Abstract
Molybdenum-rhenium (Mo/Re) and tungsten-rhenium (W/Re) alloys were investigated as substrates for thin-film, polycrystalline boron-doped diamond electrodes. Traditional, carbide-forming metal substrates adhere strongly to diamond but lose their ductility during exposure to the high-temperature (1000°C) diamond, chemical vapor deposition environment. Boron-doped semi-metallic diamond was selectively deposited for up to 20 hours on one end of Mo/Re (47.5/52.5 wt.%) and W/Re (75/25 wt.%) alloy wires. Conformal diamond films on the alloys displayed grain sizes and Raman signatures similar to films grown on tungsten; in all cases, the morphology and Raman spectra were consistent with well-faceted, microcrystalline diamond with minimal sp2 carbon content. Cyclic voltammograms of dopamine in phosphate-buffered saline (PBS) showed the wide window and low baseline current of high-quality diamond electrodes. In addition, the films showed consistently well-defined, dopamine electrochemical redox activity. The Mo/Re substrate regions that were uncoated but still exposed to the diamond-growth environment remained substantially more flexible than tungsten in a bend-to-fracture rotation test, bending to the test maximum of 90° and not fracturing. The W/Re substrates fractured after a 27° bend, and the tungsten fractured after a 21° bend. Brittle, transgranular cleavage fracture surfaces were observed for tungsten and W/Re. A tension-induced fracture of the Mo/Re after the prior bend test showed a dimple fracture with a visible ductile core. Overall, the Mo/Re and W/Re alloys were suitable substrates for diamond growth. The Mo/Re alloy remained significantly more ductile than traditional tungsten substrates after diamond growth, and thus may be an attractive metal substrate for more ductile, thin-film diamond electrodes.
1. Introduction
Although carbon thin films, containing diamond, graphene, or carbon nanotubes, have promising engineering properties, a limiting factor in the processing of carbon films is identifying appropriate substrates for film fabrication [1-9]. For example, diamond films grow conformally on high-temperature materials that pre-form a carbide interfacial layer to enhance film adhesion, e.g., tungsten and molybdenum, whereas coatings of non-carbide forming materials have often required pre-deposition of carbide-forming interlayers or altering of growth conditions, which may unavoidably compromise the diamond film quality [1, 6, 7, 9-20]. Recent interest in applying conductive diamond as an implantable electrode in biomedical devices, for use either in vitro (tissue in a dish)[7, 21, 22] or in vivo (implantation in a live animal),[1, 23] has brought renewed attention to the need for alternatives to traditional high-temperature substrates. Both the coated and uncoated regions of tungsten and molybdenum become brittle during diamond chemical vapor deposition (CVD) (1000°C hydrogen/methane environment), due to the carbide formation and hydrogen incorporation [2, 3, 9, 16]. The brittle substrates fracture when a bending stress is applied, thereby preventing their use in long-term biomedical implants that must conform to tissue movement.
Conductive diamond has potential advantages in biodevice applications, e.g., as a neural sensor, because of chemical stability, higher signal-to-noise ratio, and reduced biological fouling relative to traditional materials [2, 5-8, 21, 22, 24-26]. Diamond’s appeal for long-term biomedical implants is dependent on the electrode performance and biocompatibility. Valid assessment of the diamond films and substrate for these qualities requires in vivo testing in intact behaving animals, such that the diamond electrode structure is flexible yet maintains performance and mechanical integrity for weeks or months.
Recently developed diamond electrode sensor prototypes suitable for in vitro preparations were reviewed by Park et al. in 2008 [7]; yet, diamond electrodes suitable for chronic implantation have not been reviewed. Initially reported devices for in vivo use were diamond-on-platinum and diamond-on-tungsten wire-based sensors constructed by purposely cutting the wire after diamond growth to attach a flexible electrical lead [3, 23, 27], and device assembly with hand-made junctions that produces multiple sites for device failure. With regard to fabrication of planar devices, a first-generation, all-diamond, flexible device with an integrated electrode and lead was reported [1]. The flexibility of the all-diamond device was a result of a thin ultrananocrystalline diamond coating as the electrode, but the device exhibited a low signal-to-noise ratio of 2 during acute in vivo electrical recording [1]. Diamond-on-polymer flexible arrays also show potential, but have not yet been tested in vivo [28, 29].
Past work provides evidence that alternative substrates and adjustment of growth conditions are necessary to improve the flexibility of the growth substrate while maintaining the diamond film quality. Faceted, high-quality, uniform diamond films have been grown on rhenium, which has a similar crystal structure to most carbides [10, 30]. The rhenium substrate remained ductile after diamond coating likely in part due to a lack of carbide formation [31-33], but the diamond film readily delaminated [34].
In this report, we investigate rhenium alloyed with a traditional carbide-forming metal (e.g., molybdenum or tungsten) as a substrate for diamond growth with a focus on film adhesion and flexibility of the uncoated substrate. Wire substrates were shaped into hooks to be used in our existing in vivo tissue preparation; the resulting diamond wire electrodes were characterized using a bend-to-fracture rotation test, scanning electron microscopy (SEM), microRaman spectroscopy, and cyclic voltammetry.
2. Materials and Methods
2.1 Substrates and Growth Conditions
Conductive polycrystalline diamond film growth was compared on three wire substrate materials: tungsten (W) [Goodfellow, 99.95%], molybdenum-rhenium alloy (Mo/Re) [Rhenium Alloy Inc, 52.5 Mo: 47.5 Re wt.%], and tungsten-rhenium alloy (W/Re) [Rhenium Alloy Inc, 75.0 W: 25.0 Re wt.%]. These alloy compositions were chosen primarily based on commercial availability; molybdenum and tungsten do not alloy at the same compositions, making it impossible to compare their alloys at equal rhenium content. Almost exclusively, 125 μm diameter wire was tested, with the exception that 50 μm diameter Mo/Re wire was used for SEM fracture images.
Substrates were manually shaped into hooks (approximately 3mm long with a 2 mm diameter), seeded for diamond growth by sonication (45 min) in a diamond slurry (0.3 g, 8 nm diamond powder in 90 mL ethanol), and cleaned (15 min) by sonication in ethanol. The wires were shaped into hooks, as previously mentioned, for use in our in vivo implantation studies [3]. The straight wire section was threaded into a quartz capillary mask, providing mechanical support with a loose fit for easy removal after diamond growth, while also minimizing diamond growth inside the capillary. Each hook-shaped wire protruded 2-3 mm from the end of the capillary and was coated by hot-filament CVD at 20 torr with 0.9% CH4 in H2 gas mixture and 40 ppm trimethyl boron. (A boron-nitride filament holder also contributed to the doping of the diamond film.) The capillaries were positioned perpendicular to the hot filaments, with the hook tips 11-14 mm away. The filaments were held at 1965±5°C for 30 min as a nucleation stage and then 2030±5°C (measured by a dual-wavelength pyrometer) for a total growth time of 4 hrs, 8 hrs, or 20 hrs. The samples were then cooled under a H2 purge for 2 hours.
As control samples, analogous W, Mo/Re and W/Re substrates were exposed to CVD in pure H2 for 4 hours, using the same filament temperature scheme as for the 4-hour diamond growth runs. To minimize carbon content for this control run, the reactor components and surfaces were thoroughly cleaned of carbon residues and the tungsten hot-filaments operated without carburization.
2.2 MicroRaman Spectroscopy
MicroRaman spectroscopy was conducted with a LabRam (Jobin Yvon Horiba) microRaman spectrometer (632 nm laser), with optical imaging (Olympus microscope, 100x lens) of the hook-shaped tip. Seven scans, four seconds each (130 to 2100 cm−1 range) obtained a resolution of 1.1 cm−1. For the H2-only control samples, a longer scan time of 10 seconds was necessary to obtain a measureable signal.
2.3 Cyclic Voltammetry
Cyclic voltammograms (scan rate 300 mV/s, Bio-logic VSP® potentiostat) were obtained in a 100 μM dopamine in phosphate buffered saline (PBS) electrolyte, pH 7.2, versus an Ag/AgCl reference electrode, and with a platinum counter electrode. The cyclic voltammogram was used to verify that the diamond-coated wires had a typically large potential window, small baseline current, and sufficient conductivity of previously reported “high-quality” polycrystalline diamond electrodes [34, 35].
2.4 Bend-to-Fracture Rotation Tests
Each sample was prepared for bend-to-fracture rotation tests by attaching a clamp and a suture to the wire. For the bend test, the clamp held the wire upright, and the suture was pulled at a constant displacement horizontally with an Enduratek Axial manipulator that also measured the applied force. The wire was therefore bent at the clamped point (Point C); the maximum measureable bending angle using this setup was 90°.
Measurements for placement of the clamp and suture on the wire were made relative to a “zero” position, defined at the boundary of the capillary mask during diamond growth, and verified through an optical microscope (Fig. 1, Point B). Point B was identified based on a significant drop in diamond film thickness. Negative positions are defined as where the wire was within the capillary during diamond growth, and positive positions are defined as the wire above the capillary, which is closer to the hook-shaped tip and filaments during growth. The stationary clamp was placed at −3 mm, Fig. 1, Point C; this point was chosen because it was approximately where film growth (diamond and carbon soot) ceased along the wire’s length. A cloth suture was wrapped around the wire and glued at the border between the curved and the straight part of the substrate, an approximate position of +1.5 mm (i.e., 4.5 mm above the clamp), Fig. 1, Point A. The filaments are located +14 mm from the fracture point of the hook substrates (11-12 mm above the top of the hook).
Figure 1.

The diamond hook electrodes were grown with a loose-fit quartz capillary mask up to Point B, which is a defined “zero” position. A significant, sudden drop in film thickness was observed at Point B. For bend-to-fracture rotation tests, a clamp was used to hold the wire approximately where all deposition ended, Point C, at −3 mm. A cloth suture was attached to the wire at Point A, at +1.5 mm, and was pulled horizontally using an Enduratek Axial manipulator, bending the wire around Point C, the top of the clamp.
Fracture images along the substrate were investigated to determine if the ductility changed as a function of the distance between the wire substrate and the filaments during diamond growth. Three points of interest were investigated, identical to what were defined for the bend-to-fracture rotation testing (Fig. 1, Points A-C).
The microstructure of the as-grown diamond films and fracture surfaces generated through the bend-to-fracture rotation tests were imaged with a Hitachi S - 4500 FEG SEM. The Mo/Re wire prior to growth was imaged with a FEI Helios 650 FE SEM.
3. Results and Discussion
Conformal, conductive diamond coatings on the molybdenum or tungsten-based rhenium alloy materials did not delaminate upon cooling. High resolution images of the diamond film morphology grown on Mo/Re, W/Re and W substrates are compared in Fig. 2, also with a Mo/Re surface prior to exposure to CVD conditions (Fig. 2g). The Mo/Re substrate surface from the H2-only control runs (Fig. 2a) underwent a surface change from exposure to atomic hydrogen, as compared to the as-received wire substrate. The average diamond crystal size on Mo/Re increased from 0.5 μm to 2.0 μm with growth times from 4, 8, and 20 hours (Fig. 2 b-d), consistent with typical columnar morphology of CVD diamond grown in CH4/H2 mixtures [36, 37]. Magnified images of all films on Mo/Re, W/Re, and W respectively, from the 4-hr growth runs showed similar diamond crystal size, approximately 0.5 μm (see Fig. 2 b, e, f). Film thicknesses were not quantified, but the similar crystal sizes indicated similar growth rates on all substrate materials.
Figure 2.
The diamond crystal sizes were compared on Mo/Re for the (a) hydrogen-only control, (b) 4 hr growth, (c) 8 hr growth, and (d) 20 hr growth. High resolution images for (e) 4 hr growth on W, (f) 4 hr growth on W/Re, and (g) as-received Mo/Re prior to CVD exposure were also shown. The hydrogen-only control experienced a surface change, but with no discernible diamond crystals deposited (see Fig. 3 for Raman spectra comparison), the diamond coatings (a-d) had crystal size increasing with growth time, and the samples for the same growth time (4 hrs) on different substrates (b, e, f) had similar crystal size.
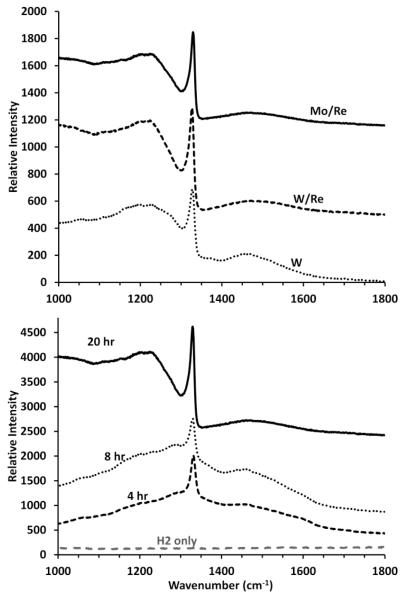
Raman spectra were obtained from diamond films after 20 hours growth on Mo/Re, W/Re, and W (Fig. 3, top), and for multiple growth times on Mo/Re substrates (Fig. 3, bottom). The spectra on different substrates had similar peaks at 1580 cm−1 (sp2 carbon), but with slightly shifted sp3 diamond peaks at 1329 cm−1, 1325 cm−1, and 1326 cm−1 from Mo/Re, W/Re, and W substrates, respectively (Fig. 3, top). The overall sp3 peak shift from 1332 cm−1 indicated that the diamond films had residual stress, with those grown on Mo/Re having less residual stress than those grown on W/Re and W; this stress variation was not further quantified. As the diamond growth time on Mo/Re increased (Fig. 3, bottom), the sp3/sp2 ratio increased, consistent with the larger grain size and more well-defined faceting observed in Fig. 2. The samples exposed to only H2 showed no Raman signal for surface carbon (Fig. 3, bottom), supporting that the observable changes (Fig. 2a) were a surface recrystallization of the metal and not carbon deposition.
Figure 3.
Raman summary of the films shown in Fig. 2. Top: Comparing the Raman spectra from 20 hr diamond growth on Mo/Re, W/Re and W. Similar sp2 peak positions and intensities were seen on each sample; the sp3- diamond signal were respectively at 1329, 1325 and 1326 cm−1, indicating less residual stress in the films on Mo/Re. Bottom: A progression in the Raman spectral change as a function of growth time (i.e. thickness). No carbon peaks were observed on the hydrogen-only control, suggesting that the observable surface changes were metal recrystallization. The sp3 - diamond signal intensity increased (1329 cm−1) compared to the sp2 - carbon signal (1580−1) as growth time increased, which is consistent with the increase in crystal size.
The cyclic voltammograms of diamond-coated Mo/Re, W/Re, and W in buffered dopamine solutions are compared in Fig. 4. The large 4.0 V voltage window (−2.0 V to +2.0 V vs. Ag/AgCl) was consistent with previous reports for “high–quality” diamond with well-defined faceting and negligible sp2 content [5, 38]. These data coupled with the Raman spectra and SEM imaging indicated that diamond growth on the Mo/Re and W/Re alloys could result in high-quality films similar to those that have been reported previously on other materials like W. The voltammograms also confirm the diamond films are highly conductive with no pin-holes, and capable of electrochemical use (e.g., dopamine detection).
Figure 4.

A wide-window cyclic voltammogram showing redox couple activity of 100 μM dopamine in phosphate buffered saline (PBS) vs. Ag/AgCl at diamond film electrodes grown for 20 hrs respectively on Mo/Re, W/Re, and W. Inset: Expanded view to show reproducible dopamine oxidation and reduction reactions.
Electrochemical detection of dopamine was compared on films grown for 4 and 20 hrs. For films grown for 4 hrs, Mo/Re substrates showed unstable redox reaction activity (activity lost after several scans), and W/Re and W films showed no activity (data not shown). However, films grown for 20 hrs on Mo/Re, W/Re, and W consistently showed long-term stable activity for the dopamine redox reaction (Fig. 4, enlarged/top). The voltammetry confirmed that 20 hours of diamond growth produced films with reproducible electrochemical behavior.
The bend-to-fracture rotation tests provided a ductility measure for the uncoated portion of the substrates after exposure to the diamond-growth conditions. These tests were not intended to demonstrate that the diamond-coated regions were ductile, as the diamond coatings were purposely grown thick; instead, the bend-to-fracture rotation tests focused on the boundary between the diamond-coated region and the uncoated metal where fracture is undesired (see Fig. 1). The Mo/Re, W/Re and W wires exposed to H2-only control runs remained flexible, bending the maximum test angle (90°) without fracture, on all three substrates (Fig. 5). The H2-only control confirmed that any fracture was due to the exposure to the methane (i.e. carbon) environment. After a 4-hour exposure to the diamond growth environment, the bend-to-fracture rotation limit was 30° for W, 67° for W/Re, and 90° (unfractured) for Mo/Re (Fig. 5). After a 20 hr growth time, the bend-to-fracture rotation was 21° for W (a 9° decrease), 27° for W/Re (a 40° decrease), and 90° (unfractured) for Mo/Re (unchanged) (Fig. 5). These data show that W, the traditional diamond-growth substrate, was consistently the most brittle material after diamond growth, with the smallest bend-to-fracture rotation angle. In contrast, Mo/Re showed similar ductility to the control samples, bending the maximum testing angle without fracture.
Figure 5.

Comparison of rhenium alloys bend-to-fracture rotation (at Point C) as a function of growth time and metal. The maximum rotation angle of the test, 90° (unfractured), was achieved for the H2-only control, indicating that changes in substrate mechanical properties from exposure to the diamond growth conditions were caused by the addition of carbon (methane). After a 4 hr growth time, the bend-to-fracture rotation was respectively 30°, 67°, and unfractured for W, W/Re, and Mo/Re. After a 20 hr growth, the bend to fracture rotation was 21°, 27°, and unfractured for W, W/Re, and Mo/Re, respectively. W was found to be the most brittle, and Mo/Re, which did not fracture during this test, was the most flexible.
Cross-sectional SEM images after fracture confirmed the trends of the bend-to-fracture rotation testing. Brittle, transgranular cleavage fractures were found on the W and W/Re fracture surfaces, Fig. 6 a-d. Larger cleavage facet size was seen for W (Fig. 6 a,b) compared to W/Re (Fig. 6 c,d), consistent with the W substrate being a more brittle material and having a smaller bending angle for even the shortest (4 hr) diamond growth times. The W/Re maintained some areas of non-defined plasticity (Fig. 6c), which also led to a larger bend angle as compared to W. The Mo/Re did not fracture during the bend test; therefore, a 50 μm diameter wire was used instead of the standard 125 μm wire to generate the fracture image, reducing the additional necessary force for this tension-induced fracture. The Mo/Re had a ductile core (Fig. 6e), shown by the dimple fracture (Fig. 6f). The microvoid coalescence in the ductile region was found to have higher rhenium content than the bulk alloy, due to formation of a sigma phase [39]. The diamond-growth environment affected the Mo/Re substrate at a much slower rate than the other materials; only the outer radius of the Mo/Re wire substrate, Fig. 6e, exhibited a layer of non-defined brittle features.
Figure 6.
Fracture surface images of the wires broken by the bend-to-fracture rotation testing. (a, b) 125 μm diameter W, (c, d) 125 μm diameter W/Re, and (e, f) 50 μm diameter Mo/Re (broken by tension separately) are shown. (b, d, f) Expanded images of the fracture surfaces show (b, d) dominant brittle, transgranular cleavage faceting for W and W/Re, compared to (f) ductile dimples observed in Mo/Re.
The substrate ductility as a function of the substrate-to-filament distance during a 4-hour diamond growth run was studied via fracture images at two points along a W substrate (Fig. 7). At Fig. 1, Point B, brittle, transgranular cleavage fractures were observed. Similar sized cleavage fractures were observed at Point C (further from the filament heat source), indicating that embrittlement of any exposed tungsten at reactor conditions was unavoidable, even though there was visually negligible diamond growth/carbon deposition on the substrate at this fracture location.
Figure 7.
The W substrate exposed to a 4 hr diamond growth was fractured at Point B, see Fig. 1. The expanded view showed transgranular cleavage fractures. A similar substrate fractured at Point C, (see Fig. 1) showed similar transgranular cleavage fractures, i.e.,at 3 mm further from the filaments during diamond growth.
Fracture images were investigated at three points along a 50 μm diameter Mo/Re wire after being exposed to 4 hours of diamond growth (Fig. 8). At Point A (Fig. 1), a ductile core with a nondescript brittle edge was observed. The diameter of the ductile core increased at fracture points located progressively farther down the wire, Point B, until at Point C, only a 2.4 μm wide (~10% of the total diameter) brittle region was observed (Fig. 8).
Figure 8.
Spatial mapping of ductility along a Mo/Re wire after 4 hr diamond growth. At Fig. 1, Point A, a ductile dimple fracture core was observed, which increased in area at Fig. 1, Point B (1. 5 mm further from filaments). At 4.5 mm from the tip, Fig. 1, Point C, the ductile core was larger. A 2.4 μm brittle region (expanded) was observed, leaving a 45.2 mm ductile core (90.4% of total diameter).
The fracture images at various points along the substrate indicated that Mo/Re remained more flexible as distance from the filaments during diamond growth increased, while tungsten stayed inflexible, regardless of growth conditions and substrate-to-filament distance (up to a substrate-to-filament distance of 17 mm). Based on these images and the bend-to-fracture rotation results, Mo/Re should be flexible enough for implantation into a live behaving animal.
As previously mentioned, it was not expected that the diamond-coated regions on the Mo/Re wires would be flexible, since typical growth conditions for a stiff diamond coating were used. Yet even for the stiff diamond coatings, the ductility of the underlying Mo/Re substrate was of interest. Several diamond-coated Mo/Re wires were bent in the diamond-coated regions. The underlying Mo/Re substrate remained highly flexible, bending 90° without fracture. Several cracks in the diamond film were observed by SEM as expected, Fig. 9, top, but the Mo/Re substrate underneath the film remained intact with no microcracks or failure, Fig. 9, bottom. Also, no delamination was observed, suggesting strong adhesion between the diamond film and the Mo/Re substrate.
Figure 9.
Upon bending the Mo/Re substrate 90° over the diamond section, (top) diamond did not bend but cracked. (bottom) The substrate underneath the diamond crack did not crack or fracture. The substrate showed no failure even directly under the diamond film, and the diamond did not delaminate.
A more comprehensive understanding of the effects of the diamond film on the alloys is still needed, with a focus on characterizing the two non-descript phases, the plasticity found in W/Re (Fig. 6c) and the smooth brittle region in Mo/Re (Fig. 6e). However, these initial data sufficiently support that the rhenium-based alloys remained substantially more ductile than traditional W substrates after exposure to the diamond-growth environment.
4. Conclusions
The 52.5 % Molybdenum / 47.5% Rhenium (wt.%) alloy is a suitable substrate for diamond growth, remaining flexible with good diamond film adhesion. The SEM, microRaman, and cyclic voltammetry analysis verified that the diamond films are analogous on Mo/Re, W/Re and the traditional W substrate. These initial findings support that rhenium-based alloys are an acceptable alternative to traditional W substrates. Furthermore, Mo/Re alloys showed advantageous flexibility to be a superior metal substrate option in diamond bioelectrode applications. Within ongoing work, we seek to further refine our understanding of the microstructure of the rhenium alloys after exposure to diamond growth conditions.
Highlights.
Rhenium alloys were investigated as substrates for diamond film growth
Mechanical bend testing and imaging compared alloy ductility and microstructure
Molybdenum-rhenium alloys stayed more flexible than tungsten and tungsten-rhenium
Embrittlement was limited to the outer shell of the molybdenum-rhenium wires
Alloys were viable high-temperature substrates for flexible diamond electrodes
Acknowledgments
The authors wish to thank Todd Leonhardt and Rhenium Alloy Inc. for rhenium alloy samples. We also gratefully acknowledge Professor John Lewandowski and Chris Tuma (Materials Science and Engineering, CWRU) for guidance in analyzing the SEM fracture images and for training in the bend-to-fracture rotation testing. Kehan Yu provided the SEM image of the as-received Mo/Re wire. This research was supported by the National Institutes of Health (R01-EB004018).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Chan HY, Aslam DM, Wiler JA, Casey B. A novel diamond microprobe for neuro-chemical and electrical recording in neural prosthesis. Journal of Microeelctromechanical Systems. 2009;18:511–521. [Google Scholar]
- [2].Fujishima A, Einaga Y, Rao TN, Tryk DA. Diamond Electrochemistry. Elsevier; Amsterdam: 2005. [Google Scholar]
- [3].Halpern JM, Cullins MJ, Chiel HJ, Martin HB. Chronic in vivo nerve electrical recordings of Aplysia californica using a boron-doped polycrystalline diamond electrodes. Diamond and Related Materials. 2010;19:178–181. [Google Scholar]
- [4].Kraft A. Doped diamond: A compact review on a new, versatile electrode material. Int. J. Electrochem. Sci. 2007;2:355–385. [Google Scholar]
- [5].Martin HB, Argoitia A, Landau U, Anderson AB, Angus JC. Hydrogen and oxygen evolution on boron-doped diamond electrodes. J. Electrochem. Soc. 1996;143:L133–L136. [Google Scholar]
- [6].Nebel CE, Rezek B, Shin D, Uetsuka H, Yang N. Diamond for bio-sensor applications. J. Phys. D: Appl. Phys. 2007;40:6443–6466. [Google Scholar]
- [7].Park J, Quaiserova-Mocko V, Patel BA, Novotny M, Liu A, Bian X, Galligan JJ, Swain GW. Diamond microelectrodes for in vitro electroanalytical measurements: Current status and remaining challenges. Analyst. 2008;133:17–24. doi: 10.1039/b710236b. [DOI] [PubMed] [Google Scholar]
- [8].Puzyr AP, Baron AV, Purtov KV, Bortnikov EV, Skobelev NN, Mogilnaya OA, Bondar VS. Nanodiamonds with novel properties: A biological study. Diamond and Related Materials. 2007;16:2124–2128. [Google Scholar]
- [9].Singh J. Novel techniques for selective diamond growth on various substrates. JMEPEG. 1994;3:378–385. [Google Scholar]
- [10].Akella J, Weir ST, Vohra YK, Prokop H, Catledge SA, Chesnut GN. High pressure phase transformations in neodymium studied in a diamond anvil cell using diamond-coated rhenium gaskets. J. Phys.: Condens. Matter. 1999;11:6515–6520. [Google Scholar]
- [11].Constant L, Speisser C, Normand FL. HFCVD diamond growth on Cu(111). Evidence for carbon phase transformations by in situ AES and XPS. Surface Science. 1997;387:28–43. [Google Scholar]
- [12].Fan QH, Pereira E, Gracio J. Diamond deposition on copper: Studies on nucleation, growth, and adhesion behaviours. Journal of Materials Science. 1999;34:1353–1365. [Google Scholar]
- [13].Gerger I, Haubner R. Gradient layers of boron-doped diamond on titanium substrates. Diamond and Related Materials, 16 (Gradient layers of boron-doped diamond on titanium substrates.) :899–904. [Google Scholar]
- [14].Neubauer E, Kladler G, Eisenmenger-Sittner C, Hell J, Prentice C, Angerer P, Ciupinski L. Interface design in copper-diamond composite by using PVD and CVD coated diamonds. Advanced Materials Research. 2009;59:214–219. [Google Scholar]
- [15].Peng XL, Clyne TW. Formation and adhesion of hot filament CVD daimond films on titanium substrates. Thin Solid Films. 1997;293:261–269. [Google Scholar]
- [16].Singh J. Nucleation and growth mechanism of diamond during hot-filament chemical vapour deposition. Journal of Materials Science. 1994;29:2761–2766. [Google Scholar]
- [17].Wang XG, Smith JR. Copper/diamond adhesion and hydrogen termination. Physical Review Letters. 2001;87:186103-186101–186103-186104. [Google Scholar]
- [18].Yu Z-M, Zhang Y-H, Wei Q-P, Liu D-Y, Meng L-C. Effects of gradient substrate temperature and reactive sputtered TiC interlayer on diamond films on Ti 6 AlV 4 alloy by HFCVD. Zhingguo Biaomian Gongcheng(China Surface Engineering) 2012;25:20–27. [Google Scholar]
- [19].Liang J, Gao C, Zhang L, Jiang L, Yang Z, Wang Z, Ji C, Le X, Rong C, Zhang J. Studies on distribution of element contents in transient layer at interface between boron-doped diamond film electrode and tantalum substrate. Applied Surface Science. 2011;257:6063–6067. [Google Scholar]
- [20].Wang XC, Shen B, Sun FH. Deposition and characterization of boron-doped HFCVD diamond films on Ti, SiC, Si and Ta substrates. Applied Mechanics and Materials. 2012;217:1062–1067. [Google Scholar]
- [21].Halpern JM, Xie S, Sutton GP, Higashikubo BT, Chestek CA, Lu H, Chiel HJ, Martin HB. Diamond electrodes for neurodynamic studies in Aplysia californica. Diamond and Related Materials. 2006;15:183–187. [Google Scholar]
- [22].Xie S, Shafer G, Wilson CG, Martin HB. In vitro adenosine detection with a diamond-based sensor. Diamond and Related Materials. 2006;15:225–228. [Google Scholar]
- [23].Suzuki A, Ivandini TA, Yoshimi K, Fujishima A, Oyama G, Nakazato T, Hattori N, Kitazawa S, Einaga Y. Fabrication characterization, and application of boron-doped diamond microelectrodes for in vivo dopamine detection. Analytical Chemistry. 2007;2007:8608–8615. doi: 10.1021/ac071519h. [DOI] [PubMed] [Google Scholar]
- [24].Park J, Show Y, Quaiserova V, Galligan JJ, Fink GD, Swain GW. Diamond electrodes for use in biological environments. Journal of Electroanalytical Chemistry. 2005;583:56–68. [Google Scholar]
- [25].Popov C, Bilznakov S, Boycheva S, Milinovik N, Apostolova MD, Anspach N, Hammann C, Nellen W, Reithmaier JP, Kulisch W. Nanocrystalline diamond/amorphous carbon composite coatings for biomedical applications. Diamond and Related Materials. 2008;17:882–887. [Google Scholar]
- [26].Schrand AM, Dai L, Schlager JJ, Hussain SM, Osawa E. Differential biocompatibility of carbon nantoubes. Diamond and Related Materials. 2007;16:2118–2123. [Google Scholar]
- [27].Sarada BV, Rao TN, Tryk DA, Fujishima A. Electrochemical characterization of highly boron-doped diamond microelectrodes in aqueous electrolyte. J. Electrochem. Soc. 1999;146:1469–1471. [Google Scholar]
- [28].Hess AE, Sabens DM, Martin HB, Zorman CA. Diamond-on-polymer microelectrode arrays fabricated using a chemical release transfer process. J. Microelectromechanical Systems. 2011;20:867–875. [Google Scholar]
- [29].Hess AE, Sabens DM, Martin HB, Zorman CA. Polycrystalline diamond-on-polymer electrode arrays fabricated using a polymer-based transfer technique. Electrochem. Solid St. Lett. 2010;13:J129–J131. [Google Scholar]
- [30].Evans EA, Hafeli U, Wusinka RAM, Morrison PW. Materials Research Society Symposium - Proceedings; 2000.pp. 433–438. [Google Scholar]
- [31].Gebhardt E, Fromm E, Roy U. In: Carbon-Rehnium Binary Phase Diagram. Villars P, Okamoto H, Cenzual K, editors. ASM Alloy Phase Diagram Center, ASM International; Materials Park: 1966. [Google Scholar]
- [32].Gonser BW. Rhenium. Elsevier; Amsterdam: 1962. [Google Scholar]
- [33].Savitskii EM, Tylkina MA, Povarova KB. Rehnium Alloys. IPST Press; Jerusalem: 1970. [Google Scholar]
- [34].Halpern JM. Chemical Engineering. Case Western Reserve University; Cleveland: 2010. p. 251. [Google Scholar]
- [35].Halpern JM, Xie S, Schreiber JL, Martin HB. Kinetic and adsorption studeis of biogenic amine neurotransmitters at polycrystalline diamond microlectrodes. ECS Transactions. 2007;3:47–57. [Google Scholar]
- [36].Trava-Airoldi VJ, Corat EJ, Pena AFV, Leite NF, Baranauskas V, Salvadori MC. Columnar CVD diamond growth structure on irregular surface substrates. Diamond and Related Materials. 1995;4:1255–1259. [Google Scholar]
- [37].Lang AR, Makepeace APW, Butler JE. Morphological and synchrotron X-ray microdiffraction studdies of large columnar CVD diamond crystallites. J. Appl. Cryst. 1999;32:924–933. [Google Scholar]
- [38].Martin HB, Argoitia A, Angus JC, Landau U. Voltammetry studies of single-crystal and polycrystalline diamond electrodes. J. Electrochem. Soc. 1999;146:2959–2964. [Google Scholar]
- [39].Leonhardt T. Personal Communication: Meeting. 2009 [Google Scholar]