Abstract
Piezo proteins have recently been identified as ion channels mediating mechanosensory transduction in mammalian cells. Characterization of these channels has yielded important insights into mechanisms of somatosensation, as well as other mechano-associated biologic processes such as sensing of shear stress, particularly in the vasculature, and regulation of urine flow and bladder distention. Other roles for Piezo proteins have emerged, some unexpected, including participation in cellular development, volume regulation, cellular migration, proliferation, and elongation. Mutations in human Piezo proteins have been associated with a variety of disorders including hereditary xerocytosis and several syndromes with muscular contracture as a prominent feature.
Keywords: Genetic Disease, Ion Channel, Mechanotransduction, Membrane Protein, Neuron, Gordon Syndrome, Arthrogryposis, Hereditary Xerocytosis, Somatosensation, Piezo1, Piezo2
Introduction
Mechanotransduction, the conversion of mechanical forces into biological signals, is a fundamental physiologic process that reveals environmental features to an organism. This process, critical for all or almost all mammalian cells, has been linked to stretch-activated ion channels (SACs)2 (1, 2). Mechanotransduction influences many important processes including embryonic development and sensory perception, such as touch, pain, proprioception and hearing, flow sensing in the kidney, regulation of vascular tone, and muscle and tendon stretch (1). In bacteria, the molecular identity of mechanosensitive ion channels has been understood at the molecular level for many years. However, until recently, the molecular identity of mechanosensory ion channels in higher organisms has been unknown (2).
Piezo Proteins
Piezo1 and Piezo2 proteins, encoded by the Piezo1/FAM38A and Piezo2/FAM38B genes, respectively, were recently identified as mechanically activated (MA) ion channels in the murine neuroblastoma cell line N2A in an siRNA screen (3, 4). They have subsequently been shown to induce MA cationic currents in numerous eukaryotic cell types, connecting mechanical forces to biological signals. Piezo proteins are predicted to be large integral membrane proteins with 24–40 transmembrane domains, making them the proteins with the largest number of transmembrane domains (Fig. 1) (4). Initially described as an essential component of an MA ion channel (4), Piezo1 was later shown to possess an ion-conducting pore (3). Piezo1 forms homotetramers, but whether the complex contains one or four pores is unknown (3).
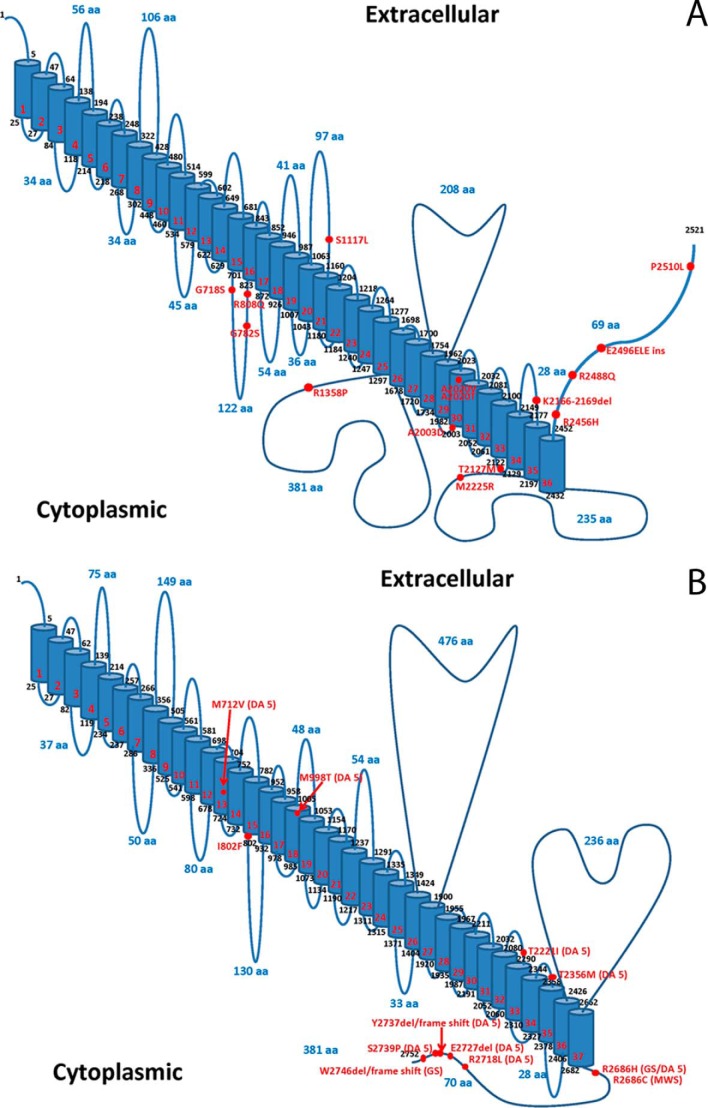
FIGURE 1.
Models of human PIEZO1 and PIEZO2. A and B, predicted membrane topology models of PIEZO1 (panel A, UniProt accession number Q92508) and PIEZO2 (panel B, UniProt accession number Q9H5I5) were created using Swiss-Prot prediction tools and the methodology of Eisenberg et al. (74). The locations of PIEZO1 mutations identified in hereditary xerocytosis (A) and PIEZO2 mutations identified in DA5, GS, and MWS (B) are marked. aa, amino acids.
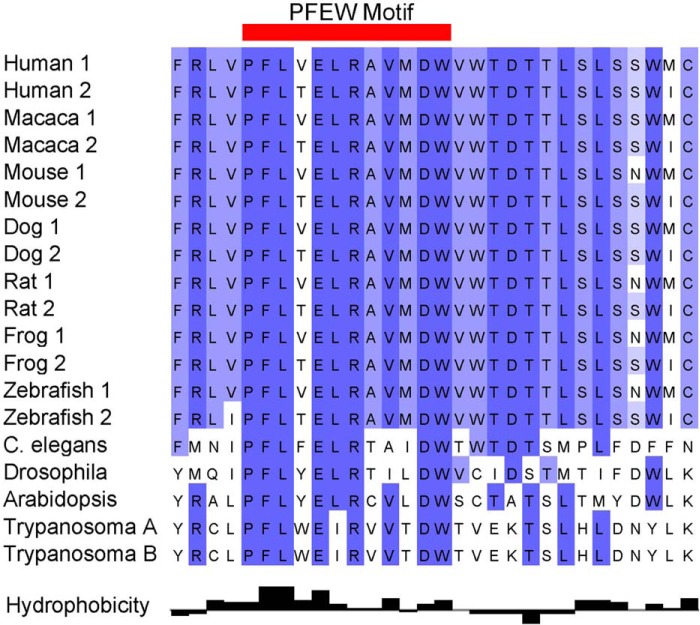
Piezo1 and Piezo2, ∼2500 and 2800 amino acids long, respectively, share ∼50% identity. Piezo homologues are found in organisms as diverse as plants, protozoa, and invertebrates. Phylogenetic analyses suggest that in vertebrates, Piezo1 diverged from Piezo2, as most lower organisms carry a single Piezo protein, whereas vertebrates have two (Fig. 2 and supplemental Fig. 1). The exception is pathogenic protozoa, where homologues of Piezo are present in two groups genetically distinct from mammalian Piezo1 and Piezo2 (5). Multispecies sequence alignments of evolutionarily distant protozoa, amoeba, plant, insect, and vertebrate Piezos reveal a remarkably conserved motif, the PFEW domain, hypothesized to be involved in channel conductance or gating (Fig. 3). Most mutations associated with human disease occur in this domain (see below).
FIGURE 2.
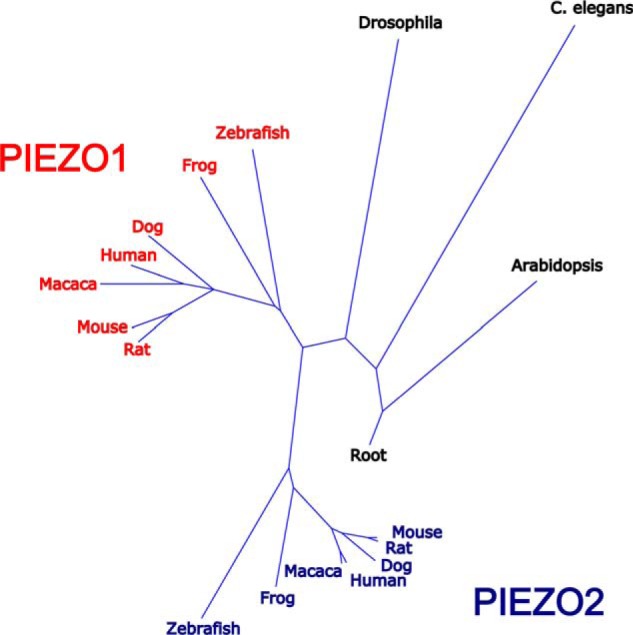
Evolutionary relationships of Piezo proteins. A phylogenetic tree based on comparison of Piezo protein sequences illustrating the relationships of Piezo proteins between species is shown. A single Piezo protein is present in lower species, including plants, worms, and flies. Near the base of the vertebrate tree, Piezo proteins were duplicated. The presence of more than one protein infers that the Piezo proteins have acquired additional functions. C. elegans, Caenorhabditis elegans.
FIGURE 3.
A highly conserved region in Piezo proteins. Multiple sequence alignment of Piezo proteins reveals a highly conserved region across species in the COOH terminus of Piezo proteins from plants to humans. This absolutely conserved region, the PFEW motif, is shown with amino acids comprising this motif shaded.
In mice and humans, many mRNA isoforms from different cell types have been identified for both Piezo loci. Whether these isoforms are translated and/or are of functional significance is unknown. Piezo1 is broadly expressed with high levels in skin, bladder, kidney, lung, endothelial cells, erythrocytes, and periodontal ligament cells (6). Piezo2 is most prominently expressed in sensory trigeminal ganglia (TG) and dorsal root ganglia (DRG), Merkel cells, lung, and bladder (4, 7–9).
Electrophysiological Properties of Piezo Channels
Piezo1 and Piezo2 mediate nonselective cationic MA currents, with rapid desensitization during the static phase of the stimulus (Fig. 4A). Single-exponential fits to the desensitization component shows that at −40 mV, Piezo1 and Piezo2 desensitize with τ ∼32 and 16 ms, respectively. Unlike peak amplitude, desensitization time is voltage-dependent and increases at positive potential (4). Experiments in heterologous systems show the absence of a systematic relationship between Piezo1- and Piezo2-mediated current amplitude and desensitization kinetics (4, 10–12), suggesting that the two parameters can be studied independently.
FIGURE 4.
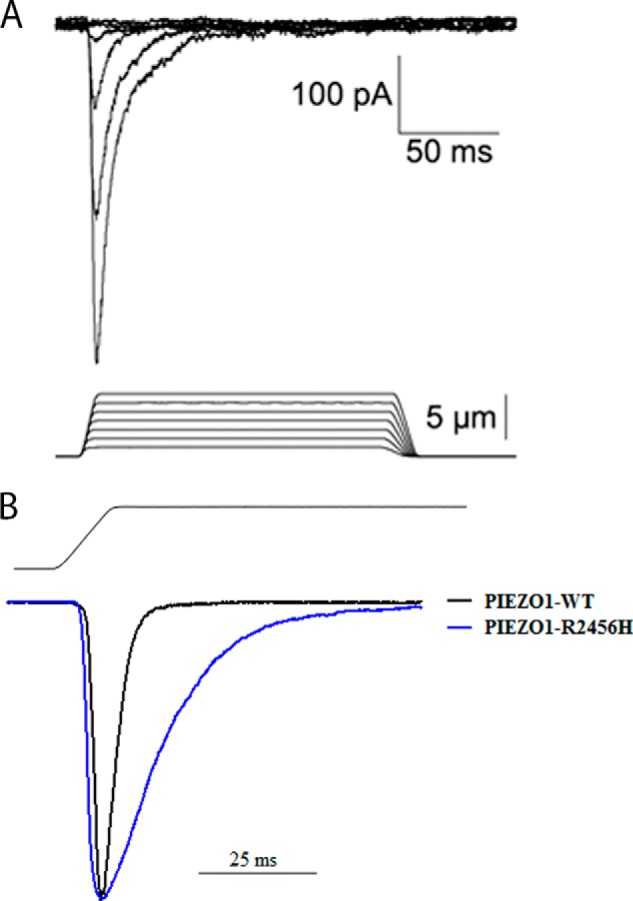
Mechano-activated Piezo currents. A, representative whole-cell currents in HEK293 cells expressing murine Piezo2. Mechanical stimulation was delivered by a glass probe affixed to a piezoelectricity-driven actuator at 800 μm/s velocity in steps of 1.0 μm (4). B, wild type and mutant R2456H PIEZO1. Representative peak-normalized traces of mechanically activated, whole-cell currents recorded from HEK293 cells expressing wild type (black trace) or mutant R2456H (blue trace) PIEZO1 at −80 mV are shown. The stimulus waveform at 11 μm is shown above the current traces. The PIEZO1 R2456H mutation exhibits slower inactivation kinetics.
Following a complete desensitization, Piezo1 cannot be efficiently opened by the application of the same or larger force without first returning the initial stimulus to baseline (11). Complete recovery from desensitization takes hundreds of milliseconds (10, 13). This mechanism is referred to as inactivation, different from the adaptation mechanism, which presupposes efficient reopening of a desensitized channel by repetitive stimulation (14). Whether Piezo2 desensitizes through inactivation or adaptation has not been fully addressed.
Pharmacological Regulation of Piezo1 and Piezo2
Piezo1 and Piezo2 are pharmacologically orphaned, with only a small number of nonspecific inhibitors available. Ruthenium Red (RuR) and gadolinium (Gd3+) reversibly block Piezo1 and Piezo2 current in C2C12 cells (4). RuR blocks murine Piezo1 at IC50 5.4 μm only when applied from the extracellular side, but fails to block the Drosophila Piezo orthologue (3). Another small molecule blocker, FM1-43, inhibits human Piezo2 current at 15 μm (15), but whether the block is reversible is unclear. In HEK293 cells, ∼80% of peak Piezo1 current is reversibly inhibited by both l-enantiomers and d-enantiomers of GsMTx4, a peptide from tarantula venom (16). GsMTx4 is effective against Piezo1 at low micromolar concentrations and inhibits the channel only when applied from the extracellular side (10, 17, 18). GsMTx4 acts as a gating modifier shifting the midpoint of activation to higher pressure values (17). Whether GsMTx4 is effective against Piezo2 is unclear; in DRG neurons, GsMTx4 fails to suppress rapidly desensitizing MA currents (19) thought to be mediated by Piezo2 (4, 20).
None of these inhibitors are Piezo-specific. RuR and Gd3+ are known as efficient blockers of various types of ion channels in native cells and heterologous systems (21–25), whereas FM1-43 effectively inhibits both Piezo2-dependent and Piezo2-independent MA current in neurons from dorsal root ganglia (26). Similarly, GsMTx4 has many off-targets (27), perhaps due to its general mechanism of action via the lipids of the plasma membrane (28).
Molecular Properties of Piezo1
Incorporation of purified Piezo1 into an asymmetric lipid bilayer triggers electrical activity suppressed by the application of 50 μm RuR to the “extracellular” side of the bilayer. In proteoliposomes, Piezo1 conducts K+ and Na+ without preference, but whether purified Piezo1 conducts divalent ions and/or retains mechanosensitivity is unclear (3). Perhaps mechanosensitivity depends on the interaction with a regulator protein, such as stomatin-like protein 3 (STOML3), which interacts with both Piezo1 and Piezo2 and positively regulates channel activity (12), or it could be an intrinsic property of the channel, similar to that reported for the bacterial mechano-gated channels MscS and MscL (29) and the eukaryotic two-pore potassium channels of the TREK group (30, 31). When compared side-by-side, Piezo1 activation requires a similar change in membrane tension as MscL (13), suggesting that lipid tension alone is, in principle, sufficient to gate Piezo1.
Biological Aspects of Piezo1
Role of Piezo1 in Vascular Biology
Piezo1 appears to function in endothelial cell types under both static and shear stress conditions. Germ line homozygous Piezo1 knock-out mice exhibited embryonic lethality at midgestation with disorganized and decreased yolk sac vasculature (32, 33). Embryos demonstrated growth retardation, pericardial effusion, and defects in vascular formation. Mice with endothelium-specific disruption of Piezo1 also exhibited embryonic lethality, with abnormal vessel formation and perturbed endothelial cell organization and alignment (32). Mice haploinsufficient for Piezo1 in endothelial cells were viable, with endothelial abnormalities in mature vessels. In Piezo1-depleted human umbilical vein endothelial cells (HUVECs) under static conditions, there was a total reduction in eNOS protein, decreased vascular endothelial growth factor (VEGF)-evoked phosphorylation of Ser-1177, a critical enhancer of eNOS activity, and suppression of endothelial cell migration, suggesting that Piezo1 drives endothelial cell migration through eNOS in the absence of shear stress (32).
Piezo1 also acts as an endothelial sensor of blood flow, promoting endothelial cell organization and alignment in the direction of flow (32, 33). Shear stress in HUVECs led to a change in subcellular localization of Piezo1, from a broad distribution under static conditions to accumulation at leading apical lamellipodia. Piezo1-depleted HUVECs exhibited defective alignment and elongation of endothelial cells in response to laminar shear stress as well as defective migration of cells toward VEGF. In wild type HUVECs under similar conditions, GsMTx4 disrupted the alignment of cells in the direction of shear stress, whereas both GsMTx4 and RuR blocked cell migration toward VEGF. Application of shear stress to endothelial cells evoked Piezo1-dependent currents with calcium influx followed by activation of calpain-2 activity. Protease activation, via proteolytic cleavage of the actin cytoskeleton and focal adhesion proteins, led to the spatial reorganization and alignment of endothelial cells to the polarity of the applied force (32).
Piezo1 in Erythrocytes
Based on its role in hereditary xerocytosis (HX, see below), numerous roles have been suggested for Piezo1 in the erythrocyte. It has been suggested that Piezo1 plays a previously unrecognized role in erythrocyte volume regulation, with HX erythrocytes gradually becoming dehydrated during repeated cycles of travel through the microcirculation, associated with changes in oxygenation/deoxygenation (34). A potential role in regulating a stretch-activated calcium pathway has also been suggested (35–38). Local deformations in the erythrocyte membrane transiently activate a calcium permeability pathway, leading to increased intracellular calcium, with activation of potassium currents through the calcium-sensitive Gardos channel and activation of secondary anion currents through other channels (39). Circulatory shear stress has been suggested to cause reversible increases in erythrocyte calcium permeability (36, 40). Erythrocyte aging has been associated with alterations in membrane permeability for calcium (41–43). Localized membrane deformation has been associated with increased calcium in the process of apical alignment in the initial steps of malaria invasion (42). Thus Piezo1 is an excellent candidate for the unidentified stretch-induced cation pathway in the erythrocyte that plays critical roles in erythrocyte aging, malaria invasion, and circulatory shear stress.
It has also been suggested that Piezo1 may act as an osmoreceptor in erythrocytes and other cells. Volume sensing, a poorly understand process in vertebrate cells, has been linked to stretch-activated currents induced by changes in cell volume (44). As soon as the Piezo proteins were identified, they were candidates for cellular osmosensors (45). GsMTx4 inhibits both mechanically activated currents and whole-cell regulatory volume decreases in NRK-49 cells, indicating a potential role for Piezo1 in volume homeostasis (46). Knockdown of Piezo1 and its homologues in zebrafish erythroid cells led to development of macrocytic anemia, sphere-shaped circulating erythrocytes with disrupted membranes, and swollen nuclei (47). These results are intriguing, suggesting that Piezo1 not only may participate in volume regulation but also may have a role in maintaining the structural integrity of the red cell. However, because zebrafish erythrocytes do not enucleate, volume homeostatic mechanisms and membrane structure may be different from that in enucleated mammalian erythrocytes. A recent genome-wide association study of red cell indices linked mean corpuscular hemoglobin concentration, a factor influenced by erythrocyte hydration, to a single nucleotide polymorphism (rs10445033) in the PIEZO1 gene locus, explaining 8% of the phenotypic variance of mean corpuscular hemoglobin concentration (48).
Piezo1 in the Genitourinary Tract
Piezo1 is expressed in cells of the genitourinary system, especially in renal tubular epithelial cells and in uroepithelial cells. Similar to endothelial cells, studies in Piezo1-expressing HEK293 cells demonstrated shear stress activation of Piezo1 followed by calcium influx. Knockdown of Piezo1 in basolateral proximal convoluted tubular cells reduced activity of SACs. Interestingly, Polycystin-2 interacts with Piezo1 and inhibits endogenous SACs in these cells, indicating that renal SACs depend on Piezo1 but are critically conditioned by Polycystin-2 (49). These results suggest a role for Piezo1 in detection of intraluminal pressure changes and urine flow sensing. In cultured uroepithelial cells, Piezo1 has a functional role in stretch-evoked calcium influx and ATP release, an effect blocked by GsMTx4, implicating a role for Piezo1 in detecting urothelial extension during bladder distention (18).
Piezo1 in Osteoclastogenesis
Static compressive loading led to Piezo1 up-regulation in human periodontal ligament cells, contributing to mechanical stress-induced osteoclastogenesis (6).
Role of Piezo1 in Cell Migration, Proliferation, and Elongation
Piezo1 was shown to participate in integrin activation, suggesting a non-MA role for Piezo1. Knockdown of Piezo1 in lung epithelial cells decreased cell adherence and promoted cell migration (50). Because it is down-regulated in many small cell lung cancer cell lines, loss of Piezo1 has been suggested to play a role in increased cell migration and distant metastases in lung cancer (50). In contrast, knockdown of Piezo1 in gastric tumor cell lines was associated with decreased cell migration (51). These studies, along with those from endothelial cells, implicate a role for Piezo proteins in cell migration, proliferation, and elongation.
Biological Aspects of Piezo2
Mechanosensation provides a necessary means of communication of an organism with its environment through the largest organ in the body of mammals, the skin. Dysregulation of somatosensory mechanosensitivity leads to peripheral neuropathies, which, in most cases, have no effective treatment. These conditions include diabetic neuropathy, peripheral vascular disease, and post-chemotherapy neuropathies. The cause of somatosensory dysfunction in most neuropathies is poorly understood. In many cases, it appears to involve aberrant sensitivity to mechanical force at the level of cutaneous mechanoreceptors or primary afferent neurons. Mechanical allodynia, associated with several neuropathies, is the disease where normally innocuous stimuli are perceived as excruciatingly painful (52). No specific treatments are available beyond palliative measures. Exemplary of these disorders is trigeminal neuralgia (53), a debilitating condition manifested as severe facial pain provoked by light touch, likely caused by aberrant mechanosensitivity of the trigeminal nerve. Despite decades of research, the molecular mechanism(s) through which we sense mechanical inputs remains poorly defined. Mechanosensitivity is the least understood of our senses at cellular and molecular level (54–56).
Piezo2 participates in the recognition of light touch and noxious stimuli at the level of both primary afferents and somatosensory components of cutaneous mechanoreceptors (see below). However, these processes require further investigation. It appears likely that Piezo2 alone is not sufficient to mediate the multitude of mechano-evoked phenomena observed in neural and somatic cells involved in cutaneous mechanotransduction. For instance, in somatosensory neurons, Piezo2 is responsible only for the rapidly desensitizing MA current (4, 20). In Merkel cells, conditional knockdown of Piezo2 is insufficient to recapitulate the effect of complete Merkel cell ablation (8). Therefore, other mechano-gated ion channels are likely to exist in both neural and somatic components of the mechanotransducing system.
Contribution of Piezo2 to Mechano-activated Current in Primary Afferent Neurons
In the skin, mechanical forces are perceived by peripheral cutaneous end organs, which contain somatic cells in complex with primary afferent nerve endings. Somata of the primary afferents are located in the somatosensory ganglia, either TG, which innervate the head, or DRG, which innervate different parts of the body. Primary afferents are intrinsically mechanosensitive, i.e. they have the ability to convert membrane stretch into depolarizing ionic current in the absence of other tissue components. Direct mechanical stimulation of somata or neurites of dissociated DRG neurons elicits MA current that exponentially desensitizes with a characteristic time constant, τ (21). τ values serve as the basis for neuronal classification as rapidly, intermediately, and slowly desensitizing (4, 12, 19–22, 24, 26, 57–59).
Piezo2 activity has been linked to mechanosensitive neurons in DRG (4, 15, 20, 60, 61) and TG (62, 63) Knockdown studies reveal that Piezo2 is responsible for a majority of the rapidly desensitizing MA current in murine DRG neurons. Piezo2-expressing neurons, however, do not seem to belong to a single functional subtype. Piezo2 is expressed in small-diameter unmyelinated nociceptors expressing TRPV1 or VGLU3 and in large-diameter myelinated neurofilament 200-positive innocuous mechanoreceptors (4, 20), suggesting the involvement of Piezo2 in both painful and innocuous mechanoreception. Intrathecal injection of Piezo2 antisense oligonucleotides into DRG neurons elevated the threshold of sensitivity to light touch (15). Because germ line knock-out of Piezo2 leads to embryonic lethality (60), a better understanding of the contribution of Piezo2-mediated MA current in neurons to physiological touch responses will require behavioral analysis of animals with primary afferents lacking Piezo2.
Role of Piezo2 in Light Touch Sensitivity
Several studies have revealed a key role for Piezo2 in mechanotransduction in Merkel cells in the skin (7–9). These cells are part of the Merkel cell-neurite complexes innervated by a specific subset of primary afferents, the Aβ slowly adapting type I low-threshold mechanoreceptors (SAI-LTMR). These complexes transmit high spatial resolution and selective sensitivity and are thought to participate in both gentle touch and the decoding of specific object features such as form, shape, and texture by the skin. Direct mechanical stimulation of dissociated murine Merkel cells with a glass probe evoked a rapidly desensitizing RuR-sensitive MA current, absent after conditional knock-out of Piezo2 in skin (7, 8). Similarly, recordings from Merkel cells in an ex vivo whisker hair follicle preparation revealed rapidly desensitizing MA current inhibited by Gd3+, RuR, anti-Piezo2 antibody, or Piezo2 shRNA knockdown (64).
Mechanical stimulation can be subdivided into two components: when the probe is in the process of deforming the membrane (the dynamic phase) and when the probe is held in the extended position (the static phase). In response to such a ramp-and-hold type of stimulation, slowly adapting type I low-threshold mechanoreceptors transmit a well defined firing pattern, consisting of a high-frequency response during the dynamic phase followed by a slowly adapting response during the static phase. Conditional knock-out of Piezo2 in Merkel cells only modestly affected firing in the dynamic phase, but profoundly attenuated the response in the static phase, decreasing the number of spikes and increasing inter-spike interval (7, 8). Piezo2 knockdown in hair follicles suppressed firing at both dynamic and static phase (64). The discrepancy between these results could be due to the different origin of Merkel cells (touch domes in Refs. 7 and 8 versus whisker hair follicles in Ref. 64). However, it appears that Piezo2 contributes to the mechanosensitivity of both neural and somatic components of the cutaneous Merkel cell organs and that the deletion of Piezo2 in Merkel cells leads to the inability of the sensory neurons to transmit afferent response in the typical slowly adapting manner. At the behavioral level, deletion of Piezo2 in Merkel cells suppresses sensitivity to gentle (<1.5 g) touch by a Von Frey filament, but does not affect sensitivity to heavier stimuli (7). Further experiments, using other types of light touch-oriented behavioral paradigms (65), are required to clarify the contribution of Piezo2 to light touch detection.
A recent study has revealed an unprecedented up-regulation of Piezo2-expressing large-diameter neurons in TG of acutely mechanosensitive species-tactile foraging ducks (63). The skin of the duck bill contains numerous mechanoreceptive end organs that are innervated by primary afferents from TG. The expansion of Piezo2-positive mechanoreceptors in duck TG, where these cells account for up to 85% of the neuronal population, further supports the notion of a key role for this protein in light touch perception (63).
Role of Piezo2 in Noxious Mechanotransduction
Co-localization of Piezo2 with small-diameter unmyelinated nociceptors in DRG suggested a role in noxious mechanosensitivity. Piezo2-mediated MA current amplitude was significantly potentiated in cells treated with the proalgesic peptide bradykinin (BK) and co-expressing the bradykinin receptor β2 (60). In addition to the effect on MA current amplitude, BK treatment prolonged desensitization, thus increasing the amount of depolarizing charge entering the cell. This effect is evident in Piezo2-expressing HEK293 cells and cultured DRG neurons exhibiting the rapidly desensitizing MA current. Physiologically, both the increase in amplitude and the prolongation of desensitization facilitate depolarization and increase the chance of action potential generation, signaling pain. The intracellular pathways connecting BK signaling and Piezo2 include G-protein-mediated signaling cascades, which converge on protein kinases C and A (PKA). Piezo2 has over 30 potential phosphorylation sites, but their functional importance awaits clarification (60).
Phosphorylation is probably not the only mechanism of Piezo2 activity regulation. For example, cAMP, an intracellular messenger linked to development of mechanical allodynia and hyperalgesia, potentiates Piezo2 activity in HEK293 cells and DRG neurons and prolongs desensitization of mouse (but not human) Piezo2 (15, 60). In addition to a direct potentiation of PKA, cAMP triggers Epac1-dependent signaling, which has numerous downstream effector targets, including phospholipases, GTPases, and ion channels (66). The involvement of the Epac1 in a Piezo2 sensitization cascade is supported by the finding that ectopic expression of Epac1 potentiates human Piezo2 activation by cAMP (15). Interestingly, upon the formation of the whole-cell recording configuration, the amplitude of the rapidly desensitizing MA current in DRG neurons and in Piezo2-expressing HEK293 cells gradually increases. This run-up process can be reversed by the omission of GTP from the intracellular solution (61), which further strengthens functional connections between Piezo2 and G-protein signaling cascades.
Intrathecal injection of Piezo2 antisense oligonucleotides significantly attenuates Epac1-mediated allodynia in two models of chronic and neuropathic pain (15). Knockdown of Piezo2 in whisker hair follicles pre-sensitized with capsaicin injection suppresses pain responses (64). In flies, full genomic or sensory neuron-specific ablation of the Piezo orthologue leads to a severe and specific defect in noxious mechanotransduction (67). Together, these data strongly link Piezo2 function with noxious mechanosensation at physiological level. Descriptive studies identified Piezo2 in a neurochemically distinct subpopulation of corneal afferent neurons that are not polymodal nociceptors or cold-sensing neurons, suggesting that this subpopulation of pure mechano-nociceptors is an excellent candidate for transducers of noxious mechanical stimuli in the cornea (62).
Piezo Proteins and Human Disease
PIEZO1 and Hereditary Xerocytosis
Combining linkage analyses, SNP typing, and exome sequencing, Zarychanski et al. (68) identified PIEZO1 as the gene for HX. HX is a dominant disorder of erythrocyte dehydration with mild to moderate compensated hemolytic anemia (69). HX erythrocytes exhibit decreased total cation and potassium content not accompanied by a proportional net gain of sodium and water. Additional HX-associated PIEZO1 missense mutations have subsequently been described, primarily in residues located in the highly conserved COOH terminus (70, 71). Functional studies demonstrate a partial gain-of-function phenotype associated with many of the mutants due to generation of MA currents that inactivate more slowly than wild type (τ increased 2–3 times, Fig. 4B), whereas R2456K or a truncation at position 2218 renders the channel non-desensitizing (10, 13, 71). This indicates that there is likely increased cation permeability that leads to HX erythrocyte dehydration. Because the channel may homo-tetramerize, this delayed inactivation may be due to a dominant negative effect. In some PIEZO1 HX-associated variants, the mechanism of cellular dehydration is unknown.
PIEZO2 and Neuromuscular Abnormalities
Coste et al. (72) identified two mutations in PIEZO2 in patients with distal arthrogryposis (DA) type 5. The arthrogryposis syndromes are a collection of disorders characterized by multiple congenital joint contractures. There is wide phenotypic variability, and numerous etiologies, inherited and acquired, have been implicated. DA5 is a dominant disorder characterized by skeletal muscle contractures, restrictive lung disease, and ophthalmoplegia. Both mutations were associated with faster recovery of PIEZO2-dependent MA currents from inactivation, whereas one was also associated with slowed inactivation (72).
McMillin et al. (73) identified 13 PIEZO2 mutations in 35 families affected by Gordon syndrome (GS), DA5, and Marden-Walker syndrome (MWS). Gordon syndrome is an uncommon dominant disorder associated with multiple contractures of the hands and feet and cleft palate. MWS is characterized by joint contractures, cleft palate, blepharophimosis, developmental delay and hindbrain malformations. All three syndromes are phenotypically similar, distinguished by a few specific characteristics. This study revealed that there is significant phenotypic and genotypic overlap between these disorders. Ten GS kindreds had PIEZO2 mutations, with nine kindreds sharing an identical mutation, R2686H. PIEZO2 mutations were found in 24 of 29 DA5 kindreds, including two kindreds with R2686H and 10 with pGlu2727del, the mutation previously identified by Coste et al. (72) One MWS kindred had a mutation in the same amino acid as GS and DA5 kindreds, R2686, with replacement of Arg by Cys instead of His. Although no functional studies were performed, the authors suggest that PIEZO2 mutations may disrupt a neuromuscular pathway that influences skeletal muscle development. Similar to HX-associated PIEZO1 mutations, most PIEZO2 mutations were located in the highly conserved COOH terminus, highlighting this region of Piezo proteins as a key element controlling channel desensitization.
All Piezo1 and Piezo2 mutations characterized so far are gain-of-function, strongly suggesting that the underlying cause of Piezo-linked disorders is excessive cation influx in response to mechanical stimulation. The phenotypic variability suggests different functional roles for the COOH terminus of Piezo proteins in varying cell types.
Future Directions
Since their discovery as mechanosensory transduction molecules, studies of Piezo proteins have revealed many unexpected roles beyond mechanotransduction. It will be exciting to unravel the numerous cell-, development-, and differentiation stage-specific and organism-specific roles of these proteins.
Supplementary Material
Acknowledgments
We thank Edyta Glogowska, Vincent Schulz, Yelena Maksimova, and Eve Schneider for assistance and Ardem Patapoutian for the mouse Piezo2 clone.
This work was supported, in whole or in part, by National Institutes of Health Grant RO1DK104046 (to P. G. G.). This work was also supported by American Heart Association Scientist Development Grant 14SDG17880015 (to S. N. B.), the Alfred P. Sloan Foundation (to E. O. G.), and the Doris Duke Foundation (to P. G. G.).

This article contains supplemental Fig. 1.
- SAC
- stretch-activated ion channel
- MA
- mechanically activated
- TG
- trigeminal ganglia
- DRG
- dorsal root ganglia
- RuR
- ruthenium red
- GsMTx4
- Grammostola spatulata mechanotoxin 4
- HX
- hereditary xerocytosis
- DA
- distal arthrogryposis
- GS
- Gordon syndrome
- MWS
- Marden-Walker syndrome
- HUVEC
- human umbilical vein endothelial cell
- eNOS
- endothelial nitric-oxide synthase
- BK
- bradykinin.
REFERENCES
- 1. Nilius B. (2010) Pressing and squeezing with Piezos. EMBO Rep. 11, 902–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsunozaki M., Bautista D. M. (2009) Mammalian somatosensory mechanotransduction. Curr. Opin. Neurobiol. 19, 362–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coste B., Xiao B., Santos J. S., Syeda R., Grandl J., Spencer K. S., Kim S. E., Schmidt M., Mathur J., Dubin A. E., Montal M., Patapoutian A. (2012) Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coste B., Mathur J., Schmidt M., Earley T. J., Ranade S., Petrus M. J., Dubin A. E., Patapoutian A. (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prole D. L., Taylor C. W. (2013) Identification and analysis of putative homologues of mechanosensitive channels in pathogenic protozoa. PLoS One 8, e66068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin Y., Li J., Wang Y., Ye R., Feng X., Jing Z., Zhao Z. (2014) Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 10.2319/123113-955.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woo S. H., Ranade S., Weyer A. D., Dubin A. E., Baba Y., Qiu Z., Petrus M., Miyamoto T., Reddy K., Lumpkin E. A., Stucky C. L., Patapoutian A. (2014) Piezo2 is required for Merkel-cell mechanotransduction. Nature 509, 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maksimovic S., Nakatani M., Baba Y., Nelson A. M., Marshall K. L., Wellnitz S. A., Firozi P., Woo S. H., Ranade S., Patapoutian A., Lumpkin E. A. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikeda R., Gu J. G. (2014) Piezo2 channel conductance and localization domains in Merkel cells of rat whisker hair follicles. Neurosci Lett. 10.1016/j.neulet.2014.05.055 [DOI] [PubMed] [Google Scholar]
- 10. Bae C., Gottlieb P. A., Sachs F. (2013) Human PIEZO1: removing inactivation. Biophys J. 105, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gottlieb P. A., Bae C., Sachs F. (2012) Gating the mechanical channel Piezo1: a comparison between whole-cell and patch recording. Channels (Austin) 6, 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Poole K., Herget R., Lapatsina L., Ngo H. D., Lewin G. R. (2014) Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nat. Commun. 5, 3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bae C., Gnanasambandam R., Nicolai C., Sachs F., Gottlieb P. A. (2013) Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1. Proc. Natl. Acad. Sci. U.S.A. 110, E1162–E1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crawford A. C., Evans M. G., Fettiplace R. (1989) Activation and adaptation of transducer currents in turtle hair cells. J. Physiol. 419, 405–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eijkelkamp N., Linley J. E., Torres J. M., Bee L., Dickenson A. H., Gringhuis M., Minett M. S., Hong G. S., Lee E., Oh U., Ishikawa Y., Zwartkuis F. J., Cox J. J., Wood J. N. (2013) A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nat. Commun. 4, 1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suchyna T. M., Johnson J. H., Hamer K., Leykam J. F., Gage D. A., Clemo H. F., Baumgarten C. M., Sachs F. (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J. Gen. Physiol. 115, 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bae C., Sachs F., Gottlieb P. A. (2011) The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4. Biochemistry 50, 6295–6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyamoto T., Mochizuki T., Nakagomi H., Kira S., Watanabe M., Takayama Y., Suzuki Y., Koizumi S., Takeda M., Tominaga M. (2014) Functional role for Piezo1 in stretch-evoked Ca2+ influx and ATP Release in urothelial cell cultures. J. Biol. Chem. 289, 16565–16575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drew L. J., Rugiero F., Cesare P., Gale J. E., Abrahamsen B., Bowden S., Heinzmann S., Robinson M., Brust A., Colless B., Lewis R. J., Wood J. N. (2007) High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PloS one 2, e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lou S., Duan B., Vong L., Lowell B. B., Ma Q. (2013) Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 33, 870–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McCarter G. C., Reichling D. B., Levine J. D. (1999) Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci. Lett. 273, 179–182 [DOI] [PubMed] [Google Scholar]
- 22. Drew L. J., Wood J. N., Cesare P. (2002) Distinct mechanosensitive properties of capsaicin-sensitive and -insensitive sensory neurons. J. Neurosci. 22, RC228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li G. R., Baumgarten C. M. (2001) Modulation of cardiac Na+ current by gadolinium, a blocker of stretch-induced arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 280, H272–H279 [DOI] [PubMed] [Google Scholar]
- 24. Drew L. J., Rohrer D. K., Price M. P., Blaver K. E., Cockayne D. A., Cesare P., Wood J. N. (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. 556, 691–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inoue R., Jian Z., Kawarabayashi Y. (2009) Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol. Ther. 123, 371–385 [DOI] [PubMed] [Google Scholar]
- 26. Drew L. J., Wood J. N. (2007) FM1–43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Mol. Pain 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Redaelli E., Cassulini R. R., Silva D. F., Clement H., Schiavon E., Zamudio F. Z., Odell G., Arcangeli A., Clare J. J., Alagón A., de la Vega R. C., Possani L. D., Wanke E. (2010) Target promiscuity and heterogeneous effects of tarantula venom peptides affecting Na+ and K+ ion channels. J. Biol. Chem. 285, 4130–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suchyna T. M., Tape S. E., Koeppe R. E., 2nd, Andersen O. S., Sachs F., Gottlieb P. A. (2004) Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430, 235–240 [DOI] [PubMed] [Google Scholar]
- 29. Sukharev S., Sachs F. (2012) Molecular force transduction by ion channels: diversity and unifying principles. J. Cell Sci. 125, 3075–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Berrier C., Pozza A., de Lacroix de Lavalette A., Chardonnet S., Mesneau A., Jaxel C., le Maire M., Ghazi A. (2013) The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. J. Biol. Chem. 288, 27307–27314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brohawn S. G., Su Z., MacKinnon R. (2014) Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc. Natl. Acad. Sci. U.S.A. 111, 3614–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J., Hou B., Tumova S., Muraki K., Bruns A., Ludlow M. J., Sedo A., Hyman A. J., McKeown L., Young R. S., Yuldasheva N. Y., Majeed Y., Wilson L. A., Rode B., Bailey M. A., Kim H. R., Fu Z., Carter D. A., Bilton J., Imrie H., Ajuh P., Dear T. N., Cubbon R. M., Kearney M. T., Prasad R. K., Evans P. C., Ainscough J. F., Beech D. J. (2014) Piezo1 integration of vascular architecture with physiological force. Nature 10.1038/nature13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ranade S. S., Qiu Z., Woo S. H., Hur S. S., Murthy S. E., Cahalan S. M., Xu J., Mathur J., Bandell M., Coste B., Li Y. S., Chien S., Patapoutian A. (2014) Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10347–10352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohandas N. (2013) To shrink or not to shrink. Blood 121, 3783–3784 [DOI] [PubMed] [Google Scholar]
- 35. Brain M. C., Pihl C., Robertson L., Brown C. B. (2004) Evidence for a mechanosensitive calcium influx into red cells. Blood Cells Mol. Dis. 32, 349–352 [DOI] [PubMed] [Google Scholar]
- 36. Johnson R. M. (1994) Membrane stress increases cation permeability in red cells. Biophys. J. 67, 1876–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma Y. L., Rees D. C., Gibson J. S., Ellory J. C. (2012) The conductance of red blood cells from sickle cell patients: ion selectivity and inhibitors. J. Physiol. 590, 2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vandorpe D. H., Xu C., Shmukler B. E., Otterbein L. E., Trudel M., Sachs F., Gottlieb P. A., Brugnara C., Alper S. L. (2010) Hypoxia activates a Ca2+-permeable cation conductance sensitive to carbon monoxide and to GsMTx-4 in human and mouse sickle erythrocytes. PLoS One 5, e8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dyrda A., Cytlak U., Ciuraszkiewicz A., Lipinska A., Cueff A., Bouyer G., Egée S., Bennekou P., Lew V. L., Thomas S. L. (2010) Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells. PLoS One 5, e9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larsen F. L., Katz S., Roufogalis B. D., Brooks D. E. (1981) Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature 294, 667–668 [DOI] [PubMed] [Google Scholar]
- 41. Lew V. L., Daw N., Perdomo D., Etzion Z., Bookchin R. M., Tiffert T. (2003) Distribution of plasma membrane Ca2+ pump activity in normal human red blood cells. Blood 102, 4206–4213 [DOI] [PubMed] [Google Scholar]
- 42. Lew V. L., Tiffert T. (2007) Is invasion efficiency in malaria controlled by pre-invasion events? Trends Parasitol. 23, 481–484 [DOI] [PubMed] [Google Scholar]
- 43. Romero P. J., Romero E. A. (1997) Differences in Ca2+ pumping activity between sub-populations of human red cells. Cell Calcium 21, 353–358 [DOI] [PubMed] [Google Scholar]
- 44. Delmas P., Hao J., Rodat-Despoix L. (2011) Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat. Rev. Neurosci. 12, 139–153 [DOI] [PubMed] [Google Scholar]
- 45. Pedersen S. F., Kapus A., Hoffmann E. K. (2011) Osmosensory mechanisms in cellular and systemic volume regulation. J. Am. Soc. Nephrol. 22, 1587–1597 [DOI] [PubMed] [Google Scholar]
- 46. Hua S. Z., Gottlieb P. A., Heo J., Sachs F. (2010) A mechanosensitive ion channel regulating cell volume. Am. J. Physiol. Cell Physiol. 298, C1424–C1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faucherre A., Kissa K., Nargeot J., Mangoni M. E., Jopling C. (2014) Piezo1 plays a role in erythrocyte volume homeostasis. Haematologica 99, 70–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van der Harst P., Zhang W., Mateo Leach I., Rendon A., Verweij N., Sehmi J., Paul D. S., Elling U., Allayee H., Li X., Radhakrishnan A., Tan S. T., Voss K., Weichenberger C. X., Albers C. A., Al-Hussani A., Asselbergs F. W., Ciullo M., Danjou F., Dina C., Esko T., Evans D. M., Franke L., Gögele M., Hartiala J., Hersch M., Holm H., Hottenga J. J., Kanoni S., Kleber M. E., Lagou V., Langenberg C., Lopez L. M., Lyytikäinen L. P., Melander O., Murgia F., Nolte I. M., O'Reilly P. F., Padmanabhan S., Parsa A., Pirastu N., Porcu E., Portas L., Prokopenko I., Ried J. S., Shin S. Y., Tang C. S., Teumer A., Traglia M., Ulivi S., Westra H. J., Yang J., Zhao J. H., Anni F., Abdellaoui A., Attwood A., Balkau B., Bandinelli S., Bastardot F., Benyamin B., Boehm B. O., Cookson W. O., Das D., de Bakker P. I., de Boer R. A., de Geus E. J., de Moor M. H., Dimitriou M., Domingues F. S., Döring A., Engström G., Eyjolfsson G. I., Ferrucci L., Fischer K., Galanello R., Garner S. F., Genser B., Gibson Q. D., Girotto G., Gudbjartsson D. F., Harris S. E., Hartikainen A. L., Hastie C. E., Hedblad B., Illig T., Jolley J., Kähönen M., Kema I. P., Kemp J. P., Liang L., Lloyd-Jones H., Loos R. J., Meacham S., Medland S. E., Meisinger C., Memari Y., Mihailov E., Miller K., Moffatt M. F., Nauck M., Novatchkova M., Nutile T., Olafsson I., Onundarson P. T., Parracciani D., Penninx B. W., Perseu L., Piga A., Pistis G., Pouta A., Puc U., Raitakari O., Ring S. M., Robino A., Ruggiero D., Ruokonen A., Saint-Pierre A., Sala C., Salumets A., Sambrook J., Schepers H., Schmidt C. O., Silljé H. H., Sladek R., Smit J. H., Starr J. M., Stephens J., Sulem P., Tanaka T., Thorsteinsdottir U., Tragante V., van Gilst W. H., van Pelt L. J., van Veldhuisen D. J., Völker U., Whitfield J. B., Willemsen G., Winkelmann B. R., Wirnsberger G., Algra A., Cucca F., d'Adamo A. P., Danesh J., Deary I. J., Dominiczak A. F., Elliott P., Fortina P., Froguel P., Gasparini P., Greinacher A., Hazen S. L., Jarvelin M. R., Khaw K. T., Lehtimäki T., Maerz W., Martin N. G., Metspalu A., Mitchell B. D., Montgomery G. W., Moore C., Navis G., Pirastu M., Pramstaller P. P., Ramirez-Solis R., Schadt E., Scott J., Shuldiner A. R., Smith G. D., Smith J. G., Snieder H., Sorice R., Spector T. D., Stefansson K., Stumvoll M., Tang W. H., Toniolo D., Tönjes A., Visscher P. M., Vollenweider P., Wareham N. J., Wolffenbuttel B. H., Boomsma D. I., Beckmann J. S., Dedoussis G. V., Deloukas P., Ferreira M. A., Sanna S., Uda M., Hicks A. A., Penninger J. M., Gieger C., Kooner J. S., Ouwehand W. H., Soranzo N., Chambers J. C. (2012) Seventy-five genetic loci influencing the human red blood cell. Nature 492, 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peyronnet R., Martins J. R., Duprat F., Demolombe S., Arhatte M., Jodar M., Tauc M., Duranton C., Paulais M., Teulon J., Honoré E., Patel A. (2013) Piezo1-dependent stretch-activated channels are inhibited by Polycystin-2 in renal tubular epithelial cells. EMBO Rep. 14, 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McHugh B. J., Murdoch A., Haslett C., Sethi T. (2012) Loss of the integrin-activating transmembrane protein Fam38A (Piezo1) promotes a switch to a reduced integrin-dependent mode of cell migration. PLoS One 7, e40346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang X. N., Lu Y. P., Liu J. J., Huang J. K., Liu Y. P., Xiao C. X., Jazag A., Ren J. L., Guleng B. (2014) Piezo1 is as a novel Trefoil factor family 1 binding protein that promotes gastric cancer cell mobility in vitro. Dig. Dis. Sci. 59, 1428–1435 [DOI] [PubMed] [Google Scholar]
- 52. Lolignier S., Eijkelkamp N., Wood J. N. (2014) Mechanical allodynia. Pflugers Arch. 10.1007/s00424-014-1532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zakrzewska J. M., Linskey M. E. (2014) Trigeminal neuralgia. Brit. Med. J. 348, g474. [DOI] [PubMed] [Google Scholar]
- 54. Abraira V. E., Ginty D. D. (2013) The sensory neurons of touch. Neuron 79, 618–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delmas P., Coste B. (2013) Mechano-gated ion channels in sensory systems. Cell 155, 278–284 [DOI] [PubMed] [Google Scholar]
- 56. Owens D. M., Lumpkin E. A. (2014) Diversification and specialization of touch receptors in skin. Cold Spring Harb. Perspect. Med. 4, a013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coste B., Crest M., Delmas P. (2007) Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J. Gen Physiol. 129, 57–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu J., Lewin G. R. (2006) Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J. Physiol. 577, 815–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCarter G. C., Levine J. D. (2006) Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Mol. pain 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dubin A. E., Schmidt M., Mathur J., Petrus M. J., Xiao B., Coste B., Patapoutian A. (2012) Inflammatory signals enhance Piezo2-mediated mechanosensitive currents. Cell Rep. 2, 511–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jia Z., Ikeda R., Ling J., Gu J. G. (2013) GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Mol. Brain 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bron R., Wood R. J., Brock J. A., Ivanusic J. J. (2014) Piezo2 expression in corneal afferent neurons. J. Comp. Neurol. 522, 2967–2979 [DOI] [PubMed] [Google Scholar]
- 63. Schneider E. R., Mastrotto M., Laursen W. J., Schulz V. P., Goodman J. B., Funk O. H., Gallagher P. G., Gracheva E. O., Bagriantsev S. N. (2014) Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc. Natl. Acad. Sci. U.S.A. 111, 14941–14946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ikeda R., Cha M., Ling J., Jia Z., Coyle D., Gu J. G. (2014) Merkel cells transduce and encode tactile stimuli to drive Aβ-afferent impulses. Cell 157, 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maricich S. M., Morrison K. M., Mathes E. L., Brewer B. M. (2012) Rodents rely on Merkel cells for texture discrimination tasks. J. Neurosci. 32, 3296–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laurent A. C., Breckler M., Berthouze M., Lezoualc'h F. (2012) Role of Epac in brain and heart. Biochem. Soc. Trans. 40, 51–57 [DOI] [PubMed] [Google Scholar]
- 67. Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A. (2012) The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zarychanski R., Schulz V. P., Houston B. L., Maksimova Y., Houston D. S., Smith B., Rinehart J., Gallagher P. G. (2012) Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120, 1908–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gallagher P. G. (2013) Disorders of red cell volume regulation. Curr. Opin. Hematol. 20, 201–207 [DOI] [PubMed] [Google Scholar]
- 70. Andolfo I., Alper S. L., De Franceschi L., Auriemma C., Russo R., De Falco L., Vallefuoco F., Esposito M. R., Vandorpe D. H., Shmukler B. E., Narayan R., Montanaro D., D'Armiento M., Vetro A., Limongelli I., Zuffardi O., Glader B. E., Schrier S. L., Brugnara C., Stewart G. W., Delaunay J., Iolascon A. (2013) Multiple clinical forms of dehydrated hereditary stomatocytosis arise from mutations in PIEZO1. Blood 121, 3925–3935 [DOI] [PubMed] [Google Scholar]
- 71. Albuisson J., Murthy S. E., Bandell M., Coste B., Louis-Dit-Picard H., Mathur J., Fénéant-Thibault M., Tertian G., de Jaureguiberry J. P., Syfuss P. Y., Cahalan S., Garçon L., Toutain F., Simon Rohrlich P., Delaunay J., Picard V., Jeunemaitre X., Patapoutian A. (2013) Dehydrated hereditary stomatocytosis linked to gain-of-function mutations in mechanically activated PIEZO1 ion channels. Nat. Commun. 4, 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coste B., Houge G., Murray M. F., Stitziel N., Bandell M., Giovanni M. A., Philippakis A., Hoischen A., Riemer G., Steen U., Steen V. M., Mathur J., Cox J., Lebo M., Rehm H., Weiss S. T., Wood J. N., Maas R. L., Sunyaev S. R., Patapoutian A. (2013) Gain-of-function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc. Natl. Acad. Sci. U.S.A. 110, 4667–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McMillin M. J., Beck A. E., Chong J. X., Shively K. M., Buckingham K. J., Gildersleeve H. I., Aracena M. I., Aylsworth A. S., Bitoun P., Carey J. C., Clericuzio C. L., Crow Y. J., Curry C. J., Devriendt K., Everman D. B., Fryer A., Gibson K., Giovannucci Uzielli M. L., Graham J. M., Jr., Hall J. G., Hecht J. T., Heidenreich R. A., Hurst J. A., Irani S., Krapels I. P., Leroy J. G., Mowat D., Plant G. T., Robertson S. P., Schorry E. K., Scott R. H., Seaver L. H., Sherr E., Splitt M., Stewart H., Stumpel C., Temel S. G., Weaver D. D., Whiteford M., Williams M. S., Tabor H. K., Smith J. D., Shendure J., Nickerson D. A., University of Washington Center for Mendelian Genomics, and Bamshad M. J. (2014) Mutations in PIEZO2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. Am. J. Hum. Genet. 94, 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Eisenberg D., Lüthy R., Bowie J. U. (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Methods Enzymol. 277, 396–404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.