Abstract
Expression of genes early during sporulation in Bacillus subtilis requires the activity of the transcription factor encoded by spo0A. The active, phosphorylated form of Spo0A is produced through the action of a multicomponent pathway, the phosphorelay. A mutant defective in the first three enzymes of the Krebs citric acid cycle was unable to express early sporulation genes, apparently because of a failure to activate the phosphorelay. Cells that produce an altered Spo0A protein that can be phosphorylated by an alternative pathway were not dependent on Krebs cycle function for early sporulation gene expression. These findings suggest that Krebs cycle enzymes transmit a signal to activate the phosphorelay and that B. subtilis monitors its metabolic potential before committing itself to spore formation.
Full text
PDF
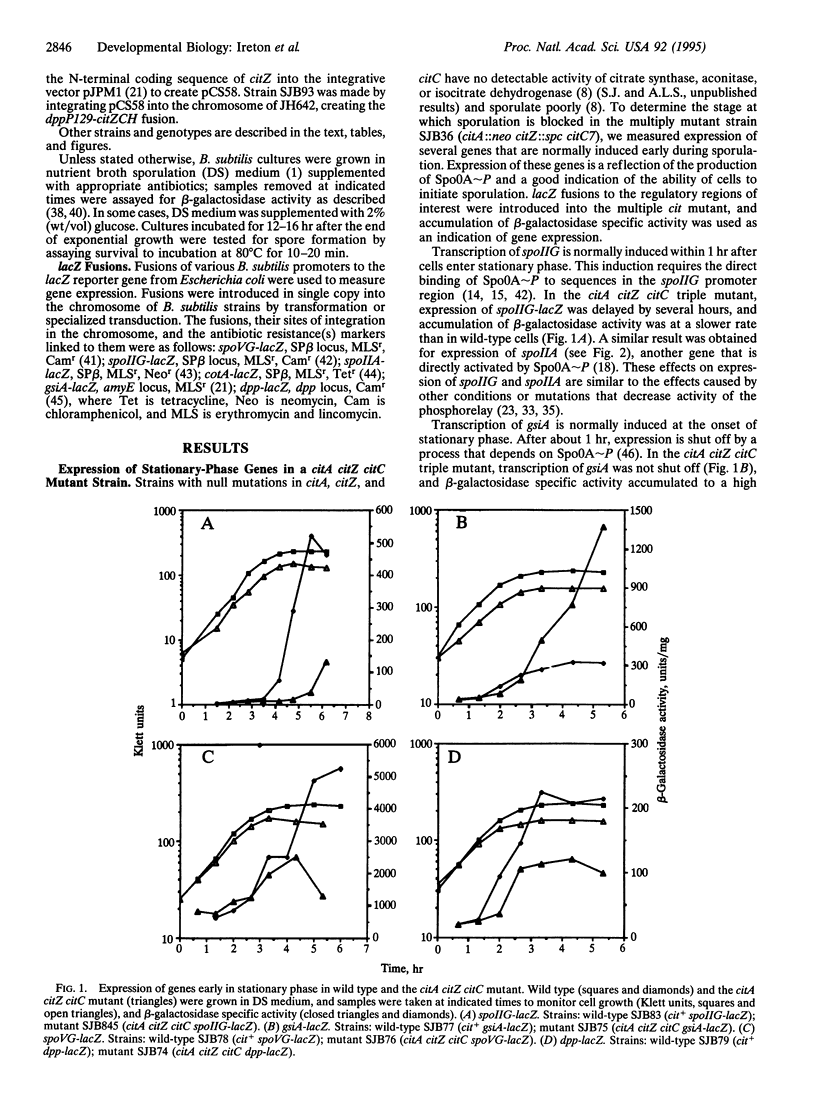
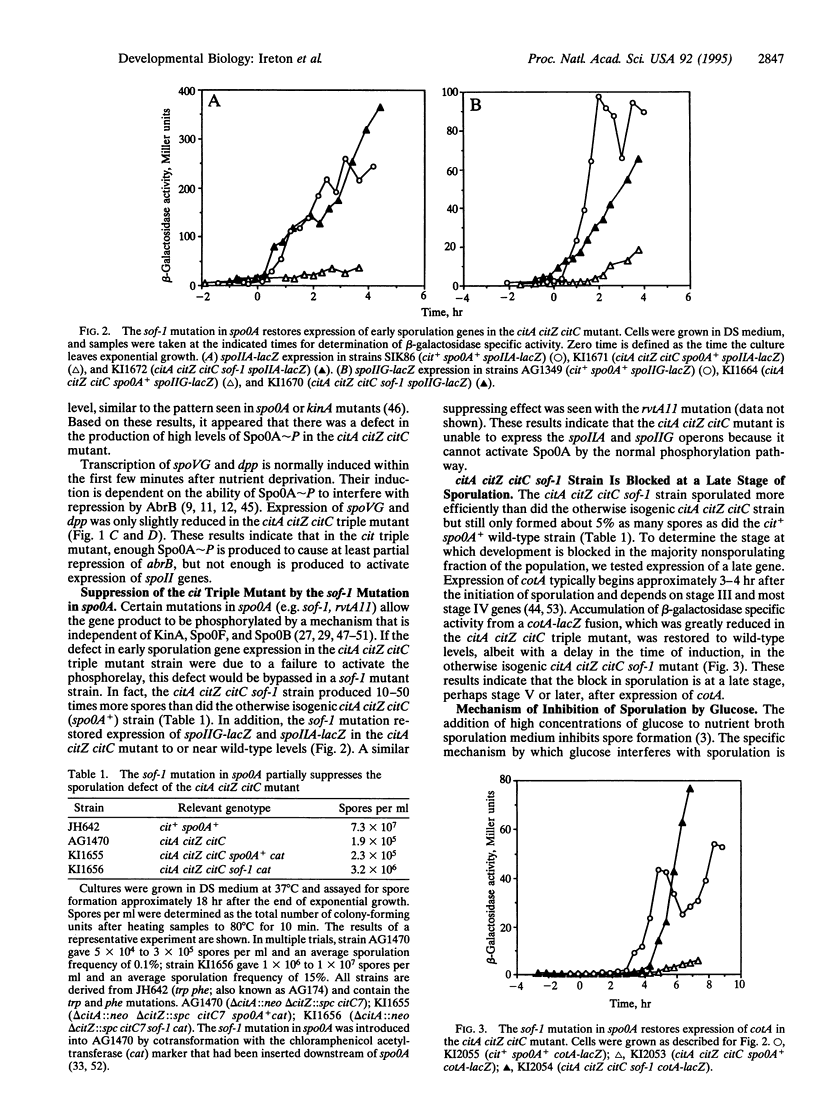


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniewski C., Savelli B., Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol. 1990 Jan;172(1):86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus J. M., Green B. D., Youngman P., Moran C. P., Jr Phosphorylation of Bacillus subtilis transcription factor Spo0A stimulates transcription from the spoIIG promoter by enhancing binding to weak 0A boxes. J Bacteriol. 1994 Jan;176(2):296–306. doi: 10.1128/jb.176.2.296-306.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. H., Grimsley J. K., Hoch J. A., Spiegelman G. B. Phosphorylation of Spo0A activates its stimulation of in vitro transcription from the Bacillus subtilis spoIIG operon. Mol Microbiol. 1993 Aug;9(4):741–749. doi: 10.1111/j.1365-2958.1993.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Burbulys D., Trach K. A., Hoch J. A. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991 Feb 8;64(3):545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Chibazakura T., Kawamura F., Takahashi H. Differential regulation of spo0A transcription in Bacillus subtilis: glucose represses promoter switching at the initiation of sporulation. J Bacteriol. 1991 Apr;173(8):2625–2632. doi: 10.1128/jb.173.8.2625-2632.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J. D., Stephanopoulos G., Ireton K., Grossman A. D. Gene expression in single cells of Bacillus subtilis: evidence that a threshold mechanism controls the initiation of sporulation. J Bacteriol. 1994 Apr;176(7):1977–1984. doi: 10.1128/jb.176.7.1977-1984.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutting S., Oke V., Driks A., Losick R., Lu S., Kroos L. A forespore checkpoint for mother cell gene expression during development in B. subtilis. Cell. 1990 Jul 27;62(2):239–250. doi: 10.1016/0092-8674(90)90362-i. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mandelstam J. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol. 1970 Sep;103(3):529–535. doi: 10.1128/jb.103.3.529-535.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortnagel P., Freese E. Analysis of sporulation mutants. II. Mutants blocked in the citric acid cycle. J Bacteriol. 1968 Apr;95(4):1431–1438. doi: 10.1128/jb.95.4.1431-1438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouet A., Sonenshein A. L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E. B., Vasantha N., Freese E. Induction of sporulation in developmental mutants of Bacillus subtilis. Mol Gen Genet. 1979 Feb 16;170(1):67–74. doi: 10.1007/BF00268581. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Lewis T., Levin N., DeVivo R. Suppressors of a spo0A missense mutation and their effects on sporulation in Bacillus subtilis. Biochimie. 1992 Jul-Aug;74(7-8):679–688. doi: 10.1016/0300-9084(92)90140-a. [DOI] [PubMed] [Google Scholar]
- Hoch J. A. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Trach K., Kawamura F., Saito H. Identification of the transcriptional suppressor sof-1 as an alteration in the spo0A protein. J Bacteriol. 1985 Feb;161(2):552–555. doi: 10.1128/jb.161.2.552-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo M., Nakayama A., Fukazawa K., Kawamura K., Ando K., Hori M., Furutani Y. A novel Bacillus subtilis gene involved in negative control of sporulation and degradative-enzyme production. J Bacteriol. 1990 Apr;172(4):1783–1790. doi: 10.1128/jb.172.4.1783-1790.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Grossman A. D. A developmental checkpoint couples the initiation of sporulation to DNA replication in Bacillus subtilis. EMBO J. 1994 Apr 1;13(7):1566–1573. doi: 10.1002/j.1460-2075.1994.tb06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Grossman A. D. Coupling between gene expression and DNA synthesis early during development in Bacillus subtilis. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8808–8812. doi: 10.1073/pnas.89.18.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Gunther N. W., 4th, Grossman A. D. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1994 Sep;176(17):5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K., Rudner D. Z., Siranosian K. J., Grossman A. D. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993 Feb;7(2):283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Jaacks K. J., Healy J., Losick R., Grossman A. D. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989 Aug;171(8):4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Sonenshein A. L. Identification of two distinct Bacillus subtilis citrate synthase genes. J Bacteriol. 1994 Aug;176(15):4669–4679. doi: 10.1128/jb.176.15.4669-4679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Sonenshein A. L. Transcriptional regulation of Bacillus subtilis citrate synthase genes. J Bacteriol. 1994 Aug;176(15):4680–4690. doi: 10.1128/jb.176.15.4680-4690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Saito H. Isolation and mapping of a new suppressor mutation of an early sporulation gene spoOF mutation in Bacillus subtilis. Mol Gen Genet. 1983;192(3):330–334. doi: 10.1007/BF00392171. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Wang L. F., Doi R. H. Catabolite-resistant sporulation (crsA) mutations in the Bacillus subtilis RNA polymerase sigma 43 gene (rpoD) can suppress and be suppressed by mutations in spo0 genes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8124–8128. doi: 10.1073/pnas.82.23.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney T. J., York K., Youngman P., Moran C. P., Jr Genetic evidence that RNA polymerase associated with sigma A factor uses a sporulation-specific promoter in Bacillus subtilis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9109–9113. doi: 10.1073/pnas.86.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Shoji K., Shimizu T., Nakano K., Sato T., Kobayashi Y. Analysis of a suppressor mutation ssb (kinC) of sur0B20 (spo0A) mutation in Bacillus subtilis reveals that kinC encodes a histidine protein kinase. J Bacteriol. 1995 Jan;177(1):176–182. doi: 10.1128/jb.177.1.176-182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDeaux J. R., Grossman A. D. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J Bacteriol. 1995 Jan;177(1):166–175. doi: 10.1128/jb.177.1.166-175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDeaux J. R., Yu N., Grossman A. D. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1995 Feb;177(3):861–863. doi: 10.1128/jb.177.3.861-863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick R., Stragier P. Crisscross regulation of cell-type-specific gene expression during development in B. subtilis. Nature. 1992 Feb 13;355(6361):601–604. doi: 10.1038/355601a0. [DOI] [PubMed] [Google Scholar]
- Mueller J. P., Bukusoglu G., Sonenshein A. L. Transcriptional regulation of Bacillus subtilis glucose starvation-inducible genes: control of gsiA by the ComP-ComA signal transduction system. J Bacteriol. 1992 Jul;174(13):4361–4373. doi: 10.1128/jb.174.13.4361-4373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J. P., Sonenshein A. L. Role of the Bacillus subtilis gsiA gene in regulation of early sporulation gene expression. J Bacteriol. 1992 Jul;174(13):4374–4383. doi: 10.1128/jb.174.13.4374-4383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmedo G., Ninfa E. G., Stock J., Youngman P. Novel mutations that alter the regulation of sporulation in Bacillus subtilis. Evidence that phosphorylation of regulatory protein SpoOA controls the initiation of sporulation. J Mol Biol. 1990 Oct 5;215(3):359–372. doi: 10.1016/s0022-2836(05)80357-2. [DOI] [PubMed] [Google Scholar]
- Perego M., Cole S. P., Burbulys D., Trach K., Hoch J. A. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989 Nov;171(11):6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Hoch J. A. Sequence analysis and regulation of the hpr locus, a regulatory gene for protease production and sporulation in Bacillus subtilis. J Bacteriol. 1988 Jun;170(6):2560–2567. doi: 10.1128/jb.170.6.2560-2567.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M., Spiegelman G. B., Hoch J. A. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988 Nov;2(6):689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Robertson J. B., Gocht M., Marahiel M. A., Zuber P. AbrB, a regulator of gene expression in Bacillus, interacts with the transcription initiation regions of a sporulation gene and an antibiotic biosynthesis gene. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8457–8461. doi: 10.1073/pnas.86.21.8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman K., Kroos L., Cutting S., Youngman P., Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988 Apr 5;200(3):461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- Satola S. W., Baldus J. M., Moran C. P., Jr Binding of Spo0A stimulates spoIIG promoter activity in Bacillus subtilis. J Bacteriol. 1992 Mar;174(5):1448–1453. doi: 10.1128/jb.174.5.1448-1453.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satola S., Kirchman P. A., Moran C. P., Jr Spo0A binds to a promoter used by sigma A RNA polymerase during sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4533–4537. doi: 10.1073/pnas.88.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji K., Hiratsuka S., Kawamura F., Kobayashi Y. New suppressor mutation sur0B of spo0B and spo0F mutations in Bacillus subtilis. J Gen Microbiol. 1988 Dec;134(12):3249–3257. doi: 10.1099/00221287-134-12-3249. [DOI] [PubMed] [Google Scholar]
- Siranosian K. J., Grossman A. D. Activation of spo0A transcription by sigma H is necessary for sporulation but not for competence in Bacillus subtilis. J Bacteriol. 1994 Jun;176(12):3812–3815. doi: 10.1128/jb.176.12.3812-3815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siranosian K. J., Ireton K., Grossman A. D. Alanine dehydrogenase (ald) is required for normal sporulation in Bacillus subtilis. J Bacteriol. 1993 Nov;175(21):6789–6796. doi: 10.1128/jb.175.21.6789-6796.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Sonenshein A. L. Mutations that relieve nutritional repression of the Bacillus subtilis dipeptide permease operon. J Bacteriol. 1993 Aug;175(15):4605–4614. doi: 10.1128/jb.175.15.4605-4614.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack F. J., Mueller J. P., Strauch M. A., Mathiopoulos C., Sonenshein A. L. Transcriptional regulation of a Bacillus subtilis dipeptide transport operon. Mol Microbiol. 1991 Aug;5(8):1915–1925. doi: 10.1111/j.1365-2958.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Spiegelman G., Van Hoy B., Perego M., Day J., Trach K., Hoch J. A. Structural alterations in the Bacillus subtilis Spo0A regulatory protein which suppress mutations at several spo0 loci. J Bacteriol. 1990 Sep;172(9):5011–5019. doi: 10.1128/jb.172.9.5011-5019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M. A., Spiegelman G. B., Perego M., Johnson W. C., Burbulys D., Hoch J. A. The transition state transcription regulator abrB of Bacillus subtilis is a DNA binding protein. EMBO J. 1989 May;8(5):1615–1621. doi: 10.1002/j.1460-2075.1989.tb03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M. A., Trach K. A., Day J., Hoch J. A. Spo0A activates and represses its own synthesis by binding at its dual promoters. Biochimie. 1992 Jul-Aug;74(7-8):619–626. doi: 10.1016/0300-9084(92)90133-y. [DOI] [PubMed] [Google Scholar]
- Strauch M., Webb V., Spiegelman G., Hoch J. A. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I. Catabolite repression-resistant mutants of Bacillus subtilis. Can J Microbiol. 1979 Nov;25(11):1283–1287. doi: 10.1139/m79-202. [DOI] [PubMed] [Google Scholar]
- Trach K. A., Hoch J. A. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol Microbiol. 1993 Apr;8(1):69–79. doi: 10.1111/j.1365-2958.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Trach K., Burbulys D., Strauch M., Wu J. J., Dhillon N., Jonas R., Hanstein C., Kallio P., Perego M., Bird T. Control of the initiation of sporulation in Bacillus subtilis by a phosphorelay. Res Microbiol. 1991 Sep-Oct;142(7-8):815–823. doi: 10.1016/0923-2508(91)90060-n. [DOI] [PubMed] [Google Scholar]
- Weir J., Predich M., Dubnau E., Nair G., Smith I. Regulation of spo0H, a gene coding for the Bacillus subtilis sigma H factor. J Bacteriol. 1991 Jan;173(2):521–529. doi: 10.1128/jb.173.2.521-529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. J., Piggot P. J., Tatti K. M., Moran C. P., Jr Transcription of the Bacillus subtilis spoIIA locus. Gene. 1991 May 15;101(1):113–116. doi: 10.1016/0378-1119(91)90231-y. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Kawamura F., Yoshikawa H., Takahashi H., Kobayashi Y., Saito H. Dissection of the expression signals of the spoA gene of Bacillus subtilis: glucose represses sporulation-specific expression. J Gen Microbiol. 1989 May;135(5):1335–1345. doi: 10.1099/00221287-135-5-1335. [DOI] [PubMed] [Google Scholar]
- York K., Kenney T. J., Satola S., Moran C. P., Jr, Poth H., Youngman P. Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J Bacteriol. 1992 Apr;174(8):2648–2658. doi: 10.1128/jb.174.8.2648-2658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Hanson R. S. Sporulation of tricarboxylic acid cycle mutants of Bacillus subtilis. J Bacteriol. 1972 Feb;109(2):886–894. doi: 10.1128/jb.109.2.886-894.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell. 1983 Nov;35(1):275–283. doi: 10.1016/0092-8674(83)90230-1. [DOI] [PubMed] [Google Scholar]


