Abstract
The molecular genetic basis of electrodermal activity (EDA) was analyzed using 527,829 single nucleotide polymorphisms (SNPs) in a large population-representative sample of twins and parents (N = 4,424) in relation to various EDA indices. Biometric analyses suggested that approximately 50% or more of variance in all EDA indices was heritable. The combined effect of all SNPs together accounted for a significant amount of variance in each index, affirming their polygenic basis and heritability. However, none of the SNPs were genome-wide significant for any EDA index. Previously reported SNP associations with disorders such as substance dependence or schizophrenia, which have been linked to EDA abnormalities, were not significant; nor were associations between EDA and genes in specific neurotransmitter systems. These results suggest that EDA is influenced by multiple genes rather than by polymorphisms with large effects.
Descriptors: Skin conductance, Electrodermal activity, Habituation, Heritability, Genome-wide association study, Molecular genetics
Beginning with the seminal work of Carl Jung in the early 1900s, electrodermal activity (EDA) has been extensively used for over 100 years to study emotional and cognitive activity (Dawson, Schell, & Filion, 2007). Abnormalities in EDA have been linked to psychiatric conditions such as schizophrenia and psychopathy (Dawson et al., 2007), leading to proposals that it be used as an endophenotype (Iacono, 1985). Endophenotypes are laboratory-measured characteristics present in individuals who are predisposed to a psychiatric disorder—regardless of the presence of overt symptoms of that disorder (Gottesman & Gould, 2003; Iacono, 1998; Iacono & Malone, 2011). Considered more proximal to the effect of genes than the psychiatric phenotypes with which they are associated, they may provide an easier route to the genetic architecture underlying that disorder. Various criteria have been proposed to identify endophenotypes, including that they are stable over time, heritable, present in affected and unaffected relatives, and able to predict the subsequent development of psychopathology in longitudinal research. EDA is particularly suited for investigating the endophenotype concept as it fits these criteria.
EDA is typically measured by passing a weak electrical current through electrodes placed on the fingertips and monitoring changes in conductance that occur in the eccrine sweat glands while subjects are at rest or engaged in a task (for a comprehensive review of EDA methods, applications, and findings, see Boucsein, 2012). EDA reflects arousal processes (Holdcraft & Iacono, 2002; Holdcraft, Iacono, & McGue, 1998) and indexes the degree of perceived stimulus significance, for example, when the stimulus has relevance to the subject, as in a conditioning study (e.g., de Geus, 2010) or in a recognition memory protocol such as the concealed information or guilty knowledge test (Iacono & Malone, 2011). EDA includes both tonic and phasic components. Tonic components include skin conductance level (SCL), a baseline measure that changes slowly with altered arousal state, and nonspecific fluctuations consisting of spontaneous responses that arise in the absence of apparent stimulation and that also index arousal. Phasic responses are stimulus elicited, and typically quantified by measuring the change in conductance (the skin conductance response, SCR) that occurs in response to a discrete stimulus. EDA is often measured in classic conditioning experiments, but as a putative endophenotype, it is probably most commonly assessed as part of a habituation task in which subjects listen to a series of intermittently presented tones that initially trigger orienting responses, followed eventually by ceased responding. EDA has also been hypothesized to index a defensive reaction in response to a stimulus that is potentially so aversive that it threatens well-being or survival. In this context, habituation of the response would not be expected, and a “fight or flight” response would ensue. In EDA protocols commonly used in psychopathology research, diminished responding, characterized by low SCL, small SCRs, and rapid habituation (or total lack of responding) has been associated with psychotic (Iacono, 1985) and externalizing (Fowles & Kochanska, 2000; Fung et al., 2005; Isen, Iacono, Malone, & McGue, 2012) psychopathology. Hyperresponding has most commonly been associated with anxiety disorders such as posttraumatic stress disorder (PTSD; Pole, 2007).
EDA as an Endophenotype
EDA can be reliably measured and shows stability across both time and situations (see Crider et al., 2004, for a brief review; also see Lacey & Lacey, 1958; O'Gorman & Horneman, 1979). For example, Iacono, Roshi, and Lacoste (1987) reported a median 1-year retest stability of .61 for six EDA measures in healthy control and unipolar and bipolar mood disorder patients. Several studies have found EDA to be heritable, with about half of its variation attributable to genetic factors (see Isen et al., 2012, for a brief review). For example, Lykken, Iacono, Haroian, McGue, and Bouchard (1988), in a study that included reared apart twins, reported that 70% of the variance in the rate of electrodermal habituation was attributable to genetic factors. Using reared together twins, others have also found substantial genetic influence for EDA measures (Crider et al., 2004; Hettema, Annas, Neale, Kendler, & Fredrikson, 2003). Hettema et al. (2008) found different EDA measures from a classic conditioning study to show 1-month retest reliabilities ranging from .37 to .85 (median = .62) and moderate heritabilities ranging from .35–.45. Using a longitudinal design, Tuvblad et al. (2012) assessed twins at the ages of 9–10, 11–13, and 14–16 years. Genetic factors accounted for 48–83% of the variance in skin conductance-orienting responses at each of the various time points; a common genetic factor accounted for anywhere between 36%–49% of the variation across time points. Thus, there appears to be extensive evidence that various EDA indices are stable and under genetic influence.
Decreased electrodermal reactivity has been reported for externalizing disorders such as antisocial behavior (Herpertz et al., 2003, 2007; Isen et al., 2012; Lorber, 2004; Raine, 2002). Decreased EDA has also been associated with alcohol use problems (Isen, Iacono, & Malone, 2013; Knott & Bulmer, 1985). Various lines of evidence point to diminished EDA indexing genetic risk for externalizing disorders in addition to manifest psychopathology. Fathers of boys with conduct disorder show smaller SCRs relative to fathers of boys without conduct disorder, and the same is true of their sons (Herpertz et al., 2007). Using a subsample of the twins included in the current investigation, Isen et al. (2012) provided strong evidence for the potential of EDA to serve as an externalizing endophenotype when they reported that genetic factors contributed to the overlap between EDA response frequency and liability to externalizing disorders. Also providing strong evidence supporting EDA as an endophenotype, developmental studies have shown that EDA predicts the subsequent development of externalizing behavior. Raine, Venables, & Williams (1990) found that decreased SC responding predicts the development of criminal behavior in male schoolchildren and that SC hyperreactivity in 15-year-olds appeared to protect against the subsequent development of criminality (Raine, Venables, & Williams, 1995). Employing a fear conditioning paradigm, Raine and colleagues (Gao, Raine, Venables, Dawson, & Mednick, 2010a) found that individuals who had become criminals by the age of 23 showed reduced electrodermal fear conditioning at age 3. In another report, this research team found that reduced fear conditioning in children assessed between the ages of 3–8 years was associated with aggressive behavior at age 8 (Gao, Raine, Venables, Dawson, & Mednick, 2010b). In a similar vein, Baker and associates (Baker, Shelton, Baibazarova, Hay, & van Goozen, 2013) found that EDA responses measured at the age of 1 predicted aggressive behaviors at age 3. Taken in the aggregate, these various reports provide strong support for EDA as an endophenotype for externalizing psychopathology.
One of the most replicable findings in schizophrenia research has come from EDA studies showing that schizophrenia patients, often about half of those examined in any one study, fail to respond at all to auditory stimuli presented as part of a habituation procedure (for reviews, see Dawson et al., 2007; Iacono, Ficken, & Beiser, 1999). In addition, among those who do respond, subgroups of hyperresponders, characterized by high SCL and many nonspecific responses, have also been identified. The patterns seen in both nonresponders and responders have been shown to be stable over periods of time as long as a year (Dawson et al., 2007), and have been found in remitted patients, indicating that the effects appear to be traitlike and independent of the presence of acute symptomatology (Iacono, 1982). First-degree relatives of those with schizophrenia also show electrodermal anomalies such as excessively high rates of nonspecific fluctuations (Iacono, Ficken, & Beiser, 1999) and other signs of EDA hyperarousal (Dawson et al., 2007).
EDA anomalies are also associated with internalizing psychopathology. Although not as well studied as schizophrenia, those with severe mood disorders characterized by recurrence or psychosis appear to show the pattern of EDA found in schizophrenia patients and their relatives, including substantial retest stability and presence in remission (Iacono, 1982; Iacono, Ficken, & Beiser, 1999; Iacono et al., 1983, 1984). PTSD has been associated with EDA hyperarousal (McTeague et al., 2010; Orr, Lasko, Shalev, & Pitman, 1995; Pole, 2007). Studies of other anxiety disorders and fear have found evidence of EDA hyperarousal, but null findings also exist. Among the earliest and most frequently cited studies, Lader and Wing (1964) found that anxious patients showed greater EDA responses than controls. More recently, Wendt and colleagues (Wendt, Lotze, Weike, Hosten, & Hamm, 2008) have found similar results in participants with spider phobia, who showed elevated EDA activity when viewing pictures of spiders. In an interesting study, Wilhelm and Roth (1998) examined EDA in subjects with a phobia of flying during flight, and showed that these subjects had greater SCLs compared to controls. In contrast, Hart (1974) did not find any differences between anxious and nonanxious subjects with respect to EDA; nor did Mayer, Merckelbach, de Jong, and Leeuw (1999) when they examined EDA responses in spider phobics presented with backwardly masked images of spiders and flowers. In summary, elevated EDA has received support as an endophenotype for mood disorders, but less is known about the potential of EDA as an endophenotype for other internalizing disorders.
EDA has also been linked to several medical conditions. As with psychiatric disorders, decreased EDA responding, or lack of differentiation in EDA responses to familiar/unfamiliar stimuli, is linked to dermatological and medical conditions such as psoriasis (Cambrai, Clar, Grosshans, & Altermatt, 1979), epidermal damage (Edelberg, 1967), neurological conditions such as coma and cerebral vascular diseases (Schuri & von Cramon, 1981), Huntington’s disease (Lawson, 1981; but also see Iacono et al., 1987), and multiple sclerosis (Kleeberg et al., 2004; Saari et al., 2008).
Neurobiology of EDA
EDA is innervated primarily by the sympathetic nervous system, with three independent pathways in the brain. The first pathway is mediated by the cortex and basal ganglia, the second by the hypothalamus and limbic system, and the third by the reticular formation in the brainstem (see Dawson et al., 2007, for overview). As EDA is operationalized in multiple ways (e.g., response frequency, rate of habituation, etc.), one or more of them may be more closely linked to one of the three circuits above. For example, an electrodermal defensive response following a noxious stimulus probe would be linked more tightly to the second circuit in the hypothalamus and limbic system, while tonic SCL indexing arousal state would be mediated more by the reticular formation in the brain. However, it is worth noting that there is much overlap among these various operationalizations of EDA, reflecting perhaps a general lability-stability dimension in which EDA responses to various types of stimuli account for individual differences in any type of experiment (Holdcraft & Iacono, 2004). Indeed, as will be seen in our analyses presented below, a factor analysis of the EDA indices used in the current study revealed a significant amount of overlap among them, suggesting that they tap into shared underlying mechanisms.
Molecular Genetics and EDA
Several molecular genetic techniques exist to investigate the associations between variations in DNA and psychiatric phenotypes. Among the most popular are candidate gene studies, which look at variations in genes of theoretical interest in relation to some phenotype. To date, two candidate gene studies have examined the association between the serotonin transporter gene (5-HTT) and electrodermal activity, with contradictory results (Gilissen, Bakermans-Kranenburg, van Ijzendoorn, & Linting, 2008; Schmitz et al., 2009). At the other end of the spectrum exist atheoretical techniques such as genome-wide association studies (GWAS), which test whether common variations across the entire genome are related to a given phenotype. No study has examined whether EDA can serve as an endophenotype directly using GWAS. This is in part because, unlike diagnostic or questionnaire data, psychophysiological data are prohibitively expensive and time consuming to collect from the thousands of subjects who are likely needed for GWAS. In between these two extremes exist other techniques that test whether particular SNPs from genes implicated in certain functions (e.g., neurotransmitter systems) show significant association with EDA (versatile gene-based association study, VEGAS), or how much phenotypic variance is accounted for by the joint effects of all SNPs together (genome-wide complex trait analyses; GCTA). Note that this is critically different from a GWAS, which examines the degree to which each individual SNP accounts for variance in a trait. In sum, then, a wide variety of techniques exists for investigating the molecular genetic bases of EDA responses. However, apart from the two candidate gene studies noted above, scant molecular genetic data exist to evaluate EDA as an endophenotype.
The size of the MCTFR samples and scope of study spanning two decades allowed us to overcome prior limitations that precluded GWAS of EDA responses and conduct the first such study. Thus, the current investigation fills an important gap in the literature by examining the potential of the electrodermal response—one of the most widely used psychophysiological indices—to serve as an endophenotype for various psychiatric disorders, by evaluating its genetic bases. To this end, the current study (a) examined the biometric heritability of EDA measures using twin and family data; (b) examined the SNP heritability, or variance accounted for by all SNPs from the GWAS together, in EDA; (c) conducted GWAS evaluating multiple EDA indices in relation to 527,829 SNPs; and (d) conducted gene-based tests of each EDA index in relation to 17,601 genes. The latter two analyses also allowed us to follow up leads in the literature by examining effects for SNPs and genes that hypothetically could be related to EDA.
We collected EDA data from a large community sample of adolescent twins and their parents (N = 4,424). To our knowledge, this represents the largest EDA database ever utilized to estimate the heritability of EDA measures. Given the promise of the endophenotype concept along with the increasing emphasis in psychology and psychiatry on finding the biological bases of psychopathology (e.g., Research Domain Criteria [RDoC], Insel et al., 2010; initiative by the National Institute of Mental Health; G. A. Miller & Rockstroh, 2013), results from the current study could provide valuable insights into the genetic correlates of psychiatric disorders.
Materials and Methods
Participants
The sample for the current study consisted of same-sex male and female twin pairs and their parents from the older and younger cohorts and enrichment sample (ES) of the Minnesota Twin Family Study (MTFS; Iacono, Carlson, Taylor, Elkins, & McGue, 1999). All twins were assessed at partially overlapping ages. For the purpose of this study, EDA data for the twins were from the age-17 assessment common to the two age cohorts and the ES. The majority of parents were also assessed using identical procedures. For further details about participants, see Iacono et al. (2014) in this issue. After a complete description of the study was given to all subjects, written informed consent or assent was obtained. The sample we drew from for this study is broadly representative of the population of Minnesota during the relevant birth years, and it is therefore primarily Caucasian (96%). In order to avoid variation in allele frequencies among different ethnic groups, which can confound molecular-genetic analyses, we restricted the sample for the present study to Caucasians. We started with an initial sample of 4,532 who had both genotype data and EDA data for at least one of the indices we are using in this manuscript (see Molecular Genetics section below and Iacono et al., 1987, for further details). One hundred and eight participants were excluded for the following reasons: equipment error; if they reported head trauma or loss of consciousness leading to hospitalization, or lasting more than a day; if participants fell asleep during experiment, or reported taking medication (especially anticholinergic medication) or any illicit substances or alcohol on the day of testing. After these exclusions, depending on the EDA index used, the total number of subjects utilized in the analyses (across all generations and cohorts) varied as follows: SCL: 3,791; SCR amplitude: 4,102; SCR response frequency: 4,299; SCR factor: 4,424. The Ns in the sample as a whole (i.e., 4,424) were as follows—males: 2,486 (56%), females: 1,938; fathers: 1,240, mothers: 609; monozygotic (MZ) twin pairs: 816 (1,632 individuals), dizygotic (DZ) twin pairs: 408 (816 individuals), singletons (twin pairs where only one twin’s data were available after data cleaning) = 127.
Habituation Task
Participants underwent a standard habituation paradigm involving a series of pure tones. Headphones were positioned over participants’ ears, and a continuous background noise of 55 dB was delivered throughout the task. Participants listened to a series of 17 pure tones while seated in front of a screen. They were instructed to focus their attention on a closed-captioned movie clip and to ignore the tones (Iacono & Lykken, 1979). After the last tone, they were asked to sit quietly with eyes closed and relax for 5 min. All stimuli had rise and fall times of 20 ms, and durations of 500 ms. Participants assessed earlier in the MCTFR heard tones of 101.5 dB, while those assessed later heard tones of 105 dB, due to minor changes in experimental paradigms, given the 20-year longitudinal nature of the study. However, each subject received tones of the same intensity, even if they varied between participants. Interstimulus intervals varied in length from 45 to 75 s (mean = 60 s). The average size of elicited responses (mean SCR amplitude), a count of the total number of responses (SCR frequency; Isen et al., 2012), and SCL at the end of the habituation session were obtained from each participant. The habituation protocol was modeled after that used in past research that uncovered EDA anomalies associated with schizophrenia and severe mood disorders (Iacono, 1982, 1983; Iacono, Ficken, & Beiser, 1999; Iacono et al., 1984).
Skin Conductance Recording
Following recommended procedures (Fowles et al., 1981), skin conductance was recorded using a pair of bipolar Ag-AgCl electrodes (1 cm in diameter) attached with electrode collars to two fingertips on each hand, which created a contact surface area of 0.79 cm2 on each fingertip. Participants washed their hands before electrodes were attached. A paste consisting of 0.5 molar NaCl electrolyte mixed with Unibase cream served as the conducting medium. Constant voltage (0.5 V) was passed through the signal conditioner for the direct recording of skin conductance. The output signal was sampled at 256 Hz (except for the final “relax” component of the protocol), and filtered with a 3 Hz low-pass filter. During the relax task, skin conductance was sampled at a rate of 8 Hz.
SCR Scoring Criteria
The digitized skin conductance signal was scored by a computer algorithm written in MATLAB. A response peak was detected when the slope of the signal changed from positive to negative. Peaks were visually inspected on raw waveforms to ensure the absence of atypical responses or nonphysiological artifacts, and adjusted accordingly. SCR amplitude was defined as the difference in microsiemens (µS) between response peak and base, and was averaged across both hands. Response onset was based on the second derivative of SCL, and specifically on the point in time at which this first became positive, while baseline was defined as the skin conductance value at stimulus onset. Correlation for twins across both hands was .91. (males = .92; females = .91). SCL was measured as the median response during the 5-min relaxation period. This was also averaged across hands. Correlation between hands for SCL was .88 (males = .90; females = .87). Reponses to the tones were considered valid as long as they occurred within 1–4 s after the tone, the rise time was 1–5 s, and the magnitude exceeded .01 µS (the minimum response detectable by the equipment was .0025 µS). Trials that did not fulfill these criteria were judged nonresponses, and scored as zero. Frequency of responding was obtained by counting the number of valid responses and was used to gauge individuals’ resistance to habituation. Mean SCR amplitude was based on the average size of all nonzero responses. Individuals consequently had undefined/missing values for this variable if they failed to respond to a single habituation trial (5.4% of sample). Mean amplitude and SCL showed substantial positive skew, and were normalized using a square-root transformation.
To obtain a global index of electrodermal activity, response frequency, amplitude, and SCL were used to estimate factor regression weights. Latent electrodermal scores were estimated for each participant using Mplus Version 6 (Muthén & Muthén, 2010). Extraction of a single factor revealed high standardized loadings for all indices on that factor (.77 for response frequency, .60 for mean amplitude, and .69 for SCL), suggesting substantial overlap among the indices.
• Molecular Genetic Data
Genetic data were mostly obtained from blood samples, except in a small percentage of cases where they were obtained from saliva instead. Data were genotyped using the Illumina Human660W-Quad array (M. B. Miller et al., 2012, for details). For details on data collection and processing, see Iacono and colleagues (2014) in this issue. After non-Caucasian subjects had been removed, principal component analysis (PCA) using EIGENSTRAT (Price et al., 2006) was conducted, and the first 10 PCs were used as covariates in all analyses in order to account for the major sources of residual genetic variation in the sample (Price et al., 2006).
Statistical Analyses
Several different statistical analyses were undertaken in order to characterize the various EDA indices from a variety of perspectives. An overview of each method is given below. For more details, consult Iacono et al. (2014). For all analyses, EDA measures were adjusted for relevant covariates: gender, generation (parent or adolescent), age effects, a dummy variable coding for variation in recording procedures over the approximately 20-year time period it took to record all the participant data, such as a change in tone intensity, ES sample (versus the two original age cohorts), and effects on mean levels of subtle genetic variation in the form of the 10 PCs from EIGENSTRAT. The ES dummy variable was included because this sample was enriched for substance abuse risk, whereas the two other age cohorts were random samples (Keyes et al., 2009).
Biometric heritability
To estimate the amount of heritable variance in the different EDA responses, standard biometric models were fit to the covariate-adjusted data. Models were fit using the OpenMx package (Boker et al., 2011) for the R statistical environment (R Development Core Team, 2010), using four member families as well as twin data. In these models, each EDA measure is assumed to reflect three latent variables: additive genetic influences (A), common environmental influences (C), and unique, or unshared, environmental influences (E). Our approach and the logic of biometric model fitting are described in Iacono et al. (2014). As noted in that paper, we also evaluated possible dominance (D) effects, reporting the results of ADE model fitting in the twin sample where a significant effect for D was found. In order to account for differences in variances between males and females and between adolescents and parents, biometric models included dummy variables coding for these two characteristics.
SNP heritability
Next, GCTA (Yang, Lee, Goddard, & Visscher, 2011) was used to estimate the amount of variance accounted for in each endophenotype by all the SNPs on the Illumina genotyping arry combined. GCTA calculates the degree of phenotypic similarity among genetically unrelated individuals that is due to shared genotypes. The degree of genetic similarity is captured in the genetic relatedness matrix (GRM), which reflects similarity over all measured SNPs between all pairs of individuals. In samples consisting of families, it is advised to filter the sample using different thresholds of genetic relatedness in order to obtain subsamples of genetically unrelated subjects and determine whether SNP heritability estimates are relatively stable across subsamples (Yang, Lee, Goddard, & Visscher, 2013). We used thresholds of .025, .05, and .10, which remove all but distant relatives. For instance, a threshold of .025 corresponds to approximately third to fourth cousins. Linkage disequilibrium (LD) with “causal” SNPs can inflate SNP heritability estimates, as well as artificially increase the precision of estimates (Speed, Hemani, Johnson, & Balding, 2012). We therefore repeated the above three analyses after weighting SNPs by local LD patterns using the program LDAK (http://dougspeed.com/ldak) (Speed et al., 2012).
Yang and colleagues have more recently recommended using the entire sample even when it consists of related subjects, while modeling the shared environmental influences within families (equivalent to C effects in biometric models; Yang et al., 2013).We conducted this third variant of GCTA, as well as one that also uses the whole sample without modeling environmental influence (i.e., by simply not filtering subjects). Comparing heritability estimates from the two methods allowed us to estimate C effects on the EDA measures from the molecular genetic data. In general, GCTA allows a comparison of biometric and SNP heritability estimates, which provides an indication of the magnitude of the influence of the common variants on the Illumina array on each EDA measure.
SNP effects: Genome-wide scan
Following this, linear regression was used to evaluate whether any of the 527,829 SNPs were individually associated with any of the EDA indices. These analyses were conducted using the R package for rapid feasible generalized least squares (RFGLS; X. Li, Basu, Miller, Iacono, & McGue, 2011). RFGLS accounts for the correlations within families in a computationally efficient manner. MZ and DZ twin correlations were estimated separately, and stepparents were treated as independent observations. All RFGLS analyses used the conventional genome-wide significance threshold of 5 × 10−8.
\3\SNP effects: Candidate SNPs
We further examined a list of 1,180 candidate SNPs that have been implicated in disorders that are linked to abnormal EDA responses (see online supporting information Tables S6–S9 for a list of the sources). Any of these endophenotype-general candidate SNPs not on the Illumina array were imputed (Iacono et al., 2014). We used a Bonferroni-corrected statistical threshold of 4.2 × 10−5 for these analyses.
Gene effects: Genome-wide scan
To test for associations between specific genes and EDA measures, we utilized the VEGAS algorithm (Liu et al., 2010), which converts p values from GWAS into chi-squared statistics, which are then aggregated into a single gene-based test statistic. The test statistic is adjusted for LD among SNPs within a given gene. Gene-based tests can be a powerful alternative to tests of individual SNPs when there are several causal SNPs in a gene. In such a case, none of the p values might be small enough to be distinguishable from noise. We evaluated 17,601 autosomal genes in total, using a Bonferroni-corrected p value threshold of 2.84 × 10−6, in a genome-wide analysis parallel to our genome-wide GWAS.
Gene effects: Candidate genes
This was followed by tests of two sets of candidate genes in relation to each of the EDA indices: (1) 204 endophenotype-general candidate genes that are part of the major neurotransmitter and neuromodulator systems (dopamine, noradrenaline, acetylcholine, GABA, glutamate, and serotonin), part of the endogenous cannabinoid or opioid systems, or that are implicated in metabolizing nicotine, alcohol, and drugs; and (2) 92 autosomal genes related to endophenotypes for schizophrenia identified by the Consortium on the Genetics of Schizophrenia (COGS; Greenwood et al., 2011; COGS candidate genes). Endophenotype-general candidate genes were evaluated using a threshold of p ≤ 5.43 × 10−4, whereas the COGS candidate genes were evaluated at a threshold of p ≤ 2.45 × 10−5.
Results
Basic descriptive statistics for each EDA index (with all covariates accounted for) are presented in the supporting information (see Table S1 and Figures S1–S4). Results are presented first for classic twin (biometrical) analyses of the various EDA indicators, including tonic (resting SCL) and phasic (SCR frequency and amplitude), as well as the composite factor score. Following this, results from GCTA analyses (i.e., the molecular genetic equivalent of biometric models) are presented. Both of these methods assume an additive genetic model. These are then followed by tests of individuals SNPs (GWAS) and then genes (VEGAS) in relation to each of the indices.
Heritability from Biometric Models
Correlations between family members for the various EDA indices (adjusted for all covariates) are presented in Table 1\t1\. All correlations between members of MZ twin pairs were of at least moderate magnitude (r = .67 for SCL being the highest). In contrast to this, DZ correlations were of generally smaller magnitude (r = .34 for SCL again being largest). Magnitude of correlations between MZ twin pairs are approximately twice that of DZs. This suggests at least a moderate additive genetic component for most EDA indices. Results from the various biometric models supported this notion and revealed that, whether we examine the entire family or used just twin pairs, all EDA indices were at least moderately heritable (see Table 2\t2\). Approximately 50% of the variation in each of the EDA indices was due to genetic effects, with the remainder mostly due to unique environment or measurement error. Common environment effects were mostly zero, or were negligible and included confidence intervals that overlapped with zero.
Table 1.
Within-Family Correlations for Electrodermal Activity (EDA) Indices.
| MZ twins | DZ twins | Mother-father | Twins-mothers | Twins-fathers | |
|---|---|---|---|---|---|
| SCL | .672 | .340 | .014 | .091 | .189 |
| SCR amplitude | .528 | .285 | .061 | .009 | .142 |
| SCR frequency | .537 | .200 | .095 | .133 | .171 |
| EDA factor | .591 | .243 | .081 | .132 | .171 |
Note. SCL and SCR = skin conductance level and response, respectively; EDA factor = the common factor extracted from the three SC measures.
Table 2.
Heritability Estimates from Biometric Model-Fitting Analyses
| Data | Measure | A | C | E |
|---|---|---|---|---|
| Family | SCL | .627 (.578–.672) | .000 (.000–.020) | .373 (.328–.422) |
| SCR amplitude | .427 (.373–.478) | .000 (.000–.017) | .573 (.522–.627) | |
| SCR frequency | .473 (.418–.521) | .000 (.000–.029) | .527 (.479–.578) | |
| EDA factor | .520 (.472–.566) | .000 (.000–.020) | .480 (.434–.528) | |
| Twins | SCL | .656 (.464–.711) | .016 (.000–.196) | .328 (.289–.372) |
| SCR amplitude | .468 (.271–.579) | .065 (.000–.243) | .468 (.420–.520) | |
| SCR frequency | .526 (.427–.573) | .000 (.000–.085) | .474 (.427–.526) | |
| EDA factor | .578 (.469–.620) | .000 (.000–.098) | .422 (.380–.468) |
Note. Point estimates of the corresponding variance components (95% confidence intervals) are given. These are standardized and sum to 1. ADE models were also fit using the twin data, but none produced a significant effect for D. Confidence intervals included 0 and D could be constrained to 0 without significantly degrading model fit (all likelihood ratio test p values > .05). SCL and SCR = skin conductance level and response, respectively; EDA factor = the common factor extracted from the three SC measures; A= additive genetic influence; C = common or shared environmental influence; E = unique or unshared environmental influence.
SNP Heritability
The effect of all SNPs that we utilized in our GWAS for each of the EDA indices is provided in Table 3\t3\ using four different cutoffs for relatedness in the GRM matrix—the conventional one of .025 (corresponding to restricting relationships among participants to third or fourth cousins or less, i.e., unrelated participants), .05, .10, and no cutoff (i.e., using all individuals in the sample). In addition, we used GRMs weighted by LD patterns. Although variability across methods and cutoffs for unrelatedness were observed as expected, estimates tended to be similar whether we used the raw GRM or GRMs weighted by LD patterns in the sample. Taking all the estimates in aggregate, the SNP heritability is generally much less than the biometric heritability for the same variable, thus indicating that not all the biometric heritability can be accounted for by the Illumina SNPs. At the lower GRM thresholds, standard errors for estimates tended to be large, reflecting the smaller sample size used when related individuals are removed from the analysis. As expected, when no GRM cutoff was used, the amount of variance accounted for in the EDA index increased for most phenotypes, approaching the heritability values in Table 2, with GCTA values slightly smaller than biometric model estimates of A, the additive genetic component. The GCTA model, which accounted for common family environment and utilized the entire sample, yielded variance estimates almost identical to that of the raw GCTA, bolstering the notion that almost no C or shared environment effects were present for any of the EDA indices in our sample, whether we examined it using GCTA or whether we used biometric models.
Table 3.
SNP Heritability of EDA Indices from GCTA Analyses Based on Variance Accounted for by All 527,829 SNPs
| \tch\Threshold | ||||
|---|---|---|---|---|
| .025 | .050 | .100 | None | |
| SCL | ||||
| Unweighted | .340 (.174) | .219 (.172) | .239 (.169) | .581 (.023) |
| Weighted, all | .352 (.224) | .189 (.220) | .224 (.217) | – |
| Family C | – | – | – | .581 (.042) |
| SCR amplitude | ||||
| Unweighted | .316 (.184) | .292 (.181) | .296 (.179) | .381 (.024) |
| Weighted, all | .211 (.240) | .189 (.235) | .213 (.232) | – |
| Family C | – | – | – | .381 (.047) |
| SCR frequency | ||||
| Unweighted | .254 (.184) | .227 (.176) | .268 (.175) | .485 (.024) |
| Weighted, all | .498 (.233) | .403 (.223) | .451 (.222) | – |
| Family C | – | – | – | .485 (.043) |
| EDA factor | ||||
| Unweighted | .351 (.177) | .313 (.172) | .347 (.170) | .511 (.022) |
| Weighted, all | .465 (.230) | .321 (.221) | .378 (.219) | – |
| Family C | – | – | – | .510 (.041) |
Note. Sample sizes for the different GRM cutoffs for unrelated people ranged from 1,903–2,099 for the unweighted estimates and from 1,765–2,028 for the weighted estimates. For the full sample, it was 4,424. SCL and SCR = skin conductance level and response, respectively; EDA factor = the common factor extracted from the three SC measures; Threshold = genetic relatedness threshold used for selecting unrelated individuals; None = no threshold was imposed and all subjects were included; Unweighted GRM = raw GRM; Weighted GRM = weights based on LD patterns to discount those SNPs in high LD (Speed et al., 2012). This is not used in the full sample, because the method was designed for samples of unrelated individuals or samples containing a small number of large pedigrees (Doug Speed, e-mail communication, May 4, 2014). Family C = uses all subjects while simultaneously modeling shared environmental influences.
SNP Effects: Genome-Wide Scan
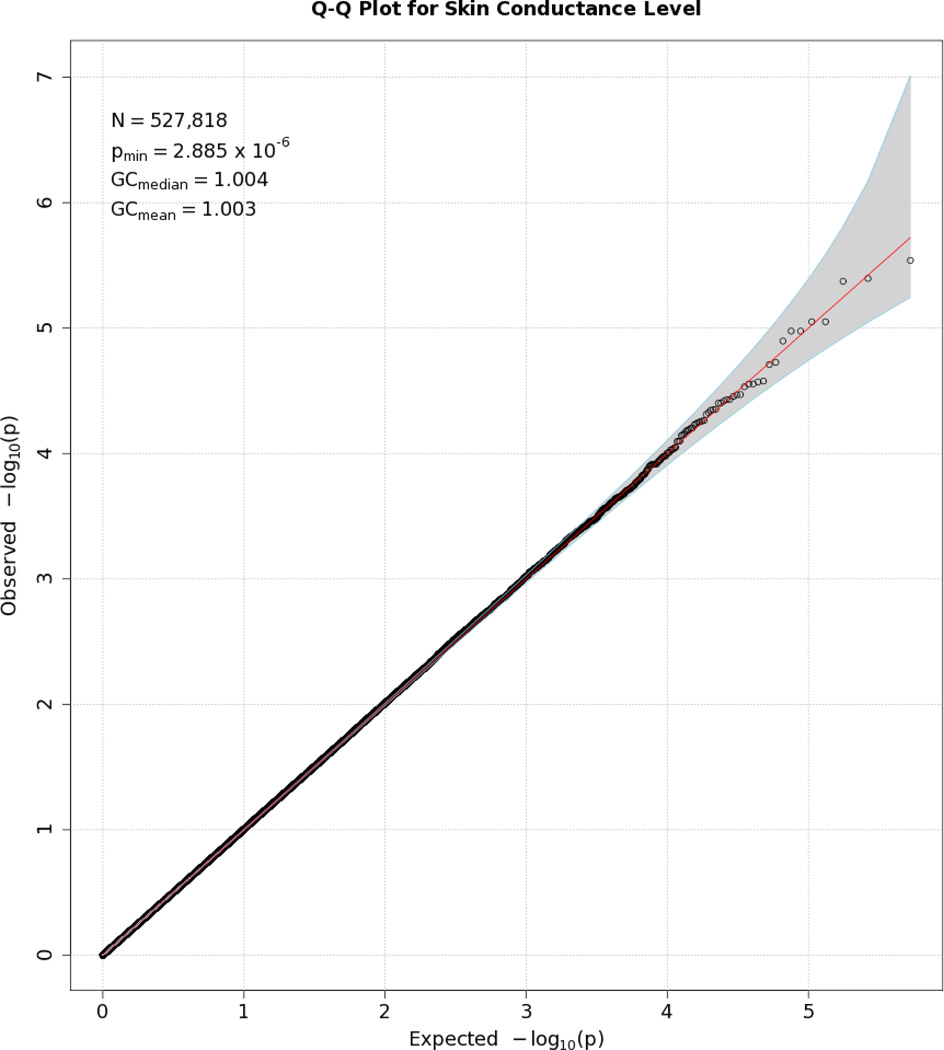
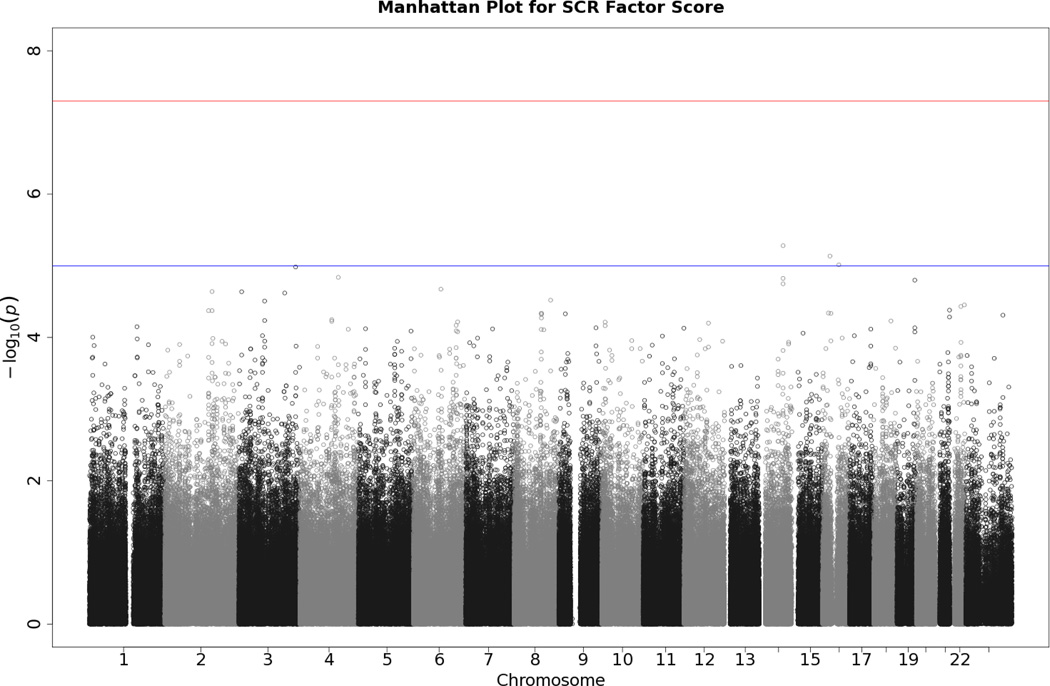
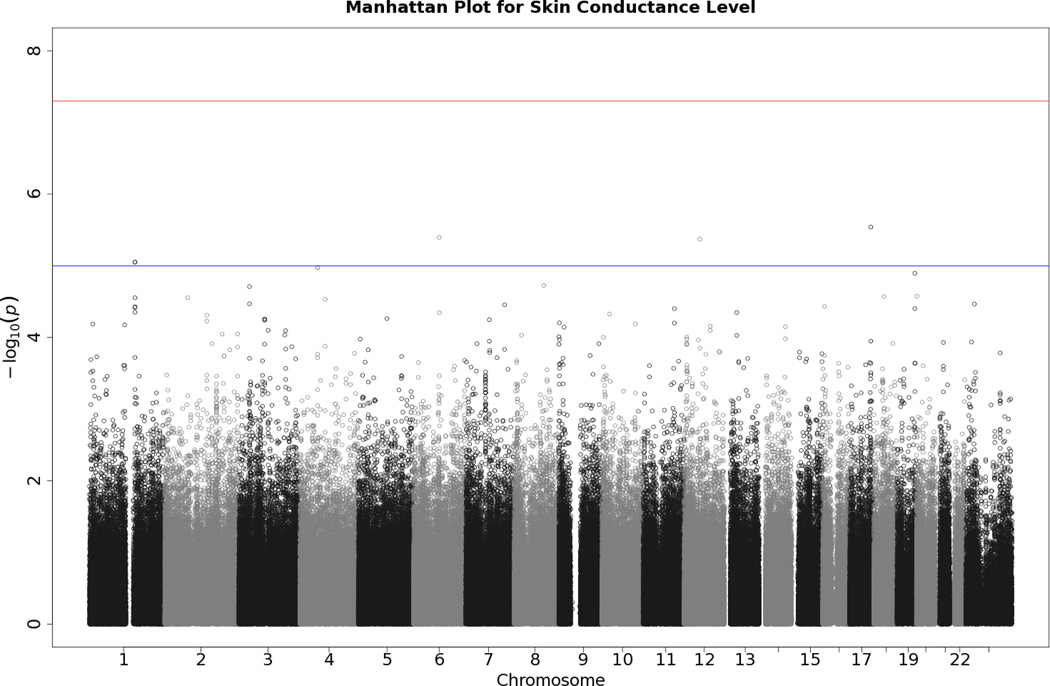
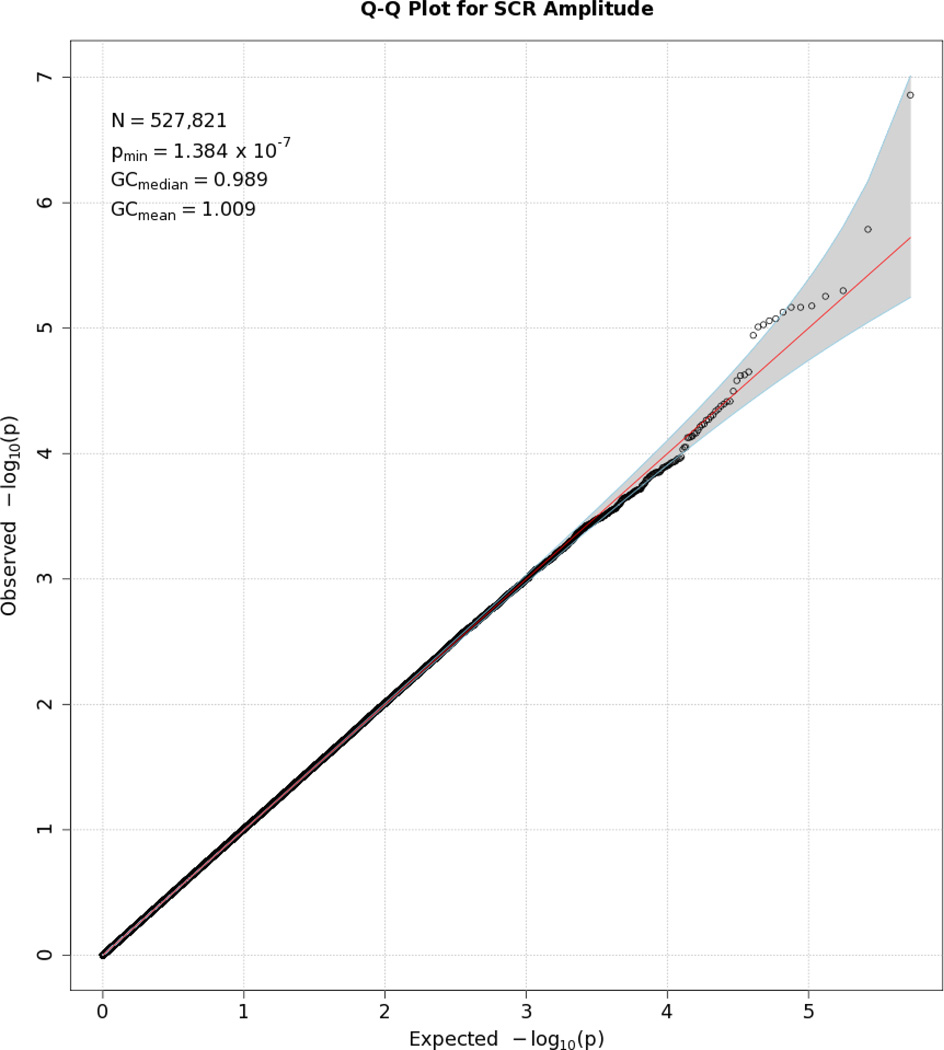
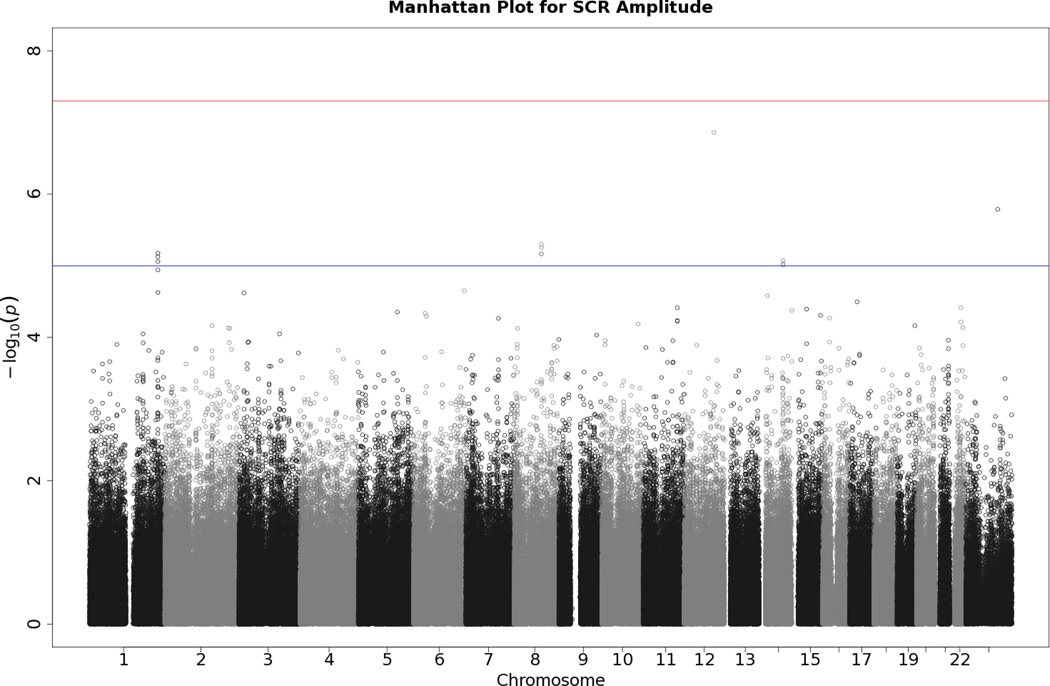
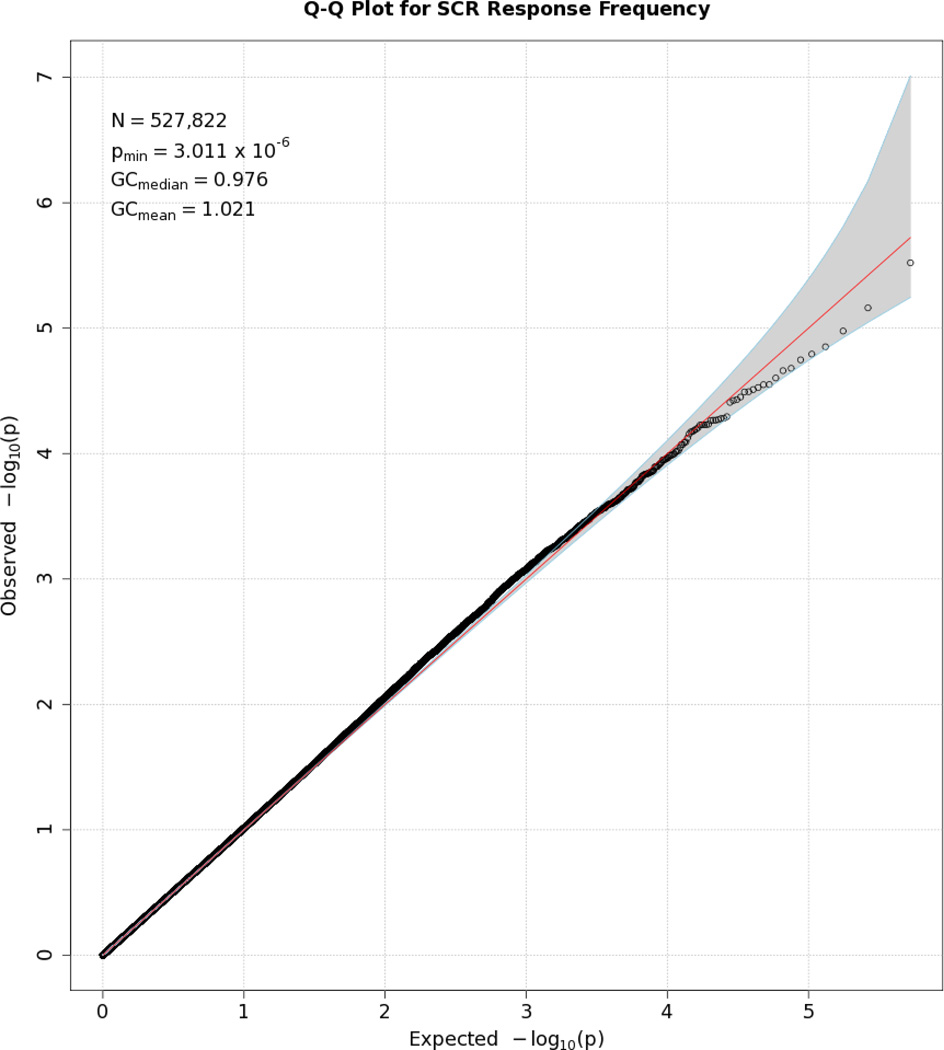
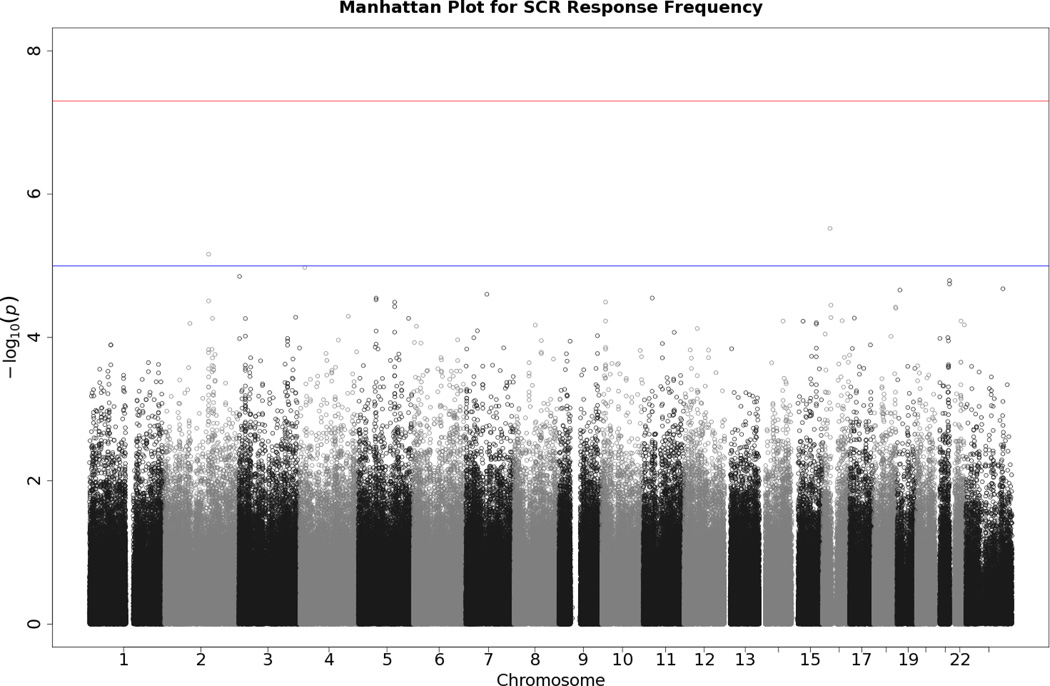
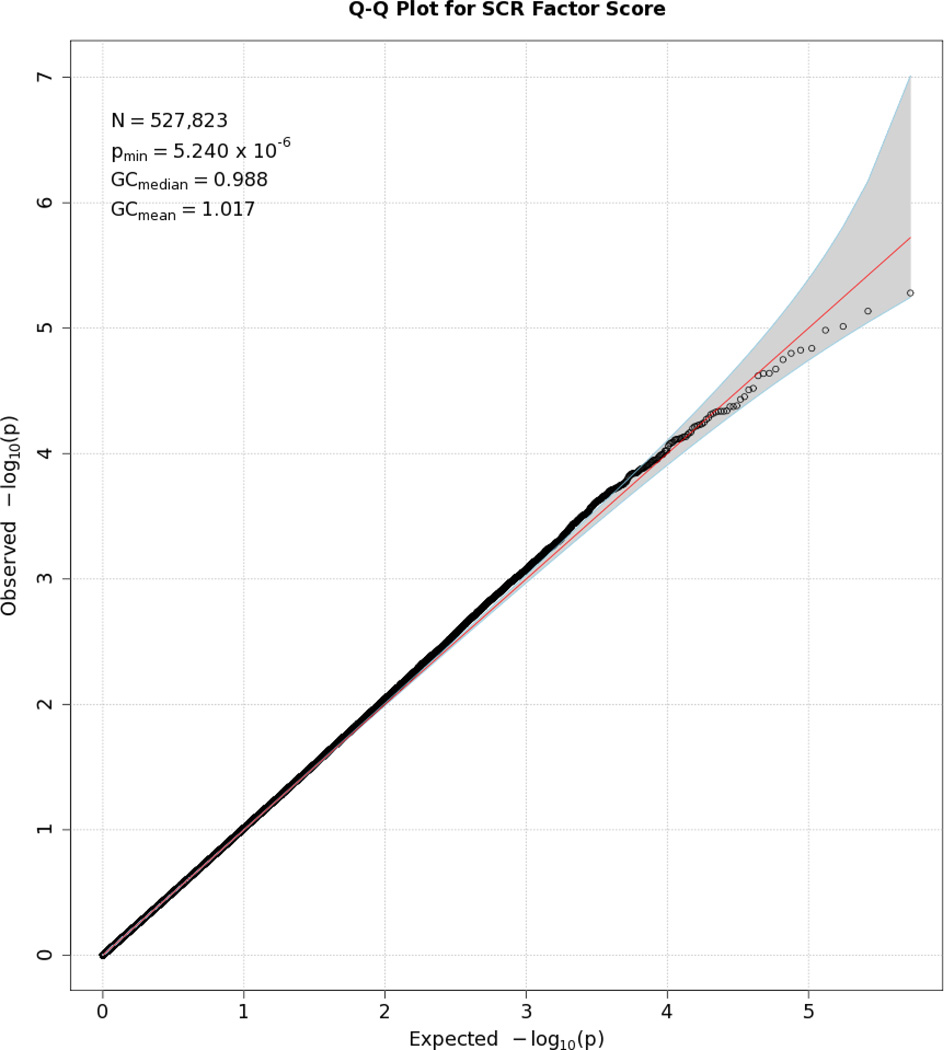
No SNP crossed the threshold for statistical significance of 5 × 10−8 for any of the EDA responses. Quantile-quantile (Q-Q) plots and Manhattan plots for all EDA indices are provided (see Figures 1–8\f1\f2\f3\f4\f5\f6\f7\f8). Q-Q plots depict p values for the various SNPs that were tested in this study plotted against the expected distribution of p values for a chi-square distribution. Extreme deviations from the diagonal x–y line could indicate problems such as population stratification, while a few at the extreme end could represent true associations among the SNPs that were tested. Manhattan plots show SNPs ordered by chromosome on the x axis, and the negative logarithm of the p values on the y axis. They provide a compact visual way of examining which SNPs from which chromosome proved statistically significant. An examination of SNPs with the p values less than 10−4 for each of the three EDA indices (see supporting information Tables S2–S4)—amplitude, response frequency, and SCL—revealed one SNP that was in common between amplitude and response frequency and a different one in common between amplitude and SCL. Not surprisingly, each of the responses did show some slight overlap (ranging from 5–13 SNPs) with SNPs with p values less than 10−4 for the EDA factor score (Table S5). However, as no single SNP was close to the statistical threshold for 5 × 10−8, we chose not to interpret these.
Figure 1.
Q-Q plot for SNP associations with SCL. The line bisecting the graph gives the expected value under the null distribution. The area shaded in gray corresponds to the 95% acceptance region. Median and mean genomic control values are given in the inset in the upper left. N refers to the number of SNPs, which is 10 fewer than the number of SNPs on the array because there was no variation for 10 SNPs in this sample. Q-Q plots in GWAS give the observed p values against the expected p values under the null distribution of no association, although the additive inverse of the common log of p values (−[p value]) is used in order to emphasize small p values. Because the vast majority of SNPs are not expected to be associated with a given phenotype, observed p values should conform closely to their expected values, falling on or very close to a 45° line, which is plotted in the center. The gray region in each plot depicts the 95% confidence region (null acceptance region).
Figure 8.
Manhattan plot of individual SNP associations with the EDA factor score. See Figure 2 for more details.
SNP Effects: Candidate SNPs
Tables S6–S9 list p values for SNPs that have been reported in the literature to be associated with various disorders linked to aberrations in EDA responses, even if EDA itself was not examined in these molecular genetic studies. These SNPs were culled from a broader list of publications including meta-analyses that were relevant to the disorders related to EDA discussed in this manuscript (e.g., Greenwood et al., 2011; C. Y. Li et al., 2011; Sullivan, Daly, & O'Donovan, 2012). As can be seen, the smallest p value attained by any SNP in these comparisons was approximately .0002—well above the Bonferroni-corrected significance threshold for 1,180 tests. SNPs with the smallest p values also tended to vary across indices with no apparent consistency in results.
Gene Effects: Genome-Wide Scan
Broadband analyses of all 17,601 genes revealed that none of them crossed the threshold for statistical significance. A gene on chromosome 1, presenilin-2 or PSEN2, came close with a p value of 9 × 10−6 for SCR amplitude. Mutations in this gene have been linked to familial forms of Alzheimer’s disease (Keyes et al., 2009).
Gene Effects: Candidate Genes
For the 204 endophenotype-general candidate gene-based analyses, statistical significance was evaluated at p ≤ .05/204 ≈ .0002. No gene proved significant at this threshold, though some were significant at the conventional p ≤ .05 (see Table S10). However, none replicated across the three indices. Finally, we examined a list of 92 COGS candidate genes hypothetically of relevance to schizophrenia. Again, no gene came close to significance for any of the EDA indices (see Tables S11–S14).
Discussion
The current study is the first to examine the behavioral and molecular genetic bases of EDA, a commonly used psychophysiological measure that indexes arousal and stimulus signal value. The size of the current population-based twin family sample (N = 4,424) made practical the first-ever application of GWAS to electrodermal system function. The current study employed a habituation protocol that was modeled closely after procedures we have used successfully in past research with schizophrenia and mood disorder patients and their first-degree relatives (Iacono, 1982; Iacono, Ficken, & Beiser, 1999; Iacono et al., 1983, 1984). This protocol, which involved the presentation of loud tones while subjects were immersed in another task (in this case, watching a movie), identified anomalous responses in both the patients and their relatives, a pattern of results that is consistent with EDA as an endophenotype for psychotic disorders. Again using this same habituation paradigm, we have also shown EDA to be stable over a one-year interval (Iacono et al., 1984), and heritable in twins reared apart (Lykken et al., 1988). Importantly, using a subset of twins also included in this investigation, we showed that the association between externalizing psychopathology and EDA was mediated by shared genetic influence, also an important criterion for an endophenotype (Isen et al., 2012). Here, we extended the biometric analysis employed in Isen et al. (2012) to the full MCTFR sample and affirmed that each of the EDA measures was at least moderately heritable.
GCTA analyses providing estimates of SNP heritability supported the biometric analyses. Using the entire MTFS sample, the GCTA heritability estimates approximated those using the biometric analyses. The two GCTA estimates using no GRM cutoff also matched each other, suggesting no effect of shared environment on EDA measures, further affirming the results of the biometric model fitting (which also found no C effect; the 95% confidence interval included zero). The weighted and unweighted GCTA analyses based on unrelated individuals were also revealing. Considering the EDA factor score, which provides an excellent summary index for EDA, and using different cutoffs to select unrelated individuals, we found that the SNP heritability approximated .35, well below all the phenotypic heritability estimates, which exceeded .50. Despite the imprecision inherent in the GCTA estimates from unrelated people, they point to the conclusion that much of the additive genetic influence in EDA is due to common genetic variants, suggesting that it was the combined effect of multiple SNPs that accounted for a substantial amount of variation in EDA. Such findings provide compelling evidence that variations in electrodermal activity are likely to result from small additive effects of many genes acting in concert, rather than any single mutation. Hence, using a proven endophenotype-relevant EDA protocol shown to be associated with several different types of psychiatric disorder, we found ample evidence to support the heritability of EDA as well as evidence that the genetic influence on EDA is due at least in part to common SNPs that we measured.
Against this backdrop, we surveyed the effects associated with all the SNPs on the Illumina array and all the genes available to us through VEGAS. In addition, we executed tests of many candidate SNPs and genes that plausibly could be related to EDA. None of these analyses produced a significant finding using our Bonferroni-adjusted p values. One gene—presenilin-2 or PSEN2 on chromosome 1—came close to significance (p = 9 × 10−6) for SCR amplitude. Mutations in this gene have been related to familial forms of Alzheimer’s disease. However, as this finding was not quite significant, future studies will be required to confirm whether this result represents a true effect.
Limitations
Although our EDA measures provided indications of both tonic and phasic activity, our results were nevertheless dependent on the limited number of EDA measures we examined. In particular, we would have liked to include a count of nonspecific fluctuations, a measure that is commonly used in psychiatric research, but that imposed an excessive processing burden when applied to a sample as large as that employed here. Miller and Rockstroh (2013) have cautioned against conceiving of endophenotypes as biological phenomena. With regard to EDA, we used a habituation paradigm, and orienting and habituation are fundamental psychological processes. Hence, our results for EDA depended on our driving our measures from this procedure. Had we used a different task, such as a conditioning paradigm, it is possible that we would have obtained different results. EDA anomalies have been reliably associated with schizophrenia, but our general population sample did not include many individuals with this disorder. Had we included a large number of individuals with psychotic disorders, it is possible we would have found more extreme deviation on our EDA measures, and this could have enhanced our chances of finding genetic associations. Comparison of our GCTA and biometric modeling results pointed to the problem of “missing heritability,” noted in other studies using GCTA with other phenotypes, which indicates that common genetic variants alone cannot account for all the genetic influence on EDA. We were unable to explore the contribution of other sources of genetic influence here, and refer readers to other papers in this special issue for results of research carried out on EDA using rare variants (Vrieze, Malone, Pankratz et al., 2014; Vrieze, Malone, Vaidyanathan et al., 2014).
Conclusion
There has been scant molecular genetic investigation of EDA. Our work largely paves the way on this topic by showing that the molecular genetics of EDA appears to be much like that for other complex phenotypes. The evidence supports the polygenicity of EDA measures. Our inability to identify any of the polygenic SNPs relevant to EDA in our GWAS indicates that the effects of any given SNP has to be quite small, requiring many more subjects than the 4,426 MTFS participants we studied. These results may seem somewhat contradictory to the notion of an endophenotype, which are considered to be simpler units of analyses than overt psychiatric symptoms, and thus presumably linked more directly to the genetic origins of psychiatric disorders. However, it is worth noting that others (Gottesman & Gould, 2003) have pointed out that endophenotypes, despite their direct ties to neurobiology, may themselves be polygenic in origin. Indeed, results such as the ones in the current study bolster the notion that the human genome and the pathways via which it exerts its effect on human behaviors are complex.
Supplementary Material
Figure 2.
Manhattan plot of individual SNP associations with SCL. Manhattan plots also depict the distribution of -log10(p values) but are ordered by SNP location on a chromosome, which provides information about the location of any SNPs associated with small p values. The horizontal upper line indicates the genome-wide significance level (5E-08). The horizontal lower line indicates E-05, which is sometimes used to indicate “suggestive” significance.
Figure 3.
Q-Q plot for SNP associations with SCR amplitude. See Figure 1 for more details.
Figure 4.
Manhattan plot of individual SNP associations with SCR amplitude. See Figure 2 for more details.
Figure 5.
Q-Q plot for SNP associations with SCR frequency. See Figure 1 for more details.
Figure 6.
Manhattan plot of individual SNP associations with SCR frequency. See Figure 2 for more details.
Figure 7.
Q-Q plot for SNP associations with the EDA factor score. See Figure 1 for more details.
Acknowledgements
This research was supported by NIH grants DA 024417, DA 05147, DA 036216, AA 09367, and DA 13240.
Footnotes
Supporting Information
Additional supporting information may be found in the online version of this article:
Table S1: Descriptive statistics for each EDA index.
Table S2: Top SNP associations with SCL.
Table S3: Top SNP associations with SCR amplitude.
Table S4: Top SNP associations with SCR frequency.
Table S5: Top SNP associations with EDA factor scores.
Table S6: SNP associations for endophenotype-general candidate SNPs for SCL.
Table S7: SNP associations for endophenotype-general candidate SNPs for SCR amplitude.
Table S8: SNP associations for endophenotype-general candidate SNPs for SCR frequency.
Table S9: SNP associations for endophenotype-general candidate SNPs for EDA factor scores.
Table S10: Gene-based associations with EDA indices at p < .05.
Table S11: Results of VEGAS gene-based tests of COGS endophenotype candidate genes for SCL.
Table S12: Results of VEGAS gene-based tests of COGS endophenotype candidate genes for SCR amplitude.
Table S13: Results of VEGAS gene-based tests of COGS endophenotype candidate genes for SCR frequency.
Table S14: Results of VEGAS gene-based tests of COGS endophenotype candidate genes for EDA factor scores.
Figure S1: Distribution of SCL residuals.
Figure S2: Distribution of SCR amplitude residuals.
Figure S3: Distribution of SCR frequency residuals.
Figure S4: Distribution of EDA factor Score residuals.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Baker E, Shelton KH, Baibazarova E, Hay DF, van Goozen SHM. Low skin conductance activity in infancy predicts aggression in toddlers 2 years later. Psychological Science. 2013;24:1051–1056. doi: 10.1177/0956797612465198. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal activity. New York: Springer; 2012. [Google Scholar]
- Cambrai M, Clar EJ, Grosshans E, Altermatt C. Skin impedance and phoreographic index in psoriasis. Archives of Dermatological Research. 1979;264:197–211. doi: 10.1007/BF00431132. [DOI] [PubMed] [Google Scholar]
- Crider A, Kremen WS, Xian H, Jacobson KC, Waterman B, Eisen SA, Lyons MJ. Stability, consistency, and heritability of electrodermal response lability in middle-aged male twins. Psychophysiology. 2004;41:501–509. doi: 10.1111/j.1469-8986.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3rd ed. New York, NY: Cambridge University Press; 2007. pp. 159–181. [Google Scholar]
- de Geus EJ. From genotype to EEG endophenotype: A route for post-genomic understanding of complex psychiatric disease? Genome Medicine. 2010;2:1–4. doi: 10.1186/gm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelberg R. Electrical properties of the skin. In: Brown EE, editor. Methods in psychophysiology. Baltimore, MD: Williams & Wilkins; 1967. pp. 1–53. [Google Scholar]
- Fowles DC, Christie MJ, Edelberg R, Grings WW, Lykken DT, Venables PH. Publication recommendations for electrodermal measurements. Psychophysiology. 1981;18:232–239. doi: 10.1111/j.1469-8986.1981.tb03024.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Kochanska G. Temperament as a moderator of pathways to conscience in children: The contribution of electrodermal activity. Psychophysiology. 2000;37:788–795. [PubMed] [Google Scholar]
- Fung MT, Raine A, Loeber R, Lynam DR, Steinhauer SR, Venables PH, Stouthamer-Loeber M. Reduced electrodermal activity in psychopathy-prone adolescents. Journal of Abnormal Psychology. 2005;114:187–196. doi: 10.1037/0021-843X.114.2.187. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Association of poor childhood fear conditioning and adult crime. American Journal of Psychiatry. 2010a;167:56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Reduced electrodermal fear conditioning from ages 3 to 8 years is associated with aggressive behavior at age 8 years. Journal of Child Psychology and Psychiatry. 2010b;51:550–558. doi: 10.1111/j.1469-7610.2009.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen R, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Linting M. Electrodermal reactivity during the Trier Social Stress Test for children: Interaction between the serotonin transporter polymorphism and children's attachment representation. Developmental Psychobiology. 2008;50:615–625. doi: 10.1002/dev.20314. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Hardiman G. Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. American Journal of Psychiatry. 2011;168:930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JD. Physiological responses of anxious and normal subjects to simple signal and non-signal auditory stimuli. Psychophysiology. 1974;11:443–451. doi: 10.1111/j.1469-8986.1974.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Wenning B, Qunaibi M, Lichterfeld C, Herpertz-Dahlmann B. Autonomic responses in boys with externalizing disorders. Journal of Neural Transmission. 2003;110:1181–1195. doi: 10.1007/s00702-003-0026-6. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Vloet T, Mueller B, Domes G, Willmes K, Herpertz-Dahlmann B. Similar autonomic responsivity in boys with conduct disorder and their fathers. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:535–544. doi: 10.1097/chi.0b013e3180306286. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Fredrikson M, Kendler KS. The genetic covariation between fear conditioning and self-report fears. Biological Psychiatry. 2008;63:587–593. doi: 10.1016/j.biopsych.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Annas P, Neale MC, Kendler KS, Fredrikson M. A twin study of the genetics of fear conditioning. Archives of General Psychiatry. 2003;60:702–708. doi: 10.1001/archpsyc.60.7.702. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cohort effects on gender differences in alcohol dependence. Addiction. 2002;97:1025–1036. doi: 10.1046/j.1360-0443.2002.00142.x. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG. Cross-generational effects on gender differences in psychoactive drug abuse and dependence. Drug and Alcohol Dependence. 2004;74:147–158. doi: 10.1016/j.drugalcdep.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Holdcraft LC, Iacono WG, McGue MK. Antisocial personality disorder and depression in relation to alcoholism: A community-based sample. Journal of Studies on Alcohol. 1998;59:222–226. doi: 10.15288/jsa.1998.59.222. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Bilateral electrodermal habituation-dishabituation and resting EEG in remitted schizophrenics. Journal of Nervous and Mental Disease. 1982;170:91–101. doi: 10.1097/00005053-198202000-00005. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Psychophysiology and genetics: A key to psychopathology research. Psychophysiology. 1983;20:371–383. doi: 10.1111/j.1469-8986.1983.tb00916.x. [DOI] [PubMed] [Google Scholar]
- Iacono WG. Psychophysiologic markers of psychopathology: A review. Canadian Psychology. 1985;26:96–112. [Google Scholar]
- Iacono WG. Identifying psychophysiological risk for psychopathology: Examples from substance abuse and schizophrenia research. Psychophysiology. 1998;35:621–637. [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Ficken JW, Beiser M. Electrodermal activation in first-episode psychotic patients and their first-degree relatives. Psychiatry Research. 1999;88:25–39. doi: 10.1016/s0165-1781(99)00071-2. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Lykken DT. The orienting response: Importance of instructions. Schizophrenia Bulletin. 1979;5:11–14. doi: 10.1093/schbul/5.1.11. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Lykken DT, Peloquin LJ, Lumry AE, Valentine RH, Tuason VB. Electrodermal activity in euthymic unipolar and bipolar affective disorders: A possible marker for depression. Archives of General Psychiatry. 1983;40:557–565. doi: 10.1001/archpsyc.1983.01790050083010. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM. Developmental endophenotypes: Indexing genetic risk for substance abuse with the P300 brain event-related potential. Child Development Perspectives. 2011;5:239–247. doi: 10.1111/j.1750-8606.2011.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, Vaidyanathan U, Vrieze SI. Genome-wide scans of genetic variants for psychophysiological endophenotypes: A methodological overview. Psychophysiology. 2014 doi: 10.1111/psyp.12343. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Peloquin LJ, Lykken DT, Haroian KP, Valentine RH, Tuason VB. Electrodermal activity in euthymic patients with affective disorders: One-year retest stability and the effects of stimulus intensity and significance. Journal of Abnormal Psychology. 1984;93:304–311. doi: 10.1037//0021-843x.93.3.304. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Roshi D, Lacoste D. Electrodermal activity in patients with Huntington's disease and their progeny. Psychophysiology. 1987;24:522–527. doi: 10.1111/j.1469-8986.1987.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Isen JD, Iacono WG, Malone SM. Characterizing electrodermal response habituation: A latent class approach with application to psychopathology. Psychophysiology. 2013;50:954–962. doi: 10.1111/psyp.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isen JD, Iacono WG, Malone SM, McGue M. Examining electrodermal hyporeactivity as a marker of externalizing psychopathology: A twin study. Psychophysiology. 2012;49:1039–1048. doi: 10.1111/j.1469-8986.2012.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, Legrand LN, McGue M, Iacono WG. The enrichment study of the Minnesota Twin Family Study: Increasing the yield of twin families at high risk for externalizing psychopathology. Twin Research and Human Genetics. 2009;12:489–501. doi: 10.1375/twin.12.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleeberg J, Bruggimann L, Annoni J-M, van Melle G, Bogousslavsky J, Schluep M. Altered decision-making in multiple sclerosis: A sign of impaired emotional reactivity? Annals of Neurology. 2004;56:787–795. doi: 10.1002/ana.20277. [DOI] [PubMed] [Google Scholar]
- Knott VJ, Bulmer DR. Effects of repetitive high intensity stimulation on electrodermal responsivity in male alcoholics and normal controls. Addictive Behaviors. 1985;10:181–185. doi: 10.1016/0306-4603(85)90025-5. [DOI] [PubMed] [Google Scholar]
- Lacey JI, Lacey BC. The relationship of resting autonomic activity to motor impulsivity. Proceedings of the Association for Research in Nervous and Mental Disease. 1958;36:144–209. [PubMed] [Google Scholar]
- Lader MH, Wing L. Habituation of the psycho-galvanic reflex in patients with anxiety states and in normal subjects. Journal of Neurology, Neurosurgery, and Psychiatry. 1964;27:210–218. doi: 10.1136/jnnp.27.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson EA. Skin conductance responses in Huntington's chorea progeny. Psychophysiology. 1981;18:32–35. doi: 10.1111/j.1469-8986.1981.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhou WZ, Zhang PW, Johnson C, Wei L, Uhl GR. Meta-analysis and genome-wide interpretation of genetic susceptibility to drug addiction. BMC Genomics. 2011;12:508. doi: 10.1186/1471-2164-12-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basu S, Miller MB, Iacono WG, McGue M. A rapid generalized least squares model for a genome-wide quantitative trait association analysis in families. Human Heredity. 2011;71:67–82. doi: 10.1159/000324839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Mcrae AF, Nyholt DR, Medland SE, Wray NR, Brown KM. A versatile gene-based test for genome-wide association studies. American Journal of Human Genetics. 2010;87:139–145. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Iacono WG, Haroian K, McGue M, Bouchard TJ. Habituation of the skin conductance response to strong stimuli: A twin study. Psychophysiology. 1988;25:4–15. doi: 10.1111/j.1469-8986.1988.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Mayer B, Merckelbach H, de Jong PJ, Leeuw I. Skin conductance responses of spider phobics to backwardly masked phobic cues. Journal of Psychophysiology. 1999;13:152–159. [Google Scholar]
- McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: Where do we stand? Annual Review of Clinical Psychology. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- Miller MB, Basu S, Cunningham J, Eskin E, Malone SM, Oetting WS, McGue M. The Minnesota Center for Twin and Family Research genome-wide association study. Twin Research and Human Genetics. 2012;15:767–774. doi: 10.1017/thg.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus: Statistical analysis with latent variables: User's guide. Muthén & Muthén; 2010. [Google Scholar]
- O'Gorman JG, Horneman C. Consistency of individual differences in non-specific electrodermal activity. Biological Psychology. 1979;9:13–21. doi: 10.1016/0301-0511(79)90019-x. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Shalev AY, Pitman RK. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:75–82. doi: 10.1037//0021-843x.104.1.75. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: A meta-analysis. Psychological Bulletin. 2007;133:725–746. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R: Foundation for Statistical Computing; 2010. Retrieved from http://www.R-project.org. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. Relationships between central and autonomic measures of arousal at age 15 years and criminality at age 24 years. Archives of General Psychiatry. 1990;47:1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Williams M. High autonomic arousal and electrodermal orienting at age 15 years as protective factors against criminal behavior at age 29 years. American Journal of Psychiatry. 1995;152:1595–1600. doi: 10.1176/ajp.152.11.1595. [DOI] [PubMed] [Google Scholar]
- Saari A, Tolonen U, Pääkkö E, Suominen K, Pyhtinen J, Sotaniemi KA, Myllylä VV. Sympathetic skin responses in multiple sclerosis. Acta Neurologica Scandinavica. 2008;118:226–231. doi: 10.1111/j.1600-0404.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Kirsch P, Reuter M, Alexander N, Kozyra E, Kuepper Y, Hennig J. The 5-HT1A C(1019)G polymorphism, personality and electrodermal reactivity in a reward/punishment paradigm. International Journal of Neuropsychopharmacology. 2009;12:383–392. doi: 10.1017/S1461145708009401. [DOI] [PubMed] [Google Scholar]
- Schuri U, von Cramon D. Electrodermal responses to auditory stimuli with different significance in neurological patients. Psychophysiology. 1981;18:248–251. doi: 10.1111/j.1469-8986.1981.tb03027.x. [DOI] [PubMed] [Google Scholar]
- Speed D, Hemani G, Johnson MR, Balding DJ. Improved heritability estimation from genome-wide SNPs. American Journal of Human Genetics. 2012;91:1011–1021. doi: 10.1016/j.ajhg.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: The emerging picture and its implications. Nature Reviews Genetics. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuvblad C, Gao Y, Isen J, Botwick T, Raine A, Baker LA. The heritability of the skin conductance orienting response: A longitudinal twin study. Biological Psychology. 2012;89:47–53. doi: 10.1016/j.biopsycho.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Malone SM, Pankratz N, Vaidyanathan U, Miller MB, Kang HM, Iacono WG. Genetic associations of nonsynonymous exonic variants with psychophysiological endophenotypes. Psychophysiology. 2014 doi: 10.1111/psyp.12349. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Malone SM, Vaidyanathan U, Kwong A, Kang HM, Zhan X, Iacono WG. In search of rare variants: Preliminary results from whole genome sequencing of 1325 individuals with psychophysiological endophenotypes. Psychophysiology. 2014 doi: 10.1111/psyp.12350. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt J, Lotze M, Weike AI, Hosten N, Hamm AO. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Roth WT. Taking the laboratory to the skies: Ambulatory assessment of self-report, autonomic, and respiratory responses in flying phobia. Psychophysiology. 1998;35:596–606. doi: 10.1017/s0048577298970196. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: A tool for genome-wide complex trait analysis. American Journal of Human Genetics. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Lee SH, Goddard ME, Visscher PM. Genome-wide association studies and genomic prediction. Vol. 1019. New York, NY: Humana Press; 2013. Genome-wide complex trait analysis (GCTA): Methods, data analyses, and interpretations; pp. 215–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.