Abstract
Background
Ganglioglioma is a rare and slowly growing benign tumor. We investigated the outcomes of patients who underwent different combination treatments.
Methods
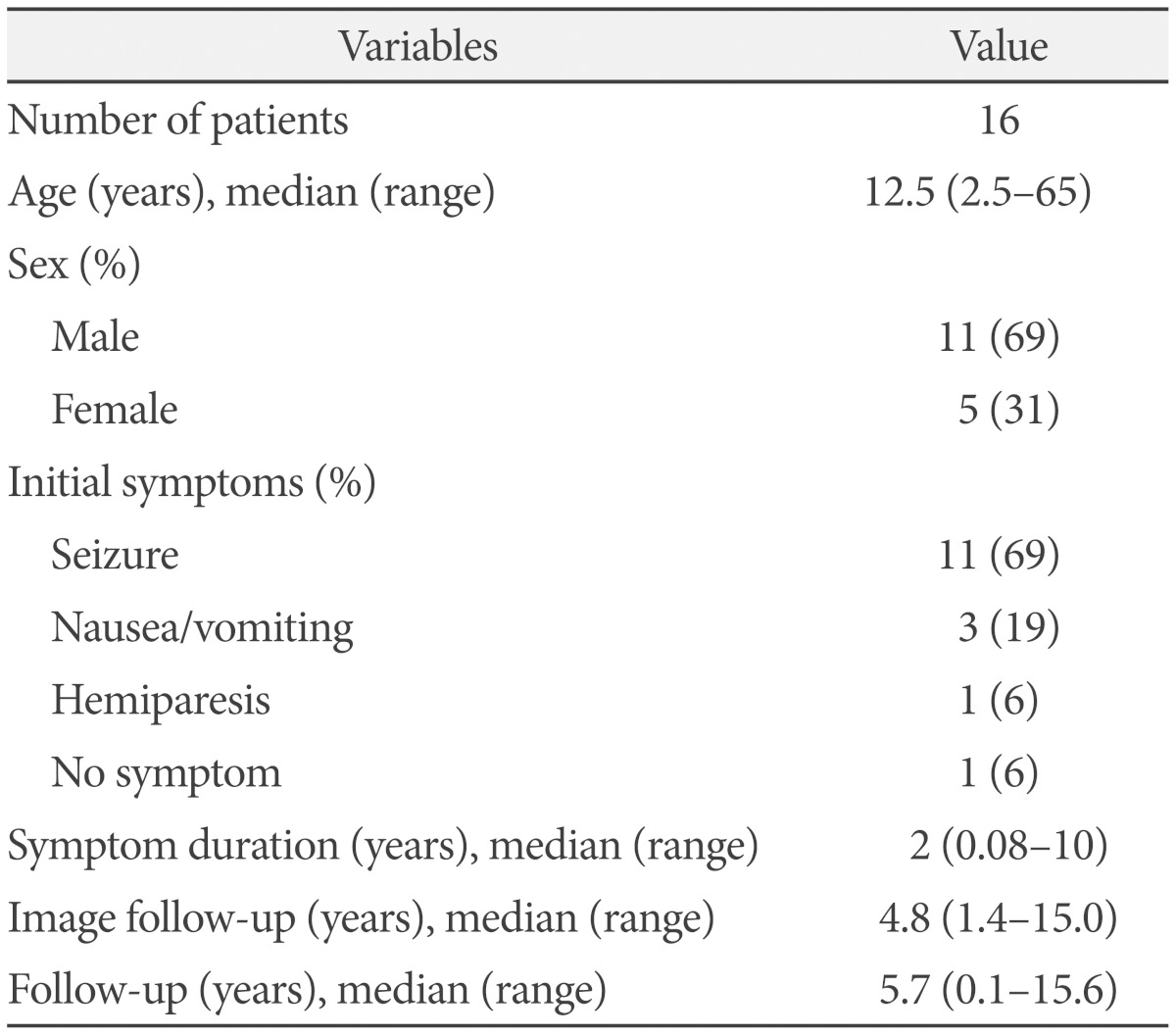
Between 1998 and 2012, 16 patients, including 11 men and 5 women, with a median age of 12.5 years (range, 2.5-65 years) were treated for intracranial gangliogliomas at our institution. The median follow-up period was 5.7 years (range, 48 days-15.6 years). Fifteen cases were included in the outcome assessment because one patient was lost to follow-up. Complete resection was achieved in 8 (53%) patients. Six (40%) patients underwent incomplete resection with or without adjuvant radiotherapy, and one patient with a brainstem tumor underwent only stereotactic biopsy.
Results
Gangliogliomas predominantly affected young (87.5%), male patients and most frequently presented with seizures (64%). Of eight patients who underwent complete resection, seven did not show recurrence, whereas only three of six with incomplete resection showed no recurrence. Four patients with recurrence received salvage treatments (two repeat surgeries and two radiosurgeries). A tumor control rate of 93% (14/15) was achieved at the last follow-up. No recurrence or malignant changes were observed after a median follow-up of 12 and 4.5 years in four patients who received gamma knife (GK) radiosurgery as adjuvant and salvage treatment.
Conclusion
Complete resection produced the best outcomes and incomplete resection followed by adjuvant or salvage treatments showed favorable outcomes. In patients who are not eligible for complete resection because of tumor location or potential neurologic deficits following surgery, GK radiosurgery should be considered for the treatment of residual or recurrent tumors.
Keywords: Ganglioglioma, Radiosurgery
INTRODUCTION
Gangliogliomas [World Health Organization (WHO) grade I] are rare tumors of the central nervous system (CNS) that account for approximately 1% of all CNS neoplasms, and they most frequently affect children and young adults, with a slight male predominance [1]. They can occur anywhere in the CNS, including the cerebrum, cerebellum, thalamus, spinal cord, hypothalamus, lateral ventricle, or brainstem, but are most commonly found in the temporal lobe (up to 85%), often associated with seizure disorders. Gangliogliomas are the most common tumor related to temporal lobe epilepsy [2,3,4].
Gangliogliomas are benign and well-differentiated neuroepithelial tumors composed of variable proportions of dysplastic neuronal elements (ganglion cells) and neoplastic glial elements [5,6]. In 5% of cases, gangliogliomas show aggressive behavior and anaplastic histopathologic features characteristic of WHO grade III tumors. These tumors are even rarer and can occur de novo or as a result of malignant transformation of a pre-existing lesion [7,8].
Low-grade gangliogliomas can be cured surgically, and complete tumor resection is the most effective treatment. Radiotherapy is reserved for progressive or malignant tumors after surgical treatment [9,10,11]. Here, we investigated the long-term clinical outcomes of 16 patients with ganglioglioma.
MATERIALS AND METHODS
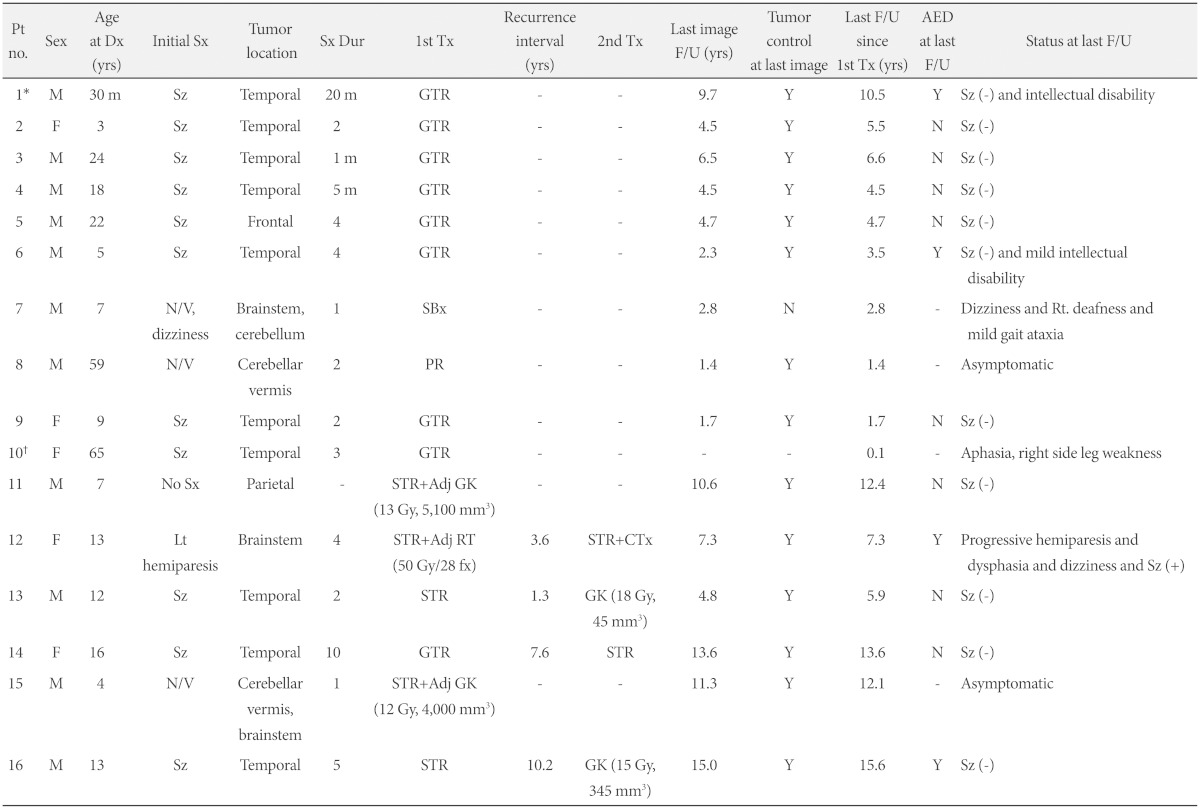
This study was approved by our local Institutional Review Board (2014-0721). The database of patients treated for intracranial tumors at our institution between 1998 and 2012 was searched, and 16 patients with intracranial ganglioglioma were analyzed retrospectively. Medical charts, surgical records, pathology reports, and pre- and post-operative imaging studies were reviewed. Patient clinical data included presenting symptoms, symptom duration before the diagnosis, symptomatic and neurological status before and after treatment, and the use of antiepileptic drugs (Table 1). A histological diagnosis of ganglioglioma was made when a tumor had a mixture of neoplastic neuronal and glial cells irrespective of which component was predominant.
Table 1.
Clinical information of all patients
*Pt #1 experienced an episode of seizure 5 years before the last follow-up, †Pt #10 lost to follow-up. Pt: patient, Dx: diagnosis, Tx: treatment, Sx: symptom, Sz: seizure, N/V: nausea and vomiting, Dur: duration, GTR: gross total resection, STR: subtotal resection, PR: partial resection, SBx: stereotactic biopsy, Adj: adjuvant, GK: gamma knife radiosurgery, RT: conventional radiotherapy, CTx: chemotherapy, F/U: follow-up, yrs: years, AED: antiepileptic drug, Tumor control: Y: no progression or recurrence on MRI, N: progression or recurrence on MRI
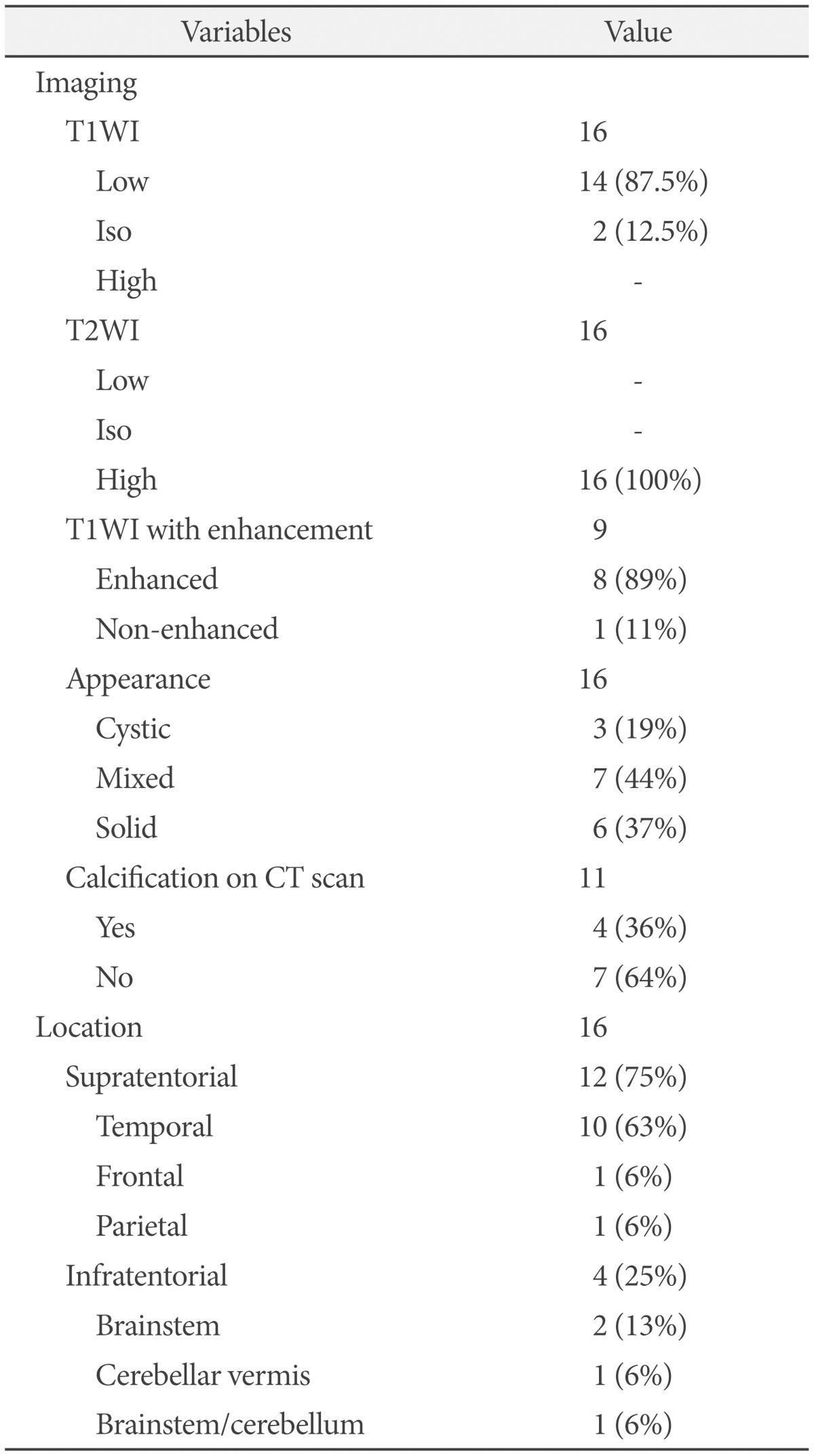
Pre-operative imaging studies included computed tomography (CT), magnetic resonance imaging (MRI), magnetic resonance spectroscopy (MRS), and positron emission tomography (PET). MRS and PET imaging results were available for only a few cases and were not included in the analysis. CT scans were performed as initial imaging studies in 11 patients to determine the presence of tumor calcification. Pre-operative MRI scans were performed in all patients and nine of them had postcontrast T1-weighted images (T1WI).
The degree of resection was described as gross total, subtotal, or partial. Gross total resection (GTR) was defined as the complete removal of the lesion without tumor remnant on post-operative MRI. Subtotal resection (STR) was defined as the removal of >80% of the tumor and partial resection (PR) as the removal of <80% of the tumor. Biopsy indicated that only a minimal amount of tumor tissue was obtained for the purpose of the diagnosis.
In four cases, gamma knife (GK) radiosurgery was performed post-operatively and marginal tumor doses were 12, 13, 15, and 18 Gy respectively. One patient received conventional radiotherapy (RT) as post-operative adjuvant treatment.
Outcome measures included tumor control and symptom status at last follow-up. Tumor control was determined by examining the growth of residual tumors or new lesions on MR and seizure outcome using the Engel Seizure Outcome Classification [12].
RESULTS
Demographics
The study population included 11 (69%) men and 5 (31%) women. The median age at diagnosis was 12.5 years (range: 2.5-65 years), with 14 patients (87.5%) aged <25 years and 2 patients aged >25 years at the time of diagnosis (Table 2).
Table 2.
Summary of patient characteristics
Tumor location and clinical presentation
Supratentorial tumors were detected in 75% (12/16) of patients and infratentorial tumors in 25% (4/16) of patients. The 12 supratentorial tumors consisted of 10 temporal, 1 frontal, and 1 parietal ganglioglioma. Among the four infratentorial tumors, two lesions were located in the brainstem, one in the cerebellum, and one in both the brainstem and cerebellum (Table 3).
Table 3.
Tumor characteristics
T1WI: T1-weighted image, T2WI: T2-weighted image
The most common presenting symptom was seizures (69%, 11/16), which occurred in all patients with supratentorial gangliogliomas except in the case showing a parietal tumor, which was asymptomatic and found incidentally. Three (19%) patients presented with dizziness, nausea, and vomiting ascribed to cerebellar and/or brainstem tumors. One patient had left hemiparesis caused by a brainstem tumor (Table 2).
Imaging findings
In general, gangliogliomas showed low signal intensity on T1WI and high signal intensity on T2-weighted images (T2WI). On enhanced MRI, heterogeneous enhancement was observed in eight of nine cases. Tumor appearance was varied and heterogeneous on MRI.
Tumors mainly composed of cystic contents and showing high signal intensity on T2WI were grouped as cystic tumors, whereas those with mainly solid contents and iso or low signal intensity were grouped as solid tumors. Tumors showing mixed signal intensity without a definite predominance were classified as a mixed tumor group. Nearly 50% of tumors had both solid and cystic components. Solid tumors were found in six patients (37%) and cystic tumors in three patients (19%). CT scans were performed in 11 patients and 4 (36%) of them showed hyperdense lesions corresponding to calcification (Table 3).
Treatment
The results of 15 cases were analyzed because one patient (Pt. #10) was lost to follow-up after she underwent burr hole trephination and subdural drainage for post-operative subdural hemorrhage, which occurred 1 month after tumor resection (Table 4).
Table 4.
Treatment outcome
*the case in which only a biopsy was performed. GTR: gross total resection, STR: subtotal resection, PR: partial resection, SBx: stereotactic biopsy, Adj: adjuvant, GK: gamma knife radiosurgery, RT: conventional radiotherapy
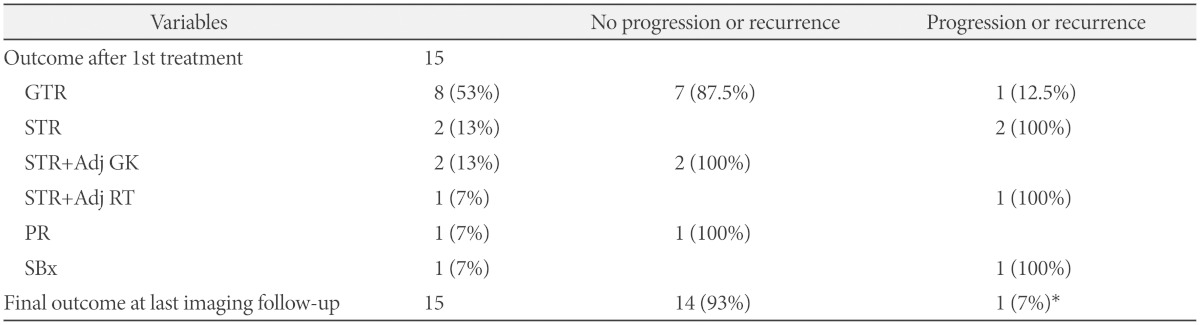
Gross total resection of the tumor was achieved in eight (53%) patients. Seven (87.5%) of them experienced no recurrence during the follow-up period. Two patients who underwent STR without any adjuvant therapy showed residual tumor regrowth at 1.3 and 10.2 years after the treatments. Subsequently, salvage GK radiosurgery was performed and no further growth was observed for 4.5 and 5.3 years after the salvage treatments. STR with adjuvant radiotherapy was performed in three patients (two GKs and one RT). The two patients who received adjuvant GK radiosurgery maintained stable disease status for more than a decade (12.4 and 12.1 years, respectively), whereas the patient who received adjuvant RT required repeat surgery followed by chemotherapy in the presence of radiographic progression of the residual tumor. One patient underwent PR of a cerebellar tumor and the residual tumor remained stable at the 1.4 year follow-up. In the other patient, the tumor was located in the brainstem and only a biopsy was performed for diagnosis because of the high risk of mortality following microsurgery. The tumor grew slightly on the last MRI 2.8 years after the diagnosis.
Outcomes
Local recurrence occurred in four cases (1 GTR, 2 STRs, and 1 STR plus RT as the first treatments), with a recurrence rate of 29% (4/14) among those who underwent surgical resection as initial treatment. Recurred tumors were detected at different times ranging from 1.3 to 10.2 years from the initial treatment (median, 5.6 years). All recurrent tumors were controlled after salvage treatments (two repeat surgeries and two GKs) at the time of last follow-up. The overall tumor control rate was 93% (14 of 15 cases, including the brainstem ganglioglioma that was only biopsied).
Ten patients with seizure presentation were seizure-free (Engel class I) at the last follow-up. One patient reported an episode of seizure after arbitrarily stopping antiepileptic medication; however, he had no seizures in the 5 years since restarting medication. Antiepileptic drugs were discontinued in seven of the ten initially epileptic patients. One patient (Pt. #12), who initially presented with hemiparesis due to brainstem ganglioglioma, developed epilepsy later after surgery and was placed on medication. Four patients with tumors in the cerebellum or brainstem showed a 50% symptom-free rate eventually. Two of these four patients showed persistent cranial nerve deficits, motor weakness, and cerebellar dysfunction symptoms, such as ataxia and dizziness.
DISCUSSION
Clinical characteristics and tumor location
Gangliogliomas mainly affect children and young adults, with a peak incidence in the second decade of life [10,13,14]. The incidence of gangliogliomas is reported to be 0.4% to 7.6% of pediatric CNS neoplasms and up to 1.3% of those in adults [5,15,16]. Our present study population included 14 young (<25 years old) patients and 2 old (around 60 years old) patients.
Gangliogliomas are typically located in the temporal lobe, and seizures are the most common presenting clinical symptom, with an incidence rate of 75-100% [10,14,17,18,19,20]. The results of the present study were in agreement with previous studies, showing 63% of patients with temporal lobe tumors and 69% of patients with seizure presentation. A recent study reported that the frontal and parietal lobes are the second most frequent site of gangliogliomas [21]. However, our data indicated that the brainstem and cerebellum were affected more often than the frontal and parietal lobes.
Infratentorial tumors were observed in 3 of the 14 young patients, and one of the two old patients. The small sample size prohibited drawing meaningful conclusions regarding the association between tumor location and patient age. In fact, several studies reported that posterior fossa gangliogliomas affect mostly patients younger than 30 years [5,15,21,22].
Treatments and outcomes
Microsurgery is the gold standard in the treatment of gangliogliomas, and complete tumor removal, when possible, is the only curative option [5,23]. Complete resection, however, is not always feasible because of tumor location at deep and critical sites adjacent to vital brain areas. In particular, brainstem gangliogliomas often require alternative or additional treatments such as radiation therapy or chemotherapy because of the limited role of microsurgery in the treatment of these lesions.
In the current series, the patients who underwent GTR showed the lowest recurrence rate at 12.5% (1/8), in contrast to a rate of 50% (3/6) in patients who underwent incomplete resection (STR or PR). Among the patients with incompletely resected tumors, those who received adjuvant treatments (2 GK and 1 RT) had a better long-term control rate than those who did not (67% vs. 33%). Consequently, GTR resulted in the best local control, as reported in previous studies [5,23], and adjuvant radiation therapy reduced the risk of recurrence. Our result is consistent with those of previous small sized studies that reported favorable results of adjuvant RT after incomplete resection of nonmalignant gangliogliomas, although GK radiosurgery was not included in these studies [24,25,26].
Recurrence occurred in 29% of surgically resected gangliogliomas (12.5% of completely resected tumors and 50% of incompletely resected tumors). Luyken et al. [23] analyzed 184 patients with supratentorial gangliogliomas and reported recurrence rates of 1% among cases with no residual tumor and 8% among cases showing residual tumor. The discrepancy in the recurrence rate between our series and Luyken's study could be explained by differences in the sample size and distribution of tumor location. For salvage purposes, both GK radiosurgery and repeat surgery were effective, accounting for the lack of further recurrence after salvage treatment.
Radiation therapy
Low-grade gangliogliomas do not require post-operative radiotherapy as long as they are completely removed. In cases of incomplete resection, as described above, adjuvant RT has yielded favorable results in terms of tumor control [24,25,26]. However, the acceptable long-term tumor control rates obtained with observation alone following STR suggest that the routine use of adjuvant RT after STR may be overtreatment [23]. Meanwhile, RT for the management of recurrence or high-grade gangliogliomas has not produced satisfactory results [24,27,28]. Furthermore, potential radiation-related malignant changes in low-grade tumors are an ongoing concern [7,25,29], despite data suggesting that radiotherapy may not be the major causative factor in malignant transformation [30]. Accordingly, there is no consensus regarding the need for radiotherapy in patients with residual or recurrent ganglioglioma. Several authors have suggested that adjuvant radiotherapy should be restricted to cases where recurrence would result in neurologic compromise or patients with anaplastic ganglioglioma in whom radical resection is not feasible because of uncertainty regarding its benefit on tumor control or the detrimental effects of radiation on the CNS [7,24,27,31].
In the current series, four patients who were treated with GK radiosurgery either as adjuvant or salvage treatment showed no recurrence after radiosurgery for a long period. Furthermore, GK radiosurgery did not result in malignant transformation of tumors despite earlier studies highlighting the potential risk of RT [7,29]. However, whether GK radiosurgery is superior to RT remains unclear in view of the malignant change of tumor cells in our small study. Future studies showing that GK radiosurgery is less likely to be associated with malignant change than RT would support an explanation based on the different radiobiologies between GK radiosurgery and RT. RT applies multiple fractions of radiation that cumulatively damage rapidly proliferating tumor cells. GK radiosurgery applies single, focused, high-dose radiation to kill or arrest all the tumor cells in the lesion. Therefore, slowly growing tumors such as nonmalignant gangliogliomas may be more susceptible to radiosurgery, whereas cumulative damage on indolent tumor cells caused by RT could induce malignant changes in benign or normal cells.
To the best of our knowledge, the effect of radiosurgery on gangliogliomas has not been investigated to date. GK radiosurgery appears to be a safe and effective treatment option for residual or recurrent gangliogliomas, particularly in deep and critical brain areas such as the brainstem.
Prognosis and prognostic factors
The prognosis of ganglioglioma is favorable, with a 10-year survival rate of 84-93% [28]. One series reported a 5-year survival rate of 93% [32], and another study showed a 7.5-year survival rate of 98% [23]. The tumor control rate of our series was 93% (14 of 15 patients on follow-up) at a median follow-up of 5.7 years.
More than half of the residual tumors after STR remain silent for a long time [27], underscoring the need to identify prognostic factors for the detection of residual tumors at risk of recurrence. Although prognostic factors are difficult to reliably define because of the low incidence of this disease, certain features such as complete resection, temporal location, epilepsy presentation [23,27,33,34], and absence of cellular atypia [28] are associated with favorable prognosis.
The degree of resection shows a strong correlation with recurrence [23,27,33], and the best tumor control and progression-free survival rates are achieved with complete tumor resection [23].
Another important prognostic factor is histologic grade. Although the criteria for WHO grade II gangliogliomas have not been established, WHO grade II tumors have atypical histological features, such as increased cellularity and increased mitotic activity, and lack unequivocal anaplastic features [33]. Several studies based on a 3-tiered histopathologic grading system reported that WHO grade II tumors accounted for approximately 10% and WHO grade III tumors accounted for 2.5-5% of all gangliogliomas [6,33,34]. Majores et al. [33] investigated the survival and recurrence rates according to tumor grade. The 5-year survival rates of patients with WHO grade I, II, and III tumors were 99%, 79%, 53%, and the recurrence rates were 2%, 33%, 60%, respectively. DeMarchi et al. [35] investigated the survival time of anaplastic gangliogliomas based on previous publications and found that nine (39%) of 23 patients with anaplastic gangliogliomas were dead at an average of 13 months after diagnosis. Although some authors reported that histological grade is not a predictor of poor outcome [32,36], it is likely to play a role in determining the clinical course and hence should be taken into consideration in choosing the treatment modalities.
Long-term epilepsy [33] and temporal tumor location [10,23,37] are among the favorable prognostic factors. Tumors in the temporal lobe commonly present with epilepsy, and in most cases, a temporal lobe location allows complete resection of the tumors. As a result, epilepsy and temporal lobe location are associated with favorable outcomes. On the contrary, infratentorial tumors, which usually cause cerebellar dysfunction or cranial nerve deficits rather than epilepsy, are frequently difficult to completely remove and consequently lead to less favorable outcomes.
Epilepsy
Epilepsy associated with ganglioglioma, even if it is medically refractory, can be controlled by tumor resection [37,38,39]. Seizure relief (Engel class I) was reported to be accomplished in 76% to 88% of epileptic patients [23,27,37,39] and 59% of seizure-free patients were off antiepileptic medication [27]. We observed a seizure-free rate of 100% at last follow-up and complete relief of seizure without antiepileptic drugs was achieved in 70% cases (7/10) after GTR or STR followed by GK radiosurgery.
In conclusion, the current series of benign gangliogliomas demonstrated the typical findings of this rare disease such as a predilection for younger patients, common temporal tumor location with seizure presentation, and a favorable prognosis after complete resection. GK radiosurgery, whether it is administered as adjuvant or salvage therapy, may be a safe and effective treatment option for gangliogliomas. In patients (especially those with high-grade tumors) who are not eligible for complete resection because of the location of the tumor or the risk of neurologic deficits after surgery, GK radiosurgery can be considered for the treatment of residual or recurrent tumors.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Zhang D, Henning TD, Zou LG, et al. Intracranial ganglioglioma: clinicopathological and MRI findings in 16 patients. Clin Radiol. 2008;63:80–91. doi: 10.1016/j.crad.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Bevilacqua G, Sarnelli R. Ganglioglioma of the spinal cord. A case with a long survival. Acta Neuropathol. 1979;48:239–242. doi: 10.1007/BF00690528. [DOI] [PubMed] [Google Scholar]
- 3.Karamitopoulou E, Perentes E, Probst A, Wegmann W. Ganglioglioma of the brain stem: neurological dysfunction of 16-year duration. Clin Neuropathol. 1995;14:162–168. [PubMed] [Google Scholar]
- 4.Scalley JR. Ganglioglioma of the cerebellum: angiographic findings. Rocky Mt Med J. 1976;73:80–82. [PubMed] [Google Scholar]
- 5.Zentner J, Wolf HK, Ostertun B, et al. Gangliogliomas: clinical, radiological, and histopathological findings in 51 patients. J Neurol Neurosurg Psychiatry. 1994;57:1497–1502. doi: 10.1136/jnnp.57.12.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blümcke I, Wiestler OD. Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol. 2002;61:575–584. doi: 10.1093/jnen/61.7.575. [DOI] [PubMed] [Google Scholar]
- 7.Rumana CS, Valadka AB. Radiation therapy and malignant degeneration of benign supratentorial gangliogliomas. Neurosurgery. 1998;42:1038–1043. doi: 10.1097/00006123-199805000-00049. [DOI] [PubMed] [Google Scholar]
- 8.Schittenhelm J, Reifenberger G, Ritz R, et al. Primary anaplastic ganglioglioma with a small-cell glioblastoma component. Clin Neuropathol. 2008;27:91–95. doi: 10.5414/npp27091. [DOI] [PubMed] [Google Scholar]
- 9.Hall WA, Yunis EJ, Albright AL. Anaplastic ganglioglioma in an infant: case report and review of the literature. Neurosurgery. 1986;19:1016–1020. doi: 10.1227/00006123-198612000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Silver JM, Rawlings CE, 3rd, Rossitch E, Jr, Zeidman SM, Friedman AH. Ganglioglioma: a clinical study with long-term follow-up. Surg Neurol. 1991;35:261–266. doi: 10.1016/0090-3019(91)90002-q. [DOI] [PubMed] [Google Scholar]
- 11.Mickle JP. Ganglioglioma in children. A review of 32 cases at the University of Florida. Pediatr Neurosurg. 1992;18:310–314. doi: 10.1159/000120681. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd ed. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 13.Isimbaldi G, Sironi M, Tonnarelli GP, Roncoroni M, Declich P, Galli C. Ganglioglioma: a clinical and pathological study of 12 cases. Clin Neuropathol. 1996;15:192–199. [PubMed] [Google Scholar]
- 14.Haddad SF, Moore SA, Menezes AH, VanGilder JC. Ganglioglioma: 13 years of experience. Neurosurgery. 1992;31:171–178. doi: 10.1227/00006123-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Koeller KK, Henry JM. From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2001;21:1533–1556. doi: 10.1148/radiographics.21.6.g01nv051533. [DOI] [PubMed] [Google Scholar]
- 16.Ildan F, Tuna M, Göçer IA, Erman T, Cetinalp E. Intracerebral ganglioglioma: clinical and radiological study of eleven surgically treated cases with follow-up. Neurosurg Rev. 2001;24:114–118. doi: 10.1007/pl00012393. [DOI] [PubMed] [Google Scholar]
- 17.Kalyan-Raman UP, Olivero WC. Ganglioglioma: a correlative clinicopathological and radiological study of ten surgically treated cases with follow-up. Neurosurgery. 1987;20:428–433. doi: 10.1227/00006123-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Ventureyra E, Herder S, Mallya BK, Keene D. Temporal lobe gangliogliomas in children. Childs Nerv Syst. 1986;2:63–66. doi: 10.1007/BF00286222. [DOI] [PubMed] [Google Scholar]
- 19.Pilcher WH, Silbergeld DL, Berger MS, Ojemann GA. Intraoperative electrocorticography during tumor resection: impact on seizure outcome in patients with gangliogliomas. J Neurosurg. 1993;78:891–902. doi: 10.3171/jns.1993.78.6.0891. [DOI] [PubMed] [Google Scholar]
- 20.Castillo M, Davis PC, Takei Y, Hoffman JC., Jr Intracranial ganglioglioma: MR, CT, and clinical findings in 18 patients. AJNR Am J Neuroradiol. 1990;11:109–114. [PMC free article] [PubMed] [Google Scholar]
- 21.Safavi-Abbasi S, Di Rocco F, Chantra K, et al. Posterior cranial fossa gangliogliomas. Skull Base. 2007;17:253–264. doi: 10.1055/s-2007-984486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagares A, Gómez PA, Lobato RD, Ricoy JR, Ramos A, de la Lama A. Ganglioglioma of the brainstem: report of three cases and review of the literature. Surg Neurol. 2001;56:315–322. doi: 10.1016/s0090-3019(01)00618-8. discussion 322-4. [DOI] [PubMed] [Google Scholar]
- 23.Luyken C, Blümcke I, Fimmers R, Urbach H, Wiestler OD, Schramm J. Supratentorial gangliogliomas: histopathologic grading and tumor recurrence in 184 patients with a median follow-up of 8 years. Cancer. 2004;101:146–155. doi: 10.1002/cncr.20332. [DOI] [PubMed] [Google Scholar]
- 24.Liauw SL, Byer JE, Yachnis AT, Amdur RJ, Mendenhall WM. Radiotherapy after subtotally resected or recurrent ganglioglioma. Int J Radiat Oncol Biol Phys. 2007;67:244–247. doi: 10.1016/j.ijrobp.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Ulutin HC, Ongürü O, Pak Y. Postoperative radiotherapy for ganglioglioma; report of three cases and review of the literature. Minim Invasive Neurosurg. 2002;45:224–227. doi: 10.1055/s-2002-36202. [DOI] [PubMed] [Google Scholar]
- 26.Zorlu F, Selek U, Onal C, Söylemezoğlu F, Gurkaynak M. Postoperative radiotherapy in cranial ganglioglioma. J Neurooncol. 2006;77:321–324. doi: 10.1007/s11060-005-9050-8. [DOI] [PubMed] [Google Scholar]
- 27.Im SH, Chung CK, Cho BK, et al. Intracranial ganglioglioma: preoperative characteristics and oncologic outcome after surgery. J Neurooncol. 2002;59:173–183. doi: 10.1023/a:1019661528350. [DOI] [PubMed] [Google Scholar]
- 28.Hakim R, Loeffler JS, Anthony DC, Black PM. Gangliogliomas in adults. Cancer. 1997;79:127–131. [PubMed] [Google Scholar]
- 29.Tarnaris A, O'Brien C, Redfern RM. Ganglioglioma with anaplastic recurrence of the neuronal element following radiotherapy. Clin Neurol Neurosurg. 2006;108:761–767. doi: 10.1016/j.clineuro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Mittelbronn M, Schittenhelm J, Lemke D, et al. Low grade ganglioglioma rapidly progressing to a WHO grade IV tumor showing malignant transformation in both astroglial and neuronal cell components. Neuropathology. 2007;27:463–467. doi: 10.1111/j.1440-1789.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 31.Rumana CS, Valadka AB, Contant CF. Prognostic factors in supratentorial ganglioglioma. Acta Neurochir (Wien) 1999;141:63–68. doi: 10.1007/s007010050267. discussion 68-9. [DOI] [PubMed] [Google Scholar]
- 32.Lang FF, Epstein FJ, Ransohoff J, et al. Central nervous system gangliogliomas. Part 2: clinical outcome. J Neurosurg. 1993;79:867–873. doi: 10.3171/jns.1993.79.6.0867. [DOI] [PubMed] [Google Scholar]
- 33.Majores M, von Lehe M, Fassunke J, Schramm J, Becker AJ, Simon M. Tumor recurrence and malignant progression of gangliogliomas. Cancer. 2008;113:3355–3363. doi: 10.1002/cncr.23965. [DOI] [PubMed] [Google Scholar]
- 34.Wolf HK, Müller MB, Spänle M, Zentner J, Schramm J, Wiestler OD. Ganglioglioma: a detailed histopathological and immunohistochemical analysis of 61 cases. Acta Neuropathol. 1994;88:166–173. doi: 10.1007/BF00294510. [DOI] [PubMed] [Google Scholar]
- 35.DeMarchi R, Abu-Abed S, Munoz D, Loch Macdonald R. Malignant ganglioglioma: case report and review of literature. J Neurooncol. 2011;101:311–318. doi: 10.1007/s11060-010-0248-z. [DOI] [PubMed] [Google Scholar]
- 36.Johannsson JH, Rekate HL, Roessmann U. Gangliogliomas: pathological and clinical correlation. J Neurosurg. 1981;54:58–63. doi: 10.3171/jns.1981.54.1.0058. [DOI] [PubMed] [Google Scholar]
- 37.Morris HH, Matkovic Z, Estes ML, et al. Ganglioglioma and intractable epilepsy: clinical and neurophysiologic features and predictors of outcome after surgery. Epilepsia. 1998;39:307–313. doi: 10.1111/j.1528-1157.1998.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 38.Park YS, Kim DS, Shim KW, Kim JH, Choi JU. Factors contributing to resectability and seizure outcomes in 44 patients with ganglioglioma. Clin Neurol Neurosurg. 2008;110:667–673. doi: 10.1016/j.clineuro.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 39.Im SH, Chung CK, Cho BK, Lee SK. Supratentorial ganglioglioma and epilepsy: postoperative seizure outcome. J Neurooncol. 2002;57:59–66. doi: 10.1023/a:1015761507357. [DOI] [PubMed] [Google Scholar]