Abstract
The radiolabeling of the liposome surface can be a useful tool for in vivo tracking of therapeutic drug loaded liposomes. We investigated radiolabeling therapeutic drug (i.e., an antibiotic, amikacin) loaded liposomes with 99mTc, nebulization properties of 99mTc-labeled liposomal amikacin for inhalation (99mTc-LAI), and its stability by size exclusion low pressure liquid chromatography (LPLC). LAI was reacted with 99mTc using SnCl2 dissolved in ascorbic acid as a reducing agent for 10 min at room temperature. The labeled products were then purified by anion exchange resin. The purified 99mTc-LAI in 1.5% NaCl solution was incubated at 4oC to assess its stability by LPLC. The purified 99mTc-LAI was subjected to studies with a clinically used nebulizer (PARI eFlow®) and the Anderson Cascade Impactor (ACI). The use of ascorbic acid at 0.91 mM resulted in a quantitative labeling efficiency. The LPLC profile showed that the liposomal peak of LAI detected by a UV monitor at both 200 nm and 254 nm overlapped with the radioactivity peak of 99mTc-LAI, indicating that 99mTc-LAI is suitable for tracing LAI. The ACI study demonstrated that the aerosol droplet size distribution determined gravimetrically was similar to that determined by radioactivity. The liposome surface labeling method using SnCl2 in 0.91mM ascorbic acid produced 99mTc-LAI with a high labeling efficiency and stability that are adequate to evaluate the deposition and clearance of inhaled LAI in the lung by gamma scintigraphy.
Keywords: 99mTc-labeled liposome-encapsulated amikacin, radiolabeling, size exclusion analysis
Introduction
Liposomes have regained a lot of attention as nano-scale drug carriers due to their ability to accommodate high drug loading and manufacturing versatility regarding types of drugs that can be loaded, introducing targeting moieties and imaging modalities into a nanoparticle platform (Torchilin, 2005, Malam et al., 2009). In vivo tracking of liposomal drug becomes a more important issue to get information on the distribution and uptake of the liposomal drug at disease sites and its efficacy in treatment (Liu et al., 2012). To facilitate tracking, radiolabeling of liposomes with a tiny amount of radionuclides is often considered because powerful imaging tools such as PET and SPECT can be used. In this study we investigated the radiolabeling of therapeutic drug loaded liposomes with 99mTc and impaction properties of 99mTc-labeled liposomal amikacin for inhalation (99mTc-LAI).
Liposome encapsulated aminoglycosides have been developed recently for inhalation delivery to treat lung infection (Minic et al., 2010, Weers et al., 2009, Antos et al., 1995, Mugabe et al., 2005, Marier et al., 2003, Omri et al., 1994, Okusanya et al., 2009). LAI, an aminoglycoside antibiotic (amikacin,70mg/ml) encapsulated within phospholipid cholesterol liposomes, is an investigational drug developed to provide a sustained benefit in the lungs with once-daily dosing (Minic et al., 2010, Geller, 2009). LAI is being evaluated in clinical trials to treat Pseudomonas aeruginosa (Pa) infections in cystic fibrosis (CF) patients and patients with non-tuberculous mycobacteria (NTM) infections. The pulmonary inhalation drug product may improve patient compliance and treatment outcomes with its reduced dosing frequency, improved drug targeting to the lung infection site, its increased penetration of drug into the Pseudomonas aeruginosa (Pa) biofilm interior, and localization into NTM infected alveolar macrophages (Okusanya et al., 2009, Moss, 2002, Weers et al., 2010, Marier et al., 2003, Omri et al., 1994, Geller, 2009). Previous studies showed many advantages in preclinical animal models and CF patients with Pa infections, including extended drug benefit and improved efficacy(Minic et al., 2010, Weers et al., 2010, Okusanya et al., 2009).
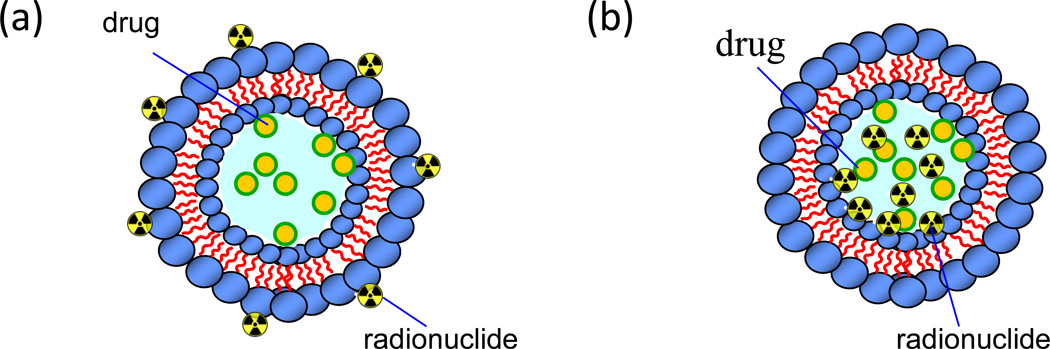
Liposome-encapsulated drugs have different biodistributions and toxicities as compared to ‘free’ drugs (e.g., amikacin)(Omri et al., 1994, Shah and Misra, 2004, Saari et al., 1999, Vidgren et al., 1995, Barker et al., 1994, Saari et al., 1998). Therefore, the radiolabeling of preformed liposomes such as 99mTc labeling using stannous chloride as a reducing agent is a good approach to track liposomal biodistribution in the body (Richardson et al., 1979). 99mTc radiolabeling of liposomes can be performed using different methods such as 99mTc radiolabeling on the outer leaflet of liposomes and encapsulation of 99mTc labeled drug molecule during liposome preparation or by a pH gradient loading method (see Figure 1) (Phillips, 1999). The encapsulation of 99mTc radiolabeled drug during liposome preparation showed less than 5% encapsulation efficiency, especially for the unilamellar vesicles (e.g., LUV (large unilamellar vesicle) and SUV (small unilamellar vesicle)) (Phillips, 1999). Moreover, it requires adulteration of the drug manufacturing process, which may be impractical, and may result in a labeled species that differs from the actual liposomal drug. In contrast, 99mTc radiolabeling on the liposome surface can be a preferable method because it can be performed on the final pharmaceutical drug product and allow encapsulated drugs to remain inert inside the liposome even though it may change in vivo behavior of liposomes due to altered surface properties.
Figure 1.
Strategies for the radiolabeling of therapeutic drug encapsulated liposomes. a) Radionuclides such as 99mTc are bound on the surface of preformed drug encapsulated liposome through a chelating reaction; b) Encapsulation of radionuclides into an enclosed compartment by a pH gradient loading method with preformed liposomes or from co-encapsulation during liposome formation using dissolved lipids and radionuclide building blocks.
Gamma scintigraphy has been used to evaluate the deposition and clearance of these inhaled 99mTc-labeled liposomes in the lungs (Richardson et al., 1979, Weers et al., 2009, Conway, 2012, Elbayoumi and Torchilin, 2006, Phillips, 1999). 99mTc-labeled LAI in the preclinical study indicated no change of in vivo behavior of LAI unlike other studies (Weers et al., 2009, Farr et al., 1985), Although 99mTc radiolabeling of the liposome surface can be a preferable method and a dialysis analysis method demonstrated the radiolabeling efficiency of 99mTc-labeled LAI was successful (Weers et al., 2009), this dialysis method takes a relatively long time (about 10 hrs) to report radiolabeling efficiency and it cannot differentiate 99mTc-labeled LAI and tin-colloid particles. Considering the short decay time of 99mTc (~6 hrs), a quick and robust assessment of 99mTc-labeled LAI is a key step toward successful clinical trials. Furthermore, the analysis reports on 99mTc radiolabeling on the liposome surface have shown inconsistent results (Baljosevic et al., 2002), so a reliable analytical method is critical to assess the labeling efficiency.
In this study, we optimized 99mTc labeling parameters to yield a high labeling efficiency without a significant impact on the liposome size and stability with drug encapsulated liposome formulation, LAI. The radiolabeling efficiency and the stability of 99mTc-LAI were investigated by size exclusion low pressure liquid chromatography (LPLC). With this analysis method, both 99mTc binding and release from the liposome outer leaflet, and encapsulated drug leakage are shown simultaneously in this study. Finally, the purified 99mTc-LAI was subjected to studies using a nebulizer instrument (PARI eFlow®) and the Anderson Cascade Impactor (ACI) to assess aerosol droplet size, an important physical parameter for the deposition and clearance of this inhaled drug.
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless specified otherwise. LAI for this study was provided by Insmed Incorporated. Oasis®Max Plus syringe cartridge was obtained from Waters corporations (Lot No. 186003517, Milford, MA, USA).
Liposomal amikacin for inhalation (LAI) preparation
LAI was prepared aseptically with dipalmitoylphosphatidylcholine (DPPC) and cholesterol in a 2:1 weight ratio using a two-stream mixing process where streams of lipid in ethanol solvent and amikacin sulfate in an aqueous solution were combined. Unencapsulated amikacin and residual solvent were removed by diafiltration to be replaced with 1.5% NaCl solution. Amikacin and total lipid concentrations in the final drug formulation were approximately 70 mg/ml and 49 mg/ml, respectively. Prior to nebulization, greater than 98% of the amikacin was encapsulated within the liposomes.
Radiolabeling of LAI
LAI was radiolabeled using sodium pertechnetate Na+[99mTcO4−] as described previously (Farr et al., 1985, Weers et al., 2009, Koizumi et al., 1992). Briefly, 0.2ml of Na+[99mTcO4−] (30mCi)) in 1.5% NaCl (Technelite® 99Mo/99mTc generator from Lantheus Medical Imaging, Inc.) was added to a sterile vial containing LAI (4 ml at 70 mg/ml amikacin) and the contents were shaken for approximately 30 sec. A volume of 0.2 ml of 2 mM SnCl2 (Lot No.452335) solution prepared in deoxygenated 20 mM ascorbic acid or 20mM HCl and filtered through a 0.22 µm sterilizing filter (MillexGV filter unit, Millipore, Billerica, MA, USA) was then added into the vial. The vial was gently mixed for approximately 30 sec and incubated at room temperature for 10 min. An aliquot of known volume was withdrawn for determination of the specific activity (mCi/mg) of the radiolabeled LAI. The reaction mixture was neutralized with 0.2 ml of sterilized 16 mM sodium carbonate and passed through a sterilized anion exchange Oasis®Max syringe cartridge to remove unlabeled 99mTc pertechnetate and potential tin-colloid from 99mTc-LAI. The specific radioactivity concentration was 5.6±0.43mCi/mL (n=3). A further aliquot of known volume was withdrawn to determine the radiochemical purity. The purified 99mTc-LAI was mixed with unlabeled LAI (i.e., mixed dose = 99mTc-LAI+ unlabeled LAI) to make a patient dose containing a total of 560 mg amikacin with 7 mCi of 99mTc in a total volume of 8.5 ml for the ACI, stability, sterility and Limulus amebocyte lysate (LAL) tests. For the sterility and LAL tests, small aliquots of the patient doses (n=3) were diluted 20 times and 10 times, respectively with sterile 1.5% NaCl solution. The sterility and LAL tests were performed at Department of Laboratory Medicine, Clinical Center, NIH and Clinical Services Program, SAIC, National Cancer Institute, respectively. The remaining product solutions were used for other studies including stability, inertial impaction and impinger studies.
Liposome analysis
Liposome size distributions were determined by dynamic light scattering (DLS) measurements using a Malvern instrument (NANO ZS, Malvern Instruments, CA, USA). The Z-average particle size was measured in triplicate. The integrity of the radiolabeled liposomes was determined by several analytical methods. The samples were assessed by size exclusion LPLC equipped with a size exclusion Sepharose CL 6B column (25×300 mm, GE healthcare, 1.5% NaCl, pH 6.8; 0.8ml/min) (modified for pressure pump use), a UV monitor, and an on-line flow radioactivity detector (Bioscan Inc., Washington, DC, USA). The radiochemical purity of 99mTc-LAI was also evaluated by ascending ITLC (Gelman Sciences, Inc., Ann Arbor, MI,USA) using 100% acetone as the solvent phase (Arulsudar et al., 2003).
Evaluation of aerosolized radiolabeled LAI
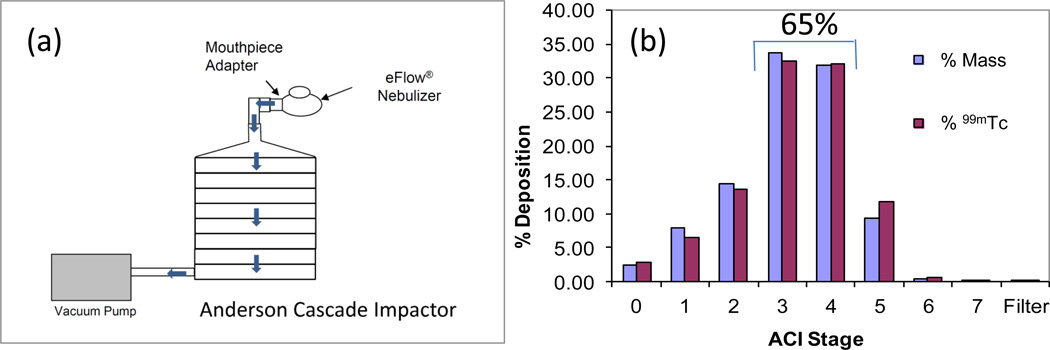
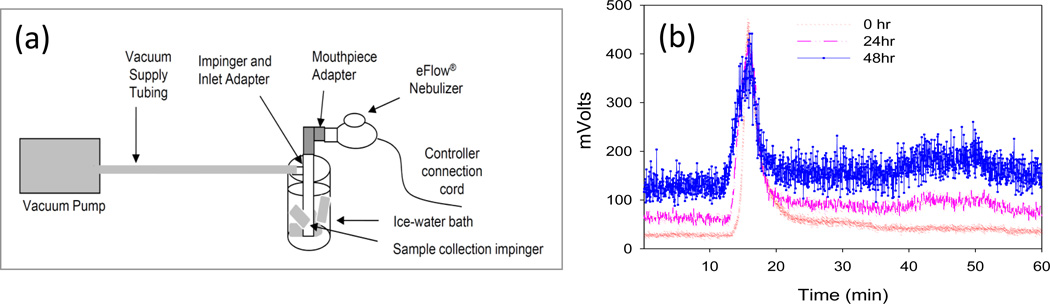
Inertial impaction tests were performed to characterize the aerosol output using an Andersen cascade impactor (ACI, Copley Instruments, UK) operated at a flow rate of 28.3 L/min under 25oC, the relative humidity (%RH) <10% and an inlet throat from a ten stage impactor (Glaxo Type, Copley Instruments, UK). An eFlow electronic nebulizer (PARI, Germany) adapted specifically for use with LAI was used to aerosolize the radiolabeled LAI. The nebulizer was operated continuously for 6 min. Deposition of the liposome preparation was determined by gravimetric assessment of the collection plates, and by dissolving the recovered stage contents in a known volume of water and counting activity with a gamma counter (Mini Gamma Counter 1275 LKB Wallac, Gaithersburg, MD). Also, samples of radiolabeled liposomes recovered from the ACI were analyzed by LPLC to determine the radiochemical and amikacin-association stability of 99mTc-LAI. Similarly, an impinger study was performed to determine the stability of 99mTc-LAI, following aerosolization of the radiolabeled LAI with eFlow electronic nebulizer. The sample collected in an ice-water bath was stored in a refrigerator at 4°C and analyzed by LPLC at different time points. The eFlow nebulizer was equipped with an exhalation filter for these studies and future patient studies to capture exhaled radioactivity.
Results and discussion
LAI analysis
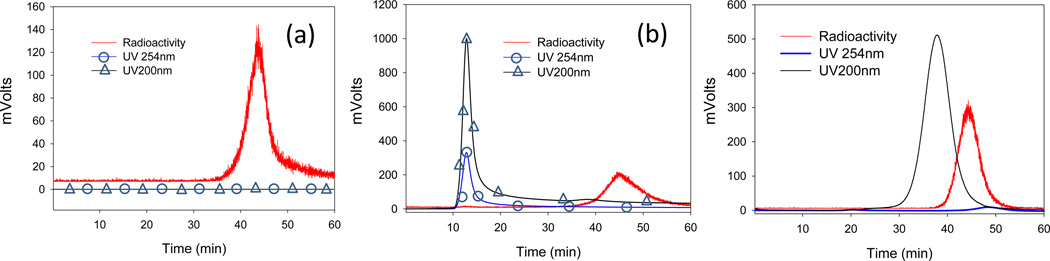
LAI was analyzed with dynamic light scattering before 99mTc radiolabeling. The size was determined to be 238.2±11.5 nm. LAI was subjected to analysis with LPLC equipped with a Sepharose CL 6B resin column. For LAI, LPLC results show that the liposome peaks were detected by a UV monitor set at 254 nm and 200 nm due to its turbidity and drug encapsulation (Figure 2). Less than 2% free amikacin was detected. The result indicates that for LAI liposome integrity is maintained during LPLC analysis. Of note, the amikacin drug peak was only observed at 200 nm, not at 254nm (Figure 2c). As a result, amikacin leakage was measured at 200 nm. The liposome contribution to 200 nm was calibrated by different concentrations of blank DPPC/CH liposomes and free drug leakage was recalculated by subtracting the contribution from the liposomal peak at 200 nm (i.e., free drug %= free drug peak intensity at 45 min/ ((free drug peak intensity at 45 min) +(encapsulated drug peak intensity at 17 min)-(blank DPPC/CH liposome contribution at 17min)).
Figure 2.
Analysis of control samples by LPLC: (a) 99mTc pertechnetate; (b) the mixture of LAI and 99mTc pertechnetate in the absence of SnCl2; and (c) the mixture of amikacin and 99mTc pertechnetate in the absence of SnCl2.
Radiolabeling chemistry and analysis of LAI
Analysis and purification of control samples
The 99mTc-radiolabeled LAI and the control LAI (a mixture of LAI with 99mTcO4−in the absence of the reducing agent, SnCl2) were evaluated by LPLC. The LPLC for the control LAI (i.e., LAI+99mTc pertechnetate) demonstrated that a single radioactivity peak eluted at a retention time of 45 min, identical to the retention time of 99mTc pertechnetate. No radioactivity (<1.0%) was observed at the liposome peak position (between 10 and 20 min) (Figure 2). This result indicates that the phosphate moiety of LAI could not make a coordination complex with pertechnetate in the absence of a reducing agent and that 99mTc pertechnetate did not undergo encapsulation inside LAI during the labeling process. The mixture of amikacin drug and 99mTc pertechnetate also demonstrates no interaction in the absence of SnCl2.
The control samples were also analyzed by the ITLC method developed with acetone as a solvent. Free 99mTcO4−eluted along with the solvent at the solvent front. Unbound 99mTc was separated from LAI in the absence of the reducing agent and moved quickly to the solvent front. In contrast, the 99mTc labeled LAI in the presence of the reducing agent remained at the origin of the sample application when the ITLC was developed with acetone.
For the purification of 99mTc-labeled LAI, we tested several cartridges and columns including Bio-Gel P-6 cartridges (BioRad), 10 DG desalting column (BioRad), PD-10 column (GE Healthcare) and Oasis Max anion exchange cartridge (Waters). We found that Oasis®Max syringe cartridge was satisfactory, quickly eluting LAI and efficiently removing 99mTc pertechnetate whereas other columns and cartridges we tested were easily clogged or the flow was very slow when LAI was eluted.
Radiolabeling efficiency and stability of 99mTc- labeled LAI
The 99mTc labeling of LAI was very efficient in the presence of stannous chloride (100 µM) in 0.91mM ascorbic acid, producing 99mTc-LAI with a high labeling yield: 100.0± 0.0% (n=3) and 89.3±3.2% (n=3), when determined by ITLC and LPLC, respectively. The radiochemical purity after the purification with Oasis Max cartridge was >99% determined by ITLC and 92.9±3.2% (n=3) by LPLC. The 99mTc labeled LAI exhibited a good radiochemical stability and a good integrity of liposome size (see below for details). In contrast, labeling in 1 mM HCl solution appeared to increase the viscosity of the liposome, thereby making it difficult to purify the labeled liposome using the anion exchange cartridge. 99mTc labeling of LAI in HCl solution can cause liposome aggregation at a HCl concentration around 1 mM.
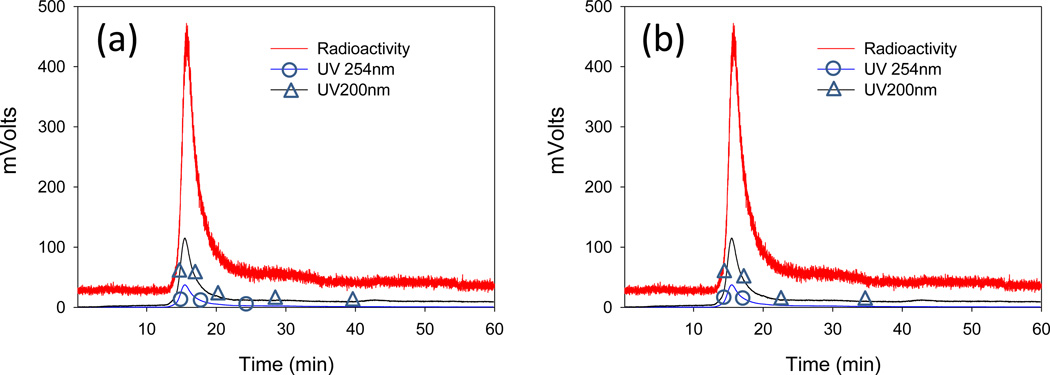
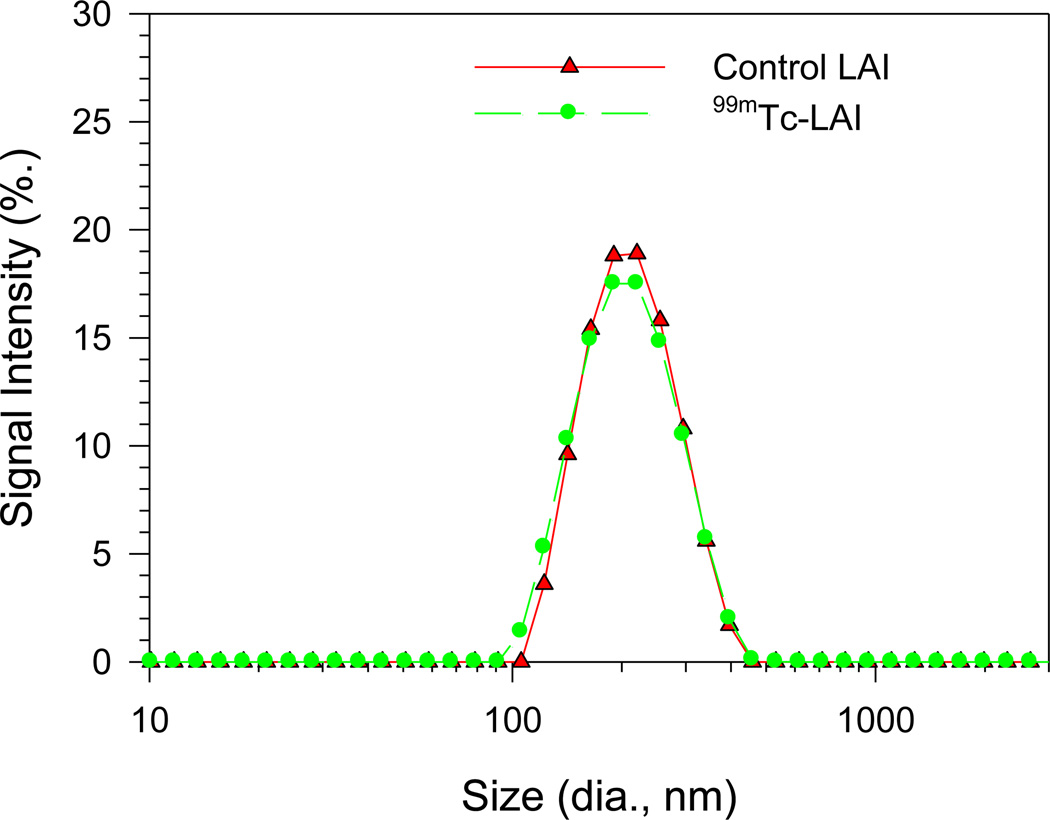
The stability of 99mTc-LAI stored at 4°C was analyzed by LPLC using a UV detector set at both 254nm and UV 200nm and an on-line radioactivity detector (Figure 3 and Table 1). The LPLC profiles of 99mTc-LAI demonstrate that a major peak detected by a UV monitor at 200 nm and 254 nm overlapped with the major radioactivity signal peak. The peak shape and retention time of these peaks was identical to those of the original LAI, indicating that the 99mTc labeling condition did not change the size of LAI. The stability data suggest that the LAI kept at 4°C provides a stable storage condition. The particle size distributions shown by dynamic light scattering demonstrate that the distribution of 99mTc-LAI sample three months after the radiolabeling overlapped with the particle size distribution of the original LAI (see Figure 4). This finding also strongly supports the LPLC data, demonstrating that the liposome integrity of LAI remains unchanged after the radiolabeling.
Figure 3.
LPLC analysis to determine the stability of 99mTc-LAI. Samples from the patient dose were stored at 4°C and analyzed at the designated time points by both a UV detector set at 254nm and 200nm and an on-line radioactivity detector connected in sequence: (a) 0 hr; (b) 3 hr. The UV peaks overlap with the radioactivity peak, indicating that the radiolabel did not change the integrity of LAI.
Table 1.
LPLC Analysis Result of Radiolabeled LAI
| % free 99mTc | % free drug | |
|---|---|---|
| Before purification | 10.7±3.2 | - * |
| After purification | 7.1±3.2 | - * |
| Mixed dose, 3hr | 10.4±0.8 | 1.4±0.0 |
| Mixed dose, 24hr | 10.1±1.5 | 1.6±0.6 |
| Mixed dose, 48hr | 31.2±9.1 | 1.9±0.6 |
| After impaction, 48hr** | 14.6 | 14 |
| After impinger, 0hr | 17.3 | 21.3 |
| After impinger, 24hr | 35.0 | 31.9 |
| After impinger, 48hr | 10.7±3.2 | 34.0 |
All the data represent mean±S.D. (n=4) except impaction and impinger studies.
Free drug was not detectable.
Data was obtained from analysis of stage 3 sample
Figure 4.
Particle size distribution determined by dynamic light scattering. The control LAI and 99mTc-LAI were stored for 3 months at 4°C. The particle size distribution measured with a dynamic light scattering instrument showed the same liposome size distribution between the control LAI and 99mTc-LAI.
Impaction study to evaluate aerosol particle size distribution
The liposome deposition in the lung that depends on aerosol droplet size can be assessed by inertial impaction tests using an Andersen cascade impactor and nebulizer. Heavier aerosol particles travel shorter distance and deposit on earlier stages, and lighter particles travel longer distance and deposit on later stages (Patton, 1996) (Figure 5a). The aerodynamic particle size distribution (PSD) was determined by both gravimetric mass and 99mTc radioactivity measurement. A great correlation was demonstrated between the two measurements (Figure 5b). No deposition on the filter was observed. About 65% of the 99mTc-LAI formulation was deposited on the middle stages: stages 3 (cut-off diameter 3.3 µm) and 4 (cut-off diameter 2.1 µm) of ACI. More than 88% was deposited between stages 2 and 5. No deposition on the filter and the increased deposition amount on the middle stage suggest that a stable aerosol of 99mTc-LAI was generated. The impinger study was also performed to determine the stability of 99mTc-LAI (Figure 6). The LPLC shows that 99mTc-LAI aerosolized through eFlow nebulizer and recaptured in theimpinge exhibited the original liposomal LPLC peak position. The LPLC analysis of impaction and impinger samples indicates that 86% and 66%, respectively, of the total amikacin remained as encapsulated drug even after storage for 48 hrs (Table 1).
Figure 5.
Schematic experimental set up for impaction study (a) and aerodynamic particle size distributions of 99mTc-LAI (b). Andersen cascade impactor (ACI) was used for this experiment in which the levels of LAI droplets on individual stages were measured gravimetrically (mass) and via quantification of radioactivity (counts per minute (CPM) of 99mTc). The aerosol particle distribution determined gravimetrically overlapped with that determined by the radioactivity measurement. About 65% of the total deposition was found in the middle stages.
Figure 6.
Impinger Study and its stability analysis: (a) Schematic diagram for impinger study to determine the stability of 99mTc-LAI after aerosolization with the eFlow electronic nebulizer; and (b) LPLC analysis for the stability of 99mTc-LAI after impinger study at different time points, showing that aerosolized 99mTc-LAI maintains its original liposomal peak position.
Discussion
The size exclusion high-performance liquid chromatography (HPLC) has been a very useful technique for liposome analysis since 1980s (Ollivon et al., 1986, Lesieur et al., 1991, Lesieur et al., 1993, Grabielle-Madelmont et al., 2003). This method permits liposomes to be separated from small solutes (i.e., free drugs) and to be subdivided into different sized-liposomes. In addition, this analysis provides information on liposome stability and drug encapsulation, as well as average size and size distribution. However, this method was found to be destructive for cholesterol rich liposomes (especially, size >300nm), which has limited its use (see also (Lesieur et al., 1993, Grabielle-Madelmont et al., 2003). The liposome associated DPPC was recovered well from the size exclusion HPLC whereas the liposome associated DPPC/cholesterol showed a poor recovery (Lesieur et al., 1991). For these reasons, LPLC method was instead chosen as the analytical method.
Comparing the stability data obtained from both ITLC and LPLC, the 99mTc-LAI was very stable for 24 h without 99mTc pertechnetate detected when the ITLC was used whereas the LPLC analysis showed about 10% 99mTc pertechnetate detected at 0, 3 and 24 h. This appears to indicate that about 10% of 99mTc is bound unstably to LAI which dissociates when eluted through the LPLC column. However, it is still associated to LAI when a gentle analysis method, ITLC is used. Although the LPLC might destabilize 99mTc binding, the LPLC profiles traced by the UV monitor indicate that the LPLC did not disrupt the integrity of LAI with respect to the leakage of amikacin, showing a minor peak (< 2% of the total) representing amikacin leaked out of LAI. This finding indicates that encapsulated amikacin remained stably inside the liposome and the liposome structure was not disrupted by the 99mTc labeling condition or the LPLC elution process. The advantage of LPLC equipped with a UV monitor and an on-line radioactivity detector in sequence was that it enabled us to simultaneously provide information on the structural integrity of liposome, the radiochemical stability of 99mTc-LAI and the liposome stability in regards to leakage of encapsulated amikacin. Of note, the LPLC stability data for 99mTc-LAI are similar to those determined by a dialysis method.(Weers et al., 2009)
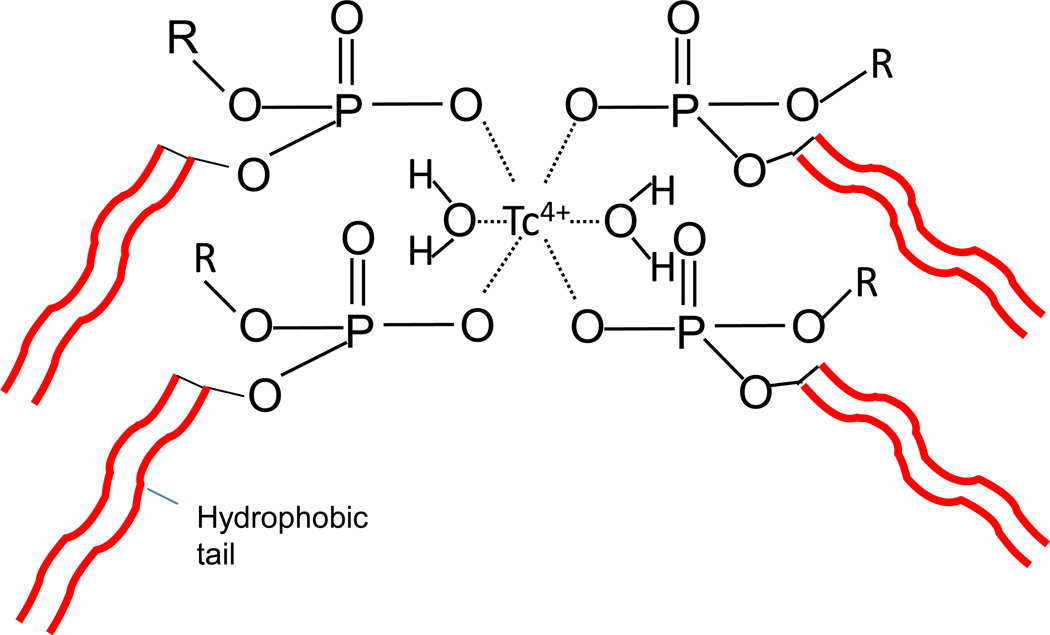
The structure of the 99mTc-LAI complex is still not well understood at the present time. It is possible that the oxidation state of 99mTc+7 was reduced to 99mTc+4 and then complexed with the phosphate moieties on the surface of the liposome (Phillips, 1999). The schematics of the radiolabeled 99mTc-DPPC structure is shown in Figure 7 by adapting a structure of 99mTc-methyl disphosphonate (Qiu, 2011). Previous studies on 99mTc labeling on preformed liposomes using SnCl2 have yielded different results with variable and inconsistent labeling efficiency (i.e., the labeling efficiency <50% in some cases)(Caride, 1990, Phillips, 1999, Baljosevic et al., 2002). We think that the high labeling efficiency and improved stability analyzed by LPLC may be the result of the following factors: 1) the presence of ascorbic acid; 2) the improvement in the removal of 99mTc pertechnetate and tin-colloid by the anion exchange cartridge; 3) a stable coordination between 99mTc4+ and liposomal surface functional groups at a high concentration, and 4) an optimum liposome size (~200 nm). The liposome size used in the previous published studies was <100 nm for SUV and about >1µm for multilamellar vesicles (MLV), and in many studies the liposome size was often not well characterized. Further structural investigation needs to quantify each factor.
Figure 7.
Schematics of the radiolabeled 99mTc-DPPC structure adapted from a structure of 99mTc-methyl disphosphonate. The stannous chloride (SnCl2) as a reducing agent changes 99mTc oxidation state from its native +7 state to a +4 state. Thus, the 99mTc binds with phosphate group of DPPC.
The results from the impaction study with a condition similar to that used in a patient study suggest that this nebulization process does not appreciably deteriorate the radiochemical stability of 99mTc-LAI or the liposome integrity with respect to the leakage of amikacin. These findings suggest that 99mTc-LAI can provide reliable monitoring of the deposition and clearance of LAI in the lung .These results together with the test results of sterility and LAL showing “no bacteria growth” and acceptable endotoxin unit of < 4.25 EU/8.5 ml (n=3) for the patient doses formulated in this study support that these methods of 99mTc labeling, purification and dose formulation under aseptic conditions are suitable for clinical trials.
Conclusions
The 99mTc labeling of LAI with stannous chloride dissolved in ascorbic acid produced 99mTc- LAI with a quantitative labeling yield without a significant impact on the liposome size and stability. The development of LPLC analysis for the LAI cholesterol rich liposome system allowed a quick and robust assessment of 99mTc-labeled LAI for radiochemical stability and liposome integrity, a key step toward successful clinical trials. The 99mTc labeling, purification and dose formulation methods under aseptic conditions provided a patient dose (7 mCi/560 mg amikacin in 8.5 ml) that is suitable for human studies. We believe that our labeling approach and evaluation methods are significantly improved methodologies important in the radiolabeling of liposomes. Establishment of these methodologies is an important key step that is required before initiating studies on the uptake of radiolabeled liposomal drugs in disease sites and studies to evaluate treatment efficacy in the clinic.
Acknowledgements
We thank Dr. Insook Kim for her critical review and editorial assistance with this manuscript.
Declaration of interest
This research was supported in part by the intramural research program of the National Institutes of Allegrgy and Infectious Disease and Clinical Center of the NIH.
References
- Antos M, Trafny EA, Grzybowski J. Antibacterial activity of liposomal amikacin against Pseudomonas aeruginosa in vitro. Pharmacol Res. 1995;32:85–87. doi: 10.1016/s1043-6618(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Arulsudar N, Subramanian N, Mishra P, Sharma RK, Murthy RS. Preparation, characterisation and biodistribution of 99mTc-labeled liposome encapsulated cyclosporine. J Drug Target. 2003;11:187–196. doi: 10.1080/10611860310001615415. [DOI] [PubMed] [Google Scholar]
- Baljosevic I, Subarevic J, Subarevic V, Minic P, Rosic R, Mircetic N. [Surgical tracheotomy in congenital anomalies of the larynx] Srp Arh Celok Lek. 2002;130(Suppl 1):37–39. [PubMed] [Google Scholar]
- Barker SA, Taylor KMG, Short MD. THE DEPOSITION AND CLEARANCE OF LIPOSOME-ENTRAPPED TC-99M-DTPA IN THE HUMAN RESPIRATORY-TRACT. International Journal of Pharmaceutics. 1994;102:159–165. [Google Scholar]
- Caride VJ. TECHNICAL AND BIOLOGICAL CONSIDERATIONS ON THE USE OF RADIOLABELED LIPOSOMES FOR DIAGNOSTIC-IMAGING. Nuclear Medicine and Biology. 1990;17:35–39. doi: 10.1016/0883-2897(90)90005-l. [DOI] [PubMed] [Google Scholar]
- Conway J. Lung imaging - Two dimensional gamma scintigraphy, SPECT, CT and PET. Advanced Drug Delivery Reviews. 2012;64:357–368. doi: 10.1016/j.addr.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:1196–1205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- Farr SJ, Kellaway IW, Parryjones DR, Woolfrey SG. TECHNETIUM-99M AS A MARKER OF LIPOSOMAL DEPOSITION AND CLEARANCE IN THE HUMAN-LUNG. International Journal of Pharmaceutics. 1985;26:303–316. [Google Scholar]
- Geller DE. Aerosol Antibiotics in Cystic Fibrosis. Respiratory Care. 2009;54:658–669. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- Grabielle-madelmont C, Lesieur S, Ollivon M. Characterization of loaded liposomes by size exclusion chromatography. J Biochem Biophys Methods. 2003;56:189–217. doi: 10.1016/s0165-022x(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Koizumi K, Uchiyama G, Arai T, Ainoda T, Yoda Y. A new liver functional study using Tc-99m DTPA-galactosyl human serum albumin: evaluation of the validity of several functional parameters. Ann Nucl Med. 1992;6:83–87. doi: 10.1007/BF03164647. [DOI] [PubMed] [Google Scholar]
- Lesieur S, Grabielle-madelmont C, Paternostre MT, Ollivon M. Size analysis and stability study of lipid vesicles by high-performance gel exclusion chromatography, turbidity, and dynamic light scattering. Anal Biochem. 1991;192:334–343. doi: 10.1016/0003-2697(91)90545-5. [DOI] [PubMed] [Google Scholar]
- Lesieur S, Grabiellemadelmont C, Paternostre M, Ollivon M. STUDY OF SIZE DISTRIBUTION AND STABILITY OF LIPOSOMES BY HIGH-PERFORMANCE GEL EXCLUSION CHROMATOGRAPHY. Chemistry and Physics of Lipids. 1993;64:57–82. [Google Scholar]
- Liu TW, Macdonald TD, Shi J, Wilson BC, Zheng G. Intrinsically copper-64-labeled organic nanoparticles as radiotracers. Angew Chem Int Ed Engl. 2012;51:13128–13131. doi: 10.1002/anie.201206939. [DOI] [PubMed] [Google Scholar]
- Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Marier JF, Brazier JL, Lavigne J, Ducharme MP. Liposomal tobramycin against pulmonary infections of Pseudomonas aeruginosa: a pharmacokinetic and efficacy study following single and multiple intratracheal administrations in rats. J Antimicrob Chemother. 2003;52:247–252. doi: 10.1093/jac/dkg317. [DOI] [PubMed] [Google Scholar]
- Minic P, Fustic S, Solyom E, Mazurek H, Antipkin Y, Feketeova A, Senatorova A, Csiszer E, Kostromina V, Takac B, Ujhelyi R, Gupta R. A Multi-cycle Open Label Study of Nebulized Liposomal Amikacin (ARIKACE™) in the Treatment of Cystic Fibrosis Patients with Chronic Pseudomonas aeruginosa Lung Infection. Pediatr Pulmonol. 2010;45(S33) [Google Scholar]
- Moss RB. Long-term benefits of inhaled tobramycin in adolescent patients with cystic fibrosis. Chest. 2002;121:55–63. doi: 10.1378/chest.121.1.55. [DOI] [PubMed] [Google Scholar]
- Mugabe C, Azghani AO, Omri A. Liposome-mediated gentamicin delivery: development and activity against resistant strains of Pseudomonas aeruginosa isolated from cystic fibrosis patients. J Antimicrob Chemother. 2005;55:269–271. doi: 10.1093/jac/dkh518. [DOI] [PubMed] [Google Scholar]
- Okusanya OO, Bhavnani SM, Hammel J, Minic P, Dupont LJ, Forrest A, Mulder GJ, Mackinson C, Ambrose PG, Gupta R. Pharmacokinetic and pharmacodynamic evaluation of liposomal amikacin for inhalation in cystic fibrosis patients with chronic pseudomonal infection. Antimicrob Agents Chemother. 2009;53:3847–3854. doi: 10.1128/AAC.00872-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivon M, Walter A, Blumenthal R. Sizing and separation of liposomes, biological vesicles, and viruses by high-performance liquid chromatography. Anal Biochem. 1986;152:262–274. doi: 10.1016/0003-2697(86)90408-2. [DOI] [PubMed] [Google Scholar]
- Omri A, Beaulac C, Bouhajib M, Montplaisir S, Sharkawi M, Lagace J. Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;38:1090–1095. doi: 10.1128/aac.38.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JS. Mechanisms of macromolecule absorption by the lungs. Advanced Drug Delivery Reviews. 1996;19:3–36. [Google Scholar]
- Phillips WT. Delivery of gamma-imaging agents by liposomes. Advanced Drug Delivery Reviews. 1999;37:13–32. doi: 10.1016/s0169-409x(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Qiu L, Lin JG, Ju XH, Gong XD, Luo SN. Structual investigation of technetium-disphosphate complex 99mTc-MDP. Chin J Chem Phys. 2011;24:295–304. [Google Scholar]
- Richardson VJ, Ryman BE, Jewkes RF, Jeyasingh K, Tattersall MN, Newlands ES, Kaye SB. Tissue distribution and tumour localization of 99m-technetium-labelled liposomes in cancer patients. Br J Cancer. 1979;40:35–43. doi: 10.1038/bjc.1979.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari M, Vidgren MT, Koskinen MO, Turjanmaa VM, Nieminen MM. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int J Pharm. 1999;181:1–9. doi: 10.1016/s0378-5173(98)00398-6. [DOI] [PubMed] [Google Scholar]
- Saari SM, Vidgren MT, Koskinen MO, Turjanmaa VMH, Waldrep JC, Nieminen MM. Regional lung deposition and clearance of Tc-99m-labeled beclomethasone-DLPC liposomes in mild and severe asthma. Chest. 1998;113:1573–1579. doi: 10.1378/chest.113.6.1573. [DOI] [PubMed] [Google Scholar]
- Shah SP, Misra A. Liposomal amikacin dry powder inhaler: effect of fines on in vitro performance. AAPS PharmSciTech. 2004;5:e65. doi: 10.1208/pt050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nature Reviews Drug Discovery. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Vidgren M, Waldrep JC, Arppe J, Black M, Rodarte JA, Cole W, Knight V. A STUDY OF TC-99M-LABELED BECLOMETHASONE DIPROPIONATE DILAUROYLPHOSPHATIDYLCHOLINE LIPOSOME AEROSOL IN NORMAL VOLUNTEERS. International Journal of Pharmaceutics. 1995;115:209–216. [Google Scholar]
- Weers J, Metzheiser B, Taylor G, Warren S, Meers P, Perkins WR. A gamma scintigraphy study to investigate lung deposition and clearance of inhaled amikacin-loaded liposomes in healthy male volunteers. J Aerosol Med Pulm Drug Deliv. 2009;22:131–138. doi: 10.1089/jamp.2008.0693. [DOI] [PubMed] [Google Scholar]
- Weers JG, Bell J, Chan HK, Cipolla D, Dunbar C, Hickey AJ, Smith IJ. Pulmonary formulations: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010;23(Suppl 2):S5–S23. doi: 10.1089/jamp.2010.0838. [DOI] [PubMed] [Google Scholar]