Background: The stoichiometry of Bax oligomers on mitochondria and the process of Bax pore formation remain unclear.
Results: Bax oligomerization occurs only if tBid is present, and its membrane insertion highly depends on cardiolipin; Bax tends to form tetramer on membrane.
Conclusion: Bax pore formation involves two independent steps: Bax oligomerization and membrane insertion.
Significance: This study may help elucidate Bax pore formation.
Keywords: Apoptosis, B-cell Lymphoma 2 (Bcl-2) Family, Bax, Lipid Bilayer, Single Molecule Biophysics
Abstract
Bax is a pro-apoptotic Bcl-2 family protein. The activated Bax translocates to mitochondria, where it forms pore and permeabilizes the mitochondrial outer membrane. This process requires the BH3-only activator protein (i.e. tBid) and can be inhibited by anti-apoptotic Bcl-2 family proteins such as Bcl-xL. Here by using single molecule fluorescence techniques, we studied the integration and oligomerization of Bax in lipid bilayers. Our study revealed that Bax can bind to lipid membrane spontaneously in the absence of tBid. The Bax pore formation undergoes at least two steps: pre-pore formation and membrane insertion. The activated Bax triggered by tBid or BH3 domain peptide integrates on bilayers and tends to form tetramers, which are termed as pre-pore. Subsequent insertion of the pre-pore into membrane is highly dependent on the composition of cardiolipin in lipid bilayers. Bcl-xL can translocate Bax from membrane to solution and inhibit the pore formation. The study of Bax integration and oligomerization at the single molecule level provides new evidences that may help elucidate the pore formation of Bax and its regulatory mechanism in apoptosis.
Introduction
Bcl-2 family proteins are the critical regulators of apoptosis in multicellular organisms (1). Members of Bcl-2 family can be classified into anti-apoptotic and pro-apoptotic proteins (2, 3). Anti-apoptotic proteins, such as Bcl-2, Bcl-xL, and Bcl-w, contain four BH domains (BH1–BH4). They prevent cell death by inhibiting the pro-apoptotic proteins, Bax and Bak. Bax and Bak are structurally similar to Bcl-xL. However, Bax and Bak can form pores on the mitochondrial outer membrane (MOM)2 and induce its permeabilization (4). The functions of the anti-apoptotic and pro-apoptotic proteins are modulated by the BH3-only Bcl-2 family proteins (e.g. tBid, Bad, Bim, Noxa, etc.).
In a healthy cell, Bax primarily exists in cytosol as an inactive monomer and travels back and forth between cytosol and MOM (5). The process is supposed to be accompanied by a reversible conformational change of Bax (6). Once the cell is stressed, Bax dramatically translocates to the MOM and oligomerizes to form pores, which initiates the mitochondrial outer membrane permeabilization (MOMP) (7–9). Notably, to form pores in MOM, Bax first needs to be recruited and activated by BH3-only proteins such as tBid (10). Further study revealed that Bax binds to tBid only in the presence of lipid membrane (11). In addition, the mitochondria-specific cardiolipin was shown to play an important role in Bax pore formation. 7% cardiolipin in membrane was sufficient for the Bax-induced membrane permeabilization (12, 13). Kuwana et al. (14) demonstrated that Bax cooperated with tBid and cardiolipin to form a supramolecular opening in MOM and lead to its permeabilization. It was suggested that membrane integration, oligomerization, and membrane insertion are the essential steps in Bax pore formation, but the order was unclear (15). However, recent cryo-EM studies argued that Bax monomer could insert into membrane in the presence of Bid BH3 domain peptide, and lead to the membrane distortion (16). This indicated that membrane-inserted Bax monomer may be the pore-forming unit and may control the kinetics of MOMP. Meanwhile, the mitochondrial Bax could be constantly retrotranslocated to cytosol by Bcl-xL (15), which shifts Bax from the activated form to the cytosolic inactive form and prevents cells from undergoing Bax-induced apoptosis.
Although much effort has been made to unravel the mechanism of Bax pore formation, there are still many unsolved issues such as the oligomeric state of Bax on membrane. Here by using single molecule fluorescence techniques, we studied the integration and oligomerization of Bax on lipid bilayers, as well as the roles of tBid, cardiolipin, and Bcl-xL in Bax pore formation. We found that tBid and cardiolipin are not required for the membrane targeting of Bax, but Bax pore formation is highly dependent on them. After activation by tBid, Bax tends to form tetramer in membrane. The oligomerization of Bax takes place before the complex inserts into membrane. Bcl-xL may translocate Bax from membrane to solution and inhibit pore formation.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Recombinant Bax (S16C, C62S, C126S) was cloned into NdeI/SapI of pTYB1 vector (New England Biolabs) and expressed in Escherichia coli BL21 (DE3). A single colony was added to LB medium with 100 μg ml−1 ampicillin and cultured at 37 °C until an optical density of 0.6 at 600 nm was reached. Cells were induced with 400 μm isopropyl-1-thio-β-d-galactopyranoside for 3 h at 30 °C. The harvested cells were lysed by sonication on ice in lysis buffer (50 mm Tris, pH 8.0, 500 mm NaCl) with cOmplete protease inhibitor cocktail tablets (Roche Applied Science, catalogue number 04693132001). The recombinant protein was isolated from the supernatant by chitin affinity chromatography according to the protocol from the vendor (New England Biolabs, catalogue number S6651L). Then the protein was purified by ion-exchange chromatography (Mono-Q column, GE Healthcare).
Recombinant full-length human Bcl-xL and Bid cloned into pTYB1 were expressed in E. coli BL21 (DE3), respectively. The proteins were purified by chitin affinity chromatography as above followed by gel filtration (Superdex 75, GE Healthcare). The purified Bcl-xL and Bid were stored in the buffer (20 mm Hepes, pH 7.5, 20% glycerol) at −80 °C for future use.
Dye Labeling
Bax mutant was labeled with cyanine-3-maleimide (Cy3) (GE Healthcare, catalogue number PA13130) in 10-fold molar excess overnight at 4 °C. Unreacted dye was removed by dialysis. The unlabeled and labeled proteins were separated by a Mono-Q column. The labeled protein was dialyzed to the buffer (20 mm Hepes, pH 7.5, 20% glycerol) and stored at −80 °C for future use. The labeling ratio was verified before experiments by dividing the fluorophore concentration by the protein concentration calculated from the absorption at 532 nm (ϵ = 150,000 m−1 cm−1) and 280 nm (ϵ = 21,168 m−1 cm−1), respectively.
Cleaning and Assembly of Sample Chamber
The glass coverslips (Fischer Scientific, catalogue number: 12-548-5M) were rigorously washed by sonication for 30 min in Alconox (Fisher Scientific) and treated by Piranha solution (3:1 mixture of sulfuric acid and 30% hydrogen peroxide) for 1 h at 90 °C. Then the cleaned coverslips were rinsed by sonication in H2O (Milli-Q, 18.2 milliohms). The hydrophilic treated coverslips were stored in H2O and freeze-dried before use. The sample chamber was assembled according to the method described by Rahul Roy et al. (17).
Preparation of Lipid Bilayers
1,2-Diphytanoyl-sn-glycero-3-phosphocholine (DPPC, Avanti Polar Lipids, catalogue number 850355) and cardiolipin (Sigma, catalogue number C0563) were dissolved in chloroform at 10 mg/ml separately. The solvent in the samples of DPPC and cardiolipin at a 93:7 mass ratio or DPPC only was evaporated using a steady nitrogen stream. Subsequently, the lipids were dissolved in PBS and agitated at a final concentration of 10 mg/ml. The lipid suspension was subjected to 10 freeze-thaw cycles. 1 μl of the small unilamellar vesicles was added to 400 μl of PBS and then injected into a clean sample chamber. After a 1-h incubation at room temperature, the small unilamellar vesicles formed supported bilayers on the coverslip. The excess small unilamellar vesicles were washed off with PBS (18).
Sample Preparation
The Cy3-labeled Bax with or without tBid or Bid in assay buffer (PBS, 0.1 mg/ml BSA) was injected into the flow chamber. After standing for 1 h at room temperature, unbound proteins were washed off with PBS. Bax bound to the bilayers was visualized in image buffer (PBS, 2 mm Trolox, 165 units/ml glucose oxidase, 2,170 units/ml catalase, 0.4% β-d-glucose) under total internal reflection fluorescence microscopy (TIRFM). To assess the membrane insertion of Bax, lipid bilayers were treated with alkaline buffer (200 mm Na2CO3, pH 11.5, 2% glycerol) for 30 min. Bax associated at the surface of the lipid bilayers was washed off by the alkaline buffer. The remaining membrane-inserted Bax was visualized in image buffer under TIRFM. To study how Bcl-xL affects the association of Bax on lipid bilayers, different concentrations of Bcl-xL in assay buffer were added into the chamber and incubated for 1 h. After incubation with Bcl-xL, the remaining Bax on the bilayers in image buffer was visualized under TIRFM.
Single Molecule Fluorescence Measurement
Total internal reflection fluorescence (TIRF) experiments were performed on an inverted microscope (Olympus IX81). An ∼1-milliwatt 528-nm laser from a laser power supply (CrystaLaser, CL-2005) was used to visualize the sample. A 30-milliwatt laser was used to cause the photobleaching of the Cy3 dye. Cy3 fluorescence was collected when total internal reflection was observed at the interface of the coverslip and the solution with a 100×/1.49 oil objective (Olympus). The fluorescence amplified by dichroic mirrors and filters was recorded with an EMCCD camera (iXonX3 Andor Technology) with a time resolution of 100 ms. Micromanager software (National Institutes of Health, version 1.4) was used to control the camera and collect the images. Five collections of 3,000 frames per sample with 512 × 512 pixels were acquired.
Data Analysis
Images were first analyzed by using ImageJ (National Institutes of Health, version 1.46). The spots were selected with the “find maximal” function. The intensity of pixels in 3 × 3 regions around the peak pixel was integrated. All spots except non-circular spots were selected because the non-circular spots were formed by several spots too close to each other. The intensities of the selected spots in each frame were converted into raw data format using the “multi measure” function. Further analysis of the trajectory data was performed visually with the aid of an in-house computer program. Only spots showing apparent photobleaching steps were counted. The number of observed spots in an image was determined by the “analyze particles” function in ImageJ. Typically, 10 full frames were co-added to improve the signal-to-noise ratio before the particle analysis. All data were expressed as mean ± S.D., derived from five experiments. An unpaired t test was used to compare the difference between the data, and statistical significance was displayed as *, p < 0.05 and ***, p < 0.001.
RESULTS
Translocation of Bax to Lipid Bilayers Does Not Depend on tBid, yet Its Oligomerization on Bilayers Depends on tBid but Not Cardiolipin
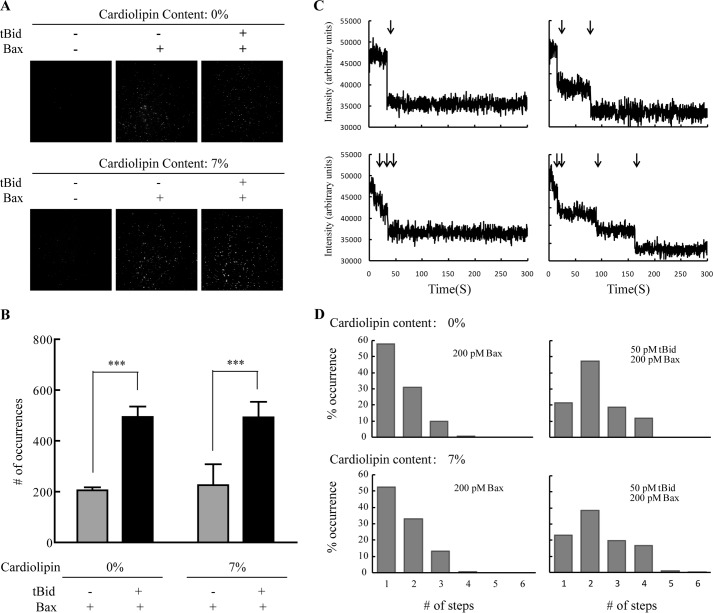
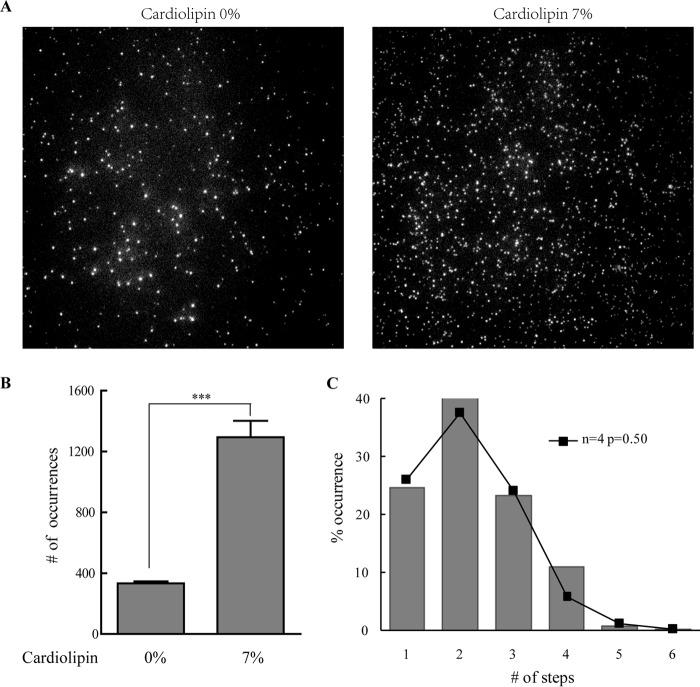
An activator protein (e.g. tBid, Bim, and Puma) and cardiolipin had been reported to be essential in Bax pore formation (14, 19). Bax, tBid, and cardiolipin cooperate to form pores in MOM and cause MOMP (14). To further investigate the specific roles of tBid and cardiolipin, we studied the integration and oligomerization of Bax on the supported lipid bilayers in the presence or absence of tBid or cardiolipin. To study these events by single molecule fluorescence techniques, we needed to label Bax with the Cy3 fluorophore. Wild-type Bax has two cysteines: Cys-62 on α2 helix and Cys-126 on α5 helix. As the α5 helix has been proved to insert into membrane (20), labeling Cys-126 would likely interfere with Bax pore formation. Meanwhile, the side chain of Cys-62 points to the inside of the Bax protein, which is inaccessible for Cy3 labeling. Therefore, we mutated Cys-62 and Cys-126 to serine and mutated Ser-16 at the N terminus to cysteine. We labeled Cys-16 of Bax mutant with Cy3, which made the mutant visible under the irradiation of a 528-nm laser. The lipid bilayers were formed on a glass coverslip either from DPPC or from 93% DPPC and 7% cardiolipin. The supported bilayers were taken as control (Fig. 1A), showing a negligible number of spots. After incubation with Cy3-labeled Bax (with tBid or not), the spots on lipid bilayers increased (Fig. 1A), indicating that Bax bound to lipid bilayers whether with or without tBid. This is in agreement with previous studies demonstrating that Bax translocated constantly to membrane even at an inactive state (5, 15). However, when comparing the number of spots on bilayers with or without tBid (Fig. 1B), we can see that the spots accumulated more readily on lipid bilayers after tBid was added. This suggested that tBid recruited Bax and caused its accumulation on the lipid bilayers. In addition, the bilayers with 7% cardiolipin had the same amount of fluorophore spots as the bilayers with DPPC only, meaning that cardiolipin did not have any effect on the accumulation of Bax to the lipid membrane.
FIGURE 1.
Translocation and oligomerization of Bax with or without tBid or cardiolipin on lipid bilayers. A, TIRF images of Cy3-labeled Bax on DPPC lipid bilayers with (lower panels) or without cardiolipin (upper panels). The left panels are blank. The central panels are the images of 200 pm Bax. The right panels are the images of 50 pm tBid and 200 pm Bax. B, number of fluorophore spots in the images of A. The error bars show the S.D. of five independent experiments. ***, p < 0.001 between the data in the absence and the presence of tBid. C, photobleaching of the fluorescence at four representative spots on lipid bilayers (93% DPPC and 7% cardiolipin) incubated with 50 pm tBid and 200 pm Cy3-labeled Bax. AU, arbitrary units. D, distributions of the photobleaching steps of the fluorophore spots on lipid bilayers (DPPC with or without cardiolipin) incubated with 200 pm Cy3-labeled Bax (with or without 50 pm tBid).
Also, we analyzed the number of subunits of Bax at each spot by photobleaching, which may help understand the roles of tBid and cardiolipin in Bax pore formation. (See supplemental Videos 1–4.) The number of photobleaching steps is related to the number of the labeled Bax subunits. Because of the incomplete labeling and previous photobleaching of Bax, not every subunit could be observed. The number of photobleaching steps presents the minimal number of subunits in each Bax oligomer. Most spots were found to display a step-like bleaching behavior (Fig. 1C). Few spots showed no bleaching, which may be caused by impurities or fast fluctuation of the fluorescence. These spots were excluded from the analysis. As shown in Fig. 1D (upper left), about 60% of the spots displayed one bleaching step for Bax without tBid. 30% of the spots showed two bleaching steps. Only a few spots (about 10%) displayed 3–4 bleaching steps. However, when Bax was incubated on lipid bilayers with tBid, the percentage of one bleaching decreased dramatically down to 20%. The amounts of 2–4 bleaching steps increased to about 45, 20, and 12%, respectively (Fig. 1D, upper right). The shift of the distribution demonstrated that Bax formed higher oligomers when tBid was added. Meanwhile, by comparing Fig. 1D (upper and lower panels), we found that cardiolipin did not affect the distribution of the oligomers. This indicated that Bax oligomerization was related to tBid and independent on cardiolipin.
Bax Tends to Form Tetramer on Lipid Bilayers
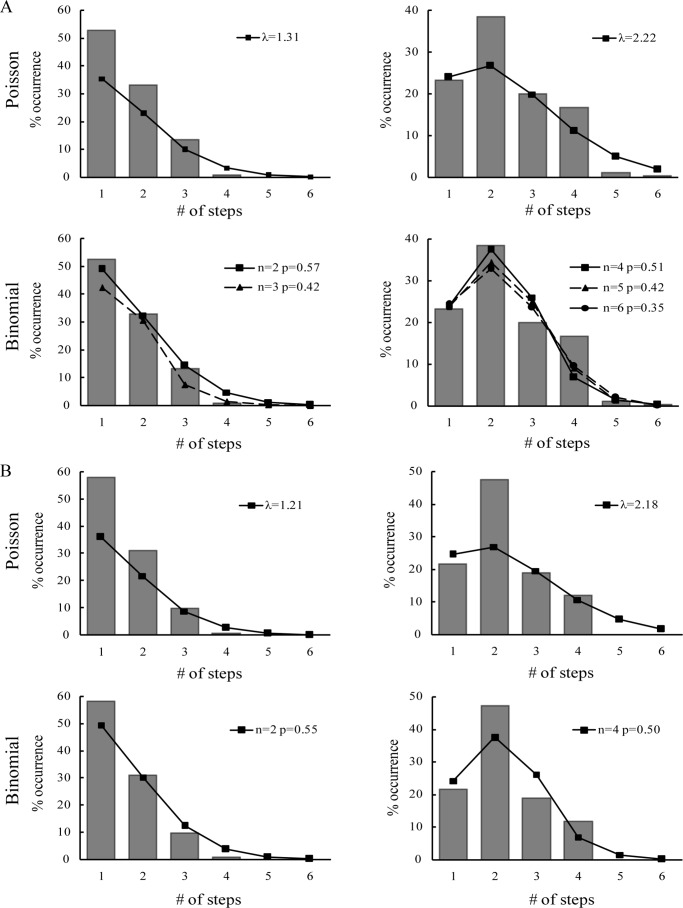
To determine the exact oligomeric state of Bax on bilayers, we carried out further analysis on the histograms as shown in Fig. 1D. Randomly oligomerized states would fit to a Poisson distribution, whereas oligomers with a defined stoichiometry followed a binomial distribution. We fitted the numbers of the photobleaching steps to both Poisson distribution and binomial distribution, respectively. For Poisson distribution, λ stands for the average number of the labeled subunits in Bax oligomers, and for the binomial distribution, p represents the probability that the labeled subunit can be observed, which corresponds to the efficiency of the labeling (EOL). We measured that the EOL for Cy3-labeled Bax was 0.67 ± 0.02. In the absence of tBid, the histogram of Bax oligomers on the bilayers with 7% cardiolipin significantly diverged from Poisson distribution (Fig. 2A, upper left). In contrast, a binominal distribution with two and three subunits fit the histogram well (Fig. 2A, lower left). However, the p value of the three subunits distribution was much lower. Moreover, there was a steep drop between the two and three bleaching steps. Both of the results suggest that Bax formed no more than dimer. Few spots showed 3–4 bleaching steps, which we presumed meant that two spots were present in a diffraction-limited area in these cases. Besides, the diversity between the p value (p = 0.57 ± 0.01) of the dimer binomial distribution and the EOL would be explained by the coexistence of monomer and dimer.
FIGURE 2.
Fitting the numbers of photobleaching steps with Poisson or binomial distribution. A, distributions of 200 pm Cy3-labeled Bax with (right panels) or without (left panels) 50 pm tBid on DPPC bilayers with 7% cardiolipin. B, distributions of 200 pm Cy3-labeled Bax with (right panel) and without (left panel) 50 pm tBid on the DPPC-only bilayers.
In the presence of tBid, we observed that the distribution was shifted toward Bax oligomers on bilayers with 7% cardiolipin (Fig. 1D, lower right). We fitted the histogram to both Poisson distribution and binomial distribution. We found that the binomial distribution with the number of subunits equal to 4, 5, and 6 (Fig. 2A, lower right) fit the histogram well, whereas Poisson distribution did not (Fig. 2A, upper right). The low p values for n = 5 and 6 and a steep drop between four and five bleaching steps suggest that the Bax oligomers contained no more than four subunits. The p value calculated from the tetramer binomial distribution was 0.51 ± 0.01, which was also lower than EOL. This may be caused by the coexistence of monomer, dimer, trimer, and tetramer, which makes the p value divergent from EOL.
We also fitted the histograms of the Bax oligomers on DPPC-only bilayers to both Poisson distribution and binomial distribution. A binomial distribution with two subunits fitted the distribution well in the absence of tBid. The p value was 0.55 ± 0.08 (Fig. 2B). In the presence of tBid, the histogram could be well fitted by a binomial distribution with four subunits (p = 0.50 ± 0.02). These results further confirmed that Bax oligomerization was dependent on tBid rather than cardiolipin.
Based on the above analysis, we proposed that the majority of Bax binds to bilayers as monomer and dimer in the absence of tBid. tBid could induce Bax to form higher oligomers, and Bax tends to form tetramer on lipid bilayers after activation by tBid.
tBid Stoichiometrically Promotes the Integration of Bax to Membrane
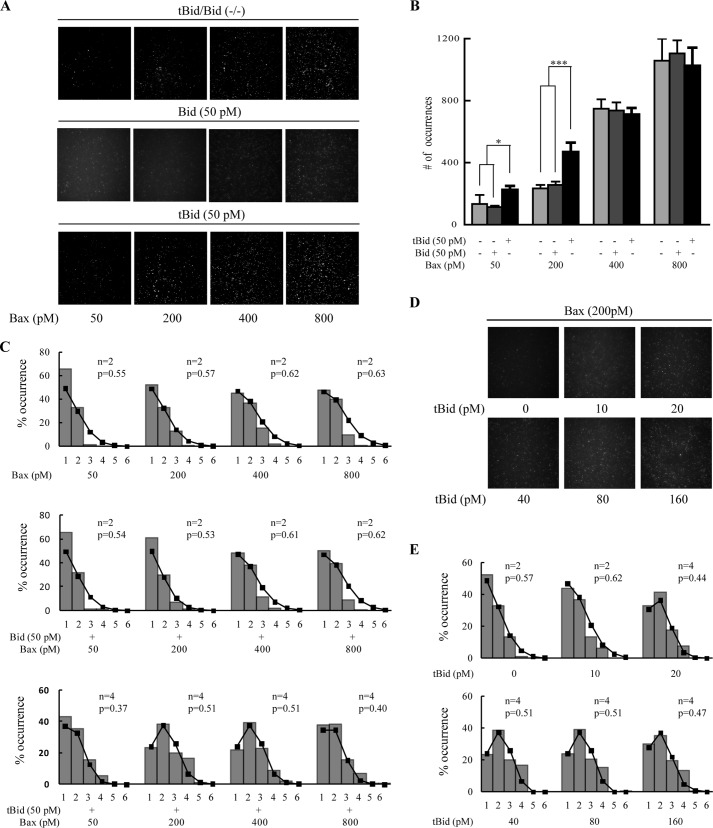
To determine whether the concentration of Bax would affect its integration to membrane, we analyzed the number of the fluorophore spots at different concentrations of Bax (Fig. 3A). We found that the number of the spots increased along with the concentration of Bax no matter whether Bid or tBid was added (Fig. 3, A and B). This is consistent with the above result that Bax alone can bind to the lipid bilayers. Interestingly, 50 pm tBid promoted the integration of 50 and 200 pm Bax to lipid bilayers (Fig. 3B). However, when the concentration of Bax increased to 400 or 800 pm, the promotion effect of tBid was not observed. At such high Bax concentrations, the numbers of spots were almost the same for the samples with or without tBid. These results suggested that tBid promotes the integration of Bax to membrane in a stoichiometric manner. Once Bax was recruited by tBid, the activated Bax might recruit other Bax molecules to the membrane, where tBid and Bax molecules formed stable multimeric complexes. When tBid was consumed, the extra amount of Bax would interact with the lipid molecules and accumulate to the membrane spontaneously. Considering that Bax tends to form tetramer on the bilayers in the presence of tBid (Fig. 2), we deduced that one tBid molecule may recruit 1–4 Bax molecules and form complexes on membrane. Meanwhile, we compared the membrane integration of Bax in the presence and absence of full-length Bid (Fig. 3B). We found that the numbers of fluorophore spots in the presence or absence of Bid had no significant difference. This suggested that full-length Bid had no effect on the integration of Bax to membrane.
FIGURE 3.
Integration and oligomerization of various concentrations of Bax in the absence and presence of 50 pm tBid or Bid. A, TIRF images at various concentrations of Cy3-labeled Bax (upper panels) and with 50 pm Bid (middle panels) or with 50 pm tBid (lower panels) on lipid bilayers (93% DPPC and 7% cardiolipin). B, numbers of the fluorophore spots at different concentrations of Cy3-labeled Bax and with 50 pm Bid or tBid. Error bars show the S.D. of five independent experiments. *, p < 0.05 and ***, p < 0.001 between the data in the absence and the presence of tBid. C, distributions of photobleaching steps of the fluorophore spots on lipid bilayers (93% DPPC and 7% cardiolipin) incubated with different concentrations of Cy3-labeled Bax and with 50 pm Bid or tBid. D, TIRF images of 200 pm Cy3-labeled Bax with various concentrations of tBid on lipid bilayers (93% DPPC and 7% cardiolipin). E, distributions of photobleaching steps of the fluorophore spots on lipid bilayers (93% DPPC and 7% cardiolipin) incubated with 200 pm Cy3-labeled Bax with various concentrations of tBid.
Further, we analyzed the oligomerization of Bax at different concentrations (Fig. 3C). (See supplemental Videos 2 and 4–14.) We found that the majority of Bax translocated to lipid bilayer as monomer and dimer in the absence of tBid or Bid. As the concentration increased, Bax tended to form into dimer. When comparing the oligomerization of 50 pm Bax in the absence and presence of 50 pm tBid, we can clearly see that the distribution was shifted from monomer and dimer to trimer and tetramer. Also, 200 and 400 pm Bax had the same results. However, when Bax concentration was increased to 800 pm, Bax monomer and dimer became dominant. This may be explained by the facts described above indicating that tBid stoichiometrically promotes Bax to form into higher oligomers, and Bax alone may integrate to lipid bilayers as monomer and dimer. Because 800 pm Bax was redundant for 50 pm tBid, the excess Bax could only accumulate to bilayers as monomer and dimer. Besides, when comparing the oligomerization of Bax in the absence and presence of full-length Bid, we found that the distribution of Bax oligomers had no significant change after Bid was added. Full-length Bid did not affect Bax oligomerization on membrane.
To confirm the above results, we fitted the histograms of Bax oligomers to binomial distribution. In the absence of tBid or Bid, the histograms were well fitted to binomial distributions with two subunits, and the p value was close to the EOL with the increasing concentrations of Bax. This confirmed that Bax tends to form dimer when it translocates to lipid membrane. Likewise, the binomial distributions with two subunits also fitted the histograms of Bax oligomers well with Bid added. In contrast, in the presence of tBid, the histograms were well fitted to tetramer binomial distributions. The p value increased with the increasing concentrations of Bax from 50 to 400 pm. At the concentration of 800 pm, the p value, however, became more deviated from the EOL. This may be explained by other oligomerization states caused by the redundant Bax, which we discussed above.
We also imaged the Cy3-labeled Bax on lipid membrane (93% DPPC and 7% cardiolipin) with increasing concentrations of tBid (Fig. 3D). (See supplemental Videos 2 and 16–19.) Analysis of the photobleaching steps showed that the distribution of Bax oligomers shifted steadily from dimer to tetramer while the concentration of tBid increased from 0 to 40 pm (Fig. 3E). As the concentration was increased to 40–160 pm, the pattern of the distribution remained almost unchanged. This result further confirmed that tBid stoichiometrically promotes Bax integration on membrane.
BH3 Domain Peptides of tBid, Bim, and Bak Can Induce Bax Integration to Membrane
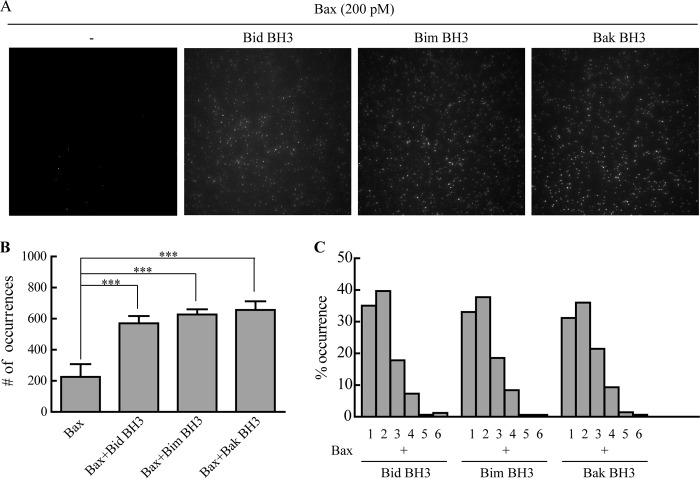
The BH3 domain peptides of pro-apoptotic Bcl-2 family proteins such as Bid, Bad, and Bim may bind to the hydrophobic pocket on the surface of anti-apoptotic proteins such as Bcl-xL (21). Meanwhile, the BH3 domain peptides of Bid also bind to Bax and induce its conformational change (22). To examine whether tBid activates the Bax translocation to membrane through its BH3 domain, we mixed 200 pm Bax with 50 pm Bid BH3 peptide and incubated the sample on lipid bilayers composed of 93% DPPC and 7% cardiolipin. The bilayers incubated with 200 pm Bax only were imaged as control. It was obvious that the fluorophore spots increased in the presence of tBid BH3 peptide (Fig. 4, A and B), indicating that the BH3 domain of tBid was able to recruit Bax to membrane. The same results were obtained with the BH3 peptides of Bim and Bak.
FIGURE 4.
Integration of Bax on bilayers may be encouraged by Bid, Bim, or Bak BH3 domain peptide. A, TIRF images of 200 pm Cy3-labeled Bax incubated with BH3 peptide on lipid bilayers (93% DPPC and 7% cardiolipin). The panel from left to right shows the images of 200 pm Bax, 200 pm Bax with 50 pm Bid, 50 pm Bim, or 50 pm Bak BH3 domain peptide, respectively. B, fluorophore spots quantifying 200 pm Cy3-labeled Bax with or without BH3 peptide. Error bars show the S.D. of five independent experiments. ***, p < 0.001 between the data in the absence and the presence of BH3 peptide. C, histograms of photobleaching steps of Cy3-labeled Bax with different BH3 peptides.
To investigate whether the BH3 peptides induced Bax oligomerization, we analyzed the photobleaching steps of the fluorophore spots as shown in Fig. 4C. (See supplemental Videos 20–22.) The distributions of Bax oligomers induced by the peptides were similar to those induced by tBid (Fig. 1D). BH3 peptides retained the ability to induce Bax oligomerization. Hence, the BH3 domain of tBid is essential for Bax oligomerization. The interaction with BH3 peptides could induce the conformational change of Bax, thus promoting its integration and oligomerization on membrane.
Cardiolipin Efficiently Promotes the Membrane Insertion of Bax
To assess whether Bax inserted into the supported bilayers, alkaline buffer (pH 11.5) was used to wash off Bax at the surface of the lipid bilayers (DPPC only or DPPC with 7% cardiolipin). To have enough fluorophore spots left for statistical analysis, the bilayers were preincubated with 500 pm tBid and 2 nm Bax. Interestingly, after wash, the number of spots on DPPC bilayers was much lower than that on bilayers with 7% cardiolipin (Fig. 5, A and B). As shown in Fig. 1, B and D, 7% cardiolipin was not essential for the attachment of Bax to bilayers or its oligomerization. However, here we showed that the insertion of Bax into membrane was highly dependent on the lipid composition. Cardiolipin was the pivotal component for promoting membrane insertion of Bax.
FIGURE 5.
Cardiolipin promotes Bax insertion into bilayers. A, TIRF images of Cy3-labeled Bax on DPPC-only bilayers (left) or with 7% cardiolipin (right). The lipid bilayers were first incubated with 500 pm tBid and 2 nm Bax for 1 h and then washed with alkali buffer (200 mm Na2CO3, pH 11.5, 2% glycerol) for 30 min. The remaining spots were Bax inserted into bilayers. B, numbers of the fluorophore spots in the images of A. Error bars show the S.D. of five independent experiments. ***, p < 0.001 between the data in the different bilayers. C, distribution of photobleaching steps of the spots left on lipid bilayers (93% DPPC and 7% cardiolipin), fitted with binomial distribution (n = 4).
We further characterized the oligomerization of the membrane-inserted Bax by photobleaching (Fig. 5C). (See supplemental Video 23.) Interestingly, the distribution was similar to the distribution before the sample was washed by alkaline buffer (Fig. 2A). A binomial distribution with four subunits fitted the histogram well, and the p value was 0.50 ± 0.001, which is lower than the EOL. As analyzed above, Bax inserted in membrane also tends to form tetramer, whereas Bax monomer and lower oligomers coexist in membrane.
As shown in Fig. 1D, Bax could oligomerize in the presence of tBid, even if cardiolipin was absent. However, only a few oligomers could insert into the bilayers without cardiolipin (Fig. 5B). These facts indicated that Bax was capable of forming oligomers before inserting into bilayers. Thus, we suggest that Bax oligomerization and membrane insertion are relatively independent steps during Bax pore formation.
Bcl-xL Retrotranslocates Bax off the Lipid Bilayers
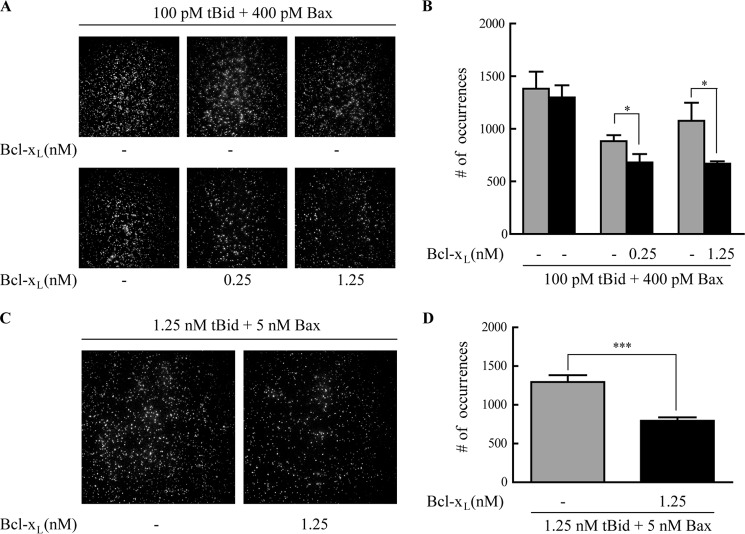
As Bcl-xL inhibits the function of Bax, we examined the effect of Bcl-xL on the association of Bax to membrane. When Bcl-xL was added to the supported bilayers preincubated with tBid and Bax, the number of the fluorophore spots was reduced (Fig. 6, A and B). This indicated that some Bax molecules were pulled off from the bilayers to solution by Bcl-xL. To check whether Bax inserted in the bilayers could be pulled back to solution, we first washed the bilayers with alkaline buffer (pH 11.5) to remove the surface-attached Bax and then added Bcl-xL to the bilayers. Interestingly, the number of the spots was decreased significantly when compared with the number of spots before Bcl-xL was added (Fig. 6, C and D), which meant that the membrane-inserted Bax could also be translocated from the bilayers to solution.
FIGURE 6.
Bcl-xL may translocate Bax from lipid bilayers to solution. A, TIRF images of 400 pm Cy3-labeled Bax on lipid bilayers (93% DPPC and 7% cardiolipin). 100 pm tBid and 400 pm Cy3-labeled Bax were incubated with bilayers for 1 h, and the TIRF images recorded are shown in the upper panels (three independent experiments). The lipid bilayers were then incubated with 0, 0.25, or 1.25 nm Bcl-xL, respectively, and the TIRF images recorded are shown in the lower panels (from left to right: 0, 0.25, and 1.25 nm Bcl-xL). B, numbers of spots before and after incubation with 0, 0.25, or 1.25 nm Bcl-xL. Error bars show the S.D. of five experiments. *, p < 0.05 between the data before and after the addition of Bcl-xL. C, Bcl-xL can translocate the membrane-inserted Bax to solution. The left panel is the TIRF image of 5 nm Cy3-labeled Bax with 1.25 nm tBid in bilayers (93% DPPC and 7% cardiolipin) after washing with alkali buffer. The right panel is the TIRF image of the sample after incubation with 1.25 nm Bcl-xL. D, numbers of the fluorophore spots in bilayers before (left column) and after (right column) 1.25 nm Bcl-xL incubation. ***, p < 0.001 according to a Student's t test between the data before and after the addition of Bcl-xL.
DISCUSSION
To investigate the mechanism of Bax pore formation, we designed single molecule fluorescence experiments to study the integration and oligomerization of Bax on lipid bilayers. A stepwise photobleaching method was employed to analyze the oligomeric states of Bax on the membrane.
As shown previously, Bax predominantly exists as a monomer in cytosol of health cell. It is not usual for Bax to be found in mitochondria (23). Yethon (6) suggested that the interaction of Bax with mitochondrial membrane is transient. However, recent studies in fibroblasts (15, 24) and epithelial cells (5) showed a dynamic equilibrium of Bax localization at cytosol and MOM, and suggested that Bax translocated constantly to mitochondria and that Bcl-xL could translocate it back to cytosol in a healthy cell. Our results demonstrated that Bax interacted spontaneously with membrane (Figs. 1B and 3B) and argued against the theory that the interaction between Bax and membrane is transient. The interaction between Bax and lipid membrane is stable even in the absence of the activator protein tBid. However, the membrane-bound Bax may be translocated back to solution by anti-apoptotic Bcl-xL protein (Fig. 6).
Our studies also revealed that Bax oligomerized only in the presence of tBid. Full-length Bid was not competent to promote Bax translocation or oligomerization. In a stressed cell, Bid is cleaved by caspase-8 into tBid (25, 26). tBid rapidly translocates to mitochondrial outer membrane and undergoes a series of conformational changes (11, 27), which leaves the BH3-containing α3 helix exposed for the interactions with Bax (28). Here our experiments showed that the BH3 domain peptides of Bid, Bim, and Bak had the ability to trigger Bax integration and oligomerization on membrane (Fig. 4). This is consistent with the previous studies demonstrating that the BH3 domain peptides of Bid and Bim could activate the Bax-induced MOMP (10). Our results proved that the BH3 domain of the activator proteins is the primary structural element for Bax activation, which facilitates Bax integration and oligomerization in membrane.
Besides, we found that cardiolipin is efficient in promoting membrane insertion of Bax, although it is not required for Bax oligomerization. This is in line with previous studies showing that 7% cardiolipin in the artificial membrane was sufficient for the Bax-induced membrane permeabilization (14). In addition, Bax oligomers were found concentrated at mitochondrial fission sites with cardiolipin enriched (29). Cardiolipin was therefore proposed to be related to Bax oligomerization and/or pore formation (30). However, in our experiments, cardiolipin had no effect on Bax oligomerization, and Bax can be recruited by tBid in the absence of cardiolipin (Fig. 1, B and C). It has been reported that cardiolipin has the ability to alter the curvature of membrane and promote the formation of non-bilayer structure (31, 32). Our results imply that cardiolipin may induce negative curvature in membrane, which promotes the Bax pore formation.
The pore formation of Bax involves the steps of targeting to membrane, oligomerization, and membrane insertion (11). However, the order of these steps is still unclear. Our results indicated that Bax oligomerization and membrane insertion are two relatively independent steps. This result is in agreement with the recent study by Kushnareva et al. (33) showing that Bax pore formation was a two-step process. We demonstrated that, in the first step, Bax was activated by tBid and promoted to form multimeric complexes. These complexes bound to the surface of the bilayers, which were identified as pre-pores. In the second step, the precursor complexes inserted into membrane with enriched cardiolipin, which facilitated the Bax pore formation.
As to the stoichiometry of interaction between tBid and Bax, one tBid molecule can recruit more than one Bax molecule directly or indirectly (34, 35). Kuwana et al. (14) suggested a tetramer-sized complex for permeabilization according to the amount of ∼100-kDa Bax oligomers correlated with the permeabilization activity. Here we showed that the membrane-integrated Bax tends to form tetramer (Figs. 1D and 3, C and E). Meanwhile, there still exist Bax monomer and lower oligomers. This supports the recent finding that Bax monomer is a key functional unit related to the pore formation (16, 33). With the aid of tBid BH3 peptide, an individual Bax molecule could insert into the lipid bilayers and form pores, thus initiating the MOM pore formation. Based on our results, we may propose that both Bax monomer and Bax oligomers may be the pore-forming units. The tetrameric structure is the highest state of oligomerization observed at the concentration range, indicating that the pore is composed no more than four subunits.
As discussed above, in a healthy cell, Bax translocates to the MOM constantly at an inactive state. Bcl-xL retrotranslocates it to cytosol to prevent the mitochondrial Bax accumulation (15). After the induction of apoptosis, Bcl-xL may also shuttle Bax back to cytosol to make MOMP, proceeding in a slower manner (24). Here our data showed that Bcl-xL can retrotranslocate the membrane-bound Bax to solution even when Bax inserts into membrane (Fig. 6). The apoptosis would be inhibited by Bcl-xL, interrupting Bax pore formation.
In summary, by using single molecule fluorescence techniques, we examined individual Bax molecule behavior at an established membrane environment. According to our results, we propose that in a healthy cell, Bax alone can translocate to the MOM (Fig. 7). It binds to membrane as monomer and dimer. After the cell is stressed, Bax undergoes two steps to form pore on MOM. In the first step, Bax is activated by the membrane-bound tBid and tends to form tetramer. The multimeric complex forms pre-pore on MOM. In the second step, the precursor complex inserts into the MOM, which is rich in cardiolipin, to lead the membrane permeabilization. As an anti-apoptotic protein, Bcl-xL could slow the process by translocating Bax to cytosol.
FIGURE 7.
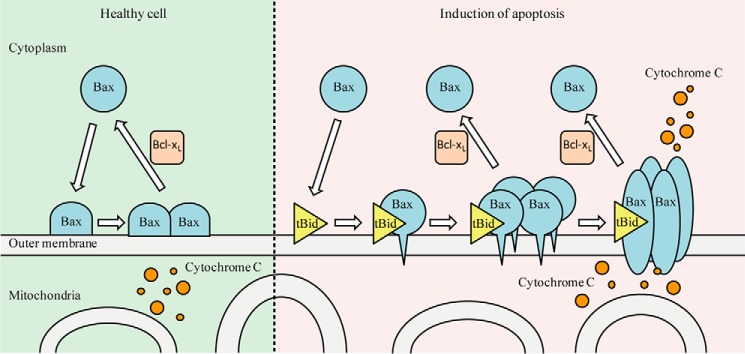
Model of integration and oligomerization of Bax on MOM. In a healthy cell, Bax can bind to the MOM as monomer and dimer. After the induction of apoptosis, Bax undergoes two steps to form pore on MOM. Bax is activated by tBid and tends to form tetramer. Subsequently, the multimeric complex inserts into the MOM to cause its permeabilization. Bcl-xL inhibits this process by translocating Bax from MOM to cytosol.
Supplementary Material
Acknowledgment
We thank Yintao Liu for the help with the data analysis.
This work was supported by the National Science Fund (Grant 21472206) and the State Key Laboratory of Drug Research.

This article contains supplemental Videos 1–23.
- MOM
- mitochondrial outer membrane
- MOMP
- mitochondrial outer membrane permeabilization
- Cy3
- cyanine-3-maleimide
- DPPC
- 1,2-diphytanoyl-sn-glycero-3-phosphocholine
- TIRF
- total internal reflection fluorescence
- TIRFM
- total internal reflection fluorescence microscopy
- EOL
- efficiency of the labeling.
REFERENCES
- 1. Green D. R., Kroemer G. (2004) The pathophysiology of mitochondrial cell death. Science 305, 626–629 [DOI] [PubMed] [Google Scholar]
- 2. Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74, 597–608 [DOI] [PubMed] [Google Scholar]
- 3. Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. (1993) Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 75, 241–251 [DOI] [PubMed] [Google Scholar]
- 4. Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schellenberg B., Wang P., Keeble J. A., Rodriguez-Enriquez R., Walker S., Owens T. W., Foster F., Tanianis-Hughes J., Brennan K., Streuli C. H., Gilmore A. P. (2013) Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol. Cell 49, 959–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yethon J. A. (2003) Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J. Biol. Chem. 278, 48935–48941 [DOI] [PubMed] [Google Scholar]
- 7. Wolter K. G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X. G., Youle R. J. (1997) Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J. C. (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144, 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eskes R., Desagher S., Antonsson B., Martinou J. C. (2000) Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell. Biol. 20, 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. (2005) BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell 17, 525–535 [DOI] [PubMed] [Google Scholar]
- 11. Lovell J. F., Billen L. P., Bindner S., Shamas-Din A., Fradin C., Leber B., Andrews D. W. (2008) Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135, 1074–1084 [DOI] [PubMed] [Google Scholar]
- 12. Schug Z. T., Gottlieb E. (2009) Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim. Biophys. Acta 1788, 2022–2031 [DOI] [PubMed] [Google Scholar]
- 13. Zhang T., Saghatelian A. (2013) Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochim. Biophys. Acta 1831, 1542–1554 [DOI] [PubMed] [Google Scholar]
- 14. Kuwana T., Mackey M. R., Perkins G., Ellisman M. H., Latterich M., Schneiter R., Green D. R., Newmeyer D. D. (2002) Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111, 331–342 [DOI] [PubMed] [Google Scholar]
- 15. Edlich F., Banerjee S., Suzuki M., Cleland M. M., Arnoult D., Wang C., Neutzner A., Tjandra N., Youle R. J. (2011) Bcl-xL retrotranslocates bax from the mitochondria into the cytosol. Cell 145, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu X. P., Zhai D., Kim E., Swift M., Reed J. C., Volkmann N., Hanein D. (2013) Three-dimensional structure of Bax-mediated pores in membrane bilayers. Cell Death Dis. 4, e683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roy R., Hohng S., Ha T. (2008) A practical guide to single-molecule FRET. Nat. Methods 5, 507–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blunck R., McGuire H., Hyde H. C., Bezanilla F. (2008) Fluorescence detection of the movement of single KcsA subunits reveals cooperativity. Proc. Natl. Acad. Sci. U.S.A. 105, 20263–20268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lucken-Ardjomande S., Montessuit S., Martinou J. C. (2008) Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ. 15, 929–937 [DOI] [PubMed] [Google Scholar]
- 20. García-Sáez A. J., Mingarro I., Pérez-Payá E., Salgado J. (2004) Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry 43, 10930–10943 [DOI] [PubMed] [Google Scholar]
- 21. Chipuk J. E., Moldoveanu T., Llambi F., Parsons M. J., Green D. R. (2010) The BCL-2 family reunion. Mol. Cell 37, 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gavathiotis E., Suzuki M., Davis M. L., Pitter K., Bird G. H., Katz S. G., Tu H.-C., Kim H., Cheng E. H. Y., Tjandra N., Walensky L. D. (2008) BAX activation is initiated at a novel interaction site. Nature 455, 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu Y. T., Youle R. J. (1998) Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273, 10777–10783 [DOI] [PubMed] [Google Scholar]
- 24. Todt F., Cakir Z., Reichenbach F., Youle R. J., Edlich F. (2013) The C-terminal helix of Bcl-xL mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 20, 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 26. Zha J., Weiler S., Oh K. J., Wei M. C., Korsmeyer S. J. (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290, 1761–1765 [DOI] [PubMed] [Google Scholar]
- 27. Shamas-Din A., Bindner S., Zhu W., Zaltsman Y., Campbell C., Gross A., Leber B., Andrews D. W., Fradin C. (2013) tBid undergoes multiple conformational changes at the membrane required for Bax activation. J. Biol. Chem. 288, 22111–22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Czabotar P. E., Westphal D., Dewson G., Ma S., Hockings C., Fairlie W. D., Lee E. F., Yao S., Robin A. Y., Smith B. J., Huang D. C. S., Kluck R. M., Adams J. M., Colman P. M. (2013) Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 152, 519–531 [DOI] [PubMed] [Google Scholar]
- 29. Montessuit S., Somasekharan S. P., Terrones O., Lucken-Ardjomande S., Herzig S., Schwarzenbacher R., Manstein D. J., Bossy-Wetzel E., Basañez G., Meda P., Martinou J. C. (2010) Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raemy E., Martinou J.-C. (2014) Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chem. Phys. Lipids 179, 70–74 [DOI] [PubMed] [Google Scholar]
- 31. Ortiz A., Killian J. A., Verkleij A. J., Wilschut J. (1999) Membrane fusion and the lamellar-to-inverted-hexagonal phase transition in cardiolipin vesicle systems induced by divalent cations. Biophys. J. 77, 2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Epand R. F., Martinou J. C., Montessuit S., Epand R. M. (2002) Membrane perturbations induced by the apoptotic Bax protein. Biochem. J. 367, 849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kushnareva Y., Andreyev A. Y., Kuwana T., Newmeyer D. D. (2012) Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol. 10, e1001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Billen L. P., Kokoski C. L., Lovell J. F., Leber B., Andrews D. W. (2008) Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 6, e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tan C., Dlugosz P. J., Peng J., Zhang Z., Lapolla S. M., Plafker S. M., Andrews D. W., Lin J. (2006) Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J. Biol. Chem. 281, 14764–14775 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.