Abstract
Objective:
To investigate whether the acetylated and propionated derivatives (LMP-09-1 and -2) of swertiamarin have anti-adipogenic effects.
Materials and Methods:
3T3-L1 pre-adipocytes were grown in Dulbecco's Modified Eagle's Medium (DMEM) containing 10% calf serum; fully confluent cells were differentiated with insulin, dexamethasone, and 3-isobutylmethylxanthine in the presence and absence of LMP-09-1 and -2 (100 μg/mL) for 10 days. Control cells received same amount of dimethylsulfoxide (DMSO). On day ten, cells were analyzed for triglycerides accumulation and the expression of genes involved in adipogenesis, lipogenesis, and lipolysis. In another set of experiment, effects of LMP-09-1 and 2 were studied for isoproterenol induced lipolysis using fully mature adipocytes.
Results:
LMP-09-1 and -2 caused a significant (P < 0.001) reduction in intracellular triglycerides accumulation. Both LMP-09-1 and -2 significantly (P < 0.001) decreased the mRNA expression of peroxisome proliferator activated receptor-γ and acetyl-CoA carboxylase-1, and increased isoproterenol induced lipolysis in adipocytes. LMP-09-1 induced lipolysis even in the absence of isoproterenol, and also showed a significant up-regulation of carnitine palmitoyl transferase-1α and hormone sensitive lipase (HSL) gene expression.
Conclusions:
These findings show that swertiamarin derivatives, LMP-09-1 and -2 have a potent anti-adipogenic effect.
Keywords: Isoproterenol, lipolysis, acetylated swertiamarin, propionated swertiamarin, peroxisome proliferator activated receptor
INTRODUCTION
Obesity is characterized by an increase in the number and size of adipocytes after differentiating from fibroblastic preadipocytes in the mature adipose tissue. An increase in adipose tissue mass occurs through the proliferation of preadipocytes followed by differentiation of these cells into mature adipocytes.[1] Adipocytes differentiation is accompanied by coordinated changes in cell morphology, hormone sensitivity, and gene expression.[1] These changes are regulated by two main transcription factors, including peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer-binding proteins (C/EBPs),[2] which are involved in the sequential expression of adipocytes-specific proteins during adipocyte differentiation.[3]
Adipocytes are metabolically active in de novo fatty acid synthesis and fat oxidation to maintain homeostasis. Acetyl-CoA carboxylase (ACC) catalyzes the rate-limiting step in de-novo fatty acid biosynthesis to produce immediate metabolite malonyl-CoA.[4] Malonyl-CoA controls mitochondrial fatty acid uptake through allosteric inhibition of carnitine palmitoyl transferase-1α (CPT-1α) that catalyzes the first committed step in fatty acid oxidation.[5] Thus, acetyl-CoA and malonyl-CoA are the key metabolic signals for the control of fatty acid production and oxidation. Inhibition of ACC offers an attractive modality to target a multitude of cardiovascular risk factors associated with obesity, diabetes, insulin resistance, and metabolic syndrome such as obesity.[6] Furthermore, obese subjects have an impaired ability to mobilize stored fat via the action of catecholamine.[7] Hormone sensitive lipase (HSL) is the chief enzyme responsible for mobilization of non-esterified free fatty acids (NEFA) from adipose tissue. HSL knockout mice are resistant to both high fat diet-induced and genetic obesity, displaying a reduction in white adipose tissue, thereby indicating the importance of HSL in adipogenesis and obesity.[8]
Various phytochemicals such as isoflavonoids, flavonoids, and catechins have been reported to inhibit adipogenesis.[9] Swertiamarin, a secoiridoid glycoside, is a bioactive phytochemical; we have previously reported anti-diabetic and anti-hyperlipidemic effects of swertiamarin in animal models.[10,11,12,13] Later on swertiamarin was found to have a low plasma half-life,[14] thereby having a low potency, which may be due to the hydrophilic nature of swertiamarin. In the current study, we synthesized less water soluble derivatives of swertiamarin (LMP-09-1 and -2) to improve the stability of swertiamarin in the blood and to increase the potency of swertiamarin. We investigated the effects of LMP-09-1 and -2 on adipogenesis.
MATERIALS AND METHODS
The 3T3-L1 cells were obtained from American Type Culture Collection (ATCC # CL-173, USA). Insulin, 3-isobutylmethylxanthine (IBMX), dexamethasone (DEX), Dulbecco's Modified Eagle's Medium (DMEM) and dimethylsulphoxide (DMSO) were from Sigma Aldrich, USA. Foetal Bovine Serum (FBS), penicillin-streptomycin, amphotericin-B, trypsin and calf serum were obtained from Gibco (USA). Oil Red O Dye and dye extracting solution was from Millipore, USA. Other reagents were: TriZol (Invitrogen, USA), random primers (Invitrogen, USA), dNTPs (Invitrogen), Taq polymerase (Invitrogen, USA), isoproterenol (Sigma Aldrich), fatty acid free bovine serum albumin (Sigma Aldrich, USA), triglycerides assay kit (236-60, SEKISUI diagnostic, USA), and NEFA-C kit (999-34691, Wako Chemicals, USA).
Synthesis of LMP-09-1 and LMP-09-2
LMP-09-1 and LMP-09-2 were synthesized from swertiamarin (isolated from Enicostemma littorale Blume) as discussed earlier.[11] Briefly, Swertiamarin (0.53 mmol) was dissolved in 5 mL of dry dimethylformamide (DMF) and stirred for 5 min at 0-8˚C. During stirring, pyridine (2.1 mmol) was added as a catalyst, followed by drop wise addition of 2.1 mmol of either acetic anhydride (for LMP-09-1) or propionic anhydride (for LMP-09-2). Stirring was continued till the reaction was complete (based on visual identification of spots appearing on thin layer chromatography, and disappearance of starting material). Reaction mixture was decomposed with crushed ice and then extracted twice with diethyl ether (6 mL). Diethyl ether was evaporated under vacuum, and the remaining solid residue was re-crystallized using hot water. Structure of the products was characterized using melting point, mass, and NMR spectroscopic techniques and purity was determined using High Pressurized Thin layer Chromatography (HPTLC), while using ethyl acetate: methanol: water (9.5:0.4:0.1) solvent system. These methods are also used by others to assess pharmaceutical product quality.[15]
Cell culture and adipocytes differentiation
The 3T3-L1 preadipocytes were cultured (37°C; 5% CO2) in DMEM supplemented with 10% calf serum, 100 U/ml penicillin and 100μg/ml streptomycin until confluent. Two days post-confluency, cell were differentiated with 1 μM insulin, 1 μM dexamethasone and 0.5 mM IBMX with or without 100 μg/ml LMP-09-1 and LMP-09-2 in DMEM containing 10% FBS. This dose was selected based on a dose response curve established in preliminary experiments.[10] After 48 hours, the medium was changed to DMEM containing 10% FBS and 10 μg/ml insulin along with the treatments; medium was replaced every 48 hours till day 10. Control cells received vehicle (DMSO, 0.05%), while untreated cells received media alone (NC). LMP-09-1 and -2 were dissolved in DMSO for treatment. On the 10th day, fully differentiated adipocytes were used for Oil Red O staining, and to measure intracellular triglycerides accumulation and gene expression.
Cell viability
Cell viability was measured using an MTT (3-(4, 5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) assay with slight modifications.[16] 3T3-L1 preadipocytes were grown in 96 well microtiter plates (10,000 cells/well), and incubated for 24 h prior to treatment with and without different concentrations of LMP-09-1 and -2 (10, 50, 100 and 250 μg/ml). After 24 h, media was removed and 20 μl of MTT (5 mg/ml) was added to each well and incubated for 2 h at 37°C. The MTT reagent was then removed and formazan crystals were solubilized in 200 μl of DMSO. The coloured solution was quantified at 570 nm and cell viability was expressed as a percentage over control.
Oil Red O staining and triglycerides assay
Lipid accumulation in the adipocytes was measured using triglycerides assay and Oil Red O staining method as per manufacturer protocol. Briefly, after removal of culture medium, cells were washed with 1X PBS (phosphate buffered saline) three times. Oil Red-O dye was added and incubated for 15 min at room temperature; adipocytes were washed and an image was captured.
To analyse intra-cellular triglycerides accumulation in adipose cells, lipids were extracted using Folch extraction method.[17] Briefly, cells were washed with 1X PBS, scraped in PBS and centrifuged at 1500 rpm for 5 minutes. Cell pellet was re-suspended in 1 ml of 1X PBS and lipids were extracted; extracted lipids were reconstituted in isopropyl alcohol to measure triglycerides. Protein concentration of cell lysate was measured using Lowry method[18] and data was expressed as mmol triglycerides per mg of protein.
Total RNA isolation and gene expression
Total RNA was isolated from the cells using TriZol method[19] and integrity of RNA was confirmed. Multiplex reversetranscription polymerase chain reaction (RT-PCR) was performed using β-actin as the housekeeping gene. Primers used for the PCR reaction were designed using National Centre of Biotechnology Information (NCBI) blast online software and are given in [Table 1]. The RT-PCR products were analysed using Chemi-Imager 4400, normalized to β-actin expression and expressed as arbitrary units.
Table 1.
Sequence of primers used for reverse transcriptase PCR reaction

Catecholamine induced lipolysis
Fully mature adipocytes (10 days after differentiation) were pre-incubated in DMEM containing 2% (v/v) fatty acid-free bovine serum albumin for 12 h. Lipolysis was stimulated by treatment with 10 μM isoproterenol for 1h, in the absence or presence of insulin (100 nM), and LMP-09-01 or -2 (100 μg/mL). After 1 h of incubation (37°C, 5% CO2), cell culture medium was collected and NEFA were measured immediately using NEFA-C kit; protein concentration of cell lysate was measured using Lowry method.[18] Data was expressed as mEq of NEFA per mg of protein.
Statistical analysis
The results were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's multiple test to determine the level of significance. P values < 0.05 were considered significant. The results were expressed as mean±SD.
RESULTS
Characterization of LMP-09-1 and -2
LMP-09-1 and 2 were characterized using melting point, mass and NMR spectroscopic techniques. The yield for LMP-09-1 and -2 after derivatization of swertiamarin was found to be 54 and 48%, respectively. The melting point of LMP-09-1 was 103-104˚ C, and the melting point of LMP-09-2 was 110-115˚ C. Mass spectra showed molecular ion peak (m + 1) at 543nm for LMP-09-1 and at 599 nm for LMP-09-2 confirming molecular weights of acetylated and propionated derivatives of swertiamarin. Furthermore, downfield shift of tertiary and secondary alcohol protons in proton NMR spectra of both compounds confirmed that esterification was successfully carried out on sugar alcoholic protons [Supplementary 1], thus swertiamarin was successfully derivatized. The purity of these compounds was found to be 99%.
Supplementary 1.
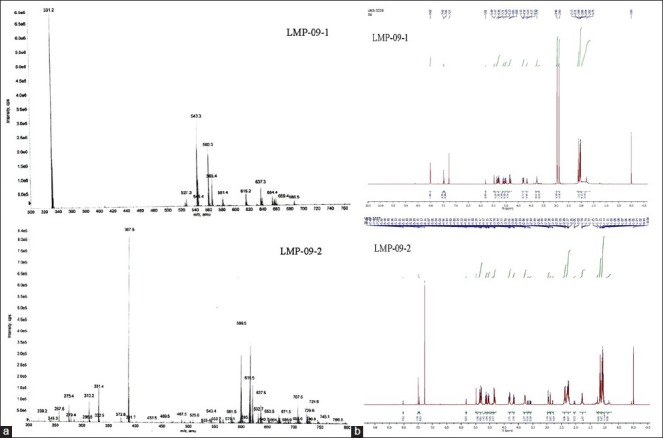
(a) Mass spectrum of LMP-09-1 and -2, (b) NMR spectrum of LMP-09-1 and -2
Effect of LMP-09-1 and LMP-09-2 on the viability of 3T3-L1 adipocytes
The viability assay was used to determine suitable dose and possible cytotoxic effect of LMP-09-1 and -2 on adipocytes. 3T3-L1 preadipocytes were incubated in the presence of different doses of LMP-09-1 and -2 (10, 50, 100 and 250 μg/ml) for 48 h. As shown in Figure 1a, treatment with LMP-09-1 and -2 at all tested concentrations did not affect cell viability.
Figure 1.
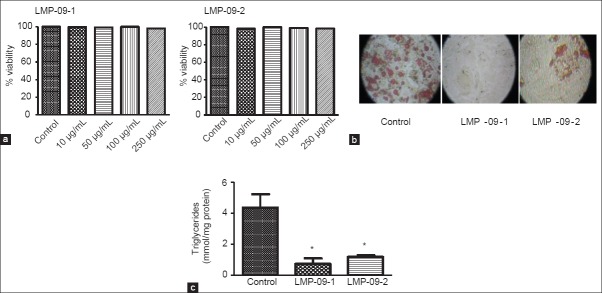
a) Effects of LMP-09-1 and LMP-09-2 on cell viability of 3T3-L1 cells, b) Oil Red O staining of 3T3-L1 cells on day 10; the original magnification was × 100, c) Intracellular triglycerides accumulation was measured using triglycerides assay kit after lipid extraction in 10 day fully differentiated 3T3-L1 cells as described in methods. Values are presented as mean ± SD, n = 3. * indicates significantly (P < 0.001) different than control
Effects of LMP-09-1 and LMP-09-2 on intracellular triglycerides accumulation
Treatment with LMP-09-1 and-2 (100 μg/ml) markedly inhibited fat accumulation in 3T3-L1 cells compared to the control cells [Figure 1b] as observed by a decrease in lipid droplet formation using Oil Red O staining [Figure 1b]. Furthermore, treatment with LMP-09-1 and -2 showed a significant (P < 0.001) decrease in intracellular triglycerides accumulation compared to the control cells [Figure 1c].
Effects of LMP-09-1 and LMP-09-2 on adipogenesis and lipogenic genes
PPAR-γ is a marker gene associated with adipogenesis. We measured the effects of LMP-09-1 and -2 on the mRNA expression of PPAR-γ to investigate whether a decrease in triglycerides accumulation corresponds with inhibition of PPAR-γ. Both LMP-09-1 and -2 significantly (P < 0.001) reduced the mRNA expression of PPAR-γ compared to the control cells [Figure 2a]; however there was no effect of the treatments on C/EBP-α mRNA expression [Figure 2b].
Figure 2.
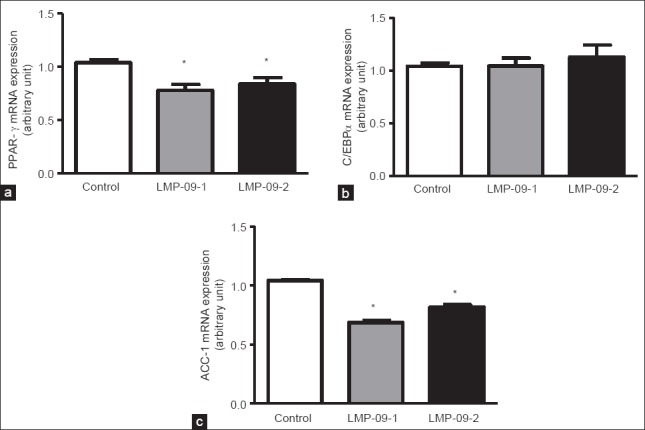
Effects of LMP-09-1 and LMP-09-2 on the mRNA expression of a) PPAR-γ, b) C/EBPα; and c) ACC-1 in 10 day fully differentiated 3T3-L1 adipocytes. Values are presented as mean ± SD, n = 3. * indicates significantly (P < 0.001) different than control
An increase in ACC-1 gene expression increases lipogenesis, thereby increasing obesity and insulin resistance; thus the effects of LMP-09-1 and -2 were measured on the mRNA expression of ACC-1. Treatment with both LMP-09-1 and -2 (100 μg/ml) significantly decreased ACC-1 mRNA expression, however the extent of decrease for LMP-09-1 was greater than LMP-09-2 (60% decrease v/s 37% decrease, respectively) [Figure 2c].
Effects of LMP-09-1 and LMP-09-2 on lipolysis
A decrease in fat accumulation may also be due to an increase in fat breakdown and release, thus isoproterenol induced lipolysis was measured in fully mature adipocytes. Isoproterenol treatment increased NEFA levels in the medium (15% increase) compared to control cells. Treatment with both LMP-09-1 and -2 increased NEFA concentrations in the presence of isoproterenol (26 and 28% respectively) [Figure 3a]. It was interesting to note that LMP-09-1 showed an increase in NEFA concentrations even in the absence of isoproterenol compared to DMSO control [Figure 3a].
Figure 3.
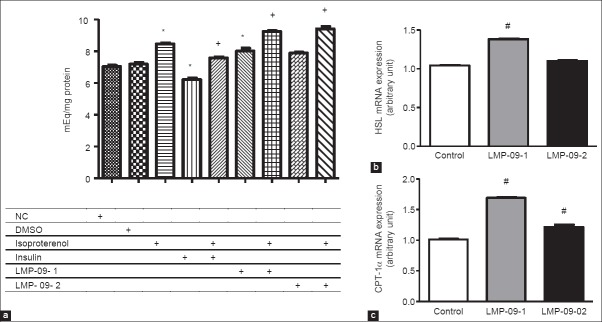
Effects of LMP-09-1 and LMP-09-2 on a) Isoproterenol induced lipolysis b) The mRNA expression of hormone sensitive lipase (HSL) and c) The mRNA expression of carnitine palmitoyl transferase (CPT)-1α; in 10 day fully differentiated 3T3-L1 adipocytes. Values are presented as mean ± SD, n = 3. * indicates significantly (P < 0.05) different than DMSO, + indicates significantly (P < 0.05) different than isoproterenol control and # indicates significantly (P < 0.05) different than control
HSL involved in fat mobilization, while CPT-1α is involved in fat oxidation; thus their expression was measured to investigate if LMP-09-1 and -2 mediated decreased in fat accumulation is due to an increased breakdown of fat. LMP-09-1 treatment showed a significant (P < 0.01) increase in the mRNA expression of HSL [Figure 3b]; while there was no effect of LMP-09-2. Moreover, we also found that treatment with both LMP-09-1 and -2 significantly increased the mRNA expression of CPT-1α [Figure 3c].
DISCUSSION
Adipose tissue is an endocrine organ that plays a crucial role in the regulation of whole-body fatty acid homeostasis thereby controlling energy balance and regulation.[20] In periods of calorie abundance, adipocytes store free fatty acids in the form of triglycerides, while releasing stored fat during times of energy shortage. In the present study, we are reporting for the first time that less water soluble derivatives of swertiamarin (LMP-09-1 and -2) possess potent anti-adipogenic effect by decreasing fatty acid synthesis and increasing release of stored fat and its oxidation.
Previously, we have shown anti-diabetic and anti-hyperlipidemic effects of swertiamarin in animal models.[11,12,13] However, recent reports have shown that swertiamarin has a very short plasma half-life,[14] thus may not be very potent. We designed less water soluble derivatives of swertiamarin namely; acetylated (LMP-09-1) and propionated (LMP-09-2) forms to improve bioavailability and potency of swertiamarin, and tested their anti-adipogenic properties using 3T3-L1 adipocytes. Treatment with LMP-09-1 and -2 markedly inhibited adipocytes differentiation and intracellular triglycerides accumulation in 3T3-L1 cells compared to the control cells. During adipogenesis process, pre-adipocytes change their morphology and convert to mature adipocytes phenotype by arresting their growth with differentiation cocktail; cells start accumulating triglycerides and become terminally differentiated adipocytes by day 8-10.[2] Adipocyte differentiation is regulated by a complex mechanism including transcriptional regulation by coordinated changes in the expression of adipocytes-specific genes, i.e. PPAR-γ and C/EBPs.[21] Both LMP-09-1 and -2 treatments significantly decreased the mRNA expression of PPAR-γ. This observation, combined with a decrease in fat accumulation, suggests that LMP-09-1 and -2 are anti-adipogenic. Previous reports have shown that adipose tissue specific inhibition of PPAR-γ protects against high fat diet induced obesity and insulin resistance.[22] Others have also reported inhibition of PPAR-γ in adipose tissue as a target for anti-obesity effect.[23,24]
Since both LMP-09-1 and -2 inhibited triglycerides accumulations in 3T3-L1 cells, it was imperative to investigate whether a decrease in triglycerides accumulation was due to inhibition of de novo lipid synthesis or an increase in lipolysis. We found that treatment with both LMP-09-1 and -2 significantly (P < 0.001) decreased the mRNA expression of ACC-1 in differentiated 3T3-L1 adipocytes compared to the control cells. Levert et al.[6] also found inhibition of ACC-1 by biotin analog that caused a decrease in adipogenesis in 3T3-L1 cells; hence inhibition of ACC-1 could be an effective means of treating lipid disorders, obesity-associated dyslipidemia and insulin resistance. Moreover ACC-1 negatively regulates CPT-1α activity, thereby increasing fatty acid oxidation.[25] CPT-1α is a rate limiting enzyme in fatty acid oxidation[26] by transporting long chain fatty acids from cytosol into mitochondrial matrix.[27] Treatment with both LMP-09-1 and -2 significantly increased the mRNA expression of CPT-1α compared to control cells; however the increase was greater for LMP-09-1 than -2 (2.9 v/s 1.65 times increase respectively). Others have also shown that stimulation of free fatty acid transport through CPT-1α in adipose tissue as well as in liver is beneficial in obesity.[28] Ejaz et al.[29] have also shown a decrease in PPAR-γ, ACC-1 and an increase in CPT-1α gene expression in 3T3-L1 adipocytes as a mechanism for anti-adipogenic and anti-obesity effects of curcumin.
Pharmacological targeting of obesity involves: (1) stimulating hydrolysis of triglycerides to diminish fat stores, and (2) increasing oxidation of newly released fatty acids; this approach led to the development of beta 3-adrenergic agonists.[30] Plasma fatty acid levels reflect lipolysis, which is strictly regulated by several hormones like catecholamine and natriuretic peptides to stimulate the catabolic pathway in humans.[30] Impairments in catecholamine-induced lipolysis have been suggested to contribute to the development or maintenance of increased adipose tissue stores and obesity.[31] Treatment with LMP-09-1 increased lipolysis even in the absence of isoproterenol, while both LMP-09-1 and -2 significantly increased lipolysis when given along with isoproterenol. There was a cumulative effect of LMP-09-1, when given with isoproterenol (26% increases v/s 5% increase when given alone). LMP-09-1 treatment also showed a significant increase in the mRNA expression of HSL, which was not observed with LMP-09-2, suggesting that LMP-09-1 has the ability to act like a catecholamine to induce lipolysis.
Overall, our findings strongly indicate that LMP-09-1 and -2 act via multiple pathways in adipocytes, that is inhibition of fat uptake and fat synthesis, breakdown of stored fat, along with an increase in fat oxidation, thus both LMP-09-1 and -2 has the potential to act as anti-adipogenic agents.
CONCLUSION
LMP-09-1 and LMP-09-2 possess potent anti-adipogenic effects by inhibiting de novo fatty acid synthesis, increasing fat breakdown and promoting fatty acid oxidation in 3T3-L1 adipocytes.
ACKNOWLEDGEMENT
We would like to thank Natural Sciences and Engineering Research Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR) for providing funding for this research. We would also like to thank the Canadian Commonwealth Exchange Program, Asia-Pacific for financial support.
Footnotes
Source of Support: We would like to thank Natural Sciences and Engineering Research Council (NSERC) and Canadian Commonwealth Exchange Program, Asia-Pacific for providing funding for this research.
Conflict of Interest: None declared.
REFERENCES
- 1.Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, et al. Fibroblast growth factor 1: A key regulator of human adipogenesis. Diabetes. 2004;53:3097–106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 3.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 4.Harwood HJ., Jr Treating the metabolic syndrome: Acetyl-CoA carboxylase inhibition. Expert Opin Ther Targets. 2005;9:267–81. doi: 10.1517/14728222.9.2.267. [DOI] [PubMed] [Google Scholar]
- 5.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Levert KL, Waldrop GL, Stephens JM. A biotin analog inhibits acetyl-CoA carboxylase activity and adipogenesis. J Biol Chem. 2002;277:16347–50. doi: 10.1074/jbc.C200113200. [DOI] [PubMed] [Google Scholar]
- 7.Lafontan M, Berlan M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J Lipid Res. 1993;34:1057–91. [PubMed] [Google Scholar]
- 8.Kraemer FB, Shen WJ. Hormone-sensitive lipase knockouts. Nutr Metab (Lond) 2006;3:12. doi: 10.1186/1743-7075-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu CL, Yen GC. Phenolic compounds: Evidence for inhibitory effects against obesity and their underlying molecular signaling mechanisms. Mol Nutr Food Res. 2008;52:53–61. doi: 10.1002/mnfr.200700393. [DOI] [PubMed] [Google Scholar]
- 10.Vaidya H, Goyal RK, Cheema SK. Anti-diabetic activity of swertiamarin is due to an active metabolite, gentianine, that upregulates PPAR-γ gene expression in 3T3-L1 cells. Phytother Res. 2013;27:624–7. doi: 10.1002/ptr.4763. [DOI] [PubMed] [Google Scholar]
- 11.Vaidya H, Rajani M, Sudarsanam V, Padh H, Goyal R. Antihyperlipidaemic activity of swertiamarin, a secoiridoid glycoside in poloxamer-407-induced hyperlipidaemic rats. J Nat Med. 2009;63:437–42. doi: 10.1007/s11418-009-0350-8. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya H, Rajani M, Sudarsanam V, Padh H, Goyal R. Swertiamarin: A lead from Enicostemma littorale Blume. for anti-hyperlipidaemic effect. Eur J Pharmacol. 2009;617:108–12. doi: 10.1016/j.ejphar.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya H, Prajapati A, Rajani M, Sudarsanam V, Padh H, Goyal RK. Beneficial effects of swertiamarin on dyslipidaemia in streptozotocin-induced type 2 diabetic rats. Phytother Res. 2012;26:1259–61. doi: 10.1002/ptr.3708. [DOI] [PubMed] [Google Scholar]
- 14.Li HL, Peng XJ, He JC, Feng EF, Xu GL, Rao Gx. Development and validation of a LC-ESI-MS/MS method for the determination of swertiamarin in rat plasma and its application in pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1653–8. doi: 10.1016/j.jchromb.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kaale E, Manyanga V, Makori N, Jenkins D, Michael Hope S, Layloff T. High-performance thin layer chromatography to assess pharmaceutical product quality. Trop Med Int Health. 2014 doi: 10.1111/tmi.12303. [DOI] [PubMed] [Google Scholar]
- 16.Marks DC, Belov L, Davey MW, Davey RA, Kidman AD. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk Res. 1992;16:1165–73. doi: 10.1016/0145-2126(92)90114-m. [DOI] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 20.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Lefterova MI, Lazar MA. New developments in adipogenesis. Trends Endocrinol Metab. 2009;20:107–14. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, et al. Deletion of PPARgamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–12. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato R, Buesa LM, Nerurkar PV. Anti-obesity effects of Emblica officinalis (Amla) are associated with inhibition of nuclear transcription factor, peroxisome proliferator-activated receptor gamma (PPAR) FASEB J. 2010;24:661–4. [Google Scholar]
- 24.Park HJ, Chung BY, Lee MK, Song Y, Lee SS, Chu GM, et al. Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPβ, C/EBPα, and PPARγ expression and the AKT signaling pathway in 3T3-L1 adipocytes. BMC Complement Altern Med. 2012;12:230. doi: 10.1186/1472-6882-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tong L, Harwood HJ., Jr Acetyl-coenzyme A carboxylases: Versatile targets for drug discovery. J Cell Biochem. 2006;99:1476–88. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010;11:380–8. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
- 27.Peyron-Caso E, Quignard-Boulange A, Laromiguiere M, Feing-Kwong-Chan S, Veronese A, Ardouin B, et al. Dietary fish oil increases lipid mobilization but does not decrease lipid storage-related enzyme activities in adipose tissue of insulin-resistant, sucrose-fed rats. J Nutr. 2003;133:2239–43. doi: 10.1093/jn/133.7.2239. [DOI] [PubMed] [Google Scholar]
- 28.Zang Y, Wang T, Xie W, Wang-Fischer YL, Getty L, Han J, et al. Regulation of acetyl CoA carboxylase and carnitine palmitoyl transferase-1 in rat adipocytes. Obes Res. 2005;13:1530–9. doi: 10.1038/oby.2005.188. [DOI] [PubMed] [Google Scholar]
- 29.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesityin C57/BL Mice. J Nutr. 2009;139:919–25. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 30.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–91. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Jocken JW, Blaak EE. Catecholamine-induced lipolysis in adipose tissue and skeletal muscle in obesity. Physiol Behav. 2008;94:219–30. doi: 10.1016/j.physbeh.2008.01.002. [DOI] [PubMed] [Google Scholar]


