Purpose and Appropriate Sample Types
This flow cytometry antibody panel was developed and optimized for the characterization of CD4+ and CD8+ T-cell memory and functional responses in adult and infant cryopreserved peripheral blood mononuclear cells (PBMC) stimulated with peptide pools to various antigens of interest (Table1). The panel has been used to evaluate Mycobacterium tuberculosis (TB) antigen-specific responses in clinical trial specimens, and is currently undergoing assay qualification.
Table 1.
Summary table for application of OMIP-022
| Purpose | T-cell phenotyping, memory categorization, cytokine production, and function following in vitro stimulation |
|---|---|
| Species | Human |
| Cell types | Cryopreserved PBMC (adult and infant) |
| Cross references | OMIP-001, OMIP-008, OMIP-009, and OMIP-014 |
Background
The lack of a correlate of immunity is a source of frustration in TB research 1. As such, it is crucial for TB vaccine researchers to “cast a wide net” when assessing immune responses to clinical trial candidates while minimizing the amount of specimen required to generate these data. This panel (Table2) began as an incremental enhancement to OMIP-014 2, and included markers for dump (fixable viability dye, CD14, and CD19), T-cell phenotype (CD3, CD4, and CD8), Th1 cytokines (IFN-γ and TNF), a Th2 cytokine (IL-4), T-cell proliferation cytokine (IL-2), degranulation (CD107a), and activation (CD154). We have also expanded the panel to include memory markers (CCR7 and CD45RO), a Th17 cytokine (IL-17A), and an IL-10 superfamily cytokine (IL-22, used in place of IL-4).
Table 2.
Reagents used for OMIP-022
| Specificity | Clone | Fluorochrome | Purpose |
|---|---|---|---|
| Viability Dye | – | AViD | Dump |
| CD14 | M5E2 | V500 | |
| CD19 | HIB19 | V500 | |
| CD3 | UCHT1 | ECD | Phenotype |
| CD4 | RPA-T4 | APC-eFluor 780 | |
| CD8 | HIT8a | Ax700 | |
| CCR7 | G043H7 | BV605 | Memory |
| CD45RO | UCHL1 | BV785 | |
| IFN-γ | B27 | V450 | Th1 |
| IL-2 | MQ1–17H12 | PE | |
| TNF | MAb11 | PE-Cy7 | |
| IL-17A | BL168 | PerCP-Cy5.5 | Th17 |
| IL-22 | IL22JOP | APC | Th22 |
| CD107a | H4A3 | Ax488 | Degranulation |
| CD154 | TRAP1 | PE-Cy5 | Activation/B-Cell Help |
We chose to combine viability dye and dump markers on the same channel using AViD and V500 (for CD14 and CD19), thus freeing additional channels on the violet laser. For phenotyping, CD3 remains on ECD and CD4 on APC-eFluor 780.
Th1 cytokines have been shown to be critical for controlling TB 3–9. To assess Th1 responses, we utilize antibodies against tumor necrosis factor (TNF) and IFN-γ. In designing this panel, we moved TNF to PE-Cy7 to improve detection of this cytokine, as fluorescein isothiocyanate (FITC) is comparatively dimmer and more prone to photobleaching. The recently introduced Brilliant Violet series of fluorochromes represents a bright and economical set of tools for detecting markers via the violet laser. Brilliant Violet 421 (BV421) was previously shown to be of similar brightness to PE 10, and was initially chosen as a replacement fluorochrome for IFN-γ. However, further investigation showed that any increase in antigen-specific response detected using the BV421 conjugate was matched with a significant increase in IFN-γ background. This increased background was observed using both the B27 (BD Biosciences, San Jose, CA) and the 4S.B3 (Biolegend, San Diego, CA) clones. When this background population was excluded from the IFN-γ gate of the BV421 conjugates, the responses detected using either the BV421 conjugates or the V450 conjugate were similar. This issue, combined with the increased cost of the reagent, led us to choose to continue to use the V450 conjugate for IFN-γ detection in this panel.
Interleukin-2 (IL-2) is a cytokine responsible for T-cell proliferation and differentiation 11,12 that also may play a role in TB immunity 13. In our panel, we use PE for bright and efficient detection of this cytokine.
As M. tuberculosis is a facultative intracellular bacteria, cytolytic activity may be necessary to help control the disease. CD107a (LAMP-1) is a marker for degranulation and an indirect marker for cytolytic function 14,15. Ideally, lytic proteins such as Perforin would be costained with the CD107a to confirm the cytotoxic potential of the CD107a+ cells. To this point, we are examining future iterations of this panel that may include a lytic marker. However, the current constraints of this panel limit our ability to add an additional marker without harming sensitivity in detecting the other markers of interest. To accommodate the change in fluorochrome for the detection of TNF, we moved CD107a to FITC initially, and then replaced FITC with the enhanced stability of Alexa Fluor 488 (Ax488).
The Brilliant Violet series of fluorochromes has also allowed us to include the memory markers CCR7 and CD45RO. CCR7 is responsible for homing T-cells to lymph nodes 16 and is expressed on naïve and central memory T-cells (TCM) 17. CD45RO is the smallest isoform of CD45, and has been shown to be expressed on effector memory (TEM) and TCM 18. The inclusion of both markers allows for T-cells to be categorized as naïve, TCM, TEM, or effector T-cells. We chose Brilliant Violet 605 for CCR7 as it was the next brightest available Brilliant Violet dye and CCR7 is expressed as a continuum (not distinct populations) 19. The binding kinetics of the CCR7 antibody has shown optimal staining when incubated at 37°C (see staining protocol in Supporting Information). Brilliant Violet 785 was chosen for CD45RO based on antibody availability and minimal spectral overlap to other markers.
IL-17A is secreted by Th17 T helper cells and acts to recruit innate immune effector cells to the site of inflammation 20. Recent works 21,22 have suggested a strong role for IL-17A in tuberculosis immunity. As such, we initially replaced the MIP-1β from OMIP-014 with and anti-IL-17A antibody. In our hands, the PerCP-Cy5.5 on CD8 causes high spectral overlap into the Ax700 channel used for IL-17A. As IL-17A is lowly expressed compared to CD8 (and as PerCP-Cy5.5 is fluorescently brighter), we exchanged the fluorochromes for these two markers to minimize the impact of the high compensation.
As an effect of changing CD8 to Ax700, a reduction in CD8+ events [perhaps an escapee phenomenon 23] was noted using Ax700 to detect CD8, but was mitigated by staining for CD8 intracellularly.
IL-4, previously included with OMIP-014, is a representative Th2 cytokine. This interleukin is extremely difficult to detect and, in our hands, is inconsistent. We continue to include this marker as an option when assessing clinical trial specimens in which Th2 is considered a desired response. Recently, however, we have substituted IL-22 on APC instead of IL-4. IL-22 is part of the IL-10 superfamily 24, and when coexpressed with IL-17 enhances production antimicrobial peptides in the mucosa 25. Although poorly characterized to this point, recent research indicates that IL-22 plays a role in TB infection 21,26.
CD154 was maintained on this panel as emerging evidence indicates its role as a specific and sensitive marker in detecting CD4 response 27, as well as its roles in upregulating antimicrobial peptides 28 and its necessity for T-cell activation of B cells 29. As indicated in OMIP-014, the inclusion of Brefeldin A in our stimulation protocol requires intracellular staining of CD154. Figure 1 shows an example staining and analysis for adult PBMC stimulated with Staphylococcal enterotoxin B (Fig. 1A) or CMV pp65 (Fig. 1B).
Figure 1.
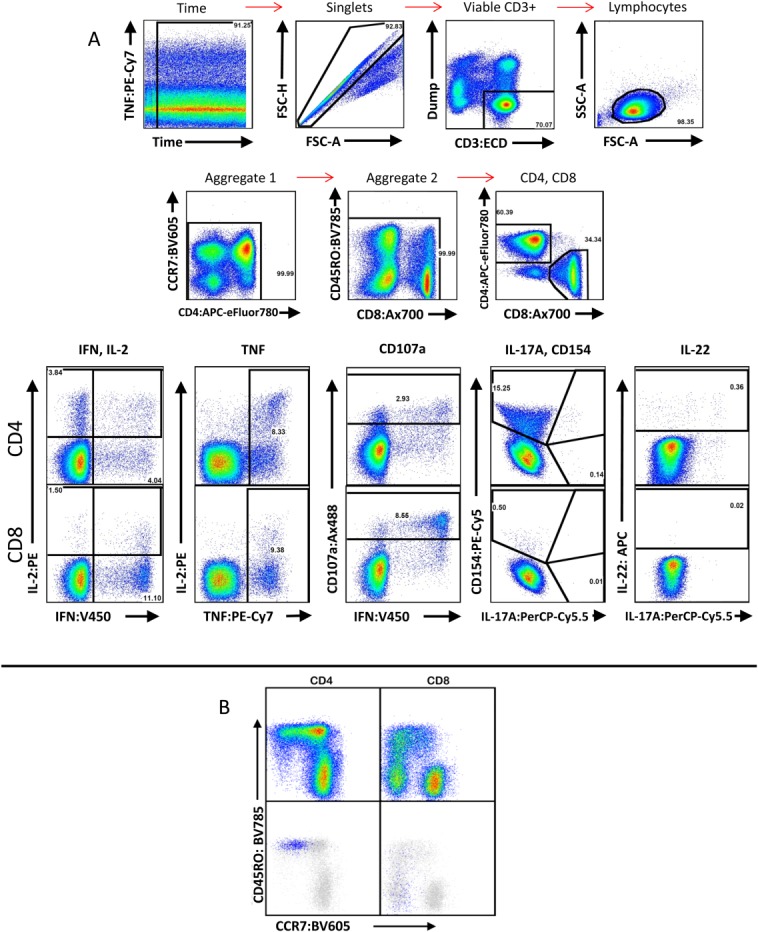
Example staining of adult human PBMC following stimulation. (A) The first two rows demonstrate the gating hierarchy from total sample to CD4/CD8 identification. A time gate is used to exclude pressure aberrations from the cytometer that may have occurred during sample acquisition. Aggregate gates are used to exclude brightly positive events that may result from antibody or cell aggregation. The bottom row demonstrates gating for cytokines and functions from CD4+ (top half) and CD8+ (bottom half) events resulting from stimulation with Staphylococcal enterotoxin B. Note that IL-17A and CD154 were gated on the same plot to avoid mischaracterization resulting from the increased spectral overlap observed from their fluorochromes. (B) Example plots showing memory profile of CCR7 versus CD45RO in CD4+ and CD8+ populations. Using CMV pp65 peptide pool-stimulated PBMC from a CMV-reactive donor, IFN-γ+ events (blue) were overlaid onto these plots (gray), confirming localization of these events to the effector memory and effector compartments.
Similarity to Published OMIPs
As this panel assesses antigen-specific T-cell responses, it is similar to OMIP-001 30, OMIP-008 31, OMIP-009 32, and OMIP-014 2. Furthermore, this panel evolved from an initial desire to implement OMIP-014. Unlike these panels, however, our panel includes T-cell memory markers as well as an extensive combination of cytokines and functions associated with tuberculosis vaccine research. Our panel was developed for use in multicenter studies under good clinical laboratory practices conditions, and is currently undergoing assay qualification.
Acknowledgments
The authors thank Mario Roederer, Stephen P. Perfetto, Stephen C. De Rosa, and Michael R. Betts for their expertise and suggestions during the development and optimization of this panel. The authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Supplementary Information Figure 1.
Supplementary Information Figure 3.
Supplementary Information Figure 3.
Supplementary Information Figure 4.
Supplementary Information Figure 5.
Supplementary Information Figure 6.
Supplementary Information Figure 7.
Supplementary Information Figure 8.
Supplementary Information Figure 9.
Literature Cited
- Hokey DA, Ginsberg A. The current state of tuberculosis vaccines. Hum Vaccin Immunother. 2013;9:2142–2146. doi: 10.4161/hv.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa SC, Carter DK, McElrath MJ. OMIP-014: Validated multifunctional characterization of antigen-specific human T cells by intracellular cytokine staining. Cytometry Part A. 2012;81A:1019–1021. doi: 10.1002/cyto.a.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez Martinez O, Ripoll Noiseux C, Carneros Martin JA, Gonzalez Lara V, Gregorio Maranon HG. Reactivation tuberculosis in a patient with anti-TNF-alpha treatment. Am J Gastroenterol. 2001;96:1665–1666. doi: 10.1111/j.1572-0241.2001.03836.x. [DOI] [PubMed] [Google Scholar]
- Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- Lammas DA, Casanova JL, Kumararatne DS. Clinical consequences of defects in the IL-12-dependent interferon-gamma (IFN-gamma) pathway. Clin Exp Immunol. 2000;121:417–425. doi: 10.1046/j.1365-2249.2000.01284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- de Jong R, Altare F, Haagen IA, Elferink DG, Boer T, van Breda Vriesman PJ, Kabel PJ, Draaisma JM, van Dissel JT, Kroon FP, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay PK, Gaylord B, Palmer A, Jiang N, Raven MA, Lewis G, Reuter MA, Nur-ur Rahman AK, Price DA, Betts MR, et al. Brilliant violet fluorophores: A new class of ultrabright fluorescent compounds for immunofluorescence experiments. Cytometry Part A. 2012;81A:456–466. doi: 10.1002/cyto.a.22043. [DOI] [PubMed] [Google Scholar]
- Smith KA. Interleukin-2: Inception, impact, and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Cantrell DA, Smith KA. The interleukin-2 T-cell system: A new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005;42:799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M, Beverley PC. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responses: Human CD8 memory CTLp selectively express CD45RO (UCHL1) Int Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- McLaughlin BE, Baumgarth N, Bigos M, Roederer M, De Rosa SC, Altman JD, Nixon DF, Ottinger J, Oxford C, Evans TG, et al. Nine-color flow cytometry for accurate measurement of T cell subsets and cytokine responses. Part I: Panel design by an empiric approach. Cytometry Part A. 2008;73A:400–410. doi: 10.1002/cyto.a.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, et al. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. J Immunol. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- Gratama JW, van der Linden R, van der Holt B, Bolhuis RL, van de Winkel JG. Analysis of factors contributing to the formation of mononuclear cell aggregates (“escapees”) in flow cytometric immunophenotyping. Cytometry. 1997;29:250–260. [PubMed] [Google Scholar]
- Fickenscher H, Hor S, Kupers H, Knappe A, Wittmann S, Sticht H. The interleukin-10 family of cytokines. Trends Immunol. 2002;23:89–96. doi: 10.1016/s1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)−22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokera R, Wilkinson KA, Meintjes GA, Skolimowska KH, Matthews K, Seldon R, Rangaka MX, Maartens G, Wilkinson RJ. Role of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosis. J Infect Dis. 2013;207:1148–1156. doi: 10.1093/infdis/jit002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- Klug-Micu GM, Stenger S, Sommer A, Liu PT, Krutzik SR, Modlin RL, Fabri M. CD40 ligand and interferon-gamma induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology. 2013;139:121–128. doi: 10.1111/imm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noelle RJ, Roy M, Shepherd DM, Stamenkovic I, Ledbetter JA, Aruffo A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnke YD, Roederer M. OMIP-001: Quality and phenotype of Ag-responsive human T-cells. Cytometry Part A. 2010;77A:819–820. doi: 10.1002/cyto.a.20944. [DOI] [PubMed] [Google Scholar]
- Zuleger CL, Albertini MR. OMIP-008: Measurement of Th1 and Th2 cytokine polyfunctionality of human T cells. Cytometry Part A. 2012;81A:450–452. doi: 10.1002/cyto.a.22035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoreaux L, Koup RA, Roederer M. OMIP-009: Characterization of antigen-specific human T-cells. Cytometry Part A. 2012;81A:362–363. doi: 10.1002/cyto.a.22042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information Figure 1.
Supplementary Information Figure 3.
Supplementary Information Figure 3.
Supplementary Information Figure 4.
Supplementary Information Figure 5.
Supplementary Information Figure 6.
Supplementary Information Figure 7.
Supplementary Information Figure 8.
Supplementary Information Figure 9.


