Abstract
Background:
HIV infection is associated with increased levels of inflammatory markers. Inflammation is hypothesized to play a role in the development of type 2 diabetes. Data addressing this issue among HIV positive participants are limited.
Methods:
A cohort of 3,695 participants without diabetes, taking antiretroviral therapy and with an average CD4+ count 523 cells/mm3 were followed for an average of 4.6 years. Diabetes risk associated with baseline levels of high sensitivity C-reactive protein (hsCRP) and interleukin-6 (IL-6) was assessed using Cox proportional hazards regression models. Analyses considered baseline levels of factors associated with diabetes risk and HIV-related measures.
Results:
137 patients developed diabetes requiring drug treatment during follow-up (8.18 per 1,000 person years). Median levels of IL-6 and hsCRP were significantly higher among those who developed diabetes compared to those who did not: 3.45 versus 2.50 pg/ml for IL-6 and 4.91 versus 3.29 μg/ml for hsCRP (P<.001). Adjusted hazard ratios (HRs) associated with a doubling of IL-6 and hsCRP were 1.29 (95% CI: 1.08-1.55; P=0.005) and 1.22 (95% CI: 1.10-1.36; P<0.001), respectively. Body mass index (P<0.001), age (P=0.013), co-infection with hepatitis B or C (P=0.03), non-smoking status (P=0.034), and use of lipid-lowering treatment (P=0.008) were also associated with an increased risk of diabetes.
Conclusions
These findings indicate that low-grade systemic inflammation is an underlying factor in the pathogenesis of diabetes.
Introduction
A growing body of evidence suggests that as compared to individuals without HIV those with HIV are at greater risk of metabolic complications, including diabetes (1). Reasons for this increased risk are unclear, but may include some antiretroviral treatments (ART) (2),(3),(4), hepatitis co-infection (5),(6), and chronic inflammation (7),(8). Untreated HIV is associated with higher levels of inflammatory markers, and initiation of ART results in a decline, but, on average, does not normalize inflammatory markers (9)(10). Insulin resistance, which is associated with the development of diabetes, was one of the first metabolic complications associated with HIV and HIV treatments (11).
It has been hypothesized that diabetes is a manifestation of an ongoing acute phase inflammatory response, and in the general population, several studies indicate that higher levels of interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hsCRP) are associated with the development of diabetes (8, 12-17). However a recent meta-analysis and an analysis of 12 inflammatory biomarkers in the Framingham Heart Study indicate that associations are diminished after adjustment for other type 2 diabetes risk factors (18)(19). Cross-sectional studies have also reported associations between CRP and insulin resistance in individuals without diabetes (20). Moreover, it has been shown that in healthy volunteers administration of subcutaneous recombinant human IL-6 induces dose dependent increases in fasting glucose (21). It has been postulated that type 2 diabetes may represent a disease of the innate immune system (22).
With the exception of a small case-control study, the relationship of inflammatory markers with risk of diabetes among HIV positive patients taking ART has not been assessed (23). Further research on this was motivated by the observation that levels of inflammatory markers are considerably higher for HIV-positive individuals, even those on suppressive ART, compared to HIV-negative age-matched men and women (9). If chronic inflammation is an important predictor of diabetes for HIV patients, this could explain, at least in part, their greater risk of diabetes compared to those without HIV. Data from the control arms of two large, international HIV trials are used to estimate the incidence of diabetes and study the association of IL-6 and hsCRP with risk of diabetes. Our hypothesis was that among patients prescribed combination ART aimed at virologic suppression higher levels of each inflammatory biomarker would be associated with an increased risk of diabetes.
Methods
Study Population
Data from the Strategies for Management of Anti Retroviral Therapy (SMART) and the Evaluation of Subcutaneous Proleukin® in a Randomized Trial (ESPRIT) were used(24)(25). Briefly, in SMART 5,472 HIV-positive adults with CD4+ cell count greater than 350 cells/mm3 were randomized by sites in 33 countries to receive uninterrupted ART with the goal of viral suppression (control group) or episodic ART guided by the CD4+ count. The SMART study was stopped early because of a safety risk in the episodic therapy arm (25). In ESPRIT, 4,111 HIV-positive adults with CD4+ cell counts greater than or equal to 300 cells/mm3 were randomized by sites in 25 countries to receive continuous ART alone (control group) or continuous ART in combination with subcutaneous interleukin-2. ESPRIT failed to demonstrate a clinical benefit of interleukin-2 (24).
Since continuous ART represents the current standard of care for people with HIV, this investigation is restricted to patients in the control groups of these two studies who did not have a diagnosis of diabetes at study entry and who consented to storing plasma for the biomarker measurements.
In SMART, patients could be enrolled while on or off ART. Those not on ART were to receive combination ART aimed at virologic suppression if they were assigned to the control group.
Inflammatory Biomarkers
The biomarkers, hsCRP and IL-6, were centrally measured on stored plasma collected at baseline (prior to randomization) for all patients who provided written consent. These two biomarkers were prospectively chosen, initially for studying their association for all-cause mortality and cardiovascular disease (CVD)(26) (27). In those investigations, both markers were associated with an increased risk of mortality and CVD. They were chosen for those investigations because they have high laboratory and biological reproducibility and have been associated with all-cause mortality and CVD in the general population (28)(29)(30) .
The institutional review board at the University of Minnesota approved plans for the analysis of stored specimens. For SMART participants, biomarkers were measured at the Laboratory for Clinical Biochemistry Research at the University of Vermont (Burlington). In the ESPRIT trial, laboratory measurements were performed by SAIC-Frederick (Frederick, MD). IL-6 was measured by the same method at each laboratory (Chemiluminescent Sandwich ELISA, R&D Systems). hsCRP was measured by ELISA by both laboratories. For SMART participants, a NBTMII nephelometer, N Antiserum to Human CRP (Siemens Diagnostics) was used. For ESPRIT participants, an R&D Systems ELISA assay was used. The hsCRP assays, while different, compared very well on duplicate samples. The lower limit of detection for IL-6 was 0.16 pg/ml, for CRP, the lower limit was 0.16 μg/mL for SMART and 0.078 μg/mL for ESPRIT participants. All samples were analyzed blinded to clinical information about the patients.
The year-to-year consistency of IL-6, but not hsCRP, was assessed in a random sample of 235 patients in the control arm of ESPRIT. IL-6 was measured at baseline, year 1 and year 3. The reliability coefficient (ratio of between subject variability to total variability) of log IL-6 was 0.39. This is similar to that reported in a systematic overview of IL-6 and risk of coronary heart disease (0.41)(31). The short-term reliability of hsCRP was estimated using 324 patients in the control arm of SMART who were seen at baseline and 6 months. It was 0.78. Based on a systematic review of CRP and coronary heart disease, the reliability coefficient for log CRP considering year to year variability was 0.58(32).
Follow-up and Diabetes Determination
In SMART, follow-up visits were scheduled at 1 month and 2 months, every 2 months thereafter in the first year, and every four months in the second and subsequent years. In ESPRIT, follow-up visits were scheduled every 4 months following randomization.
In both SMART and ESPRIT, dates of diabetes diagnosis and use of drugs for diabetes were recorded. A patient was considered to have developed diabetes if they received a diagnosis of diabetes requiring drug treatment during follow-up. Diabetes was ascertained in the SMART study at baseline and reported during follow-up as soon as the diagnosis was made. The date of diagnosis, the drug prescribed and plasma glucose laboratory value were collected. In the ESPRIT study, treatment for diabetes, including date of treatment initiation, were reported annually.
Fasting glucose measurements were not obtained in ESPRIT. In SMART, fasting glucose measurements were only obtained for 111 participants who also enrolled in a substudy on body composition.
Statistical Analysis
Crude incidence rates of diabetes, defined as a diagnosis of diabetes requiring drug treatment, are reported as rates per 1000 person years. Kaplan-Meier estimates of the cumulative probability of developing diabetes are also cited, both overall and by levels of the biomarkers. For patients with biomarker levels below the level of detection (4 patients for IL-6 and 33 patients for hsCRP), the value of the biomarker was imputed as the lower level of detection. Baseline measurements of hsCRP and IL-6 were used to predict the development of type 2 diabetes after adjusting for other risk factors for type 2 diabetes which were measured in the two studies. In both studies, age, race, body mass index (BMI), blood pressure-lowering drugs, lipid-lowering drugs, co-infection with hepatitis, prior history of cardiovascular disease (CVD), type of ART, CD4+ count and HIV RNA level were collected at baseline. Patients in SMART also had information available on cigarette smoking, HDL cholesterol, LDL cholesterol and triglycerides at baseline.
Cox regression models, with stratification by study (SMART and ESPRIT), were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) associated with higher biomarker levels. In addition to models that categorized the biomarkers into quartiles with lowest quartile as reference, models with log2 transformed biomarker levels were considered. A log transformation was used because the markers were skewed to the right. With a log2 transformation, exponentiation of the parameter estimate from the Cox regression gives the increased risk of diabetes associated with a doubling of the biomarker level. Patients were censored by death and last annual visit attended. We checked the proportional hazards assumption for each of the biomarkers.
The association of incident diabetes with each biomarker was first studied in a model with no covariates and then in 3 regression models with the following baseline covariates (I) age, race, sex, co-infection with hepatitis C or B, CD4+ count and HIV RNA level; (II) age, race, sex, co-infection with hepatitis C or B, CD4+ count, HIV RNA level, body mass index (BMI), use of lipid-lowering drugs, use of blood pressure –lowering drugs, use of ART, and if taking ART, use of a stavudine (d4T) - containing ART regimen, use of a zidovudine (ZDV), but not d4T – containing ART regimen, and use of an ART regimen that did not contain d4T or ZDV; (III) all of the aforementioned covariates plus cigarette smoking status, total cholesterol, LDL, HDL, and triglycerides (SMART only). Model I considers demographic and HIV factors; model II adds variables significant in univariate analyses and which have been associated with risk of diabetes in other studies; and model III considers other potential confounding factors that were only measured in SMART.
Subgroup analyses for baseline variables that were significant predictors of diabetes were carried out to assess whether the association of each biomarker with diabetes was consistent across the subgroups. For these analyses, p-values corresponding to the interaction between each biomarker and the subgrouping variable are cited.
Statistical analyses were performed using SAS software (version 9.2; SAS Institute). P values are 2-sided and 95% confidence intervals (CIs) are cited.
Results
Characteristics of Study Cohort
Among the 4,792 patients in the control arms of SMART and ESPRIT 240 (5%) reported taking drugs for diabetes at entry. IL-6 and hsCRP were available for 214 of those taking drugs for diabetes at entry and median (IQR) levels were 2.44 (1.62, 4.10) and 2.63 (1.14, 5.78), respectively.
Among those not taking drug treatment for diabetes at entry (n=4,552), 3,965 (87%) had IL-6 and hsCRP measurements and attended at least one annual follow-up visit (1,719 in ESPRIT and 2,246 in SMART). These 3,965 patients, who had an average CD4+ count of 523 cells/mm3, were considered to be at risk for developing diabetes during follow-up and are the subject of this report. Using fasting glucose measurements made on a sample of SMART patients, we were able to assess the extent to which the cohort was free of diabetes. Among the 2,246 patients in SMART, 111 had fasting glucose measurements at entry. None of these 111 patients had levels of 126 mg/dl or higher; 22 (19.8%) had levels between 100 and 125 mg/dl; and 89 (80.1%) had fasting glucose levels < 100 mg/dL. We also assessed the correlation of each biomarker with fasting glucose. Correlations with IL-6 and hsCRP were 0.24 (p=0.01) and 0.17 (p=0.07), respectively.
Attendance at annual visits ranged from 89.5% to 97.7% for these 3,965 patients. The numbers of patients who missed 2, 3 and 4 or more annual visits were 72, 29 and 26 respectively.
Over an average follow-up of 4.6 years (2.9 years in SMART and 6.8 years in ESPRIT), 137 (68; 3.0 % in SMART and 69; 4.0% in ESPRIT) of the 3,965 patients in the cohort developed diabetes (3.5%, 8.18 per 1000 person years). Cumulative percents developing diabetes after 2, 4 and 6 years were 1.4, 3.5 and 4.8%
For this cohort on continuous ART, the percent with an HIV RNA level of 500 copies/mL or lower and average CD4+ cell count did not change appreciably during follow-up. At 12, 24, 36 and 48 months, percents with HIV RNA of 500 copies/mL or lower were 81.9%, 81.2%, 80.8%, and 82.6%. Average levels of CD4+ cell count at these visits were 610, 604, 592, and 593 cells/mm3.
Table 1 summarizes baseline characteristics for patients who developed diabetes (i.e. initiated drug treatment for diabetes) with those who did not. In contrast to those who did not develop diabetes, incident cases were more likely to be non-white, be older in age, be non-smokers, have higher CD4+ cell count, BMI, and triglycerides, and were more likely to be taking lipid lowering and blood pressure lowering drugs at baseline. Those who developed diabetes on ART were less likely to have a viral load of 500 copies/mL or less (Table 1).
Table 1.
Baseline characteristics for participants in the control arms of ESPRIT and SMART according to the development of diabetes during follow-up
| Incident diabetes | ||||
|---|---|---|---|---|
|
| ||||
| Total (n=3965) |
No (n=3,828) |
Yes (n=137) |
P-value* | |
| Study | 0.002 | |||
| ESPRIT | 43.3 | 43.1 | 50.4 | |
| SMART | 56.7 | 56.9 | 49.6 | |
| Age (years ± SD) | 42.7 ± 9.4 | 42.5 ± 9.4 | 47.5 ± 9.1 | <0.001 |
| Gender (% female) | 22.9 | 23.0 | 20.4 | 0.79 |
| Race (%) | <0.001 | |||
| White | 66.4 | 66.8 | 53.3 | |
| Nonwhite | 33.6 | 33.2 | 46.7 | |
| Lipid Lowering drug (% ) | 12.8 | 12.3 | 27.9 | <0.001 |
| Blood pressure lowering drug (%) | 11.0 | 10.5 | 25.0 | <0.001 |
| Co-infected with hepatitis B or C (%) | 16.4 | 16.3 | 18.2 | 0.60 |
| Prior MI, stroke or CAD requiring surgery (%) | 2.2 | 2.2 | 1.5 | 0.74 |
| Mean BMI (kg/m2 ± SD) | 25.0 ± 4.9 | 24.8 ± 4.7 | 28.6 ± 6.9 | <0.001 |
| Mean D-Dimer (μg/ml ± SD) | 0.35 ± 0.51 | 0.35 ± 0.52 | 0.35 ± 0.28 | 0.47 |
| HIV RNA ≤ 500 (%) | 76.4 | 76.6 | 69.1 | 0.04 |
| Baseline ART Regimen | 0.07 | |||
| No ART (%) | 9.2 | 9.3 | 7.3 | |
| Stavudine containing regimen (%) | 27.6 | 27.0 | 43.1 | |
| Zidovudine containing regimen, no
Stavudine (%) |
42.2 | 42.4 | 36.5 | |
| Other ART, no Zidovudine or Stavudine (%) | 21.0 | 21.3 | 13.1 | |
| Mean CD4 count ( per mm3 ± SD) | 590 ± 241 | 589 ± 240 | 603 ± 276 | 0.02 |
| Smoking (%)† | 41.1 | 41.5 | 27.9 | 0.02 |
| Mean HDL cholesterol (mg/dL ± SD)† | 43.6 ± 14.9 | 43.7 ± 14.9 | 41.3 ±15.5 | 0.19 |
| Mean LDL cholesterol (mg/dL ± SD)† | 114.6 ± 35.4 | 114.7 ± 35.4 | 112.2 ± 35.9 | 0.87 |
| Mean Total cholesterol (mg/dL ± SD)† | 195.3 ± 47.1 | 195.3 ± 47.0 | 195.5 ± 49.7 | 0.94 |
| Mean Triglycerides (mg/dL ± SD)† | 211.9 ± 194.9 | 209.7 ± 190.4 | 282.8 ± 298.3 | 0.006 |
Only available in the SMART study.
P-value from univariate Cox proportional hazards models with the exception of baseline ART regimen which is the p-value from the 3df Χ2 test.
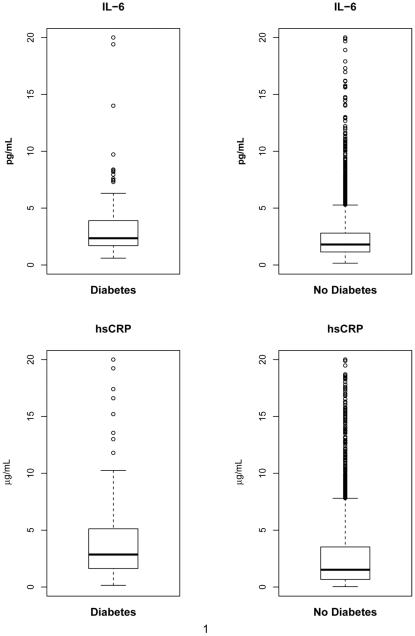
Median baseline levels of both IL-6 and hsCRP were significantly higher among those who developed diabetes: 3.45 versus 2.50 pg/ml for IL-6 and 4.91 versus 3.29 μg/ml for hsCRP (P<.001 for each marker) (Figure 1). In the sample of 111 patients from SMART with fasting glucose measurements, IL-6 and hsCRP were positively correlated with fasting glucose: rank correlation 0.24 for IL-6 (p=0.01) and 0.17 for hsCRP (p=0.07). Median levels of the two biomarkers for those with fasting glucose 100-125 (n=22) versus < 100 mg/dl (n=89) were 2.35 versus 1.74 pg/ml for IL-6 (p=0.01) and 2.12 versus 2.55 μg/ml for hsCRP (p=0.40).
Figure 1.
Boxplots of IL-6 and hsCRP according to the development of diabetes during follow-up. Percentiles of the IL-6 distribution for those with diabetes are: 10th percentile=1.14, 25th percentile=1.70, median = 2.35, 75th percentile=3.90, 90th percentile=6.30. For those without diabetes, the percentiles are: 10th percentile=0.80, 25th percentile=1.14, median = 1.80, 75th percentile=2.80, 90th percentile=4.46. Percentiles of the hsCRP distribution for those with diabetes are: 10th percentile=0.84, 25th percentile=1.63, median = 2.86, 75th percentile=5.12, 90th percentile=11.80. For those without diabetes, the percentiles are: 10th percentile=0.31, 25th percentile=0.67, median = 1.52, 75th percentile=3.52, 90th percentile=7.35.
Baseline levels of IL-6 and hsCRP and the development of diabetes requiring drug treatment
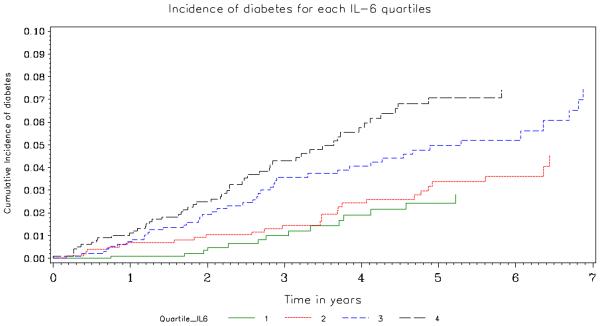
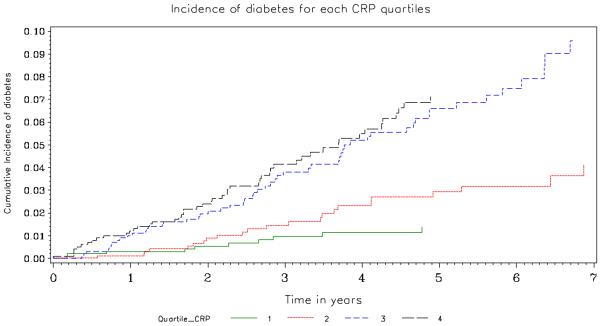
Table 2 and Figures 2 and 3 summarize the association of IL-6 and hsCRP with the development of diabetes. In crude (unadjusted) analyses, both biomarkers were associated with an increased risk of developing diabetes (HR associated with a doubling of IL-6 and hsCRP were 1.47; 95% CI 1.26-1.70 and 1.32; 95% CI 1.20 -1.45, respectively). Those in the highest quartile of baseline plasma hsCRP had rates more than 5 times greater than those in the lowest quartile (HR=5.13; 95% CI 2.60-10.1); for those in the highest quartile of IL-6 rates were more than 3 times greater than those in the lowest quartile (HR 3.45; 95% CI 1.91-6.23). Figures 2 and 3 give the cumulative percent developing diabetes by quartile of each biomarker. There is a clear separation in risk of diabetes for the upper two versus lower 2 quartiles.
Table 2.
Risk of diabetes associated with IL-6 and CRP levels at entry
| Type of adjustment | Quartile 1 HR (95% CI) |
Quartile 2 HR (95% CI) |
Quartile 3 HR (95% CI) |
Quartile 4 HR (95% CI) |
HR associated with doubling of biomarkers | |
|---|---|---|---|---|---|---|
| HR (95%CI) | P-value | |||||
| IL-6 | ||||||
| IL6 range (pg/ml) | (0.15-1.15) | (1.16-1.80) | (1.81-2.82) | (2.83-88.63) | - | - |
|
No-patients with
Incident diabetes (n) |
14 | 27 | 44 | 52 | - | - |
|
Crude Analysis
(n=3,965; 3.45%) |
1.00 (Ref) | 1.70 (0.89-3.25) | 2.86 (1.56-5.24) | 3.45 (1.91-6.23) | 1.47 (1.26-1.70) | <0.001 |
| Model 1: | 1.00 (Ref) | 1.53 (0.80-2.93) | 2.23 (1.20-4.13) | 2.52 (1.37-4.63) | 1.35 (1.15-1.58) | <0.001 |
| Model 2: | 1.00 (Ref) | 1.30 (0.67-2.50) | 1.74 (0.93-3.24) | 1.93 (1.04-3.59) | 1.29 (1.08-1.55) | 0.005 |
| Model 3: | 1.00 (Ref) | 1.18 (0.46-3.03) | 1.76 (0.74-4.21) | 1.89 (0.81-4.42) | 1.36 (1.07-1.72) | 0.01 |
| hsCRP | ||||||
| hs-CRP range (μg/ml) | (0.03-0.68) | (0.69-1.56) | (1.57-3.56) | (3.57-98.00) | - | - |
|
No-patients with
Incident diabetes (n) |
10 | 23 | 55 | 49 | - | - |
|
Crude Analysis
(n=3,965; 3.45%) |
1.00 (Ref) | 2.32 (1.10-4.87) | 5.52 (2.82-10.84) | 5.13 (2.60-10.14) | 1.32 (1.20-1.45) | <0.001 |
| Model 1: | 1.00 (Ref) | 2.17 (1.03-4.57) | 4.64 (2.35-9.16) | 4.15 (2.08-8.28) | 1.28 (1.16-1.42) | <0.001 |
| Model 2: | 1.00 (Ref) | 2.16 (1.00-4.70) | 4.21 (2.06-8.60) | 3.47 (1.67-7.20) | 1.22 (1.10-1.36) | <0.001 |
| Model 3: | 1.00 (Ref) | 2.84 (1.01-8.04) | 3.15 (1.17-8.49) | 3.20 (1.19-8.61) | 1.22 (1.05-1.41) | 0.01 |
Model 1: Adjusted for age, sex, race, square root baseline CD4 count , co-infection with hepatitis B or C, viral load and type of ART (stavudine, zidovudine (no stavudine), other ART or no ART); stratified by study. 10 participants including 1 event are excluded due to missing baseline covariates.
Model 2: Model 1 plus use of lipid lowering drugs, use of blood pressure lowering drugs and BMI; stratified by study. 32 participants including 2 events are excluded due to missing baseline covariates.
Model 3: Model 2 plus cigarette smoking status, LDL, HDL, and triglycerides; SMART participants only. 17 participants including 0 events are excluded due to missing baseline covariates.
Figure 2.
Cumulative Percent Developing Diabetes by Quartile of IL-6. The number at risk in Quartiles 1, 2, 3 and 4 was 989, 1023, 962 and 991, respectively at baseline, 746, 841, 812 and 822 at year 2, 395, 570, 564 and 464 at year 4 and 194, 302, 282 and 211 at year 6. The p-value corresponding to a logrank test with 3 degrees of freedom that compares the incidence of diabetes by quartile is <0.001.
Figure 3.
Cumulative Percent Developing Diabetes by Quartile of hsCRP. The number at risk in Quartiles 1, 2, 3 and 4 was 995, 987, 993, 990, respectively at baseline, 801, 822, 807 and 791 at year 2, 511, 522, 519 and 441 at year 4 and 268, 284, 246 and 191 at year 6. The p-value corresponding to a logrank test with 3 degrees of freedom that compares the incidence of diabetes by quartile is <0.001.
Covariate adjustment diminished the associations but they remained significant. For the three models considered, HRs associated with a doubling of biomarkers for IL-6 ranged from 1.29 to 1.36; for hsCRP the HRs ranged from 1.22 to 1.28. The lower HRs in models 2 and 3 were due primarily to the adjustment for BMI. Supplemental Table 1 gives unadjusted and adjusted HRs for each of the covariates considered in Model 3. A 1 kg/m2 higher BMI was associated with a 10% increased risk of diabetes (<0.001). Other significant predictors of diabetes in the multivariable model were older age (p=0.013), hepatitis B or C infection (p=0.032), use of lipid lowering therapy (p=0.008), and non-smoking status (p=0.03). Consistent with other studies (33), d4T and ZDV were associated with an increased risk of diabetes, but these associations did not achieve statistical significance.
We investigated the higher risk of diabetes with non-smoking status further by dividing the non-smokers into two groups, former smokers (n=561) and never smokers (n=762). The unadjusted HRs for diabetes for smokers versus former smokers and for smokers versus never smokers were 0.51 (95% CI: 0.28 to 0.93) and 0.56 (95% CI: 0.31 to 1.01). With adjustment for the covariates in supplemental Table 1 plus hsCRP and IL-6, HRs for former versus current smokers and for never versus current smokers were reduced to 0.63 (95% CI: 0.33 to 1.19) and 0.53 (95% CI: 0.27 to 1.00), respectively.
For comparison with a recent meta-analysis (18), we also considered the risk of diabetes associated with 1 natural log higher levels of IL-6 and hsCRP. With the Model II covariates, these HRs for IL-6 and hsCRP were 1.45 (95% CI: 1.12 to 1.88) and 1.34 (95% CI: 1.15 to 1.56), respectively.
To assess whether the risk of diabetes associated with elevated IL-6 and hsCRP was similar in baseline defined subgroups, we carried out additional analyses. These subgroup analyses showed no interaction between the diabetes risk factors considered and the biomarkers except for race and hsCRP (Supplemental Tables 2 and 3). A higher level of hsCRP was associated with significantly higher risk of developing diabetes for whites and other races, but there was no association in blacks (P=0.009 for interaction).
The test for proportional hazard ratio assumption showed no evidence that the assumption was violated for hsCRP (P-value=0.34). For IL-6 there was evidence that the proportional hazards assumption may not hold (P-value=0.03). The association between IL-6 and incident diabetes was stronger early in the follow-up period compared to later (see Supplemental Tables 4 and 5). In the multivariable analysis (Model 2), a doubling of IL-6 was associated with a HR of 1.45 (95% CI: 1.18-1.79; P<0.001) in the first 3 years of follow-up when 87 of the 137 diabetes events occurred, and a HR of 1.01 (95% CI: 0.72-1.42 P=0.94) after 3 years of follow-up.
Discussion
In this cohort study of HIV positive patients, higher baseline levels of IL-6, and hsCRP were significantly associated with the risk of developing diabetes over an average of 4.6 years of follow-up. These associations were independent of BMI, age and other established diabetes risk factors.
Our findings are similar to a recent systematic review of studies in the general population (19). That review included 10 cohort studies with a total of 19,709 participants and 4,480 cases of diabetes for IL-6, and 22 cohort studies with a total of 40,735 participants and 5,753 cases of diabetes for hsCRP. In that review, relative risks associated with a 1 natural log higher IL-6 and hsCRP level were 1.31 (95% CI: 1.17-1.46) and 1.26 (95% CI 1.16-1.37), respectively. Several of the studies in this overview adjusted for measures of glycemia. With adjustment for measures of glycemia, the associations of IL-6 and hSCRP with diabetes were attenuated but remained significant. For the subgroup of studies in this systematic review that did not adjust for measures of glycemia (as in this investigation), the relative risk estimates associated with a 1 natural log higher IL-6 and hsCRP were 1.46 (95% CI: 1.25-1.71) and 1.33 (95% CI: 1.10-1.62). These latter estimates are very similar to HRs for IL-6 and hsCRP in this HIV cohort: 1.45 and 1.34 for IL-6 and hsCRP, respectively.
We cannot establish in our study whether inflammation increased levels of glycemia or vice versa. The baseline cross-sectional correlations we observed between IL-6 and hsCRP with fasting glucose in the small sample of SMART patients with these biomarkers (0.24 and 0.17) are similar to those reported in other studies (34) (20) (35). The findings from the aforementioned systematic review (19), suggest that these inflammatory markers provide independent information for predicting the development of diabetes. Experimental studies aimed at reducing inflammation are needed to establish a causal relationship.
The study of the systemic inflammation and diabetes among HIV positive patients is limited to a single case-control study (23). Brown et al. examined the association of hsCRP, IL-6 and soluble receptors of tumor necrosis factor-α (sTNFR1 and sTNFR2) measured 48 weeks after ART initiation with subsequent development of diabetes among HIV positive patients. They carried out a nested case-control study that included 55 patients who developed diabetes a median of 1.9 years after the 48 week blood measurements and 55 control patients matched on BMI at ART initiation, age and race. Higher levels of each of the inflammatory markers were associated with an increased risk of diabetes and associations were significant for hsCRP, sTNFR1, and sTNFR2 (23).
In the Atherosclerosis Risk in Communities Study a significant association between a score based on several inflammatory markers and diabetes was found for nonsmokers but not smokers (36). Therefore we considered the possibility of interaction with smoking in our investigation. We did not observe an interaction between smoking and hsCRP (P=0.91) or IL-6 (P=0.48). We did find a significant interaction between race and hsCRP levels. hsCRP was significantly associated with diabetes in whites and other race groups but not in blacks. An interaction with race was not found in the MESA study (37). There was no evidence that the association of IL-6 and hsCRP with diabetes varied by age, gender, BMI, use of lipid lowering drug or use of blood pressure lowering drug. Associations were also similar in the SMART and ESPRIT studies.
The association between higher levels of IL-6, but not hsCRP, was reduced with longer follow-up. Weaker associations of diabetes with IL-6 compared to hsCRP were also observed in the Women’s Health and Nurses’ Health Study (10)(13). This finding could be a chance finding or could be due to some patients at study entry having levels of blood glucose indicative of diabetes or pre-diabetes that led to raised levels of IL-6. While inclusion of a large percent of patients with baseline fasting glucose levels of 126 mg/dl or higher in this cohort seems unlikely given the results from the sample of patients from SMART who had fasting glucose measurements, about 20% did have pre-diabetes levels (100-125 mg/dl). Similarly, in the study by Brown et al. fasting glucose levels at study entry were significantly higher for patients who later developed diabetes compared to controls (23). As in the previously mentioned overview (19), if it had been possible in our investigation to adjust for measures of glycemia at entry in our analyses, the strength of the associations of IL-6 and hsCRP with diabetes incidence would likely have been attenuated.
Consistent with a recent review of risk prediction models for type 2 diabetes that found age, BMI and race to be among the most common predictors reported for type 2 diabetes (38), these were also important predictors in our analyses. In the HIV population, these are also important predictors (3). Inconsistent with a recent overview of studies in the general population which found that smoking was associated with a 44% increased risk of diabetes (39), we found that smoking was associated with a reduced risk of diabetes. This was also observed in the D:A:D and Swiss Cohort studies (not significant in the latter) (3)(38). Smoking cessation has been associated with an increased risk of diabetes (40), and this may explain this finding, in part, although we did HRs for former and never smokers versus smokers were similar in our investigation.
The strengths of our study include the large sample size, the standardized biomarker measurements and the long follow-up. Patients in these trials had frequent contact with medical providers, thereby reducing the likelihood that diabetes would not be diagnosed if it developed during the follow-up period. Several limitations should be considered, however. First of all, measures of glycemia used to define diabetes were not available at baseline and during follow-up except for a small sample of participants. Thus, we have likely underestimated the incidence of diabetes and also the strength of the association between the inflammatory markers and diabetes since those with diabetes defined by glucose measurements only are included in the no diabetes group. Also, we could not consider covariate adjustment for baseline fasting glucose. Second, the inflammatory biomarkers were only measured once. This also results in an underestimation of risk due to measurement error and the variability in these markers over time (41). Third, some important baseline covariates were not available for ESPRIT study therefore limiting our ability to adjust for smoking and lipoprotein levels in all participants. Nevertheless, when analyses were restricted to patients in SMART and these factors were included in the multivariate analysis (Model 3), HRs were not strongly affected. Fourth, while BMI was available, information was not available for measures of adiposity such as waist circumference in either SMART or ESPRIT. Information on family history of diabetes was also not available.
As for individuals in the general population (42), diabetes is a strong risk factor for CVD among HIV positive patients (33). Thus, our findings have important clinical and public health implications. First, elevated hsCRP and IL-6 levels in HIV patients even on suppressive ART are likely to lead to increased numbers with diabetes as the HIV population ages unless preventive measures are increased. Second, these data may offer some clues as to why HIV-positive individuals appear to have an increased risk of CVD and other serious non-AIDS diseases. Inflammation may be a common factor leading to increased risk of diabetes and serious non-AIDS conditions.
Conclusion
In conclusion, we found that elevated plasma levels of hsCRP and IL-6 were associated with diabetes among HIV-positive patients taking ART. Our findings support the hypothesis that low-grade systemic inflammation is an underlying factor in the pathogenesis of type 2 diabetes.
Supplementary Material
Acknowledgments
We acknowledge the SMART and ESPRIT participants and the SMART and ESPRIT study teams (see references 24 and 25).
Funding sources: National Institute of Allergy and Infectious Diseases (NIAID) Conflict of interest and sources of funding: The SMART and ESPRIT studies were funded by NIAID [grant numbers U01AI042170 and U01AI046362 (SMART); U01AI46957 and U01AI068641 (ESPRIT)].
Footnotes
All authors declare no conflicts of interest.
References
- 1.Samaras K. The burden of diabetes and hyperlipidemia in treated HIV infection and approaches for cardiometabolic care. Curr HIV/AIDS Rep. 2012 Sep;9(3):206–217. doi: 10.1007/s11904-012-0124-x. [DOI] [PubMed] [Google Scholar]
- 2.Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005 May 23;165(10):1179–1184. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 3.De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, El-Sadr W, Monforte A, Fontas E, Law MG, Friis-Moller N, Phillips A. Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Incidence and risk factors for new-onset diabetes in HIV-infected patients: The data collection on adverse events of anti-HIV drugs (D:A:D) study. Diabetes Care. 2008 Jun;31(6):1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tien PC, Schneider MF, Cole SR, Levine AM, Cohen M, DeHovitz J, Young M, Justman JE. Antiretroviral therapy exposure and incidence of diabetes mellitus in the women's interagency HIV study. AIDS. 2007 Aug 20;21(13):1739–1745. doi: 10.1097/QAD.0b013e32827038d0. [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Neuhaus J, Peters L, Ryom L, Bickel M, Grint D, Koirala J, Szymczak A, Lundgren J, Ross MJ, Wyatt CM, INSIGHT SMART Study Group, ESPRIT Study Group Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS One. 2012;7(7):e40245. doi: 10.1371/journal.pone.0040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reingold J, Wanke C, Kotler D, Lewis C, Tracy R, Heymsfield S, Tien P, Bacchetti P, Scherzer R, Grunfeld C, Shlipak M. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: The fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48(2):142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stankov M, Behrens GMN. Contribution of inflammation to fat redistribution and metabolic disturbances in HIV-1 infected patients. Curr Pharm Des. 2010;16(30):3361–3371. doi: 10.2174/138161210793563473. [DOI] [PubMed] [Google Scholar]
- 8.Donath M. Type 2 diabetes as an inflammatory disease. Nature Reviews.Immunology. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 9.Neuhaus J, Jacobs D, Baker J, Calmy A, Duprez D, La Rosa A, Kuller L, Pett S, Ristola M, Ross M, Shlipak M, Tracy R, Neaton J. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso WH, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon DE, Paton NI, Neaton JD, INSIGHT SMART Study Group Changes in inflammatory and coagulation biomarkers: A randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. J Acquir Immune Defic Syndr. 2011 Jan 1;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney ER, Mallon PW. Insulin resistance in treated HIV infection. Best Pract Res Clin Endocrinol Metab. 2011 Jun;25(3):443–458. doi: 10.1016/j.beem.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Hu F, Meigs J, Li T, Rifai N, Manson J. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 14.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based european prospective investigation into cancer and nutrition (EPIC)-potsdam study. Diabetes. 2003 Mar;52(3):812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 15.Sjoholm A, Nystrom T. Inflammation and the etiology of type 2 diabetes. Diabetes Metab Res Rev. 2006 Jan-Feb;22(1):4–10. doi: 10.1002/dmrr.568. [DOI] [PubMed] [Google Scholar]
- 16.Akash MS, Rehman K, Chen S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013 Mar;114(3):525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 17.Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabak AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: The whitehall II study. Diabetes Care. 2009 Mar;32(3):421–423. doi: 10.2337/dc08-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallmeier D, Larson MG, Wang N, Fontes JD, Benjamin EJ, Fox CS. Addition of inflammatory biomarkers did not improve diabetes prediction in the community: The framingham heart study. J Am Heart Assoc. 2012 Aug;1(4):e000869. doi: 10.1161/JAHA.112.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Bao W, Liu J, Ouyang Y, Wang D, Rong S, Xiao X, Shan Z, Zhang Y, Yao P, Liu L. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. doi: 10.2337/dc12-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The insulin resistance atherosclerosis study (IRAS) Circulation. 2000 Jul 4;102(1):42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 21.Tsigos C, Papanicolaou DA, Kyrou I, Defensor R, Mitsiadis CS, Chrousos GP. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J Clin Endocrinol Metab. 1997 Dec;82(12):4167–4170. doi: 10.1210/jcem.82.12.4422. [DOI] [PubMed] [Google Scholar]
- 22.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41(10):1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 23.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010 Oct;33(10):2244–2249. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.INSIGHT-ESPRIT Study Group. SILCAAT Scientific Committee. Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, Fox L, Gordin F, Lane HC, Lundgren JD, Mitsuyasu R, Neaton JD, Phillips A, Routy JP, Tambussi G, Wentworth D. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009 Oct 15;361(16):1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ, Cohn D, Cooper D, Darbyshire J, Emery S, Fatkenheuer G, Gazzard B, Grund B, Hoy J, Klingman K, Losso M, Markowitz N, Neuhaus J, Phillips A, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, Paton NI, Neaton JD, INSIGHT SMART Study Group Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duprez D, Neuhaus J, Kuller L, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Nixon D, Paton N, Prineas R, Neaton J. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS ONE. 2012;7(9):e44454–e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuller LH, Tracy RP, Shaten J, Meilahn EN. Relation of C-reactive protein and coronary heart disease in the MRFIT nested case-control study. multiple risk factor intervention trial. Am J Epidemiol. 1996 Sep 15;144(6):537–547. doi: 10.1093/oxfordjournals.aje.a008963. [DOI] [PubMed] [Google Scholar]
- 29.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999 May;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000 Apr 18;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 31.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: Two new prospective studies and a systematic review. PLoS Med. 2008 Apr 8;5(4):e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010 Jan 9;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worm SW, De Wit S, Weber R, Sabin CA, Reiss P, El-Sadr W, Monforte AD, Kirk O, Fontas E, Dabis F, Law MG, Lundgren JD, Friis-Moller N. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: The data collection on adverse events of anti-HIV drugs (D:A:D study) Circulation. 2009 Feb 17;119(6):805–811. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, Markiewicz W, Brook GJ, Levy Y. Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabet Med. 2004 Jan;21(1):39–44. doi: 10.1046/j.1464-5491.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 35.Cardellini M, Andreozzi F, Laratta E, Marini MA, Lauro R, Hribal ML, Perticone F, Sesti G. Plasma interleukin-6 levels are increased in subjects with impaired glucose tolerance but not in those with impaired fasting glucose in a cohort of italian caucasians. Diabetes Metab Res Rev. 2007 Feb;23(2):141–145. doi: 10.1002/dmrr.679. [DOI] [PubMed] [Google Scholar]
- 36.Duncan B, Schmidt M, Pankow J, Ballantyne C, Couper D, Vigo A, Hoogeveen R, Folsom A, Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: The atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- 37.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: The multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2010 Apr;33(4):804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, Cavassini M, Bernasconi E, Schmid P, Egger M, Weber R, Swiss HIV Cohort Study Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the swiss HIV cohort study. Clin Infect Dis. 2007 Jul 1;45(1):111–119. doi: 10.1086/518619. [DOI] [PubMed] [Google Scholar]
- 39.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: A systematic review and meta-analysis. JAMA. 2007 Dec 12;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 40.Davey Smith G, Bracha Y, Svendsen KH, Neaton JD, Haffner SM, Kuller LH, Multiple Risk Factor Intervention Trial Research Group Incidence of type 2 diabetes in the randomized multiple risk factor intervention trial. Ann Intern Med. 2005 Mar 1;142(5):313–322. doi: 10.7326/0003-4819-142-5-200503010-00006. [DOI] [PubMed] [Google Scholar]
- 41.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999 Aug 15;150(4):341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 42.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the multiple risk factor intervention trial. Diabetes Care. 1993 Feb;16(2):434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.