Abstract
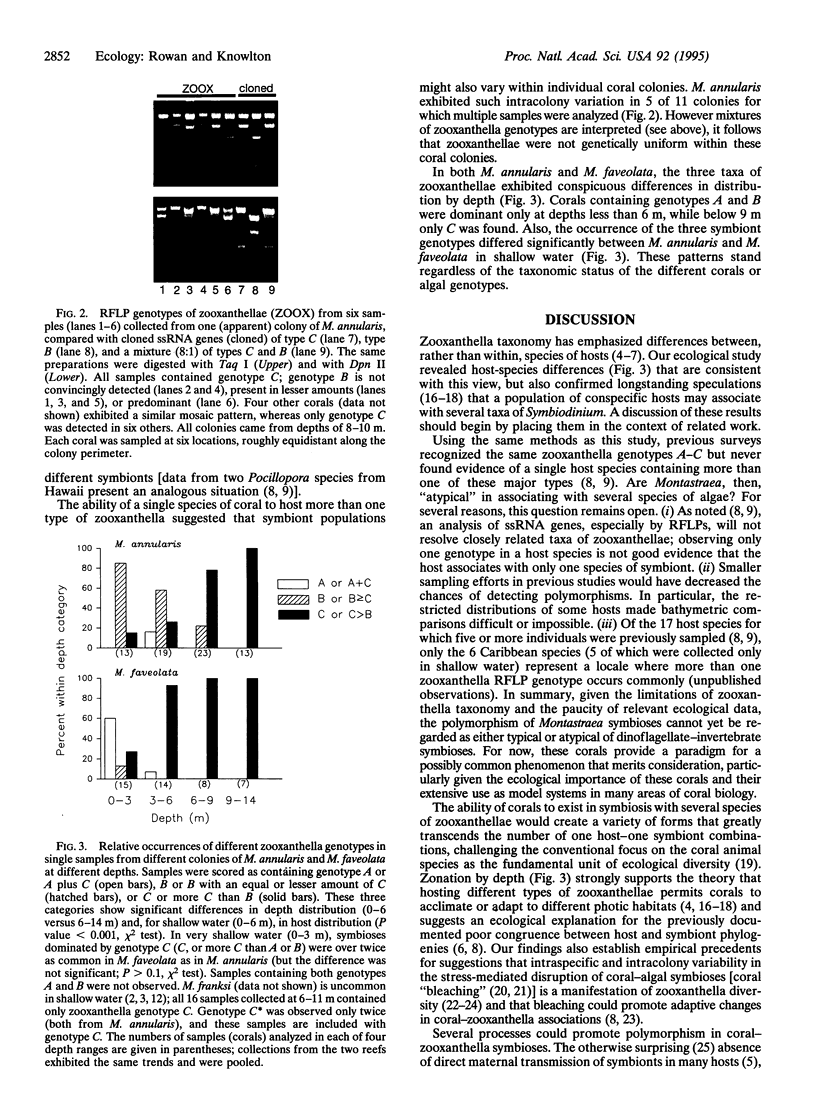
All reef-building corals are obligately associated with photosynthetic microalgal endosymbionts called zooxanthellae. Zooxanthella taxonomy has emphasized differences between species of hosts, but the possibility of ecologically significant zooxanthella diversity within hosts has been the subject of speculation for decades. Analysis of two dominant Caribbean corals showed that each associates with three taxa of zooxanthellae that exhibit zonation with depth--the primary environmental gradient for light-dependent marine organisms. Some colonies apparently host two taxa of symbionts in proportions that can vary across the colony. This common occurrence of polymorphic, habitat-specific symbioses challenges conventional understanding of the units of biodiversity but also illuminates many distinctive aspects of marine animal-algal associations. Habitat specificity provides ecological explanations for the previously documented poor concordance between host and symbiont phylogenies and the otherwise surprising lack of direct, maternal transmission of symbionts in many species of hosts. Polymorphic symbioses may underlie the conspicuous and enigmatic variability characteristic of responses to environmental stress (e.g., coral "bleaching") and contribute importantly to the phenomenon of photoadaptation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank R. J., Trench R. K. Speciation and symbiotic dinoflagellates. Science. 1985 Aug 16;229(4714):656–658. doi: 10.1126/science.229.4714.656. [DOI] [PubMed] [Google Scholar]
- Kinzie R. A., 3rd, Chee G. S. The effect of different zooxanthellae on the growth of experimentally reinfected hosts. Biol Bull. 1979 Jun;156(3):315–327. doi: 10.2307/1540920. [DOI] [PubMed] [Google Scholar]
- Knowlton N., Weil E., Weigt L. A., Guzmán H. M. Sibling Species in Montastraea annularis, Coral Bleaching, and the Coral Climate Record. Science. 1992 Jan 17;255(5042):330–333. doi: 10.1126/science.255.5042.330. [DOI] [PubMed] [Google Scholar]
- Porter J. W., Fitt W. K., Spero H. J., Rogers C. S., White M. W. Bleaching in reef corals: Physiological and stable isotopic responses. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9342–9346. doi: 10.1073/pnas.86.23.9342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan R., Powers D. A. A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbioses. Science. 1991 Mar 15;251(4999):1348–1351. doi: 10.1126/science.251.4999.1348. [DOI] [PubMed] [Google Scholar]
- Rowan R., Powers D. A. Ribosomal RNA sequences and the diversity of symbiotic dinoflagellates (zooxanthellae). Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3639–3643. doi: 10.1073/pnas.89.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H. Structural diversity of eukaryotic small subunit ribosomal RNAs. Evolutionary implications. Ann N Y Acad Sci. 1987;503:125–139. doi: 10.1111/j.1749-6632.1987.tb40603.x. [DOI] [PubMed] [Google Scholar]