Abstract
Extraintestinal manifestations occur commonly in inflammatory bowel diseases (IBD). Pulmonary manifestations (PM) of IBD may be divided in airway disorders, interstitial lung disorders, serositis, pulmonary vasculitis, necrobiotic nodules, drug-induced lung disease, thromboembolic lung disease and enteropulmonary fistulas. Pulmonary involvement may often be asymptomatic and detected solely on the basis of abnormal screening tests. The common embryonic origin of the intestine and the lungs from the primitive foregut, the co-existence of mucosa associated lymphoid tissue in both organs, autoimmunity, smoking and bacterial translocation from the colon to the lungs may all be involved in the pathogenesis of PM in IBD. PM are mainly detected by pulmonary function tests and high-resolution computed tomography. This review will focus on the involvement of the airways in the context of IBD, especially stenoses of the large airways, tracheobronchitis, bronchiectasis, bronchitis, mucoid impaction, bronchial granulomas, bronchiolitis, bronchiolitis obliterans syndrome and the co-existence of IBD with asthma, chronic obstructive pulmonary disease, sarcoidosis and a1-antitrypsin deficiency.
Keywords: Inflammatory bowel diseases, Airways, Bronchiolitis
Core tip: The lung is commonly involved in inflammatory bowel diseases; however, airway involvement is often overlooked. This review will help gastroenterologists recognize the involvement of the airways in the context of inflammatory bowel diseases (IBD), especially stenoses of the large airways, tracheobronchitis, bronchiectasis, bronchitis, mucoid impaction, bronchial granulomas, bronchiolitis, bronchiolitis obliterans syndrome and the co-existence of IBD with asthma, chronic obstructive pulmonary disease, sarcoidosis and a1-antitrypsin deficiency, and appropriately manage their patients.
INTRODUCTION
Extraintestinal manifestations (EIM) commonly occur in inflammatory bowel diseases (IBD), with a prevalence rate between 21%-41% reported in various series. Crohn’s disease (CD) presents with EIM more frequently than ulcerative colitis (UC)[1]. The most common EIM are erythema nodosum, pyoderma gangrenosum, arthritis, uveitis, episcleritis, mouth ulcers, renal stones, thromboembolic disease and primary sclerosing cholangitis. Pulmonary involvement complicating IBD was originally considered rare (with a frequency rate < 1%), but the first case series published in 1976 assisted in better recognition, evaluation and description of IBD related respiratory disease[2].
Pulmonary manifestations (PM) of IBD have been studied in the literature by small in size case-control studies, case reports and epidemiological population-based studies. Depending on the anatomic site involved, PM in IBD may be divided in to airway disorders, interstitial lung disorders, serositis, pulmonary vasculitis, necrobiotic nodules, drug-induced lung disease, thromboembolic lung disease and enteropulmonary fistulas. Concomitant occurrence of IBD with specific respiratory diseases [granulomatosis with polyangiitis (GPA), asthma, chronic obstructive pulmonary disease (COPD), alpha 1 antitrypsin deficiency and sarcoidosis] is not uncommon. Pulmonary involvement is often asymptomatic and may be detected solely on the basis of abnormal screening tests. This review will focus on the involvement of the airways in the context of IBD.
EPIDEMIOLOGY
The exact incidence and prevalence of PM in IBD is not known; however, airway involvement constitutes a large proportion, responsible for 40%-63% of overall respiratory incidents[3,4]. PM in total and specifically airway involvement seem to occur more commonly in UC than in CD.
Although IBD related PM were originally considered rare, certain population-based studies have revealed significant interrelationships between the lungs and IBD. Bernstein and colleagues in a large population-based study in North America in 2005 reported that airway disorders in general (including asthma and bronchitis) were the most common extraintestinal manifestation in subjects with CD and the second most common in subjects with UC, with the prevalence of asthma in this population between 7%-8%[5]. In another retrospective study from the opposite view, Birring reported that IBD was 4 times more prevalent among patients with airway diseases, particularly non-asthmatic patients with productive cough, than in the general population[6].
These epidemiological studies along with the observation of concordant EIM among siblings and first degree relatives suffering from IBD led to genome-wide studies that showed certain genetic predispositions for various EIM. Hence, in CD, HLA-A2 and HLA-DR1 and in UC, haplotypes HLA-B27, HLA-B58 and HLA-B8/DR3 are all linked to IBD related skin, joint and eye disorders[7,8]. To our knowledge, such a genetic predisposition for respiratory involvement in IBD has not been demonstrated yet.
PATHOGENESIS
Current theory for the pathogenesis of IBD postulates that in genetically susceptible individuals, environmental triggering factors cause a local immunologically aberrant intestinal injury and repair as a response to commensal bacteria. Environmental factors implicated are smoking (for CD), stress, infection, drugs and diet. Polymorphisms in NOD2-CARD15 (caspase recruitment domain family member 15) are found in 25%-35% of patients of European descent with CD; haplotype HLA DRB1*0103 within major histocompatibility complex (MHC) is associated with susceptibility to and extensive UC. Various genetic mutations (ATG16L1, IL-23 receptor, TNF polymorphisms) confer to result in disease progression by loosening of the intestinal epithelial barrier, decreasing of microbial clearance, loss of tolerance of the mucosa to enteric microflora (autophagy) and immunological dysregulation. Innate and adaptive immunity recognize microbial antigens and through pattern recognition receptors [toll-like receptors (TLR), nucleotide binding domain like receptors (NLR)] initiate an abnormal T-cell expansion, in the form of Th-1 and Th-17 pathways in CD and mainly Th-2 pathway in UC, that lead to chronic mucosal inflammation and disease[9,10].
Although significant progression is noted in the understanding of the pathogenesis of IBD, the exact mechanisms responsible for the cross-talk between bowel disease and airway disorders are not clearly elucidated. This association is important since clinical observations have repeatedly pointed out the phenomenon of a respiratory exacerbation occurring suddenly in patients with IBD after a therapeutic intervention (enterectomy) for their colon disease, with respiratory disease being completely unresponsive to this intervention[11]. However, the common embryonic origin of the intestine and the lungs from the primitive foregut should be considered the basis for this association. Their common origin reflects their common structural features: an extensive luminal surface area, protected by a tight epithelial barrier that covers a submucosal layer of goblet cells, glands and, most importantly, lymphoid tissue responsible for homing of lymphocytes, as well as innate and adaptive immunity[12].
Bronchus associated lymphoid tissue (BALT) and gut associated lymphoid tissue (GALT) are both parts of the mucosa associated lymphoid tissue (MALT). Lymphocytes become activated according to the inflammatory stimuli they receive and mis-homing of lymphocytes may provide an explanation for the migration of inflammation[13,14]. Furthermore, as we described earlier, apart from immune-mediated phenomena, autoimmunity [pANCA, anti-Saccharomyces cerevisiae antibodies (ASCA) and antibodies against intestinal epithelial antigens] also contributes to the pathogenesis of IBD[15]. It is therefore likely that based on common structures and the affinities of the lymphoid tissue, circulating immune complexes, stimuli and autoantibodies migrate from the intestine to bronchial and possibly alveolar epithelium, leading to airway inflammation and disease. This shift of inflammation may become more dramatic when the colon is removed after colectomy. In line with this, Adenis and colleagues in a scintigraphy study demonstrated increased pulmonary permeability in patients with CD[16].
Certain contributing factors to such presumed pathogenesis may be proposed. Smoking is a well-known risk factor both for airway diseases and CD[17]. Bacterial translocation occurring in IBD may well affect the lung microbiome and confer to result in airway disorders since we already know that abnormal microbiome of the lungs carries implications in the pathogenesis of COPD[18]. Common TLR molecules (TLRs 2 and 4) participate in the pathogenesis of both COPD and IBD[19,20]. Lastly, matrix metalloproteinases (MMP) and anti-proteases like alpha-1 antitrypsin, well known as a cause of pulmonary emphysema, have been increasingly studied in the pathogenesis of IBD where their expression and balance seems to be disrupted, offering another potential link between the two systems[21]. A proposed schema for the pathogenesis of IBD related airway diseases is shown in Figure 1.
Figure 1.
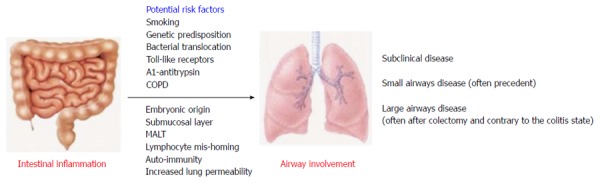
Possible pathogenesis of airway involvement and disease in the event of inflammatory bowel diseases. COPD: Chronic obstructive pulmonary disease; MALT: Mucosa associated lymphoid tissue.
PATHOLOGY
As described earlier in this article, in the context of IBD, pathology examination may reveal abnormalities in different lung compartments, namely the airways, the interstitial tissue, the pleura, the parenchyma and the vessels. Interstitial pneumonitis and drug related eosinophilic pneumonia have been described; however, most intriguing for the pathologist is the differentiation between CD related pulmonary involvement and GPA in the case of pulmonary nodules, particularly since CD and GPA may coincide, as shown in several reports[22]. In this review, we shall focus on subclinical disease and airway pathology findings.
Bronchoalveolar lavage (BAL) studies have shown that chronic inflammation is common in the bronchi and alveoli of patients with CD. Wallaert et al[23] reported that 61% of asymptomatic patients with CD exhibit BAL features of an overt lymphocytic alveolitis. The clinical significance of this phenomenon, which provides further evidence for a systemic immunological manifestation of IBD to the lungs, is unknown. In our opinion, this alveolitis will not necessarily progress to clinical stage disease.
Small case series have described all types of biopsy proven bronchiolitis, documented with wedge and transbronchial biopsies mostly but also with open lung biopsies. Granulomatous bronchiolitis is more common; acute bronchiolitis with peribronchiolar inflammation, concentric small airways fibrosis, constrictive bronchiolitis and diffuse panbronchiolitis are also reported[4]. It should be highlighted that in the larger series by Casey and colleagues, bronchiolitides usually present before or concurrently with bowel disease, unlike other respiratory disorders that commonly follow bowel disease by a considerable time[24].
SCREENING FOR AIRWAY DISEASES
Airway disease, either latent and subclinical or clinically active, should be recognized for several reasons: (1) airway disease may complicate and follow a course independent of the course of the primary IBD; (2) IBD related airway diseases necessitate appropriate treatment and follow-up; (3) certain pulmonary function tests (PFT) may have a role as potential markers of disease activity; and (4) screening for respiratory disease may add to the recognition of concurrent diseases such as asthma and sarcoidosis. Screening for airway disease in the context of IBD may include medical history and clinical examination, PFT and radiological examinations.
Symptoms - clinical examination
Patients examined in an IBD clinic should be regularly asked about respiratory symptoms as nearly half of them report at least one symptom which they may attribute to anything but their primary disease. The most common symptoms reported are cough, sputum production, breathlessness mainly while exercising and wheezing. Stridor and hoarseness may develop in cases of upper airway narrowing.
Camus and colleagues reported that respiratory symptoms follow IBD presentation by months or even decades in nearly 85% of patients. In 10%-15%, respiratory symptoms precede and rarely (5%-10%) coincide with inflammatory bowel disease[3]. Respiratory symptom prevalence ranges from 25.6% in a recent study of 30 UC and 9 CD patients to 50% in an older study of 11 CD and 19 UC patients[25,26]. In a case control study of 64 IBD patients compared to 1346 controls, after adjustment for age, sex and smoking status, IBD patients were more likely to report shortness of breath and sputum production and to a lesser degree, cough [odds ratio (OR) respectively 3.4, 2.5, 1.8], highlighting the importance of relative clinical awareness[27].
Moreover, Higenbottam et al[28] described a respiratory exacerbation with cough and shortness of breath several years after coloproctectomy with the primary IBD in remission. This finding has been consistently described by other authors and several case reports. Thus, respiratory symptoms may occur independently of the course and activity of the bowel disease and are not responsive in parallel to its surgical treatment; on the contrary, pulmonary disease may be exacerbated. Most of these cases are reported with ulcerative colitis.
When respiratory symptoms occur acutely, the differential diagnosis includes pulmonary embolism, infectious pneumonia and drug toxicity. When respiratory disease follows an indolent course, airway disorders are more likely. Prompt evaluation in the latter scenario should include detailed medical history (with an emphasis on smoking habit, history of asthma and medication used), pulmonary function tests and radiological exams, including high resolution computed tomography scan (HRCT) of the chest with expiratory maneuvers. Bronchoscopy, as we will discuss further, is mandatory when there is evidence of upper airway involvement.
PFT
Symptomatic IBD patients may have normal PFT; this is apparently due to the anatomic site and the recruiting capacity of the airway system (bigger for small airways, smaller for large airways). On the other hand, up to two thirds of asymptomatic IBD patients have been found to demonstrate PFT abnormalities in recent studies, a surprising finding in light of the past reports of infrequent pulmonary involvement in IBD.
A prospective study of 40 IBD patients reported a 55% frequency of abnormal PFT in active IBD, equally distributed between UC and CD. This finding fell to 17.5% when IBD went into remission[29]. Herrlinger et al[30] reported 39% frequency of abnormal PFT in CD patients and 45% in UC, all asymptomatic, with values more affected during IBD activity but persisting in remission. Yilmaz et al[25] found abnormal PFT in 56% of IBD patients, with results directly affected by disease activity in UC cases. Another case-control study of 23 UC and 13 CD patients demonstrated abnormal PFT in 58% [75% of total events included low diffusing capacity for carbon monoxide (DLCO)], 81% of these patients with active IBD[31]. Lastly, in the larger study of 83 UC and 12 CD patients, Desai et al[32] reported abnormal PFT in 28.5% and low DLCO in 18%. Most of these studies included a mixed CD and UC population. UC patients, however, usually outnumbered CD patients, perhaps making PFT abnormalities seem more frequent in UC.
Decreased forced expiratory volume in the 1st second (FEV1), decreased FEV1/forced vital capacity (FVC) ratio, increased residual volume (RV)/total lung capacity (TLC) ratio, low forced expiratory flow (FEF25-75) and, more importantly, decreased DLCO are the parameters noted to be abnormal in IBD patients in the existing literature. While FEV1 and FEV1/FVC ratio have been found to be normal in certain older studies, other studies and recent data suggest mild functional compromise[33,34]. Results should be interpreted with caution since different criteria have been used to define abnormal and certain studies included smoking patients or an inappropriate control population. In current studies, when an obstructive pattern has been noted, it demonstrates only partial reversibility which helps to differentiate it from asthma. RV/TLC ratio elevation has been demonstrated to correlate with bowel disease activity in several studies[35]. Tzanakis et al[36] have thoroughly described small airways disease, particularly in the event of active UC or CD.
The most consistent finding is a reduction in DLCO that commonly correlates with disease activity. In a large study of 47 CD and 85 UC patients, decreased DLCO was found in 19% and 17.6% respectively, with values being worse when the primary disease was active[37]. In another major study, DLCO was abnormal in 53% of inactive UC and in 81% of active UC patients; DLCO also correlated with pathological intestinal disease activity grading[38]. In children with IBD where PFT abnormalities are rather rare, reduced DLCO was the only finding in 53% of 26 children with active CD[39]. In conclusion, PFT and DLCO abnormalities in asymptomatic IBD are frequent but particularly so in active UC. It is believed that, as a systemic inflammatory disease, IBD affects the lung, creating a mild pulmonary inflammation corresponding to bowel inflammation. It is currently unknown, however, whether DLCO could serve as a disease activity marker.
Bronchial hyperresponsiveness (BHR) seems to occur more frequently in IBD than in a control population. This was demonstrated in 71% of children with CD and in 41% non-atopic IBD patients[40,41]. In line with this, airway eosinophilia and deranged induced sputum have also been reported in IBD[42]. These features may be associated with concomitant asthma or be subclinical and attributed solely to the underlying IBD. Thus, the etiology of BHR IBD may be two-fold: atopic and secondary to intestinal mucosal inflammation via the increased lung permeability observed in IBD[16].
It is currently unclear if asymptomatic patients with PFT abnormalities will progress to clinical respiratory disease and if so what defines this progression. Until more knowledge is acquired, PFT abnormalities should dictate closer follow-up of these patients.
Radiology - high resolution computed tomography
Studies show that HRCT in patients with IBD often demonstrates abnormal findings. The most common findings include bronchial wall thickening and bronchiectasis, cysts, emphysema, ground glass opacities and reticulonodular opacities. Centrilobular nodules, “tree-in-bud” opacities, air trapping and a mosaic pattern in expiratory scans all constitute a bronchiolitis radiological appearance. The prevalence ranges from 22% to 89% and radiological findings may be independent of symptoms, PFT results and primary disease intestinal activity[32]. This is because these findings may not be solely attributed to primary IBD related pulmonary disease. Alternative diagnoses include COPD, smoking related bronchiolitis, smoking related interstitial lung abnormalities, drug toxicities, thromboembolic disease and infections.
AIRWAY DISEASES IN IBD
Airway inflammation and disease are the most prevalent and distinctive pattern of respiratory involvement in IBD and accounts for 40%-63% of the total of clinically significant pulmonary complaints. Airway diseases (AD) usually follow IBD presentation by many years or even decades and the opposite presentation is the exception; when AD occur, IBD is rather inactive. If left untreated, AD can lead to irreversible stenoses in the airway. The clinical manifestations depend on the exact anatomic site involved. A classification for AD is shown in Table 1.
Table 1.
| Site of involvement | Manifestations | Percent of total PM |
| Upper extrathoracic and intrathoracic airways (larynx/glottis, trachea, mainstem bronchi) | Stenoses, tracheobronchitis, acute respiratory failure | 7%-8% |
| Large airways | Bronchiectasis | 23%-26% |
| Simple chronic bronchitis without suppuration | 10%-20% | |
| Mucoid impaction | ||
| Bronchial granulomas | ||
| Suppurative bronchitis | 3%-8% | |
| Small airways | Granulomatous bronchiolitis | 3%-10% |
| Acute bronchiolitis | ||
| Diffuse panbronchiolitis | ||
| Bronchiolitis obliterans syndrome | ||
| Concomitant diseases involving the airways | Asthma | |
| Chronic obstructive pulmonary disease | ||
| Sarcoidosis | ||
| A1 antitrypsin deficiency |
PM: Pulmonary manifestations.
Upper airways
Upper airway disease (UAD) in IBD is a rare entity that may involve pharynx, larynx, trachea and mainstem bronchi. A total of 24 cases have been reported in the literature. Exudative lesions affect the bronchial mucosa and may cause subglottic stenosis and tracheobronchitis. UAD has been described in both UC and CD, with UC being predominant. Usually, it occurs years after diagnosis of the bowel disease, with IBD stable or in remission. UAD can occur after colectomy, with the time interval being as short as 30 d.
UAD may present with hoarseness, stridor and severe respiratory distress or just with cough, phlegm and shortness of breath[43,44]. Physical examination may reveal wheezing during inspiration, expiration or both. Because the clinical presentation may mimic infection or asthma, a certain degree of clinical suspicion is required to suspect an insult to the upper airway as a consequence of IBD. PFT and radiology are helpful in the diagnosis. A flow-volume loop demonstrates variable extrathoracic obstruction or fixed upper airway obstruction with plateau at both phases of respiration. While chest radiography has subtle findings, a CT scan of the chest may show circumferential or nodular narrowing of the trachea and bronchi. Bronchoscopy is the diagnostic procedure of choice[45]. Mucosal inflammation with exuberant pseudotumoral lesions, deformities, whitish lesions and narrowing of the lumen have all been described. Histology shows neutrophilic inflammation, granulation tissue, ulcerations, squamous cell metaplasia and plasma cell submucosal infiltrates. Noncaseating bronchial granulomas have also been reported in CD.
The differential diagnosis of IBD related UAD includes sarcoidosis, tuberculosis, amyloidosis and GPA[46]. The clinical presentation may be confusing if there is no bowel disease activity or other extraintestinal manifestations of IBD or if there is anti-neutrophil cytoplasmic antibodies (ANCA) positivity. ANCA are found to be positive in 50%-90% of UC and 10%-20% of CD patients but usually are neither cytoplasmic nor perinuclear in location[47]. The ANCA type specificity and histology of the airway lesions may help differentiate IBD-related UAD from GPA. Interestingly, isolated ANCA positivity without vasculitis has been associated with isolated subglottic stenosis in one study[48].
Empirical data suggest initial treatment with systemically administered high doses of corticosteroids (prednisone 1 mg/kg of lean body weight administered orally or methylprednisolone 60-80 mg intravenously per day); these suggestions are drawn from case reports, however, and not from randomized controlled trials, and should therefore be critically viewed[3]. In refractory cases, rigid bronchoscopy and interventional bronchoscopy procedures (laser beam, balloon dilation, stent placement) may be required in order to maintain an adequate airway[49,50].
Large airways
Large airways include the bronchi from the level of lobar bronchi to the level of terminal bronchioles. Large airways are the most common anatomic site of respiratory involvement in IBD, accounting for about 50% of total PM. Bronchial disease is more common in non-smoking females in their 5th decade of life in UC, particularly when other extraintestinal manifestations are present. Bronchial disease occurs many years after IBD in 8%-85% of patients, precedes IBD in younger patients (10%-15%) and less often coincides with active IBD (5%-10%). In 79% of cases, IBD is inactive and 50% of reported bronchial disease has followed colectomy[3,4].
The main large airway disorders are bronchiectasis (BE), chronic bronchitis (CB), suppurative bronchitis and mucoid impaction (Table 1). The classification depends on the patient’s symptoms (the presence or absence of copious purulent sputum in the absence of BE classifies patient as suppurative bronchitis or CB, respectively) and HRCT features. Such features include luminal dilatation with bronchial wall thickening (definition of BE), bronchial wall thickening alone (CB) and mucoid impaction.
PFT usually reveal an obstructive pattern non-reversible to bronchodilators, or occasionally a mixed obstructive and restrictive pattern. CB diagnosis may be difficult to establish, particularly in long standing respiratory illnesses or in the presence of smoking. However, it should be noted that in most studies the reported patients were never smokers. Moreover, UC is epidemiologically connected to non-smoking individuals; in this setting, diagnosis of CB becomes more straightforward.
BE is the most important IBD related lung disorder. Typically, UC patients appear to present more commonly than CD. The bowel disease is often inactive and patients present with subacute symptoms of sputum production, cough and shortness of breath. In half of IBD-BE cases, a curative surgery (colectomy) has preceded the diagnosis of BE. In this setting, a close temporal relationship of weeks to months is well documented[11]. Spira and colleagues reported 6 UC and 1 CD patient with BE and CB in half of them manifesting after colectomy[51]. In another study of 14 UC and 3 CD patients, 76% developed BE, 41% shortly after enterectomy; such features are verified by other studies as well[3,52]. The most popular explanation for this sequence implicates a shift of mediators from the resected bowel to the lung, based on their common embryonic origin. In addition, withdrawal of immune-modulatory medication like corticosteroids after IBD remission may play a role in the flare up of pulmonary disease. Autoimmunity, as postulated by other authors, may also play a role as antinuclear antibodies have been discovered in some cases[53].
Importantly, bronchial disease is treated separately and independently from the bowel disease. As such, colectomy would aggravate rather than palliate bronchial disease. Treatment is the same as with any other cause of non-cystic fibrosis bronchiectasis. Antibiotics, bronchial toilet and bronchodilators should be offered as usual in all BE. Historically, certain authors advocate the use of corticosteroids in IBD-BE, based on case series and personal experience. These authors suggest inhaled corticosteroids should be used initially and if response is poor suggest prednisone administered orally at a dose of 0.5 mg/kg. Methylprednisolone has been also lavaged through the bronchoscope directly into the airways. Since no hard evidence supports the use of corticosteroids in IBD-BE or in bronchiectasis in general, and because of the concern for long term treatment with corticosteroids (CS), it is the authors’ opinion that CS should not be administered in primary IBD related airway diseases[3,46,54].
Small airways
Small airways refer to the transitional airway zone from terminal bronchioles to alveolar ducts. Although larger case series attribute bronchiolitides as only 3%-10% of total IBD related pulmonary manifestations, their true involvement may be greater[4]. Kelly and colleagues evaluated 10 patients with IBD and bronchiectasis and found that 70% of them had abnormal FEF25-75, suggesting that subclinical small airways disease is more frequent[55].
Bronchiolitides, the main form of small airway disorders, share a different clinical presentation than the clinical characteristics of upper and large airways involvement. They occur at a younger age, earlier in the disease course and in 1/3 of cases, pulmonary disease precedes intestinal disease[4]. As such, patients with bronchiolitis who have yet to be diagnosed with IBD often undergo invasive diagnostic procedures (bronchoscopy, open lung biopsy). In contrast to other pulmonary manifestations of IBD, the bronchiolitides are equally relevant in Crohn’s disease and ulcerative colitis, as opposed to rest of airway disorders that are more prevalent in UC.
Pulmonary pathology in small airway involvement has been described earlier in this article. Granulomatous bronchiolitis is the most common finding, accounting for 59% of cases, and relates to CD as a systemic granulomatous disease[24]. PFT commonly demonstrates an obstructive pattern that may derange FEV1 or only FEF25-75. DLCO is often abnormal as well[36]. HRCT features are the same as for all bronchiolitides (as already described).
This secondary bronchiolitis may be acute or, more commonly, chronic. Chronic bronchiolitis that persists untreated significantly worsens prognosis since it may eventually progress to diffuse airway narrowing and bronchiolitis obliterans syndrome (BOS). It may also lead to the progressive formation of bronchiolectasis and bronchiectasis[56]. This development is important because it explains physiologically the coexistence of small airways disease with larger airway involvement observed in patients with IBD. Since CS have a modest effect on small airways disease, we suggest the use of macrolides in the setting of IBD related bronchiolitis. Macrolides have shown clinical benefit in diffuse panbronchiolitis and BOS; azithromycin is shown to inhibit epithelial to mesenchymal transition and fibrosis to the small airways[57]. Nevertheless, in cases of BOS and despite therapy, transplantation may eventually be needed.
Concomitant diseases involving the airways
Asthma: After arthritis, asthma is the most common comorbidity in both UC and CD. A large population-based epidemiological study at the University of Manitoba compared 3873 UC cases with 38674 matched controls and found a 7.88% incidence of asthma in UC (especially in males); similarly, they studied 4187 CD cases with 41815 matched controls and found a 7.09% prevalence ratio of asthma in CD (especially in females)[5].
We have already discussed the increased prevalence of BHR and atopy in IBD. It should be noted that when a clinical phenotype of asthma is established in a patient with IBD, appropriate treatment is mandatory since there is epidemiological evidence of increased mortality in the asthma-UC population and laboratory evidence of more severe BHR in asthma-UC patients[58,59].
COPD: Cigarette smoke is known to protect against UC but promotes CD progression. Another population-based study investigated the relationship between COPD and IBD. Investigators found that COPD cases had a 1.83 hazard ratio (HR) for UC and a 2.72 HR for CD; this hazard extended to first degree relatives. As such, an inflammatory vulnerability in COPD patients has been postulated[60]. A very recent study evaluating the intestinal function of COPD patients demonstrated that COPD, regarded as a systemic inflammatory disease, causes intestinal hyperpermeability and enterocyte damage leading to intestinal compromise. The latter potentially provides an explanation for this coincidence from an etiological and environmental point of view[61].
COPD in IBD patients should be investigated, recognized and treated appropriately. COPD increases all-cause mortality and specific cause mortality in patients with CD. A meta-analysis by Duricova and colleagues reported a standardized mortality rate of 2.55 for CD-COPD subjects[62].
Sarcoidosis: Sarcoidosis shares many common characteristics with IBD, especially CD, as they both are granulomatous diseases. Sarcoidosis and IBD may coincide, as shown by case reports and population-based studies. They both share multi-organ manifestations (joints, eye, skin). Sarcoidosis and IBD seem to share a genetic overlap regarding cytoplasmic nucleotide oligomerization domains 1 and 2 and certain polymorphisms in chromosomes 1 (loci IL-23R and 1q.24.3) and 15 (locus HERC2)[63].
IBD, as discussed earlier in this article, may exhibit granulomatous lung disease, mainly bronchiolitis. If infections have been ruled out, it is intriguing to differentiate between an IBD pulmonary localization and concomitant sarcoidosis. Histopathological features pointing to sarcoidosis are a lymphangitic distribution of granulomas and the absence of interstitial pneumonia and chronic bronchiolitis[24]. A clinical approach, however, is mandatory as the diagnosis of sarcoidosis apart from granuloma pathology demands compatible clinical and radiological findings.
A1 antitrypsin deficiency: Heterozygosity for the PiZ allele of a1 antitrypsin (AAT) deficiency (AATD) has been found to be more prevalent in patients with UC than in the general Swedish population (8.5% vs 4.7%)[64]. More importantly, a recent study from the United Kingdom confirmed a higher prevalence of UC among subjects suffering from emphysema due to homozygous PiZZ allele and AATD in comparison to the general population (1.5% vs 0.4%)[65]. Consequently, a blood test for AATD should be ordered when emphysema and IBD coincide in a young patient. It is unknown if AAT supplementation could be of use in the therapy of IBD.
CONCLUSION
Patients with IBD may present sometime during the course of their disease with various pulmonary incidents. The clinical approach relies upon the doctor’s knowledge and judgment to attribute the patient’s symptoms to the primary disease, comorbidities or to other complications after a thorough investigation. A proposed diagnostic algorithm for the evaluation of respiratory disease in IBD is shown in Figure 2. Table 2 contains the key messages that, in our opinion, summarize the pulmonary-intestinal interrelationships in inflammatory bowel diseases.
Figure 2.
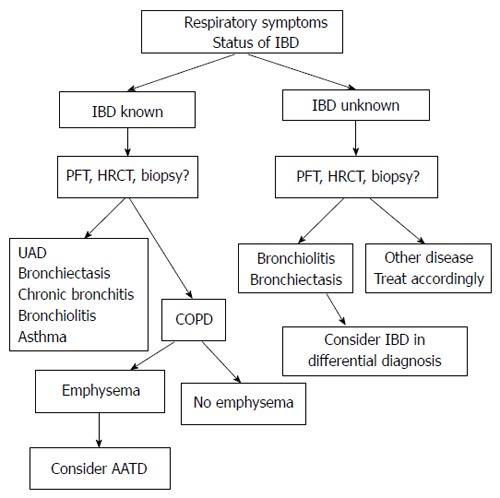
Proposed diagnostic algorithm for the evaluation of airway disease in inflammatory bowel diseases. IBD: Inflammatory bowel disease; PFT: Pulmonary function tests; HRCT: High resolution computed tomography; UAD: Upper airway disease; COPD: Chronic obstructive pulmonary disease; AATD: A1 antitrypsin deficiency.
Table 2.
Key messages
| In a patient with IBD and respiratory symptoms, symptoms should be initially attributed to the primary disease because of significant lung-intestine interference |
| IBD, asthma and COPD often coincide |
| IBD should be always remembered in the differential diagnosis of bronchiectasis and bronchiolitis |
| PFT and HRCT are necessary to evaluate a symptomatic patient |
| IBD related airway disease does not necessarily follow the course of colitis |
IBD: Inflammatory bowel disease; COPD: Chronic obstructive pulmonary disease; PFT: Pulmonary function tests; HRCT: High resolution computed tomography.
Footnotes
P- Reviewer: Ibrahim M, Kozarek R, Ljubicic N, Seow-Choen F S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Wang CH
References
- 1.Hoffmann RM, Kruis W. Rare extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:140–147. doi: 10.1097/00054725-200403000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Kraft SC, Earle RH, Roesler M, Esterly JR. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med. 1976;136:454–459. [PubMed] [Google Scholar]
- 3.Camus P, Piard F, Ashcroft T, Gal AA, Colby TV. The lung in inflammatory bowel disease. Medicine (Baltimore) 1993;72:151–183. [PubMed] [Google Scholar]
- 4.Black H, Mendoza M, Murin S. Thoracic manifestations of inflammatory bowel disease. Chest. 2007;131:524–532. doi: 10.1378/chest.06-1074. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein CN, Wajda A, Blanchard JF. The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology. 2005;129:827–836. doi: 10.1053/j.gastro.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 6.Raj AA, Birring SS, Green R, Grant A, de Caestecker J, Pavord ID. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med. 2008;102:780–785. doi: 10.1016/j.rmed.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Roussomoustakaki M, Satsangi J, Welsh K, Louis E, Fanning G, Targan S, Landers C, Jewell DP. Genetic markers may predict disease behavior in patients with ulcerative colitis. Gastroenterology. 1997;112:1845–1853. doi: 10.1053/gast.1997.v112.pm9178675. [DOI] [PubMed] [Google Scholar]
- 8.Orchard TR, Chua CN, Ahmad T, Cheng H, Welsh KI, Jewell DP. Uveitis and erythema nodosum in inflammatory bowel disease: clinical features and the role of HLA genes. Gastroenterology. 2002;123:714–718. doi: 10.1053/gast.2002.35396. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 10.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 11.Eaton TE, Lambie N, Wells AU. Bronchiectasis following colectomy for Crohn’s disease. Thorax. 1998;53:529–531. doi: 10.1136/thx.53.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 13.Holt PG. Development of bronchus associated lymphoid tissue (BALT) in human lung disease: a normal host defence mechanism awaiting therapeutic exploitation? Thorax. 1993;48:1097–1098. doi: 10.1136/thx.48.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012;5:7–18. doi: 10.1038/mi.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen Z, Fiocchi C. Inflammatory bowel disease: autoimmune or immune-mediated pathogenesis? Clin Dev Immunol. 2004;11:195–204. doi: 10.1080/17402520400004201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adenis A, Colombel JF, Lecouffe P, Wallaert B, Hecquet B, Marchandise X, Cortot A. Increased pulmonary and intestinal permeability in Crohn’s disease. Gut. 1992;33:678–682. doi: 10.1136/gut.33.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Sethi S. Chronic obstructive pulmonary disease and infection. Disruption of the microbiome? Ann Am Thorac Soc. 2014;11 Suppl 1:S43–S47. doi: 10.1513/AnnalsATS.201307-212MG. [DOI] [PubMed] [Google Scholar]
- 19.Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, Sethi S. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014;69:811–818. doi: 10.1136/thoraxjnl-2013-203669. [DOI] [PubMed] [Google Scholar]
- 20.Bank S, Andersen PS, Burisch J, Pedersen N, Roug S, Galsgaard J, Turino SY, Brodersen JB, Rashid S, Rasmussen BK, et al. Associations between functional polymorphisms in the NFκB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharmacogenomics J. 2014:Epub ahead of print. doi: 10.1038/tpj.2014.19. [DOI] [PubMed] [Google Scholar]
- 21.Matusiewicz M, Neubauer K, Mierzchala-Pasierb M, Gamian A, Krzystek-Korpacka M. Matrix metalloproteinase-9: its interplay with angiogenic factors in inflammatory bowel diseases. Dis Markers. 2014;2014:643645. doi: 10.1155/2014/643645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaszar LT, Orzechowski NM, Specks U, Ytterberg SR, Loftus EV, Mark EJ, Tazelaar HD. Coexistent pulmonary granulomatosis with polyangiitis (Wegener granulomatosis) and Crohn disease. Am J Surg Pathol. 2014;38:354–359. doi: 10.1097/PAS.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 23.Wallaert B, Dugas M, Dansin E, Perez T, Marquette CH, Ramon P, Tonnel AB, Voisin C. Subclinical alveolitis in immunological systemic disorders. Transition between health and disease? Eur Respir J. 1990;3:1206–1216. [PubMed] [Google Scholar]
- 24.Casey MB, Tazelaar HD, Myers JL, Hunninghake GW, Kakar S, Kalra SX, Ashton R, Colby TV. Noninfectious lung pathology in patients with Crohn’s disease. Am J Surg Pathol. 2003;27:213–219. doi: 10.1097/00000478-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz A, Yilmaz Demirci N, Hoşgün D, Uner E, Erdoğan Y, Gökçek A, Cağlar A. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol. 2010;16:4952–4957. doi: 10.3748/wjg.v16.i39.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceyhan BB, Karakurt S, Cevik H, Sungur M. Bronchial hyperreactivity and allergic status in inflammatory bowel disease. Respiration. 2003;70:60–66. doi: 10.1159/000068407. [DOI] [PubMed] [Google Scholar]
- 27.Birring SS, Morgan AJ, Prudon B, McKeever TM, Lewis SA, Falconer Smith JF, Robinson RJ, Britton JR, Pavord ID. Respiratory symptoms in patients with treated hypothyroidism and inflammatory bowel disease. Thorax. 2003;58:533–536. doi: 10.1136/thorax.58.6.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higenbottam T, Cochrane GM, Clark TJ, Turner D, Millis R, Seymour W. Bronchial disease in ulcerative colitis. Thorax. 1980;35:581–585. doi: 10.1136/thx.35.8.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ateş F, Karincaoğlu M, Hacievlıyagıl SS, Yalniz M, Seçkın Y. Alterations in the pulmonary function tests of inflammatory bowel diseases. Turk J Gastroenterol. 2011;22:293–299. doi: 10.4318/tjg.2011.0215. [DOI] [PubMed] [Google Scholar]
- 30.Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol. 2002;97:377–381. doi: 10.1111/j.1572-0241.2002.05473.x. [DOI] [PubMed] [Google Scholar]
- 31.Songür N, Songür Y, Tüzün M, Doğan I, Tüzün D, Ensari A, Hekimoglu B. Pulmonary function tests and high-resolution CT in the detection of pulmonary involvement in inflammatory bowel disease. J Clin Gastroenterol. 2003;37:292–298. doi: 10.1097/00004836-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Desai D, Patil S, Udwadia Z, Maheshwari S, Abraham P, Joshi A. Pulmonary manifestations in inflammatory bowel disease: a prospective study. Indian J Gastroenterol. 2011;30:225–228. doi: 10.1007/s12664-011-0129-1. [DOI] [PubMed] [Google Scholar]
- 33.Godet PG, Cowie R, Woodman RC, Sutherland LR. Pulmonary function abnormalities in patients with ulcerative colitis. Am J Gastroenterol. 1997;92:1154–1156. [PubMed] [Google Scholar]
- 34.Johnson NM, Mee AS, Jewell DP, Clarke SW. Pulmonary function in inflammatory bowel disease. Digestion. 1978;18:416–418. doi: 10.1159/000198228. [DOI] [PubMed] [Google Scholar]
- 35.Neilly JB, Main AN, McSharry C, Murray J, Russell RI, Moran F. Pulmonary abnormalities in Crohn’s disease. Respir Med. 1989;83:487–491. doi: 10.1016/s0954-6111(89)80132-5. [DOI] [PubMed] [Google Scholar]
- 36.Tzanakis N, Samiou M, Bouros D, Mouzas J, Kouroumalis E, Siafakas NM. Small airways function in patients with inflammatory bowel disease. Am J Respir Crit Care Med. 1998;157:382–386. doi: 10.1164/ajrccm.157.2.97-04075. [DOI] [PubMed] [Google Scholar]
- 37.Tzanakis N, Bouros D, Samiou M, Panagou P, Mouzas J, Manousos O, Siafakas N. Lung function in patients with inflammatory bowel disease. Respir Med. 1998;92:516–522. doi: 10.1016/s0954-6111(98)90301-8. [DOI] [PubMed] [Google Scholar]
- 38.Marvisi M, Borrello PD, Brianti M, Fornarsari G, Marani G, Guariglia A. Changes in the carbon monoxide diffusing capacity of the lung in ulcerative colitis. Eur Respir J. 2000;16:965–968. doi: 10.1183/09031936.00.16596500. [DOI] [PubMed] [Google Scholar]
- 39.Munck A, Murciano D, Pariente R, Cezard JP, Navarro J. Latent pulmonary function abnormalities in children with Crohn’s disease. Eur Respir J. 1995;8:377–380. doi: 10.1183/09031936.95.08030377. [DOI] [PubMed] [Google Scholar]
- 40.Mansi A, Cucchiara S, Greco L, Sarnelli P, Pisanti C, Franco MT, Santamaria F. Bronchial hyperresponsiveness in children and adolescents with Crohn’s disease. Am J Respir Crit Care Med. 2000;161:1051–1054. doi: 10.1164/ajrccm.161.3.9906013. [DOI] [PubMed] [Google Scholar]
- 41.Louis E, Louis R, Drion V, Bonnet V, Lamproye A, Radermecker M, Belaiche J. Increased frequency of bronchial hyperresponsiveness in patients with inflammatory bowel disease. Allergy. 1995;50:729–733. doi: 10.1111/j.1398-9995.1995.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 42.Fireman Z, Osipov A, Kivity S, Kopelman Y, Sternberg A, Lazarov E, Fireman E. The use of induced sputum in the assessment of pulmonary involvement in Crohn’s disease. Am J Gastroenterol. 2000;95:730–734. doi: 10.1111/j.1572-0241.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 43.Kuźniar T, Sleiman C, Brugière O, Groussard O, Mal H, Mellot F, Pariente R, Malolepszy J, Fournier M. Severe tracheobronchial stenosis in a patient with Crohn’s disease. Eur Respir J. 2000;15:209–212. doi: 10.1034/j.1399-3003.2000.15a38.x. [DOI] [PubMed] [Google Scholar]
- 44.Lamblin C, Copin MC, Billaut C, Marti R, Tacq V, Riviere O, Wallaert B. Acute respiratory failure due to tracheobronchial involvement in Crohn’s disease. Eur Respir J. 1996;9:2176–2178. doi: 10.1183/09031936.96.09102176. [DOI] [PubMed] [Google Scholar]
- 45.Bayraktaroglu S, Basoglu O, Ceylan N, Aydın A, Tuncel S, Savas R. A rare extraintestinal manifestation of ulcerative colitis: tracheobronchitis associated with ulcerative colitis. J Crohns Colitis. 2010;4:679–682. doi: 10.1016/j.crohns.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Camus P, Colby TV. The lung in inflammatory bowel disease. Eur Respir J. 2000;15:5–10. doi: 10.1183/09031936.00.15100500. [DOI] [PubMed] [Google Scholar]
- 47.Roozendaal C, Kallenberg CG. Are anti-neutrophil cytoplasmic antibodies (ANCA) clinically useful in inflammatory bowel disease (IBD)? Clin Exp Immunol. 1999;116:206–213. doi: 10.1046/j.1365-2249.1999.00905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries N, Gans RO, Donker AJ, Goldschmeding R, Hoorntje SJ, Snow GB. Autoantibodies against constituents of neutrophils in the diagnosis and treatment of (isolated) subglottic stenosis. Arch Otolaryngol Head Neck Surg. 1992;118:1120–1123. doi: 10.1001/archotol.1992.01880100112021. [DOI] [PubMed] [Google Scholar]
- 49.Wilcox P, Miller R, Miller G, Heath J, Nelems B, Muller N, Ostrow D. Airway involvement in ulcerative colitis. Chest. 1987;92:18–22. doi: 10.1378/chest.92.1.18. [DOI] [PubMed] [Google Scholar]
- 50.Plataki M, Tzortzaki E, Lambiri I, Giannikaki E, Ernst A, Siafakas NM. Severe airway stenosis associated with Crohn’s disease: case report. BMC Pulm Med. 2006;6:7. doi: 10.1186/1471-2466-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spira A, Grossman R, Balter M. Large airway disease associated with inflammatory bowel disease. Chest. 1998;113:1723–1726. doi: 10.1378/chest.113.6.1723. [DOI] [PubMed] [Google Scholar]
- 52.Mahadeva R, Walsh G, Flower CD, Shneerson JM. Clinical and radiological characteristics of lung disease in inflammatory bowel disease. Eur Respir J. 2000;15:41–48. doi: 10.1183/09031936.00.15104100. [DOI] [PubMed] [Google Scholar]
- 53.Butland RJ, Cole P, Citron KM, Turner-Warwick M. Chronic bronchial suppuration and inflammatory bowel disease. Q J Med. 1981;50:63–75. [PubMed] [Google Scholar]
- 54.Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax. 2010;65 Suppl 1:i1–58. doi: 10.1136/thx.2010.136119. [DOI] [PubMed] [Google Scholar]
- 55.Kelly MG, Frizelle FA, Thornley PT, Beckert L, Epton M, Lynch AC. Inflammatory bowel disease and the lung: is there a link between surgery and bronchiectasis? Int J Colorectal Dis. 2006;21:754–757. doi: 10.1007/s00384-006-0094-9. [DOI] [PubMed] [Google Scholar]
- 56.Papiris SA, Malagari K, Manali ED, Kolilekas L, Triantafillidou C, Baou K, Rontogianni D, Bouros D, Kagouridis K. Bronchiolitis: adopting a unifying definition and a comprehensive etiological classification. Expert Rev Respir Med. 2013;7:289–306. doi: 10.1586/ers.13.21. [DOI] [PubMed] [Google Scholar]
- 57.Banerjee B, Musk M, Sutanto EN, Yerkovich ST, Hopkins P, Knight DA, Lindsey-Temple S, Stick SM, Kicic A, Chambers DC. Regional differences in susceptibiity of bronchial epithelium to mesenchymal transition and inhibition by the macrolide antibiotic azithromycin. PLoS One. 2012;7:e52309. doi: 10.1371/journal.pone.0052309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Persson PG, Bernell O, Leijonmarck CE, Farahmand BY, Hellers G, Ahlbom A. Survival and cause-specific mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. 1996;110:1339–1345. doi: 10.1053/gast.1996.v110.pm8613037. [DOI] [PubMed] [Google Scholar]
- 59.Kanazawa H, Yoshikawa J. A case-control study of bronchial asthma associated with ulcerative colitis: role of airway microvascular permeability. Clin Exp Allergy. 2005;35:1432–1436. doi: 10.1111/j.1365-2222.2005.02358.x. [DOI] [PubMed] [Google Scholar]
- 60.Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung. 2008;186:167–172. doi: 10.1007/s00408-008-9080-z. [DOI] [PubMed] [Google Scholar]
- 61.Rutten EP, Lenaerts K, Buurman WA, Wouters EF. Disturbed intestinal integrity in patients with COPD: effects of activities of daily living. Chest. 2014;145:245–252. doi: 10.1378/chest.13-0584. [DOI] [PubMed] [Google Scholar]
- 62.Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm Bowel Dis. 2010;16:347–353. doi: 10.1002/ibd.21007. [DOI] [PubMed] [Google Scholar]
- 63.Fischer A, Nothnagel M, Franke A, Jacobs G, Saadati HR, Gaede KI, Rosenstiel P, Schürmann M, Müller-Quernheim J, Schreiber S, et al. Association of inflammatory bowel disease risk loci with sarcoidosis, and its acute and chronic subphenotypes. Eur Respir J. 2011;37:610–616. doi: 10.1183/09031936.00049410. [DOI] [PubMed] [Google Scholar]
- 64.Elzouki AN, Eriksson S, Löfberg R, Nässberger L, Wieslander J, Lindgren S. The prevalence and clinical significance of alpha 1-antitrypsin deficiency (PiZ) and ANCA specificities (proteinase 3, BPI) in patients with ulcerative colitis. Inflamm Bowel Dis. 1999;5:246–252. doi: 10.1097/00054725-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 65.Stone H, Pye A, Stockley RA. Disease associations in alpha-1-antitrypsin deficiency. Respir Med. 2014;108:338–343. doi: 10.1016/j.rmed.2013.10.006. [DOI] [PubMed] [Google Scholar]


