrated suite of characters’ across nine macaque (Macaca) species.
Chimpanzees hunt red colobus monkeys wherever the two species are sympatric (Uehara 1997; Mitani 2009). At all sites where predation by chimpanzees has been studied in detail, the probability of a hunt occurring is positively correlated with the number of adult male chimpanzees present in the subgroup that encounters red colobus monkeys (Stanford et al. 1994a; Boesch & Boesch-Achermann 2000; Hosaka et al. 2001; Mitani & Watts 2001; Gilby et al. 2006; Gilby & Wrangham 2007). Thus, while chimpanzees do sometimes hunt alone (Gilby et al. 2006, 2008), hunting more often involves several individuals. While there is debate over the degree to which hunters coordinate their actions (Boesch 1994; Gilby & Connor 2010), the probability of a kill is positively correlated with the number of male chimpanzees present at a hunt (Mitani & Watts 2001; Gilby et al. 2006, 2008). During a hunt, males exhibit signs of great excitement, including piloerection, loud vocalizations, grimaces and embraces (Goodall 1986). When a chimpanzee captures a large monkey that fights back (posing a threat to its captor), the hunter typically bites, pounds and drags the victim until it is incapacitated. Goodall (1986, page 334) describes such behaviour as ‘retaliatory aggression’, noting similarities with attacks on chimpanzees from neighbouring communities (Goodall 1986, pp. 529–530).
Male chimpanzees jointly defend group territories (Wilson & Wrangham 2003; Boesch et al. 2008; Mitani 2009), by advertising territory ownership with vocalizations and by attempting to repel or kill any strangers that they encounter (except for reproductively active females, especially those without infants). Encounters with neighbours (‘intergroup encounters’ hereafter) occur most often in boundary areas (Wilson et al. 2012) and may include lethal aggression, which can account for a substantial proportion of total mortality (e.g. 9.3% at Gombe, 3.8% at Mahale Mountains National Park, Tanzania; Wilson 2013). Similar to hunting red colobus monkeys, the outcome of an intergroup encounter depends on the number of participants; parties with more males are more likely to call in response to vocalizations from simulated (Wilson et al. 2001) and real (Wilson et al. 2012) intruders. During an intergroup encounter in which the numerical odds are favourable or even, males behave in much the same way as they do during hunts of red colobus monkeys (e.g. bristling, embracing and vocalizing loudly). Killings appear most likely to occur when one side has an over-whelming numerical advantage (Manson & Wrangham 1991), reducing the risk of injury for attackers (Manson & Wrangham 1991). Like large red colobus monkey prey, chimpanzee victims are dragged, pummelled and bitten until incapacitated. The aggressors twist limbs and tear flesh, behaviours that are typically not seen during intracommunity aggression (Goodall 1986, page 529).
Given the importance of numerical odds in intergroup encounters, chimpanzees are more likely to visit the periphery when in parties with more males (Wilson et al. 2007, 2012). Visits to the periphery may include boundary patrols (Goodall et al. 1979; Watts & Mitani 2001), in which males spend more time travelling and less time feeding than usual (Amsler 2010), apparently searching for neighbours to attack. Therefore, we treat such patrols as examples of intraspecies aggression; by joining a patrol, each participant is committing to a potential conflict with hostile conspecifics.
Understanding the relationship between hunting and intergroup aggression has important implications for studies of aggression in general. For example, some argue that the considerable psychological and developmental differences between chimpanzees and their closest genetic relative, the bonobo, Pan paniscus, are due to a physiological link between aggressive and predatory behaviour. Bonobos exhibit considerably lower rates of both between-group aggression and hunting than chimpanzees (Surbeck & Hohmann 2008; Surbeck et al. 2009; Hare et al. 2012), a difference that has been proposed to result either from selection against within-group aggression in the bonobo lineage, with an associated (but unselected) reduction in between-group aggression (Wrangham & Peterson 1996; Hare et al. 2012), or from selection specifically against lethal raiding due to larger, more stable parties in bonobos (Wrangham 1999). This has been proposed to explain the considerable psychological (Hare et al. 2007), physiological (Wobber et al. 2010) and morphological (Wrangham & Pilbeam 2001) differences between the two species. To evaluate this proposal, we must understand to what extent, and why, hunting and intergroup aggression are related in these species.
We begin by demonstrating that among the Gombe chimpanzees, hunting and territoriality are temporally related: over 32 years, hunts were more likely to occur on days with patrolling and vice versa. We then test the hypothesis that this correlation can be explained by a behavioural syndrome. However, we find that extrinsic, ecological factors explain the co-occurrence of hunting and patrolling in this population.
Hypotheses and Predictions
H1: species-level behavioural syndrome
Several investigators have argued that hunting by chimpanzees is part of a species-wide behavioural syndrome, in which hunting emerged as a by-product of selection for other traits (Kortlandt 1972; Eibl-Eibesfeldt 1975; Goodall et al. 1979; van Hooff 1990; Wrangham 1999). Kortlandt (1972) suggested that hunting is a redirection of intraspecies aggression towards another species. More recently, Wrangham (1999) hypothesized that communal predation by chimpanzees evolved as a by-product of intraspecific coalitionary killing. For example, increases in testosterone associated with aggression (Muller & Wrangham 2004) may lead to an increase in hunting. Accordingly, males should be ‘primed’ to kill monkeys after patrolling or encountering hostile conspecifics. This predicts that, upon encountering red colobus monkeys, chimpanzees will be more likely to hunt them (1) after a patrol and/or an intergroup interaction than before such events, and (2) after an intergroup interaction in which they approached the strangers than after an intergroup interaction in which they did not approach the strangers (Table 1).
Table 1.
Summary of hypotheses and predictions
| Hypothesis | Logic | Predictions | Supported? | |
|---|---|---|---|---|
|
Behavioural
syndrome |
H1: Species-level | Hunting is a redirection of intraspecies aggression |
Hunting will be more likely: (1) after a patrol or an intergroup interaction than before |
No |
| (2) after an intergroup interaction in which chimpanzees approach strangers |
No | |||
| H2: Individual-level | Certain ‘impact’ males act as catalysts for both hunting and patrolling |
(1) Hunting will be more likely when particular males are present |
Yes | |
| (2) Patrolling will be more likely when particular males are present |
Yes | |||
| (3) The same individuals will be impact males in both contexts |
No | |||
|
Extrinsic
factors |
H3: Many males | Hunting and patrolling both require many adult males to be effective, but the proximate causes are different |
Hunting and patrolling will be driven solely by variation in male chimpanzee party size |
No |
| H4: Distance/location | Patrolling increases the probability of encountering (vulnerable) colobus monkeys |
(1) Likelihood of patrolling/periphery visit increases with distance travelled |
Yes | |
| (2) Probability of encountering colobus increases with travel distance |
Yes | |||
| (3) Likelihood of encountering colobus in woodland increases with travel distance |
Yes | |||
| (4) Hunting probability higher at range periphery |
No | |||
| (5) Hunting success higher at range periphery |
Yes |
H2: individual-level behavioural syndrome
If hunting and intergroup aggression share underlying physiological or psychological mechanisms, this should be evident in the behaviour of individuals. Some individuals appear particularly motivated to hunt, raising the possibility that such individuals may also be inclined to participate in intergroup aggression. Moreover, such highly motivated individuals may play a catalytic role in the occurrence of group-level predation and aggression. For example, at Kanyawara (Kibale National Park, Uganda), the presence and behaviour of two particular chimpanzees affected hunting probability (Gilby et al. 2008). Upon encountering prey, a chimpanzee party almost never hunted unless one or both of these males (AJ or MS) were present. When at least one of them was present, other adult males did not hunt unless either AJ or MS did. At Ngogo (Kibale National Park, Uganda), MO was usually one of the first male chimpanzees to hunt, apparently prompting others to follow (D. P. Watts, personal communication). Boesch & Boesch (1989) attributed an increase in group hunting success at Taï National Park (Côte d’Ivoire) to the maturation of one particularly persistent hunter. Gilby et al. (2008) and Gilby & Connor (2010) proposed that such ‘impact’ hunters have a catalytic effect on other potential hunters via a simple by-product mutualism: the actions of particularly motivated individuals create opportunities for others to hunt in circumstances when they would normally refrain. For example, an ‘average’ hunter might be initially wary of being the sole target of male red colobus defenders. However, once a hunt is in progress, red colobus defences must be spread among the hunters, thus reducing the costs for each individual hunter.
As with hunting, the individual costs of patrolling appear to decrease as the number of males increases. If an aggressive encounter occurs, a given male is less likely to be injured if he has several companions. Therefore, an individual should be more likely to join a patrol if one or more others have already demonstrated a willingness to participate. In support of this idea, Goodall (1986, page 518) described individual differences in patrolling frequency among Gombe males. For example, SH was ‘almost always in the forefront of patrols and several times was the leader when parties travelled to peripheral areas to feed’. Similarly, at Ngogo, males EL and HO had unusually high patrolling rates (Watts & Mitani 2001). Recent playback experiments in captivity showed ‘intra-individual consistency and inter-individual variation in behavioural reactions…to vocalisations by unfamiliar chimpanzees’ (Kutsukake et al. 2012, page 269). Although Wilson et al. (2001) found no difference among males at Kanyawara in the likelihood of responding to simulated calls of strangers (except when impact hunter MS stayed behind to mate-guard a female), low-ranking males were less likely to travel to the periphery of the community range (Wilson et al. 2012). If hunting and intergroup aggression are components of an individual-level behavioural syndrome, we predict that (1) the same males that are prone to hunting should also be prone to intergroup aggression and (2) the temporal correlation between hunting and patrolling is due to the presence of ‘impact males’ that increase the probability of both behaviours (Table 1).
H3: extrinsic factors: many males
Chimpanzees have a fission–fusion social system in which members of a community form temporary ‘parties’ that change in size and composition over the course of hours or days (Nishida 1968; Wrangham & Smuts 1980; Goodall 1986). There is considerable seasonal variation in average party size (Wrangham 1977) in response to the distribution and availability of food and sexually receptive females (Wrangham 1977; Newton-Fisher 2000; Anderson et al. 2002; Mitani et al. 2002). As noted earlier, several studies have demonstrated a positive relationship between the number of adult males in a party (‘male party size’ hereafter) and the likelihood of hunting (Stanford et al. 1994a; Mitani & Watts 2001; Gilby et al. 2006; Gilby & Wrangham 2007), patrolling (Mitani & Watts 2005) and aggressively approaching strangers, either real (Wilson et al. 2012) or experimentally simulated (Wilson et al. 2001). The most parsimonious explanation for a temporal correlation between hunting and patrolling is that they both occur when male party size is large, but are otherwise independent; other than occurring in large parties, the proximate mechanisms/motivations for participating are different. Hence, the ‘many males hypothesis’ predicts that the occurrence of hunting and patrolling will be primarily driven by variation in male party size. That is, there will no longer be a temporal association between hunting and patrolling once male party size is statistically controlled for (Table 1).
H4: extrinsic factors: travel distance/location
Males travel further during patrols compared to regular foraging (Amsler 2010). Since the probability of encountering a red colobus group should increase with travel distance, patrolling may simply provide more opportunities to hunt, even if hunting probability (hunts/encounter) stays the same (Gilby 2004). Alternatively, patrolling behaviour may increase the chances of encountering particularly vulnerable prey groups. At Gombe, the mean size of red colobus groups in the centre of the Kasekela chimpanzee community range is 46% smaller than in the periphery, where there are significantly more infants and juveniles (Stanford 1995). This is probably because the Gombe chimpanzees prey primarily on infant and juvenile red colobus (Goodall 1986; Stanford et al. 1994b) and encounter central red colobus groups more frequently than peripheral ones (Stanford 1995). Since most patrols occur at the periphery of the community range (Goodall 1986; Watts & Mitani 2001; Amsler 2010), the likelihood of encountering a particularly large red colobus group with many infants is expected to be high during a patrol.
Similarly, the vulnerability of a red colobus group also depends on the physical attributes of the habitat where chimpanzees encounter it. Gombe is characterized by a series of steep river valleys covered with thick evergreen forest, separated by ridges of deciduous woodland (Clutton-Brock & Gillett 1979). Hunts of red colobus are more likely to occur (and succeed) in woodland (Gilby et al. 2006), where visibility is greater and there are fewer prey escape routes. To get to the periphery of the range from the centre, a party must cross several of these ridges, again increasing the probability that a patrolling party will encounter vulnerable prey. Alternatively, searching for vulnerable monkey groups might take a chimpanzee party to the periphery, where they then patrol. Wilson et al. (2012) demonstrated that the location of food resources was an important predictor of visits to the extreme periphery at Kanyawara. However, there is no evidence that the Gombe chimpanzees actively seek red colobus groups, as they are reported to do at Ngogo (Watts & Mitani 2002).
Thus, the ‘travel distance/location hypothesis’ predicts that daily path length will be positively associated with the probability of (1) a patrol/periphery visit, (2) an encounter with red colobus and (3) an encounter with red colobus in woodland. Additionally, the probability of (4) hunting and (5) killing will be higher at the periphery than at the centre (Table 1).
METHODS
Study Site and Long-term Data Collection
Gombe National Park, Tanzania consists of 35 km2 of semi-deciduous habitat that transitions from riverine forest in the valleys to woodland and grassland on the ridges (Clutton-Brock & Gillett 1979). It contains three communities of chimpanzees (from north to south): Mitumba, Kasekela and Kalande. Jane Goodall began habituating the Kasekela community to the presence of human researchers in 1960 (Goodall 1986), and demographic records have been continuously kept ever since. Since the early 1970s, observers have conducted almost-daily dawn-to-dusk focal follows (Altmann 1974) of adult chimpanzees, during which they systematically recorded changes in party composition and location (Goodall 1986; Wilson 2012). The data obtained from each such focal follow constitute a ‘follow’. Locations were recorded at 15 min intervals on a paper map and then converted to UTM coordinates using ArcInfo and ArcGIS software. Ground-truthing using hand-held GPS units indicated a mean error of 133 m (Gilby et al. 2006). Throughout each follow, field assistants recorded narrative notes, in which they described the ongoing activities of the focal subject and other chimpanzees, including all observations of key events such as tool use, hunting, mating and intergroup interactions. For more detail on the study site and different aspects of the long-term data collection, see Wilson (2012).
Data Extraction
All long-term data from Gombe are housed and maintained in a relational database at the Jane Goodall Institute Research Center at Duke University. Here we focus on a 32-year period from 1976 to 2007, for which all relevant data have been digitized and thoroughly checked for consistency and accuracy.
Hunting
Following Gilby et al. (2006), we identified all encounters with red colobus monkeys recorded in the narrative notes. The observers are trained to record all cases when they observed red colobus monkeys within approximately 50 m of the focal chimpanzee, regardless of any hunting behaviour. We determined the number and identity of adult male chimpanzees and sexually receptive (maximally tumescent, ‘swollen’) females present in the party at the start of each encounter, ±15 min. ‘Adult’ males were at least 12 years old, the age at which males at Gombe begin to consistently hunt successfully (Gilby et al. 2006). For each encounter, we determined whether or not at least one male hunted. In the narrative notes, ‘hunting’ sometimes included simply running along the ground showing intense interest in the prey. To avoid counting these ambiguous instances as ‘true’ hunts, we limited our analysis to encounters where it was clear whether or not any chimpanzees climbed in clear pursuit (Gilby et al. 2006, 2008; Gilby & Wrangham 2007) of prey.
Patrols, intergroup interactions and periphery visits
We identified all patrols described in the narrative notes using the following criteria: (1) chimpanzees travelled cautiously and (2) they appeared to be watching or listening for chimpanzees from neighbouring communities. In most such cases, field assistants explicitly recorded that the chimpanzees were patrolling. We determined the number and identity of adult male chimpanzees present at the 15 min scan sample nearest in time to the start of each patrol. We estimated start time based on the first instance in which chimpanzees were identified as patrolling, or when they first showed signs of the behavioural criteria described above.
Observers indicated in the narrative notes when intergroup interactions were known or suspected to have taken place, by writing (for example) that vocalizations were heard from strangers, or that an unfamiliar chimpanzee or group of chimpanzees was seen. We read through the narrative notes and extracted (insofar as possible) data for each case, including the date, start and end time of the interaction, the location, whether the encounter involved acoustic, visual or physical contact, the number and age–sex class of any strangers seen, and the outcome of the interaction (e.g. whether the Kasekela chimpanzees approached the strangers).
We used the 15 min location data to identify all visits to the periphery of the Kasekela chimpanzee community range. While parties travel to the periphery for reasons other than patrolling, such as searching for food or mates, a periphery visit serves as an objective indicator of a male’s willingness to risk encountering members of the neighbouring community. For each year of the study, we determined the centre of the community range by taking the mean X and Y coordinates of all points recorded during focal follows of adult males. We then calculated the distance of each point from the centre. We defined any point as being at the periphery if it was further from the centre than the mean distance + 1 standard deviation for that year. This method allowed us to account for fluctuating range size (i.e. areas that might be considered safe in one year might be ‘dangerous’ in the next).
Vegetation type
Gilby et al. (2006) used satellite imagery to classify the type of vegetation for each red colobus encounter between 1976 and 2001. Specifically, they plotted the location of each encounter on a vegetation map derived from a 4 m multispectral IKONOS satellite image acquired on 30 June 2000. They classified the vegetation using the normalized difference vegetation index (NDVI) threshold in ERDAS Imagine (Leica Geosystems, Heerbrugg, Switzerland), and recorded whether each red colobus encounter occurred in evergreen forest or woodland. We used these published data for the current analysis, updating with new data from 2002 to 2007.
Statistical Analyses
We used SAS 9.2 (SAS Institute, Cary, NC, U.S.A.) for all statistical analyses. Our general approach was to use multivariate regression models, using the generalized estimating equations (GEE) technique (PROC GENMOD) to control for repeated sampling of subjects and/or time periods (Diggle et al. 2002). This method adjusts estimated parameter variance based on sampling frequency and is equivalent to incorporating the variable as a random effect in a generalized linear mixed model. In several cases, in order to statistically control for the potential confounding effects of additional variables (e.g. male party size), we included these variables as main effects in a model. For simplicity, we describe the specifics of each test in the Results.
RESULTS
Temporal Variation in Hunting and Patrolling
We identified 1782 occasions between 1976 and 2007 when chimpanzees encountered red colobus monkeys and it was clear whether or not at least one male chimpanzee hunted. A hunt occurred in 1159 (65.0%) of these encounters, and 719 (62.0%) hunts resulted in at least one kill. The probability that at least one male hunted was positively associated with male party size (multiple logistic regression, odds ratio = 1.13, , P < 0.0001) and negatively associated with the presence of swollen females (one swollen female: odds ratio = 0.67, , P 0.004; two or more swollen females: odds ratio = 0.55, , P < 0.0001).
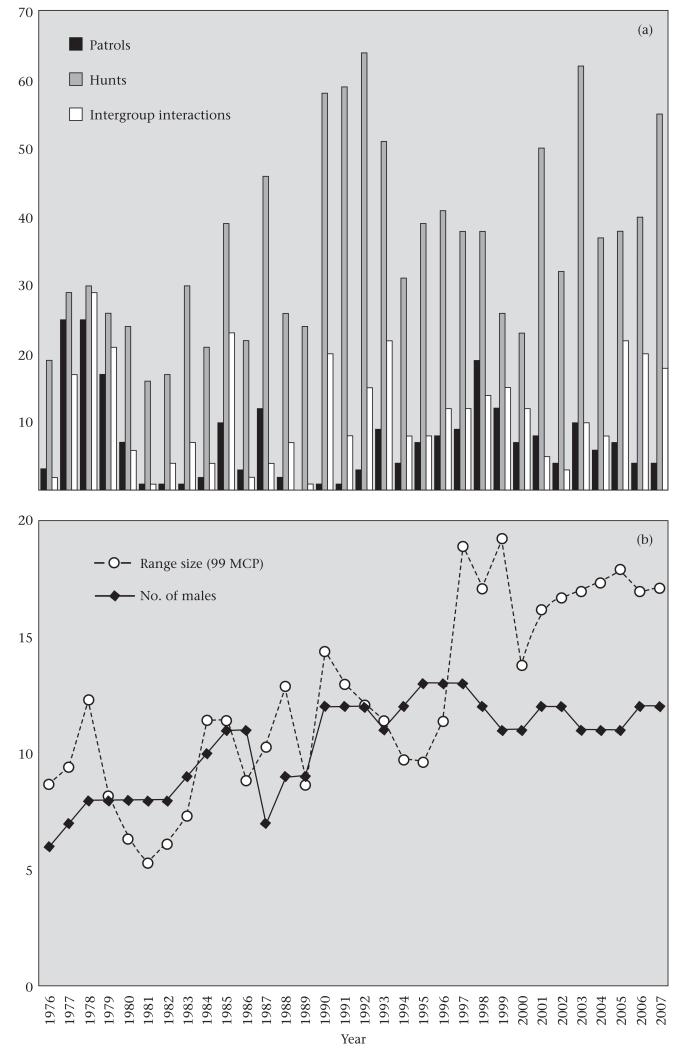
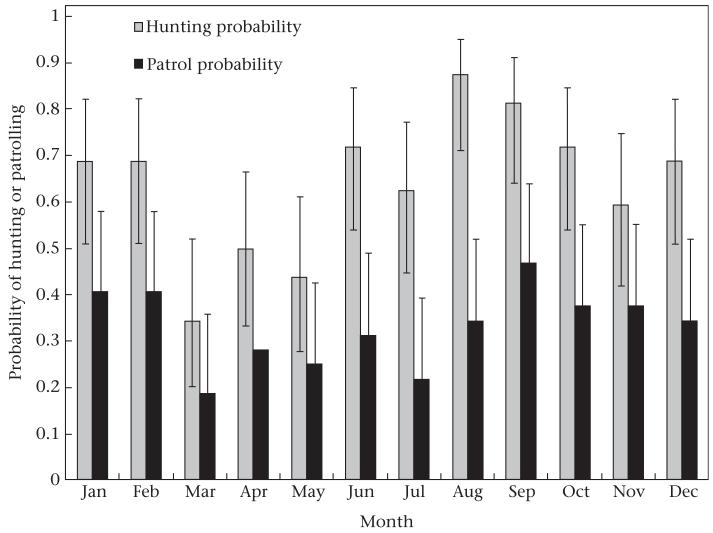
There was considerable variation in the number of hunts per year, ranging from 16 in 1981 to 64 in 1992 (Fig. 1). The number of hunts per year was positively correlated with the number of adult males in the community (Pearson correlation: r30 = 0.55, P = 0.001) and with community range size (r30 = 0.48, P = 0.006). Monthly hunting totals also varied considerably, ranging from 0 (N = 62 months) to 17 (September 1987). This resulted in a non-normal distribution of monthly hunting rates (Kolmogorov–Smirnov test: D = 0.12, P < 0.01), even after log transformation (D = 0.16, P < 0.01). Therefore, we used a GEE logistic regression to test whether hunting frequency varied over time, including ‘year’ as a repeated measure. There was significant monthly variation in the probability of a hunt occurring (Fig. 2). Hunts were most likely to occur in August, significantly more so than in April (P = 0.001), November (P = 0.02), May (P = 0.002) or March (P = 0.0004). September was also a peak hunting month, with a hunting probability significantly greater than in April (P = 0.01), May (P = 0.003) or March (P = 0.0006). March saw the lowest probability of hunting, significantly less than all months except April.
Figure 1.
(a) Number of chimpanzee patrols, hunts and intergroup interactions during the study period (1976–2007). (b) Number of adult males in the community in a given year and the yearly size of the community’s range (99% minimum convex polygons, km2).
Figure 2.
Mean monthly probabilities of hunting and patrolling (from logistic regressions) by chimpanzees, 1976–2007. Error bars indicate 95% confidence intervals.
We identified 232 patrols between 1976 and 2007 (Table 2). Chimpanzees on patrol encountered chimpanzees from a neighbouring community on 90 occasions (58 auditory, 16 visual, 16 physical). There were an additional 270 intergroup interactions that were not preceded by patrolling behaviour. Like hunting, there was considerable yearly variation in both patrolling and intergroup interactions (Fig. 1). There was no correlation between the number of males in the community in a given year and the number of patrols (Pearson correlation: r30 = −0.20, P = 0.26) or intergroup interactions (r30 = 0.19, P = 0.3). Yearly community range size was positively correlated with the number of intergroup interactions (r30 = 0.36, P = 0.05), but not with the number of patrols (r30 = 0.15, P = 0.41). Patrolling rates per month were not normally distributed (Kolmogorov–Smirnov test: D = 0.37, P < 0.01), even after log transformation (D = 0.42, P < 0.01). Similar to the hunting data, a GEE logistic regression (repeated measure = year) revealed that there was significant monthly variation in the probability of a patrol occurring (Fig. 2). The probability of a patrol occurring was significantly higher in September than in May (P = 0.03) or March (P = 0.02). A patrol was also more likely to occur in either January (P = 0.05) or February (P = 0.05) than in March. Note that the month with the highest probability of patrolling (September) was the month with the second-highest hunting probability. Also, March and May were least likely to see either a patrol or a hunt. This suggests that hunting and patrolling are temporally correlated.
Table 2.
Yearly totals of chimpanzee patrols and intergroup encounters
| Year | Patrols | Intergroup encounters | Both |
|---|---|---|---|
| 1976 | 3 | 2 | 1 |
| 1977 | 25 | 17 | 9 |
| 1978 | 25 | 29 | 9 |
| 1979 | 17 | 21 | 8 |
| 1980 | 7 | 6 | 3 |
| 1981 | 1 | 1 | 1 |
| 1982 | 1 | 4 | 1 |
| 1983 | 1 | 7 | 1 |
| 1984 | 2 | 4 | 2 |
| 1985 | 10 | 23 | 5 |
| 1986 | 3 | 2 | 2 |
| 1987 | 12 | 4 | 5 |
| 1988 | 2 | 7 | 2 |
| 1989 | 0 | 1 | 0 |
| 1990 | 1 | 20 | 0 |
| 1991 | 1 | 8 | 1 |
| 1992 | 3 | 15 | 2 |
| 1993 | 9 | 22 | 3 |
| 1994 | 4 | 8 | 1 |
| 1995 | 7 | 8 | 2 |
| 1996 | 8 | 12 | 1 |
| 1997 | 9 | 12 | 1 |
| 1998 | 19 | 14 | 7 |
| 1999 | 12 | 15 | 7 |
| 2000 | 7 | 12 | 3 |
| 2001 | 8 | 5 | 1 |
| 2002 | 4 | 3 | 2 |
| 2003 | 10 | 10 | 3 |
| 2004 | 6 | 8 | 1 |
| 2005 | 7 | 22 | 4 |
| 2006 | 4 | 20 | 2 |
| 2007 | 4 | 18 | 0 |
| Total | 232 | 360 | 90 |
| Mean | 7.3 | 11.3 | 2.8 |
‘Both’ indicates the number of patrols during which there was an intergroup encounter.
Supporting this expectation, the occurrence of a patrol in a given month (yes/no) was positively associated with the number of red colobus hunts observed during that month (GEE logistic regression: odds ratio = 1.22, , P < 0.0001, repeated measure = year), even after statistically controlling for total monthly observation time using multiple regression (odds ratio = 1.17, , P = 0.002). To provide a more fine-grained analysis, which is particularly important given the relative rarity of patrolling, we conducted further analyses by ‘follow’ (day). If similar proximate mechanisms promote both behaviours, then the occurrence of a red colobus hunt should increase the likelihood of a patrol occurring during the same follow, and vice versa. We present analyses of focal follows on adult males, although we found the same results using all follows (including those when the focal chimpanzee was female). The odds of a patrol occurring were significantly greater during follows when hunting occurred (42 patrols/783 male follows with hunting = 5.4%) than when hunting did not occur (121 patrols/4434 male follows without hunting = 2.7%; GEE multiple logistic regression, odds ratio = 1.71, , repeated year, P = 0.006, repeated measures = year, focal ID). Similarly, the odds of a hunt were significantly greater during male follows with a patrol (42 hunts/163 male follows with a patrol = 25.7%) than during male follows without a patrol (741 hunts/5054 male follows without a patrol = 14.6%; odds ratio = 1.57, , P = 0.005).
H1: Species-level Behavioural Syndrome
If hunting monkeys is a form of redirected aggression (Kortlandt 1972), then hunting should be more likely to occur when chimpanzees have been primed for aggression by territorial behaviour, including patrols and direct encounters with neighbours. Regarding patrols, our results showed a nonsignificant pattern in the opposite direction: hunting was more likely to occur before a patrol (89.6%, 26 hunts in 29 prepatrol colobus encounters) than after (69.7%, 30 hunts in 43 postpatrol colobus encounters; GEE logistic regression: odds ratio = 3.74, , P = 0.06, repeated measure = focal ID). Likewise, the occurrence of intergroup interactions (including those not preceded by a patrol) did not have a statistically significant effect on the probability of hunting. Chimpanzees hunted in 77% (49/64) of colobus encounters that occurred after an intercommunity interaction compared to 70% (49/70) of those that occurred before, but this difference was not statistically significant (GEE logistic regression: , P = 0.45, repeated measure = focal ID). The results were the same when we excluded 20 encounters with lone female chimpanzees (, P = 0.70), which may have represented pre-immigration events and were therefore not hostile.
Finally, this hypothesis also predicted that a hunt would be more likely to occur after an intergroup encounter in which the Kasekela chimpanzees approached the strangers than when they did not approach. Again, this prediction was not supported. The chimpanzees hunted in 19 of 26 (73%) red colobus encounters that occurred after approaching strangers versus 29 of 37 (78%) that occurred after they did not approach strangers (GEE logistic regression: , P = 0.67, repeated measure = focal ID).
H2: Individual-level Behavioural Syndrome
Hunting
We used multiple logistic regression to test whether a hunt was more likely to occur when a particular male was present in a party that encountered red colobus. For example, we asked whether parties containing male AL were more likely to hunt than parties without AL. We considered only those red colobus encounters that occurred on or after the male’s 12th birthday and before his death (or the end of the study period). We included male party size and the presence of swollen females as main effects in all regressions. We ran one regression for each of the 27 males that reached adulthood during the study period. The data set for each male was unique (e.g. 1034 encounters during AL’s adult life (1979–1999), compared to 235 for FE (2004–2007)); therefore, we considered P values less than 0.05 to be statistically significant, rather than apply a correction for multiple tests.
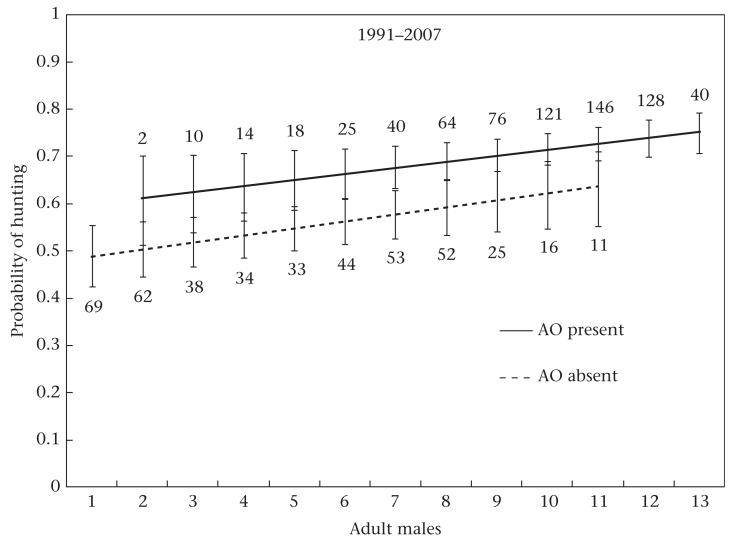
Over the course of the entire study, there were two ‘impact hunters’ (AO and FG) whose presence was associated with increased hunting probability, even after statistically controlling for male party size and the presence of swollen females (Table 3). For all colobus encounters that occurred during AO’s adult life (N = 1121, 1991–2007, , P = 0.02), the odds that a hunt occurred were 50% higher if AO was present than if he was absent (Table 3, Fig. 3). The presence of FG increased the odds of hunting by 131% (N = 230, 1976–1982, , P = 0.02; Table 3). Note that the adult lives of the two males did not overlap. Therefore, there was an impact hunter in the community for 24 of the 32 years of study.
Table 3.
Summary data by male chimpanzee
| ID | Birth year |
Start year of study |
End year of study |
Red colobus encounters |
Periphery visits |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hunting probability when present |
Hunting probability when absent |
Effect of presence on hunting probability |
P | Patrol probability when present |
Patrol probability when absent |
Effect of presence on patrol probability |
P | ||||
| AL | 1967 | 1979 | 1999 | 0.69 (451/649) | 0.65 (250/385) | 0.09 (31/330) | 0.11 (20/185) | ||||
| AO | 1979 | 1991 | 2007 | 0.71 (488/684) | 0.55 (240/437) | + | 0.02 | 0.01 (43/320) | 0.1 (24/248) | ||
| BE | 1969 | 1981 | 2002 | 0.68 (542/797) | 0.61 (248/407) | 0.12 (42/361) | 0.11 (23/217) | ||||
| EV | 1952 | 1976 | 1993 | 0.68 (306/449) | 0.66 (252/382) | 0.13 (36/288) | 0.07 (10/147) | ||||
| FD | 1971 | 1983 | 2007 | 0.68 (664/975) | 0.59 (325/548) | 0.11 (54/471) | 0.11 (29/262) | ||||
| FE | 1992 | 2004 | 2007 | 0.68 (139/204) | 0.45 (14/31) | 0.11 (9/85) | 0 (0/23) | * | |||
| FG | 1953 | 1976 | 1982 | 0.74 (l24/167) | 0.54 (34/63) | + | 0.02 | 0.22 (27/123) | 0.02 (1/55) | + | 0.06 |
| FO | 1989 | 2001 | 2007 | 0.62 (206/331) | 0.53 (99/186) | 0.13 (19/142) | 0.06 (6/97) | + | 0.06 | ||
| FR | 1976 | 1988 | 2007 | 0.68 (601/885) | 0.58 (227/392) | 0.11 (43/407) | 0.11 (26/236) | ||||
| GB | 1964 | 1976 | 2004 | 0.67 (684/1024) | 0.61 (312/512) | 0.15 (84/550) | 0.07 (l8/254) | + | 0.009 | ||
| GL | 1977 | 1989 | 2007 | 0.68 (497/736) | 0.61 (256/418) | 0.13 (41/326) | 0.11 (26/228) | ||||
| HM | 1946 | 1976 | 1981 | 0.77 (72/93) | 0.66 (61/93) | 0.22 (15/68) | 0.14 (13/93) | ||||
| JG | 1971 | 1983 | 1986 | 0.65 (52/80) | 0.6 (32/53) | 0.3 (10/33) | 0 (0/16) | * | |||
| JJ | 1956 | 1968 | 1987 | 0.7 (153/219) | 0.61 (128/210) | 0.26 (35/136) | 0.05 (6/121) | + | 0.02 | ||
| KS | 1982 | 1994 | 2007 | 0.67 (385/575) | 0.53 (163/305) | 0.17 (45/270) | 0.09 (l6/187) | + | 0.03 | ||
| MM | 1973 | 1985 | 1986 | 0.7 (7/10) | 0.58 (15/26) | 0.14(1/7) | 0.28 (5/18) | ||||
| MU | 1965 | 1977 | 1987 | 0.67 (119/177) | 0.6(115/193) | 0.18 (18/102) | 0.11 (12/112) | ||||
| PF | 1971 | 1983 | 1998 | 0.71 (409/580) | 0.67 (177/263) | 0.09 (25/269) | 0.08 (10/121) | ||||
| PX | 1977 | 1989 | 2007 | 0.69 (609/882) | 0.55 (181/329) | 0.12 (52/436) | 0.09 (16/169) | ||||
| SD | 1976 | 1988 | 1990 | 0.58 (32/55) | 0.65 (61/94) | 0.03 (1/30) | 0 (0/44) | * | |||
| SH | 1961 | 1976 | 1979 | 0.75 (54/72) | 0.68 (47/69) | 0.34 (20/58) | 0.11 (7/65) | ||||
| SL | 1983 | 1995 | 2007 | 0.65 (333/511) | 0.56(174/310) | 0.15 (34/230) | 0.13 (24/191) | ||||
| ST | 1955 | 1976 | 1987 | 0.64 (149/233) | 0.67 (132/196) | − | 0.06 | 0.22 (33/149) | 0.07 (8/108) | ||
| TB | 1977 | 1989 | 2007 | 0.69 (584/846) | 0.57 (223/391) | 0.12 (51/411) | 0.08 (17/208) | ||||
| TN | 1994 | 2006 | 2007 | 0.66 (63/95) | 0.73 (11/15) | 0.02 (1/47) | 0.06(1/16) | ||||
| WL | 1972 | 1984 | 2007 | 0.67 (680/1018) | 0.61 (269/443) | 0.13 (62/495) | 0.09 (20/213) | ||||
| ZS | 1993 | 2005 | 2007 | 0.66 (75/113) | 0.59 (20/34) | 0.02 (1/56) | 0.1 (2/21) | − | 0.02 | ||
Hunting probability when present: proportion of colobus encounters with hunting when a given male was present (sample sizes in parentheses); hunting probability when absent: proportion of colobus encounters with hunting when a given male was absent; effect of presence on hunting probability: whether the presence of a given male at a colobus encounter increased (+), decreased (−) or did not affect (null) the probability that at least one male in the party hunted, based on the regression models described in the text; patrol probability when present: patrols/periphery visits for parties containing a given male; patrol probability when absent: patrols/periphery visits for parties without a given male; effect of presence on patrol probability: whether the presence of a given male increased (+), decreased (−) or did not affect (null) the probability that a patrol occurred during a visit to the periphery of the community range, based on the regression models described in the text. Bold font indicates statistical significance (α = 0.05). Asterisks indicate that a model did not converge.
Figure 3.
Relationship between the probability of hunting, number of males and presence/absence of adult male AO in chimpanzee parties that encountered red colobus monkeys. Regression lines are from the GEE multiple logistic regression described in the text. Error bars represent 95% confidence intervals. Numbers indicate sample sizes.
Patrolling
On average, each male was present on 75.4% of all patrols that were documented during his adult life (N = 21 males with at least 10 patrol opportunities, median = 73.6%, range 54–100%). Six males (FE, FG, GB, JG, JJ, WL), had participation rates greater than 0.5 SD (5.6%) above the mean. One of these males (FG) was one of the impact hunters identified in the previous section. FG was present on 84% of the patrols that were recorded during his adult lifetime. This finding provides weak preliminary evidence supporting the hypothesis that the same males may act as catalysts for both hunting and patrolling. However, as the other impact hunter, AO, had lower-than-average patrolling rates (61%), we cannot conclude that the presence of certain males is the primary reason why hunting and patrolling were correlated.
Periphery visits
Next, we examined the tendency of parties (and certain individuals) to travel to the periphery of the community range. While parties may travel to the periphery for reasons other than patrolling, such visits reflect a willingness to risk dangerous encounters with hostile neighbouring groups. Of the 5217 focal male follows that occurred between 1976 and 2007, 896 (17.2%) reached the periphery of the community range (as defined in the Methods). The probability of reaching the periphery was strongly positively associated with the maximum number of males in the focal male’s party that day (GEE logistic regression: odds ratio = 1.17, , P < 0.0001, repeated measure = year, focal ID).
Next, we ran a series of multiple logistic regressions, asking whether the probability of reaching the periphery (yes/no) was affected by the presence of a certain male. We included daily maximum male party size as a main effect in each model, with year and focal ID as repeated measures. For each male, we analysed all focal follows of adult males that occurred during his adult lifetime. There were six males over the course of the whole study whose presence was associated with an increased probability of a focal male follow reaching the periphery of the range (AL: P = 0.02; EV: P = 0.001; GB: P = 0.05; MU: P = 0.05; SH: P = 0.05; WL: P = 0.03). However, this result may simply reflect the fact that certain males have preferred ranging areas (Murray et al. 2008) nearer to the periphery than others. Therefore, we asked whether there were any males whose presence increased the probability of reaching both the southern and northern peripheral areas of the range (where the threat of an intergroup interaction is greatest). No males satisfied these criteria. These results did not change if we used a more conservative definition of periphery (>mean distance + 2 SD from the centre).
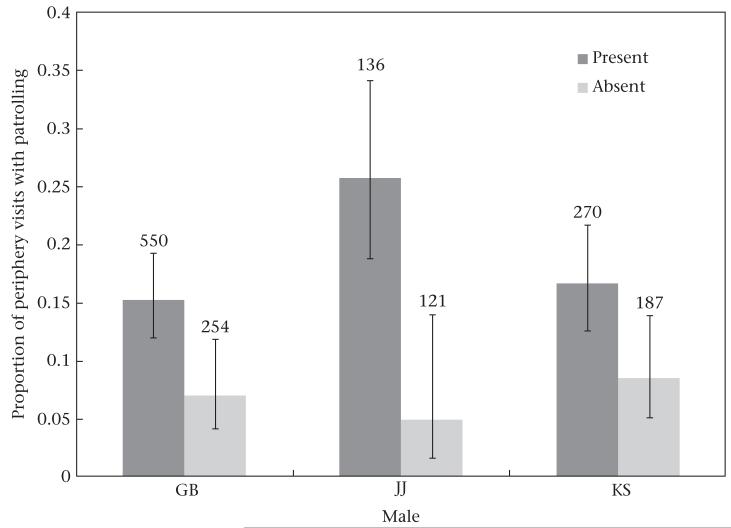
Finally, we asked whether periphery visits were more likely to involve a patrol if certain males were present. That is, when presented with an opportunity (a periphery visit), were particular males more likely to catalyse a patrol? We ran a series of GEE logistic regressions with patrol (yes/no) as the dependent variable and the presence of a given male (yes/no) and maximum male party size as main effects. We included year and focal ID as repeated measures. For each male, we analysed all focal follows of adult males that occurred during his adult lifetime. There were three males whose presence in a group that visited the periphery was significantly associated with an increased probability of patrolling: GB (odds ratio = 2.27, , P = 0.009), JJ (odds ratio = 4.13, , P = 0.02) and KS (odds ratio = 2.11, , P = 0.03) (Table 3, Fig. 4). None of these males were impact hunters. While the presence of FG (an impact hunter) was associated with a substantial increase in patrolling probability (odds ratio = 9.58), the effect was not statistically significant at α = 0.05 (, P = 0.06). There was a similar nonsignificant effect of male FO on patrolling probability (odds ratio = 2.16, , P = 0.06).
Figure 4.
Relationship between chimpanzee patrolling and presence of three male ‘impact patrollers’ (GB, JJ and KS) during the study period (1976–2007). Numbers indicate sample sizes. Error bars represent 95% confidence intervals based on the GEE logistic regression model described in the text.
H3: Extrinsic Factors: Many Males
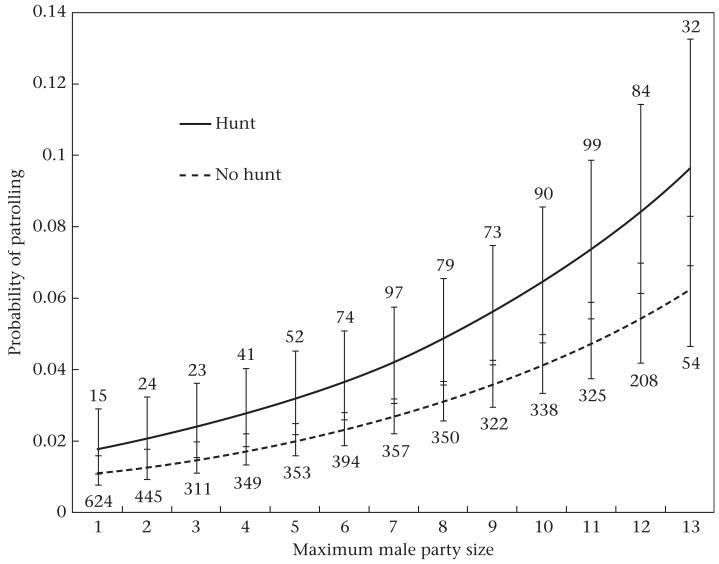
To test the hypothesis that the temporal correlation between hunting and patrolling arose as a simple by-product of the fact that both behaviours are promoted by the presence of several adult males, we reran the earlier GEE logistic regression of hunting probability by follow, this time including maximum male party size as a main effect in the model. As before, we included follow duration and patrol occurrence (yes/no) as main effects, with year and focal ID as repeated measures. As expected, the probability that a hunt occurred on a given day increased significantly with maximum male party size (odds ratio = 1.15, , P < 0.0001). However, as before, the positive association between hunting and patrolling remained significant, after controlling for the effects of maximum male party size (odds ratio = 1.82, , P = 0.03). Similarly, the probability that a patrol occurred on a given day increased significantly with maximum male party size (odds ratio = 1.17, , P < 0.0001). Nevertheless, patrol probability was higher if a hunt also occurred after controlling for maximum male party size (odds ratio = 1.50, , P = 0.03; Fig. 5). Together, these results show that while patrolling and hunting were both more likely to occur on days when many adult males travelled together, there was an additional positive effect of hunting on the likelihood of patrolling, and vice versa. Therefore, the many males hypothesis cannot be the sole explanation for the temporal correlation between hunting and patrolling.
Figure 5.
Relationship between the probability of chimpanzee patrolling, male party size and the occurrence of a hunt. Regression lines are from the GEE multiple logistic regression described in the text. Error bars represent 95% confidence intervals. Numbers indicate sample sizes.
H4: Extrinsic Factors: Travel Distance/Location
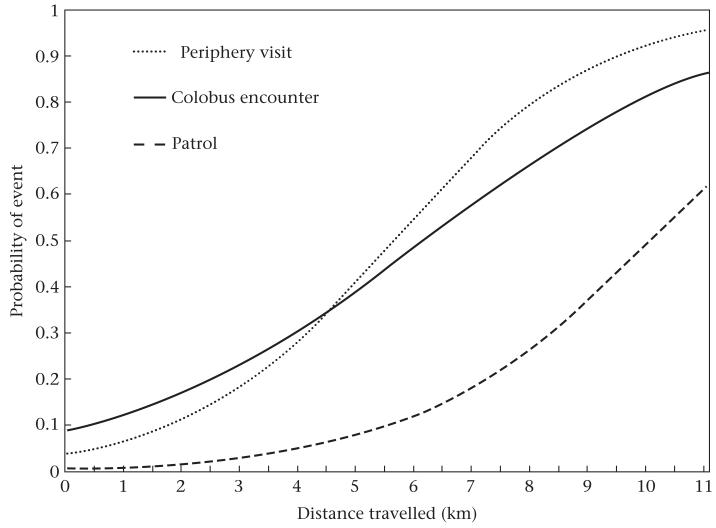
With each kilometre travelled by the focal male, there was a significant increase in the probability of patrolling (odds ratio = 1.63, , P < 0.0001), visiting the periphery of the community range (odds ratio = 1.77, , P < 0.0001) and encountering red colobus (odds ratio = 1.46, , P < 0.0001; Fig. 6). We included year and focal ID as repeated measures in each of these GEE logistic regressions. When we added travel distance as a main effect to the earlier regression of patrol probability (yes/no) versus hunting and maximum male party size (see H3, Fig. 5), the effect of hunting was no longer statistically significant (Table 4). Similarly, the probability of a hunt was not associated with the occurrence of a patrol (, P = 0.38) when travel distance was included in the model. Together, these results support the hypothesis that the correlation between hunting and patrolling arises because patrols tend to cover large distances, increasing the probability of encountering red colobus.
Figure 6.
Relationship between the distance chimpanzees travelled on a given follow and the probability that they reached the periphery of the range, encountered red colobus monkeys and patrolled. Regression lines are from analyses described in the text.
Table 4.
Output from the GEE logistic regression of the probability of chimpanzee patrolling on a given day
| Parameter | Category | Estimate | Wald χ2 | P |
|---|---|---|---|---|
| Intercept | −5.5 | 532.7 | <0.0001 | |
| Maximum male party size | 0.1 | 16 | <0.0001 | |
| Distance travelled (km) | 0.43 | 107 | <0.0001 | |
| Hunt | Yes | 0.21 | 0.9 | 0.33 |
| No | – | – | – |
Patrolling was most likely to occur on days with large male parties that travelled a long distance. When distance travelled was included in the model, the occurrence of a red colobus hunt no longer increased the likelihood of patrolling.
The probability of encountering red colobus monkeys in woodland (where hunting is more likely; Gilby et al. 2006) was positively associated with focal male travel distance (GEE logistic regression: odds ratio = 1.40, , P < 0.0001, repeated measures = year, focal ID). To account for the possibility that this result was due to the fact that travel distance increased the probability of encountering red colobus in any habitat, we reran this analysis, including only those days on which chimpanzees encountered red colobus monkeys at least once. Again, focal male travel distance increased the probability that the encounter(s) occurred in woodland (odds ratio = 1.06, , P = 0.04). This supports the notion that patrolling increases the probability of encountering particularly vulnerable prey. However, hunting was no more likely at the periphery of the range than at the centre (GEE logistic regression, including adult male party size and the presence of swollen females as main effects: odds ratio = 1.23, , P = 0.09, repeated measures = year, focal ID). Interestingly, when a hunt did occur at the periphery, a kill was significantly more likely than in hunts at the centre of the range (GEE logistic regression controlling for adult male party size and the presence of swollen females: odds ratio = 2.41, , P < 0.0001).
DISCUSSION
We report a temporal correlation between two group-level behaviours practised by chimpanzees in Gombe National Park, Tanzania. Over 32 years, adult males were more likely to patrol the border of their range on days when they also hunted red colobus monkeys (and vice versa). As both behaviours involve groups of males searching for and potentially attacking and killing other primates, we tested the hypothesis that territoriality and predation are components of a species-level aggressive behavioural syndrome. If both behaviours have similar proximate psychological causes, then the occurrence of one should promote the other. Specifically, some have argued that predation by chimpanzees evolved as a by-product of selection for intraspecific territorial aggression (Kortlandt 1972; Eibl-Eibesfeldt 1975; Wrangham 1999). As such, hunting should be more likely after a patrol or intergroup interaction than before. However, we found no association between the relative timing of hunting, patrolling and intergroup interactions on a given day. These results are consistent with a recent study at Ngogo, showing that elevated testosterone was associated with territorial patrolling but not hunting (Sobolewski et al. 2012). Sobolewski et al. (2012) concluded that different proximate mechanisms are responsible for territoriality and predation, since the latter has ‘no immediate link to male reproduction’. However, in contrast to our study, the occurrence of a hunt was not associated with patrolling at Ngogo (Mitani & Watts 2005). It remains to be seen whether this represents a methodological or biological difference between these populations.
Next, we tested, and rejected, the hypothesis that an individual-level behavioural syndrome explains why hunting and patrolling tend to occur on the same day. Based on the observation that male chimpanzees vary consistently in their tendency to participate in hunts and patrols (Goodall 1986; Watts & Mitani 2001), Gilby et al. (2008) and Gilby & Connor (2010) proposed that the actions of certain individuals may reduce the potential costs for others to participate in collective action. For example, the costs of joining a hunt that has already started are expected to be much lower than those of initiating one, as red colobus will be easier to catch if they are already fleeing or occupied defending themselves against other hunters. Therefore, if one or two individuals take on the initial hunting costs, then others will be more likely to join. Gilby & Connor (2010) suggested that the same males may act as both impact hunters and patrollers, which would explain the temporal correlation between the two behaviours.
At Gombe, we identified two impact hunters (AO and FG) whose presence in a party that encountered red colobus increased the probability of a hunt occurring. This is consistent with results from Kanyawara, where Gilby et al. (2008) also identified two impact hunters (MS and AJ). However, at Kanyawara, the effect of these males was much more striking: parties without either MS or AJ almost never hunted (Gilby et al. 2008). This may be because hunting costs are lower at Gombe, where individual success rates are relatively high (Boesch 1994), perhaps due to different forest structure at the two sites. Tall trees and a relatively continuous canopy at Kanyawara provide ample refuge for fleeing monkeys, whereas at Gombe the canopy is lower and, in woodland areas, frequently interrupted. We also found the first definitive evidence that certain males increase the likelihood of a patrol occurring. Periphery visits by parties that included at least one of three males (GB, JJ or KS) were more likely to patrol. This suggests that their presence somehow stimulated a patrol, but the mechanism is unclear. Perhaps they are the ones to initiate movement towards neighbours, thereby assuming the most risk. Like hunting, the costs of joining a patrol are expected to be lower than initiating one, as the presence of other companions reduces the chances of being injured in a hostile interaction with neighbours. To test this idea, detailed and systematic data on the behaviour of each individual before and during a patrol are needed.
The fact that the impact hunters and patrollers were different individuals does not support the hypothesis that hunting and patrolling at Gombe were correlated because of the presence of males that catalyse both behaviours. This suggests that with respect to the costs and benefits of participating, hunting and patrolling are fundamentally different. While hunters face some risk of being injured, hunting costs are primarily energetic, and the benefits are immediate and nutritional. Therefore, impact hunters may be more risk-prone in the ecological sense; that is, they may be more likely than others to choose a foraging option with a high chance of failure (Stephens 1981; Gilby & Wrangham 2007). In contrast, patrolling entails a different kind of risk (being injured or killed by conspecifics) and involves intraspecific aggression. The benefits of patrolling (e.g. obtaining a larger feeding territory for self, mates and offspring) may be more delayed, and more contingent on mating success (at Ngogo, males with higher mating success patrolled more often; see below).
Understanding the causes of individual variation in aggression and risk-prone behaviour is a fertile research area. At Ngogo, the males with the highest patrolling rates were those who achieved more copulations with parous females than expected for their rank, suggesting that territory defence was contingent upon reproductive investment (Watts & Mitani 2001). Two of the impact patrollers in our study (GB and KS) became the alpha male and fathered several offspring during their adult lives (Wroblewski et al. 2009; Gilby et al. 2013). Note, however, that the two males with the highest reproductive success to date (FR and WL, with 8 and 10 offspring, respectively; Wroblewski et al. 2009; Gilby et al. 2013, Jane Goodall Institute Research Center, unpublished data) did not exhibit high patrolling rates. A growing number of studies highlight the importance of primate genomics for understanding behavioural variation (Bradley & Lawler 2011). Targeted studies of genes associated with aggression (e.g. MAOA: Inoue-Murayama et al. 2006) or risk-seeking behaviour (e.g. DRD4: Seaman et al. 2000; Eisenberg et al. 2008) may indicate a genetic explanation for differences in hunting and patrolling behaviour. Additionally, more detailed analyses of within-individual variation in patrolling and hunting effort are needed. For example, our current analyses identified males that impacted hunting and patrolling over the course of their adult lives. It is entirely plausible that their impact was greater during some periods than during others, or that we failed to identify other individuals that hunted or patrolled particularly often during certain years.
An alternative explanation for the temporal correlation between hunting and patrolling is that both behaviours involve large groups of males. Therefore, even if the proximate causes are entirely different, both will be more frequent during periods when the formation of large parties is favoured. Our results did not support this hypothesis as the sole explanation, because the correlation remained significant after statistically controlling for male party size. For example, on a day when the maximum male party size was 10, there was a 6.5% chance of a patrol occurring if a hunt also occurred, compared with 4.1% if there was no hunt (Fig. 5). This pattern suggests that an additional variable promotes both types of group-level behaviour. This variable was daily travel distance: the probability of encountering red colobus increased with the number of kilometres travelled, and parties travelled significantly further on patrol days than on nonpatrol days. When we controlled for travel distance, the correlation between hunting and patrolling was no longer statistically significant. Additionally, daily travel distance was positively associated with the probability of encountering red colobus in woodland habitat, where chimpanzees in this population are more likely to hunt and make a kill (Gilby et al. 2006). Also, hunts that occurred at the periphery of the chimpanzee range were more likely to succeed, probably because these red colobus groups are larger and contain more infants (Stanford 1995). Surprisingly, however, hunting probability did not increase at the periphery.
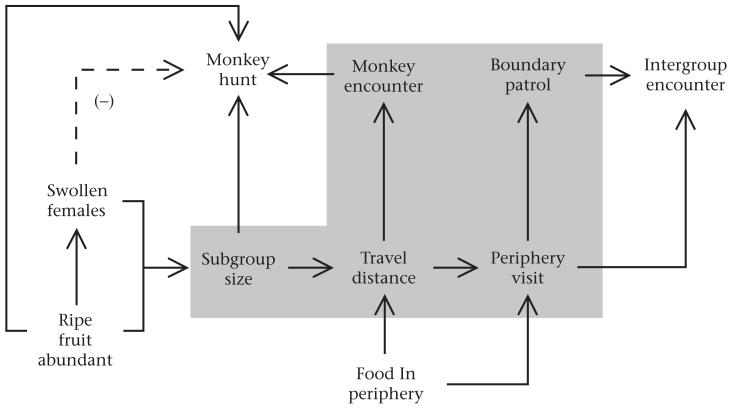
In this paper we have focused on explanations for why hunting and patrolling are correlated. In addition to the variables we have addressed here, previous studies have shown that hunting and patrolling depend on other factors, particularly the abundance and distribution of plant foods, and the presence of swollen females (Fig. 7). The large subgroups that promote both hunting and patrolling form when food is abundant (Wrangham 1977; Mitani et al. 2002; Itoh & Nishida 2007) and/or distributed in large patches (Newton-Fisher 2000) and/or when one or more swollen females are present (Hashimoto et al. 2001; Anderson et al. 2002). Chimpanzees are more likely to hunt when more high-quality food is available, presumably because abundant food reduces the risks of engaging in a high-risk foraging strategy such as hunting (Gilby & Wrangham 2007). Hunts are also more likely to occur in areas with broken forest canopy (Watts & Mitani 2002; Gilby et al. 2006), where monkeys can be isolated more easily. The presence of swollen females reduces both the probability of hunting (Gilby et al. 2006; this study) and the probability of visiting the range periphery (Wilson et al. 2012), presumably because males focus their efforts on competing for mating opportunities at the expense of other activities. Parties with many males may be more likely to visit the periphery for multiple reasons, including safety in numbers (Mitani & Watts 2005; Wilson et al. 2007) and the more rapid depletion of food patches, which requires them to travel further (Wrangham et al. 1993; Williams et al. 2002). The distribution and abundance of food affect not only the size of subgroups, but also where they travel. Chimpanzees spend much of their time searching for and eating food, and they concentrate their foraging effort in areas where preferred foods occur. At Kanyawara, when preferred fruits were in season, chimpanzees spent more time in parts of their range where that fruit species was more abundant (Wilson et al. 2012). At Kanyawara, the majority (62%) of intergroup interactions occurred during the few months when fruits of a single species, Uvariopsis congensis, were abundant (Wilson et al. 2012). Extensive groves of these trees happen to be located in the periphery of Kanyawara’s range, and thus attract large feeding parties from neighbouring communities.
Figure 7.
Diagram of variables affecting the probability of hunting and territorial aggression in chimpanzees. Solid arrows indicate a positive effect of the source variable on the target variable. The dashed arrow indicates a negative relationship. Grey shading demonstrates the findings of this study.
Implications
This study casts doubt on the idea that hunting by chimpanzees arose as a ‘psychological by-product’ of aggression against neighbouring chimpanzee groups. Instead, our results point to a simpler, ecological explanation for the temporal association between territorial border patrols and hunting: patrolling involves travelling greater distances, which in turn, increases the probability of encountering potential prey. Our results also suggest that, for both hunting and patrolling, males take advantage of low-cost opportunities to participate: the presence of impact males promoted group-level action. However, those who catalysed hunts did not have the same effect on patrolling, suggesting that different psychological mechanisms, and different evolutionary costs and benefits, underlie these two behaviours.
Acknowledgments
Data collection was funded by the Jane Goodall Institute (JGI). Digitization and analysis of behavioural data were supported by grants from the National Science Foundation (DBS-9021946, SBR-9319909, BCS-0452315, BCS-0648481, LTREB-1052693), the National Institutes of Health (R01 AI50529, R01 AI58715, P30 AI 27767), the University of Minnesota, the Harris Steel Group, the Windibrow Foundation, the Jane Goodall Institute, the Carnegie Corporation, Minnesota Base Camp and Duke University. We thank TANAPA, TAWIRI and COSTECH for permission to work in Gombe National Park, and the tireless Gombe Stream Research Center staff for maintaining data collection. Many thanks to Ross Bernstein, Jason Beyer, Burke Bourne, Kelly Hughes, Katie Lee, Doug Ludeman, Deus Mjungu, Kim Neu, Alphonce Nicholaus, Nathan O’Neil, Miranda Oliver, Kevin Potts, Anne Scheuerman, Joann Schumacher-Stankey, Elma Stapic, Andrea Vidmar, Marta Wernikiewicz and Anna Wynn for assistance with data extraction. Finally, we are extremely grateful to Lilian Pintea for development of the vegetation map.
References
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Amsler SJ. Energetic costs of territorial boundary patrols by wild chimpanzees. American Journal of Primatology. 2010;72:93–103. doi: 10.1002/ajp.20757. [DOI] [PubMed] [Google Scholar]
- Anderson DP, Nordheim EV, Boesch C, Moermond TC. Factors influencing fission–fusion grouping in chimpanzees in the Taï National Park, Côte d’Ivoire. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge University Press; Cambridge: 2002. pp. 90–101. [Google Scholar]
- Boesch C. Cooperative hunting in wild chimpanzees. Animal Behaviour. 1994;48:653–667. [Google Scholar]
- Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest. Behavioural Ecology and Evolution. Oxford University Press; Oxford: 2000. [Google Scholar]
- Boesch C, Boesch H. Hunting behavior of wild chimpanzees in the TaïNational Park. American Journal of Physical Anthropology. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. [DOI] [PubMed] [Google Scholar]
- Boesch C, Crockford C, Herbinger I, Wittig R, Moebius Y, Normand E. Intergroup conflicts among chimpanzees in Tai National Park: lethal violence and the female perspective. American Journal of Primatology. 2008;70:519–532. doi: 10.1002/ajp.20524. [DOI] [PubMed] [Google Scholar]
- Bradley BJ, Lawler RR. Linking genotypes, phenotypes, and fitness in wild primate populations. Evolutionary Anthropology. 2011;20:104–119. doi: 10.1002/evan.20306. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH, Gillett JB. A survey of forest composition in the Gombe National Park, Tanzania. African Journal of Ecology. 1979;17:131–158. [Google Scholar]
- Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. 2nd edn. Oxford University Press; New York: 2002. [Google Scholar]
- Eibl-Eibesfeldt I. Krieg und Frieden aus der Sicht der Verhaltensforschung. Piper Verlag; Munich: 1975. [Google Scholar]
- Eisenberg DTA, Campbell B, Gray PB, Sorenson MD. Dopamine receptor genetic polymorphisms and body composition in undernourished pastoralists: an exploration of nutrition indices among nomadic and recently settled Ariaal men of northern Kenya. BMC Evolutionary Biology. 2008;8:173. doi: 10.1186/1471-2148-8-173. http://dx.doi.org/10.1186/1471-2148-8-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC. Ph.D. thesis. University of Minnesota; 2004. Hunting and meat sharing among the chimpanzees of Gombe National Park, Tanzania. [Google Scholar]
- Gilby IC. Cooperation among nonkin: reciprocity, markets and mutualism. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB, editors. Evolution of Primate Societies. University of Chicago Press; Chicago: 2012. pp. 514–530. [Google Scholar]
- Gilby IC, Connor RC. The role of intelligence in group hunting: are chimpanzees different from other social predators? In: Lonsdorf EV, Ross SR, Matsuzawa T, editors. The Mind of the Chimpanzee: Ecological and Experimental Perspectives. University of Chicago Press; Chicago: 2010. pp. 220–233. [Google Scholar]
- Gilby IC, Wrangham RW. Risk-prone hunting by chimpanzees (Pan troglodytes schweinfurthii) increases during periods of high diet quality. Behavioral Ecology and Sociobiology. 2007;61:1771–1779. [Google Scholar]
- Gilby IC, Eberly LE, Pintea L, Pusey AE. Ecological and social influences on the hunting behaviour of wild chimpanzees (Pan troglodytes schweinfurthii) Animal Behaviour. 2006;72:169–180. [Google Scholar]
- Gilby IC, Eberly LE, Wrangham RW. Economic profitability of social predation among wild chimpanzees: individual variation promotes cooperation. Animal Behaviour. 2008;75:351–360. [Google Scholar]
- Gilby IC, Brent L, Wroblewski E, Rudicell RS, Hahn BH, Goodall J, Pusey AE. Fitness benefits of coalitionary aggression in male chimpanzees. Behavioral Ecology and Sociobiology. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Harvard University Press; Cambridge, Massachusetts: 1986. [Google Scholar]
- Goodall J, Bandora A, Bergmann E, Busse C, Matama H, Mpongo E, Pierce A, Riss D. Intercommunity interactions in the chimpanzee population of Gombe National Park. In: Hamburg DA, McCown ER, editors. The Great Apes. Benjamin/Cummings; Menlo Park, California: 1979. pp. 12–53. [Google Scholar]
- Hashimoto C, Furuichi T, Tashiro Y. What factors affect the size of chimpanzee parties in the Kalinzu Forest, Uganda? Examination of fruit abundance and number of estrous females. International Journal of Primatology. 2001;22:947–959. [Google Scholar]
- Hare B, Melis AP, Woods V, Hastings S, Wrangham R. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology. 2007;17:619–623. doi: 10.1016/j.cub.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Hare B, Wobber V, Wrangham R. The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Animal Behaviour. 2012;83:573–585. [Google Scholar]
- van Hooff JARAM. Intergroup competition and conflict in animals and man. In: van der Dennen JMG, Falger VSE, editors. Sociobiology and Conflict: Evolutionary Perspectives on Competition, Cooperation, Violence, and Warfare. Chapman & Hall; London: 1990. pp. 23–54. [Google Scholar]
- Hosaka K, Nishida T, Hamai M, Matsumoto-Oda A, Uehara S. Predation of mammals by the chimpanzees of the Mahale Mountains, Tanzania. In: Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. All Apes Great and Small. Vol. I: African Apes. Klewer Academic; New York: 2001. pp. 107–130. [Google Scholar]
- Itoh N, Nishida T. Chimpanzee grouping patterns and food availability in Mahale Mountains National Park, Tanzania. Primates. 2007;48:87–96. doi: 10.1007/s10329-006-0031-0. [DOI] [PubMed] [Google Scholar]
- Inoue-Murayama M, Mishima N, Hayasaka I, Ito S, Murayama Y. Divergence of ape and human monoamine oxidase A gene promoters: comparative analysis of polymorphisms, tandem repeat structures and transcriptional activities on reporter gene expression. Neuroscience Letters. 2006;405:207–211. doi: 10.1016/j.neulet.2006.06.069. [DOI] [PubMed] [Google Scholar]
- Kortlandt A. New Perspectives on Ape and Human Evolution. Stichting voor Psychobiologie; Amsterdam: 1972. [Google Scholar]
- Kutsukake N, Teramoto M, Homma S, Mori Y, Matsudaira K, Kobayashi H, Ishida T, Okanoya K, Hasegawa T. Individual variation in behavioural reactions to unfamiliar conspecific vocalisation and hormonal underpinnings in male chimpanzees. Ethology. 2012;118:269–280. [Google Scholar]
- Manson JH, Wrangham RW. Intergroup aggression in chimpanzees and humans. Current Anthropology. 1991;32:369–390. [Google Scholar]
- Mitani JC. Cooperation and competition in chimpanzees: current understanding and future challenges. Evolutionary Anthropology. 2009;18:215–227. [Google Scholar]
- Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Animal Behaviour. 2001;61:915–924. [Google Scholar]
- Mitani JC, Watts DP. Correlates of territorial boundary patrol behaviour in wild chimpanzees. Animal Behaviour. 2005;70:1079–1086. [Google Scholar]
- Mitani JC, Watts DP, Lwanga JS. Ecological and social correlates of chimpanzee party size and composition. In: Boesch C, Hohmann G, Marchant L, editors. Behavioural Diversity in Chimpanzees and Bonobos. Cambridge University Press; Cambridge: 2002. pp. 102–111. [Google Scholar]
- Muller MN, Wrangham RW. Dominance, aggression, and testosterone in wild chimpanzees: a test of the ‘challenge hypothesis’. Animal Behaviour. 2004;67:113–123. [Google Scholar]
- Murray CM, Gilby IC, Mane SV, Pusey AE. Adult male chimpanzees inherit maternal ranging patterns. Current Biology. 2008;18:20–24. doi: 10.1016/j.cub.2007.11.044. [DOI] [PubMed] [Google Scholar]
- Newton-Fisher NE. Food supply and chimpanzee (Pan troglodytes schweinfurthii) party size in the Budongo Forest Reserve, Uganda. International Journal of Primatology. 2000;21:613–628. [Google Scholar]
- Nishida T. The social group of wild chimpanzees in the Mahali Mountains. Primates. 1968;9:167–224. [Google Scholar]
- Parmigiani S, Palanza P. Fluprazine inhibits intermale attack and infanticide, but not predation, in male mice. Neuroscience and Biobehavioral Reviews. 1991;15:511–513. doi: 10.1016/s0149-7634(05)80141-1. [DOI] [PubMed] [Google Scholar]
- Seaman MI, Chang FM, Deinard AS, Quinones AT, Kidd KK. Evolution of exon 1 of the dopamine D4 receptor (DRD4) gene in primates. Journal of Experimental Zoology. 2000;288:32–38. [PubMed] [Google Scholar]
- Siegel A, Victoroff J. Understanding human aggression: new insights from neuroscience. International Journal of Law and Psychiatry. 2009;32:209–215. doi: 10.1016/j.ijlp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM. Insights for behavioral ecology from behavioral syndromes. Advances in the Study of Behavior. 2008;38:227–281. doi: 10.1016/S0065-3454(08)00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih A, Bell A, Johnson JC. Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution. 2004a;19:372–378. doi: 10.1016/j.tree.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. Quarterly Review of Biology. 2004b;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Sobolewski ME, Brown JL, Mitani JC. Territoriality, tolerance and testosterone in wild chimpanzees. Animal Behaviour. 2012;84:1469–1474. [Google Scholar]
- Stanford CB. The influence of chimpanzee predation on group size and anti-predator behaviour in red colobus monkeys. Animal Behaviour. 1995;49:577–587. [Google Scholar]
- Stanford CB, Wallis J, Mpongo E, Goodall J. Hunting decisions in wild chimpanzees. Behaviour. 1994a;131:1–18. [Google Scholar]
- Stanford CB, Wallis J, Matama H, Goodall J. Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, 1982-1991. American Journal of Physical Anthropology. 1994b;94:213–228. doi: 10.1002/ajpa.1330940206. [DOI] [PubMed] [Google Scholar]
- Stephens DW. The logic of risk-sensitive foraging preferences. Animal Behaviour. 1981;29:628–629. [Google Scholar]
- Surbeck M, Hohmann G. Primate hunting by bonobos at LuiKotale, Salonga National Park. Current Biology. 2008;18:R906–R907. doi: 10.1016/j.cub.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Surbeck M, Fowler A, Deimel C, Hohmann G. Evidence for the consumption of arboreal, diurnal primates by bonobos (Pan paniscus) American Journal of Primatology. 2009;71:171–174. doi: 10.1002/ajp.20634. [DOI] [PubMed] [Google Scholar]
- Thierry B, Aureli F, Nunn CL, Petit O, Abegg C, de Waal FBM. A comparative study of conflict resolution in macaques: insights into the nature of trait covariation. Animal Behaviour. 2008;75:847–860. [Google Scholar]
- Uehara S. Predation on mammals by the chimpanzee (Pan troglodytes) Primates. 1997;38:193–214. [Google Scholar]
- Watts DP, Mitani JC. Boundary patrols and intergroup encounters in wild chimpanzees. Behaviour. 2001;138:299–327. [Google Scholar]
- Watts DP, Mitani JC. Hunting behavior of chimpanzees at Ngogo, Kibale National Park, Uganda. International Journal of Primatology. 2002;23:1–28. [Google Scholar]
- Watts DP, Muller MN, Amsler SJ, Mbabazi G, Mitani JC. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. American Journal of Primatology. 2006;68:161–180. doi: 10.1002/ajp.20214. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Christiansen M, Young WS. Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain and Behavior. 2007;6:653–660. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Liu H, Pusey AE. Costs and benefits of grouping in female chimpanzees at Gombe. In: Boesch C, Hohmann G, Marchant L, editors. Behavioral Diversity in Chimpanzees and Bonobos. Cambridge University Press; Cambridge: 2002. pp. 192–203. [Google Scholar]
- Wilson ML. Long-term studies of the chimpanzees of Gombe National Park, Tanzania. In: Kappeler PM, Watts DP, editors. Long-term Field Studies of Primates. SpringereVerlag; Berlin: 2012. pp. 357–384. [Google Scholar]
- Wilson ML. Chimpanzees, warfare and the invention of peace. In: Fry DP, editor. War, Peace, and Human Nature. Oxford University Press; Oxford: 2013. pp. 361–388. [Google Scholar]
- Wilson ML, Wrangham RW. Intergroup relations in chimpanzees. Annual Review of Anthropology. 2003;32:363–392. [Google Scholar]
- Wilson ML, Hauser MD, Wrangham RW. Does participation in intergroup conflict depend on numerical assessment, range location or rank for wild chimpanzees? Animal Behaviour. 2001;61:1203–1216. [Google Scholar]
- Wilson MW, Hauser MD, Wrangham RW. Chimpanzees (Pan troglodytes) modify grouping and vocal behaviour in response to location-specific risk. Behaviour. 2007;144:1621–1653. [Google Scholar]
- Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Animal Behaviour. 2012;82:277–291. [Google Scholar]
- Wobber V, Hare B, Maboto J, Lipson S, Wrangham R, Ellison PT. Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences, U.S.A. 2010;107:12457–12462. doi: 10.1073/pnas.1007411107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangham RW. Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In: Clutton-Brock TH, editor. Primate Ecology: Studies of Feeding and Ranging Behaviour in Lemurs, Monkeys and Apes. Academic Press; London: 1977. pp. 503–538. [Google Scholar]
- Wrangham RW. Evolution of coalitionary killing. Yearbook of Physical Anthropology. 1999;42:1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Peterson D. Demonic Males: Apes and the Origins of Human Violence. Houghton Mifflin; Boston, Massachusetts: 1996. [Google Scholar]
- Wrangham RW, Pilbeam D. African apes as time machines. In: Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. All Apes Great and Small. Vol. I: African Apes. Kluwer Academic/Plenum; New York: 2001. pp. 5–17. [Google Scholar]
- Wrangham RW, Smuts B. Sex differences in the behavioral ecology of chimpanzees in the Gombe National Park, Tanzania. Journal of Reproduction and Fertility, Supplement. 1980;28:13–31. [PubMed] [Google Scholar]
- Wrangham RW, Gittleman JL, Chapman CA. Constraints on group size in primates and carnivores: population density and day-range as assays of exploitation competition. Behavioral Ecology and Sociobiology. 1993;32:199–209. [Google Scholar]
- Wroblewski EE, Murray CM, Keele BF, Schumacher-Stankey JC, Hahn BH, Pusey AE. Male dominance rank and reproductive success in chimpanzees, Pan troglodytes schweinfurthii. Animal Behaviour. 2009;77:873–885. doi: 10.1016/j.anbehav.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]