Abstract
Severe skin reactions occur less frequently with eslicarbazepine (ESL) than with the other aromatic anticonvulsants. We report the first case of cutaneous adverse drug reaction (CADR) to ESL and co-sensitization between ESL and betalactams. A 41-year-old white woman developed focal epilepsy due to a meningioma that was removed. As post-operatory complication, she suffered meningitis as well as a maculo-papular erythema caused by the treatment with meropenem. Subsequently, ESL was started and gradually increased until 800 mg/day. Twenty-five days later, the patient developed an Erythema Multiforme Major (EMM). Strong positive immediate reaction was induced by prick test with carbamazepine (CBZ) and ESL at 0.01 and 0.1% within 15 and 30 minutes; however the delayed reading at 48 hours was negative. The patient was not carrier of the HLA alleles A3101 and B1502 associated with CBZ induced EMM. The hypersensitivity pathogenic mechanism of EMM is unclear and a delayed hypersensitivity process is speculated. However, the patch and intradermal tests in our patient did not show a delayed reaction but an immediate cutaneous one. A first allergic episode may elicit a massive nonspecific activation of the immune system, providing an enhanced expression of co-stimulatory molecules that decreases the level of tolerance to other drugs. When prescribing ESL, we suggest ruling out previous CADR, especially to CBZ and oxcarbazepine but also other chemically unrelated drugs such as beta-lactams.
Keywords: Allergic reaction, cutaneous adverse drug reaction, erythema multiforme major, eslicarbazepine, rash
INTRODUCTION
A cutaneous adverse reaction caused by a drug (CADR) is any undesirable change in the structure or function of the skin, its appendages, or mucus membranes. It can range from a mild localized skin rash to severe mucocutaneous reactions such as erythema multiforme major (EMM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). CADR may affect 2-16% of patients taking aromatic anticonvulsants (AA) such as carbamazepine (CBZ), oxcarbazepine (OXC), phenobarbital, and phenytoin, although severe forms have a lower incidence.[1]
Although the diagnosis is primarily clinical, a diagnostic cutaneous biopsy is required. A classification of these syndromes based on the type and distribution of skin lesions and estimated affected skin surface has been proposed. When the extent of the epidermal involvement is less than 10%, it is classified as SJS and if the epidermal detachment is greater than 30%, it is classified as TEN. Controversy exists with regard to whether erythema multiforme and SJS/TEN are distinct entities or represent a spectrum of one disease process.[2]
Eslicarbazepine acetate (ESL) is a new AA that has been demonstrated to be highly effective in the treatment of focal epilepsies.[3] ESL shares the basic chemical structure of a dibenzazepine nucleus bearing the 5-carboxamide substituent with CBZ and OXC, but is structurally different at the 10, 11-position and shows a longer elimination half-life of 20-24 h.[4] Rash and pruritus was described in 0.4-1.5% of patients receiving ESL; its severe forms led to discontinue the treatment in 0.1% of cases.[5]
Allergic cross-reactivity has often been reported between anticonvulsant drugs, but there are limited data regarding the development of anticonvulsant drug hypersensitivity preceded by a CADR induced by chemically unrelated drugs.
Here we present a case of EMM due to ESL in a patient with previous CADR caused by meropenem. To our knowledge, this is the first reported case of severe CADR to ESL and of co-sensitization between ESL and beta-lactams.
CASE REPORT
A 41-year-old white woman developed epilepsy with focal seizures secondary to a left fronto-parietal atypical meningioma (grade II World Health Organization). The initial seizure was focal, characterized by speech arrest followed by right hemifacial clonic movement that propagated to the right arm and ended with a generalized tonic-clonic phase. The meningioma was removed. The patient suffered meningitis as a postoperative complication, which was treated with meropenem. Twenty-five days later, the patient developed a skin reaction characterized by generalized maculopapular erythema attributed to meropenem. This CADR was confirmed by skin biopsy and the meropenem imputability by a delayed intradermal test.
The patient's medical and family history was otherwise unremarkable except for an atopic constitution. The patient had allergic rhinoconjunctivitis to dust mites, Gramineae, and olive trees. Among cutaneous diseases, the patient suffered from seborrheic dermatitis with recurrent episodes of eczema.
After the surgical removal of the tumor, the seizures persisted with lower frequency and without generalization despite treatment with Levetiracetam 4000 mg/day.
Lacosamide was added, reaching a dose of 600 mg/day. Finally, ESL was started at doses of 400 mg/day and increased gradually until 800 mg/day. Twenty-five days later, the patient complained of a cutaneous eruption characterized by painful and itching lesions. The eruption started over the palms and soles. Afterward, the cutaneous reaction spread to the upper trunk, legs, and face. The conjunctiva, oral and vaginal mucosa were also involved. The patient complained of throat pain, dysphagia, and dyspnea. At that time, she was admitted to a hospital. Physical examination was in accordance with EMM, showing round erythematoviolaceus plaques with central and peripheral vesicles of 3-4 cm diameter [Figure 1], bilateral conjunctival injection, inflamed erythematous lips with vesicula, erythematous and violaceous annular plaques at the oral mucosa and pharynx, as well as griseous vaginal exudate. A painful submandibular adenopathy was also present. Laboratory investigations showed liver dysfunction with elevated liver enzyme activities [aspartate transaminase (AST), 94 U/L; alanine transaminase (ALT), 226 U/L; gamma-glutamyl transpeptidase (GGT), 263 U/L], lymphocytosis (46%), and eosinophilia (3.9 × 103/mm3). Enzyme-linked immunosorbent assay (ELISA) for HIV was negative.
Figure 1.
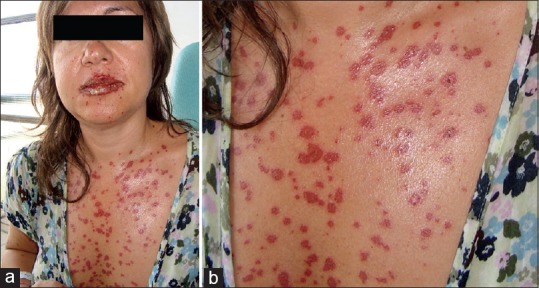
Generalized maculopapular rash in accordance with erythema multiforme major after introduction of eslicarbazepine
ESL was withdrawn, and the patient was treated with prednisone, topical betamethasone, and gentamicine. The rash and mucosae lesions disappeared in 2 weeks. The usual seizure frequency persisted over the subsequent months of follow-up.
A strong positive immediate reaction could be induced by prick test and intradermic test with ESL and CBZ at 0.01 and 0.1% concentrations in aqua. The reaction was observed at 15 and 30 mins. but, paradoxically, not in the delayed reading at 48 hrs [Figure 2]. Epicutaneous pharmacological testing with ESL and CBZ was negative. Patch testing with the European series just showed delayed hypersensitivity to nickel sulfate.
Figure 2.
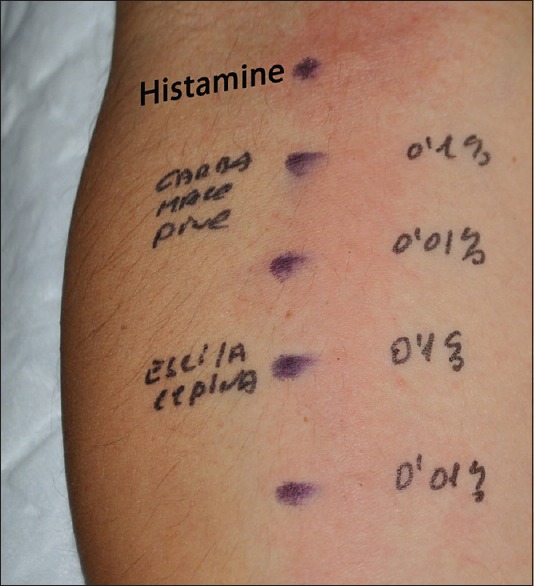
Prick test showing a strong positive immediate reaction with carbamazepine and eslicarbazepine at 0.1 and 0.01% concentrations
Previous cutaneous provocation test and oral challenge with penicillins and cephalosporins were performed in another hospital, which gave negative results.
High-resolution human leukocyte antigen (HLA) analysis was performed sequencing the specific oligonucleotide (SSO) probes. HLA-A alleles 1 * 02:01 and 2 * 26:01 and HLA-B alleles 1 * 08:01 and 2 * 38:01 were observed.
DISCUSSION
CADR to CBZ involves 2-3% of patients, with most of such reactions being mild.[6] Because of its pharmacological structure, CADR occurs less frequently with other AA such as OXC and ESL. However, 0.1-0.5% of patients stop ESL due to having severe skin symptoms.[5,7] To our knowledge, this is the first case reported of EMM induced by ESL.
Serious cutaneous reactions occur typically after 1-12 weeks of treatment. The corresponding time for EMM is 1-14 days and tends to be maximum at day 4.[8] When the rash develops, the severity of cutaneous changes does not necessarily reflect the severity of internal organ involvement; therefore, early diagnosis, immediate withdrawal of the suspected drug, and prompt treatment are important to minimize the associated morbidity and mortality.
The skin reaction of our patient showed the classic features of EMM. A clear temporal relationship between the administration of ESL and the onset of symptoms was observed, as well as a clinical improvement after ESL removal. ESL imputability was confirmed by cutaneous provocation test.
The hypersensitivity pathogenic mechanisms involved in the development of EMM are under research. A delayed hypersensitivity mechanism was speculated, as well as the implication of genetically determined abnormalities in the metabolic patterns of the aromatic anticonvulsants (such as epoxide hydrolases), critical to the detoxification of arene oxide metabolites. The reactive metabolites irreversibly modify cellular proteins, which then either initiate or serve as targets for an autoimmune attack in target organs.[1] Immune mechanisms, particularly T-cell–dependent reactions such as cell-mediated cytotoxicity, likely play an important role in this process.[8] However, the immunological mechanisms of CADR are also controversial and often not clearly defined. In this regard, it is interesting to emphasize that in our patient, the prick and intradermal tests did not show delayed, but an immediate cutaneous reaction to ESL.
Cross-reactivity between AA and aromatic or non-aromatic drugs has been widely described in the literature.[9] An intrinsic hypersensitivity has been postulated as the cause of such cross-reactivity.[1] Interestingly, our patient has had a previous episode of skin allergic reaction induced by beta-lactams (meropenem).
Some authors have suggested that the cross-reactions between drugs without chemical or antigenic similarities are due to the fact that the drug responsible for the second reaction was administered during the immunological depression occurring during a first episode.[9] However, in the present case, ESL was not administered during the first episode. It has also been suggested that an allergic episode may elicit a massive nonspecific activation of the immune system, which will provide the enhanced expression of co-stimulatory molecules and pro-inflammatory cytokines. The latter will allow a more efficient presentation of chemical antigens to antigen-presenting cells and, consequently, decrease the level of tolerance to other drugs. By this model, both pharmacogenetic and immunologic mechanisms play an important role and might explain alterations in a patient's immunologic status over time, in response to a variety of factors including drug exposure.[8]
A higher prevalence of SJS and TEN due to CBZ was demonstrated in patients carrying HLA alleles A3101 and B1502. In our case, the patient was not a carrier of any of these risk alleles.[10]
In conclusion, this is a case of co-sensitization of two chemically unrelated drugs, ESL and meropemen. CADR is an unpredictable, but relatively frequent complication of AA. When ESL should be prescribed, we suggest ruling out previous allergic reactions to not only the drugs like CBZ and OXC but also to other drugs such as beta-lactams.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Klassen BD, Sadler RM. Induction of hypersensitivity to a previously tolerated antiepileptic drug by a second antiepileptic drug. Epilepsia. 2001;42:433–5. doi: 10.1046/j.1528-1157.2001.33400.x. [DOI] [PubMed] [Google Scholar]
- 2.Roujeau JC. Stevens-Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol. 1997;24:726–9. doi: 10.1111/j.1346-8138.1997.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 3.Massot A, Vivanco R, Principe A, Roquer J, Rocamora R. Post-authorisation study of eslicarbazepine as treatment for drug-resistant epilepsy: Preliminary results. Neurologia. 2014;29:94–101. doi: 10.1016/j.nrl.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA 2-093) Neurotherapeutics. 2007;4:88–96. doi: 10.1016/j.nurt.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen RT. Eslicarbazepine acetate: A novel agent for the adjunctive treatment of epilepsy. Drugs Today (Barc) 2010;46:23–31. doi: 10.1358/dot.2010.46.1.1437709. [DOI] [PubMed] [Google Scholar]
- 6.Pelekanos J, Camfield P, Camfield C, Gordon K. Allergic rash due to antiepileptic drugs: Clinical features and management. Epilepsia. 1991;32:554–9. doi: 10.1111/j.1528-1157.1991.tb04692.x. [DOI] [PubMed] [Google Scholar]
- 7.Buggy Y, Layton D, Fogg C, Shakir SA. Safety profile of oxcarbazepine: Results from a prescription-event monitoring study. Epilepsia. 2010;51:818–29. doi: 10.1111/j.1528-1167.2009.02489.x. [DOI] [PubMed] [Google Scholar]
- 8.Söğüt A, Yilmaz A, Kilinç M, Söğüt AG, Demiralay E, Uzar H. Suspected lamotrigine-induced toxic epidermal necrolysis. Acta Neurol Belg. 2003;103:95–8. [PubMed] [Google Scholar]
- 9.Ben Fredj N, Aouam K, Chaabane A, Toumi A, Ben Rhomdhane F, Boughattas N, et al. Hypersensitivity to amoxicillin after drug rash with eosinophilia and systemic symptoms (DRESS) to carbamazepine and allopurinol: A possible co-sensitization. Br J Clin Pharmacol. 2010;70:273–6. doi: 10.1111/j.1365-2125.2010.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gidal BE. Carbamazepine hypersensitivity: Progress toward predicting the unpredictable. Epilepsy Curr. 2011;11:189–91. doi: 10.5698/1535-7511-11.6.189. [DOI] [PMC free article] [PubMed] [Google Scholar]


