Background: DEPTOR is a negative regulator of mTOR activity and thus plays a role in the regulation of cell metabolism and growth.
Results: DEPTOR levels decrease upon differentiation of embryonic stem cells, and reduction of DEPTOR levels is sufficient to promote differentiation.
Conclusion: DEPTOR is a stemness factor that regulates pluripotency.
Significance: Manipulation of DEPTOR activity provides a means of influencing embryonic stem cell renewal and differentiation.
Keywords: Cell Differentiation, Cell Signaling, Embryonic Stem Cell, Mammalian Target of Rapamycin (mTOR), Neurogenesis
Abstract
The mammalian target of rapamycin (mTOR) pathway regulates stem cell regeneration and differentiation in response to growth factors, nutrients, cellular energetics, and various extrinsic stressors. Inhibition of mTOR activity has been shown to enhance the regenerative potential of pluripotent stem cells. DEPTOR is the only known endogenous inhibitor of all known cellular mTOR functions. We show that DEPTOR plays a key role in maintaining stem cell pluripotency by limiting mTOR activity in undifferentiated embryonic stem cells (ESCs). DEPTOR levels dramatically decrease with differentiation of mouse ESCs, and knockdown of DEPTOR is sufficient to promote ESC differentiation. A strong decrease in DEPTOR expression is also observed during human ESCs differentiation. Furthermore, reduction in DEPTOR level during differentiation is accompanied by a corresponding increase in mTOR complex 1 activity in mouse ESCs. Our data provide evidence that DEPTOR is a novel stemness factor that promotes pluripotency and self-renewal in ESCs by inhibiting mTOR signaling.
Introduction
Stem cells undergo symmetric and asymmetric cell division resulting in self-renewal and differentiation. Both microenvironmental cues and intrinsic signals are required for the maintenance of stem cell populations, and dysregulation of these signals is associated with a decline in their regenerative potential. Mammalian target of rapamycin (mTOR)4 is a serine/threonine kinase that responds to extracellular cues to regulate a variety of cellular processes, including metabolism, protein expression, autophagy, and growth (1). Due to its key role in integrating growth factor and nutrient signaling to cell growth and metabolism, mTOR is a critical regulator of cell differentiation and tissue regeneration (1). Although mTOR activity is essential for stem cell (SC) maintenance, modest reduction in mTOR activity enhances self-renewal of adult SC populations by preventing uncontrolled growth and cell exhaustion (2). It has been reported that mTOR activity is increased in hematopoietic stem cells in aged mice and that elevated levels of mTOR activity can lead to premature aging in hematopoietic stem cells in young mice (3). Inhibition of mTOR improved the regenerative potential of hematopoietic stem cells in old mice. Additionally, negative regulators of mTOR, USP9X, PTEN, and Fbw7, are important in maintenance of quiescence and prevention of SC exhaustion (4–6). mTOR has also been shown to regulate proliferation and self-renewal of human and mouse embryonic stem cells and to have a required role in reprogramming of somatic cells into induced pluripotent stem cells (7–9).
DEPTOR (DEP domain-containing mTOR interacting protein, also known as DEPDC6) was recently identified as a negative regulator of mTOR signaling that inhibits the activities of both mTOR complexes 1 and 2 (mTORC1/2) (10). Through its effects on mTOR signaling, DEPTOR plays an important role in various cellular processes, including cell growth, apoptosis, autophagy, and adipogenesis (10–12). Recently, it was shown that reduction of DEPTOR expression promotes muscle hypertrophy and increased myoblast size (13). DEPTOR levels are in turn regulated by mTOR activity; mTOR activation results in DEPTOR phosphorylation and ubiquitination, leading to its degradation by the ubiquitin proteasome pathway (14). DEPTOR levels are high in multiple myelomas, resulting in decreased mTORC1 activity, loss of mTORC1-mediated inhibition of phosphoinositide-3 kinase (PI3K) signaling, and increased cell survival (10).
Here we describe a novel function for DEPTOR as a stemness factor that is important for maintenance of the pluripotent state of embryonic stem cells. We show that the expression of DEPTOR is high in both mouse and human embryonic stem cells (mESCs and hESCs), and its levels are dramatically reduced in differentiated cells. No other known regulators of mTOR activity/signaling showed a similar decrease in expression during differentiation. Decrease in DEPTOR levels during differentiation of mESCs was accompanied by increased mTORC1 activity, as measured by S6 phosphorylation. More importantly, we show that knockdown of DEPTOR promoted differentiation of mESCs, even in the presence of small molecules required for maintenance of the undifferentiated state of stem cells. Our findings indicate that pluripotent stem cells employ high levels of DEPTOR to maintain stem cell renewal by limiting mTOR activity in undifferentiated cells. Upon initiation of differentiation, DEPTOR expression is down-regulated, resulting in increased mTOR activity required for efficient differentiation of stem cells.
EXPERIMENTAL PROCEDURES
Cell Culture and Differentiation
Mouse R1 ESCs cells (ATCC) and human H1 ESCs were maintained as described previously (15–17). The cells were maintained at 37 °C in an atmosphere of 90% N2, 5% CO2, and 5% O2. HEK293T cells (ATCC) were grown in Dulbecco's modified Eagle's medium (DMEM; Cellgro) supplemented with 10% fetal bovine serum (FBS; Invitrogen). Cells were grown under 5% CO2 at 37 °C. Differentiation of mESCs was done in neuronal stem cell medium (17). For mRNA extractions, cells were removed from the plate using Accutase, and for protein extractions, cells were lysed directly in 1× gel loading buffer. Differentiation was done for 26 days and with medium change on alternate days. Samples were collected at the indicated times and analyzed. H1 hESCs were differentiated as described previously (16).
Lentiviral Infection
Lentiviral particles were produced in HEK293T cells. R1 mESCs were infected with virus in the presence of 8 μg/ml Polybrene (EMD Millipore Corp.), and the medium was changed into normal growth medium 2 days after infection. Drug selection (puromycin, 2 μg/ml) was started 3 days after infection, and the cells were selected in the drug medium for at least 3 days prior to use.
Quantitative PCR (qPCR)
mRNA from R1 mESCs was extracted using an RNeasy kit (Qiagen). 1 μg of mRNA was amplified (Taqman RT-PCR kit from Applied Biosystems), and 1% of this reaction was analyzed in qPCR (Bioline SensiFAST Probe No-ROX mix). Primers and probe pairs for each gene were used based on recommendations from Roche Applied Science. mRNA from H1 hESCs was extracted using the RT-PCR kit from Roche Applied Science and amplified using a Bio-Rad cDNA kit. qPCR was done using gene-specific primers and SYBR Green mix from Bio-Rad. Actin was used as an internal control.
Western Blotting and Immunofluorescence
Protein samples were boiled in 1× LDS sample buffer (Invitrogen), and Western blot analysis was done as described previously (6). R1 mESCs were plated on Matrigel-coated coverslips, and immunofluorescence analysis was done as described previously, except permeabilization and blocking was done using 0.3% Triton X-100, 5% FBS in PBS (6).
Bioinformatic Analysis of Promoter Regions
LASAGNA-Search was used to identify OCT4 binding sites in the mouse and human promoter regions up to 950 bp upstream of the transcription start sites of the DEPTOR, PRAS40, and TSC2 genes. Only sequences with p values of <0.001 were analyzed and confirmed with the UCSC Genome Browser.
RESULTS
DEPTOR Levels Decrease with mESC Differentiation
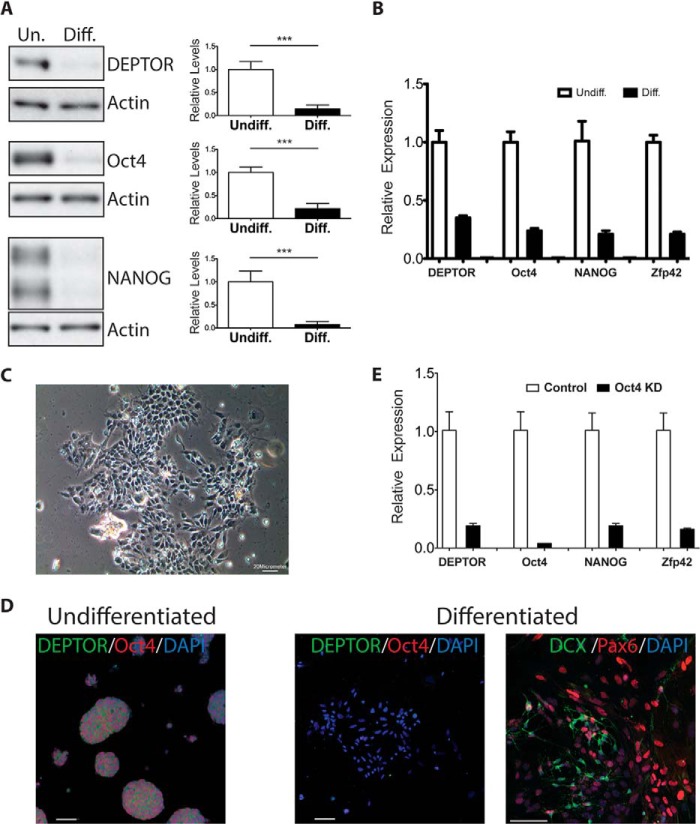
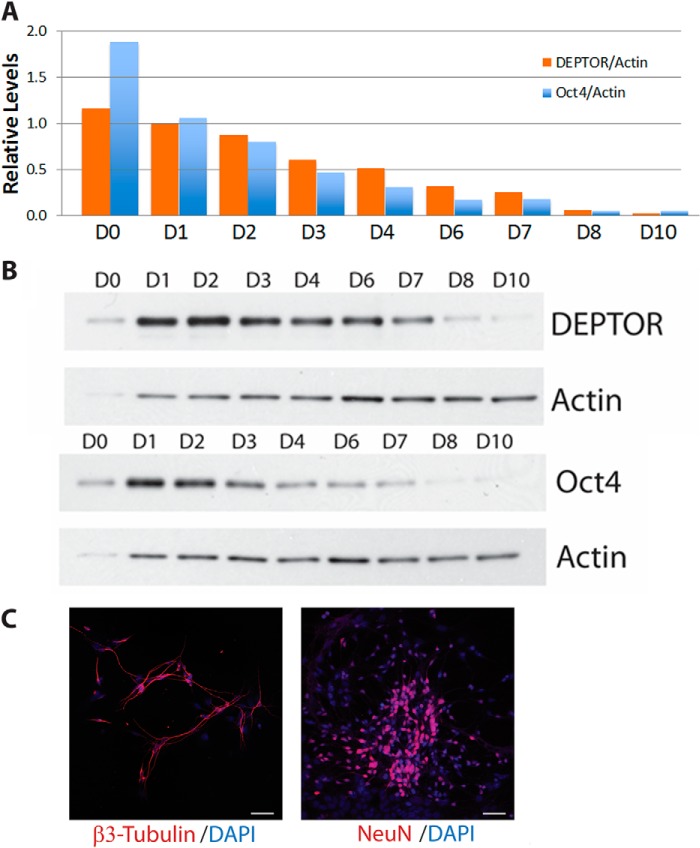
DEPTOR is a negative regulator of both mTORC1 and mTORC2. To determine whether DEPTOR plays a role in regulating mTOR signaling in stem cells, we measured changes in DEPTOR levels upon mESC differentiation. We differentiated mESCs toward neuronal fate using our previously published protocol using Wnt and TGF-β inhibitors along with IGF1 for 10 days as monolayers (Figs. 1 and 2) (18). As shown in Fig. 1A, differentiation of R1 mESCs resulted in a dramatic decrease in DEPTOR protein levels. Similar decreases were observed for the pluripotency markers OCT4 and NANOG (Fig. 1A). We also observed a decrease in Deptor mRNA levels (Fig. 1B), suggesting that DEPTOR transcription is turned off during differentiation. Differentiation of R1 mESCs into neurons was confirmed by changes in cell morphology and immunofluorescence (Fig. 1, C and D). The differentiation was efficient, as can be seen from the loss of pluripotency marker OCT4 and the presence of differentiation markers PAX6 and DCX (Fig. 1D). Consistent with Western blot and qPCR analysis, DEPTOR was absent from the differentiated cells, whereas a high level of cytoplasmic DEPTOR was detected in mESCs (Fig. 1D). It is known that knockdown of pluripotency markers, including Oct4, results in differentiation of mESCs. Interestingly, DEPTOR transcript levels were decreased upon Oct4 knockdown in mESCs, as measured by microarray expression profiling (19). We confirmed this by qPCR using gene-specific primers. As shown in Fig. 1E, knockdown of Oct4 using shRNA in R1 mESCs resulted in decreased DEPTOR expression in the cells. There was a concomitant decrease in two other pluripotency markers, Nanog and Zfp42. More importantly, the decrease in DEPTOR levels was similar in magnitude to that of Nanog and Zfp42. Fig. 2 shows time courses for DEPTOR and OCT4 mRNA (A) and protein (B) expression during differentiation toward a neural lineage over a 10-day period. Expression of the differentiation markers β3-tubulin and NeuN of these cells at day 10 is shown in Fig. 2C.
FIGURE 1.
DEPTOR is down-regulated during mESCs differentiation. A, representative image of DEPTOR, OCT4, and NANOG protein levels in undifferentiated and differentiated R1 mESCs with quantitation of at least three biological replicates. B, Deptor, Oct4, Nanog, and Zfp42 mRNA expression in undifferentiated and differentiated mESCs analyzed using qPCR. C, morphological changes of differentiated R1 mESCs analyzed under phase contrast. Scale bar, 20 μm. D, representative immunofluorescence image showing the loss of DEPTOR and Oct4 and the presence of differentiation markers Pax6 and Doublecortin with differentiation. Scale bar, 50 μm. E, Deptor, Oct4, Nanog, and Zfp42 mRNA expression upon Oct4 knockdown in mESCs. ***, p < 0.0005. Error bars, S.E.
FIGURE 2.
Kinetics of DEPTOR and Oct4 expression during mESCs differentiation. R1 mESCs were differentiated for 10 days on Matrigel-coated plates in the presence of IGF and inhibitors of TGF-β and BMP. Cells were collected before the start of differentiation (D0) each day for 10 days after the initiation of differentiation (D1–D10). A, Deptor and Oct4 mRNA expression were measured by qPCR. B, protein expression was measured by Western blot analysis. In both cases, actin was used as a control. C, differentiation of R1 mESCs was further analyzed at day 10 by immunofluorescence for the presence of differentiation markers β-tubulin, and NeuN. Scale bar, 50 μm.
mTORC1 Activity and Proteins Involved in mTOR Signaling Change during Differentiation
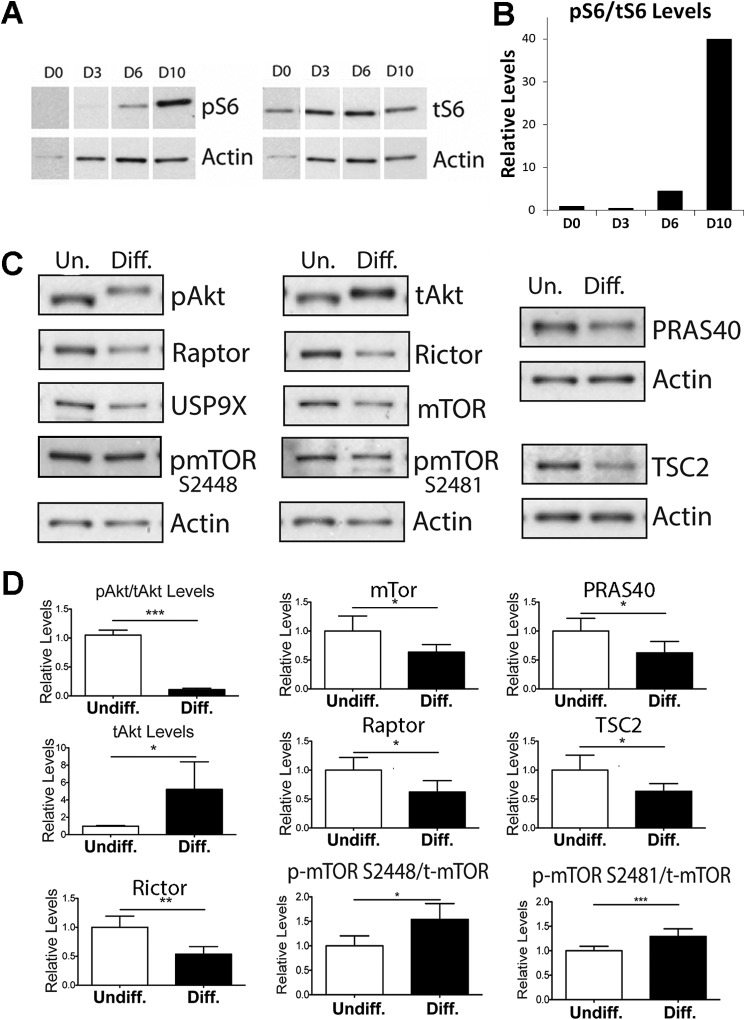
To further characterize changes in mTOR signaling associated with ESC differentiation, we assayed mTORC1 and mTORC2 activity in cells by measuring the phosphorylation of ribosomal protein S6 and Akt (protein kinase B), respectively. Differentiation of R1 mESCs resulted in a dramatic increase in S6 phosphorylation, indicating increased mTORC1 activity. We also observed increased phosphorylation of the mTORC1 substrate 4E-BP upon differentiation (not shown). Fig. 3A shows a time course of S6 phosphorylation after 3, 6, and 10 days of differentiation. The ratios of phosphorylated S6 (pS6) to total S6 (tS6) protein at these time points are shown in Fig. 3B.
FIGURE 3.
mTORC1 kinase activity and levels of mTOR interacting proteins change upon mESCs differentiation. A, a time course of mTORC1 activity during differentiation was analyzed by measuring S6 phosphorylation. B, the ratios of phospho-S6 (pS6) to total S6 (tS6) protein levels at these time points is shown. C, representative Western blots of mTOR and specific interacting protein levels in undifferentiated and differentiated R1 mESCs. Protein samples collected for undifferentiated and differentiated R1 mESCs were also analyzed for phospho-mTOR (pmTOR) Ser-2448 and Ser-2481 and total mTOR (tmTOR) levels. Actin was used as a loading control for all of the gels. The phospho-mTOR level was further normalized to total mTOR. D, quantitation of at least three biological replicates of experiments shown in C (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). Error bars, S.E.
Fig. 3C shows representative Western blots for mTOR substrates and interacting proteins at day 1 (Undiff.) and 10 days (Diff.) following directed differentiation. We did not observe increased AKT phosphorylation at the mTORC2 site Ser-473. However, we observed a change in AKT migration on SDS-PAGE. In differentiated cells, AKT migrated slowly, suggesting that it had other post-translational modifications. We also observed an increase in total AKT upon differentiation. There was also a decrease in mTOR and a number of its key interacting proteins upon differentiation. RAPTOR and RICTOR, components of mTORC1 and mTORC2, respectively, showed modestly decreased protein levels (∼40%) with differentiation. PRAS40, an mTOR-interacting protein that negatively regulates mTOR activity, and TSC2, an upstream inhibitor of mTOR signaling, also decreased with differentiation to a similar extent, suggestive of a tight control in mTOR signaling. However, none of these regulators of mTOR decreased to the same degree as DEPTOR during differentiation. Although we observed a decrease in mTOR levels, the fraction of mTOR phosphorylated at both Ser-2448 and Ser-2481 showed a modest increase with differentiation (Fig. 3C). Phosphorylation of mTOR at Ser-2448 and Ser-2481 has been shown to correlate with mTOR activation (20, 21). Fig. 3D shows quantification of these observations from multiple experimental replicates (n = 3 in each case). Our data indicate that during differentiation of mESCs toward neuronal fate, there is a modest decrease in levels of mTOR signaling components associated with an increase in mTORC1 activity. However, DEPTOR uniquely is turned off completely, indicating its critical requirement in pluripotent stem cells.
Decrease in DEPTOR Levels Is Associated with H1 hESCs Differentiation
To determine whether DEPTOR has a conserved role in human ESCs, we analyzed DEPTOR levels in undifferentiated or 2-week differentiated H1 hESCs. Cells were differentiated toward a neuronal lineage using DKK, IGF1, and Noggin (15, 16). After 2 weeks of differentiation, cells were analyzed for OCT4, PAX6, and DEPTOR expression using qPCR. As seen in Fig. 4, differentiation of H1 hESCs was accompanied by down-regulation of DEPTOR expression. This was associated with loss of OCT4 expression and a gain of PAX6 expression indicative of neural lineage. Reduction in DEPTOR levels with hESC differentiation, in a manner similar to that observed in mESCs, indicates that this mechanism for regulating mTOR signaling during stem cells differentiation is conserved between mice and humans.
FIGURE 4.
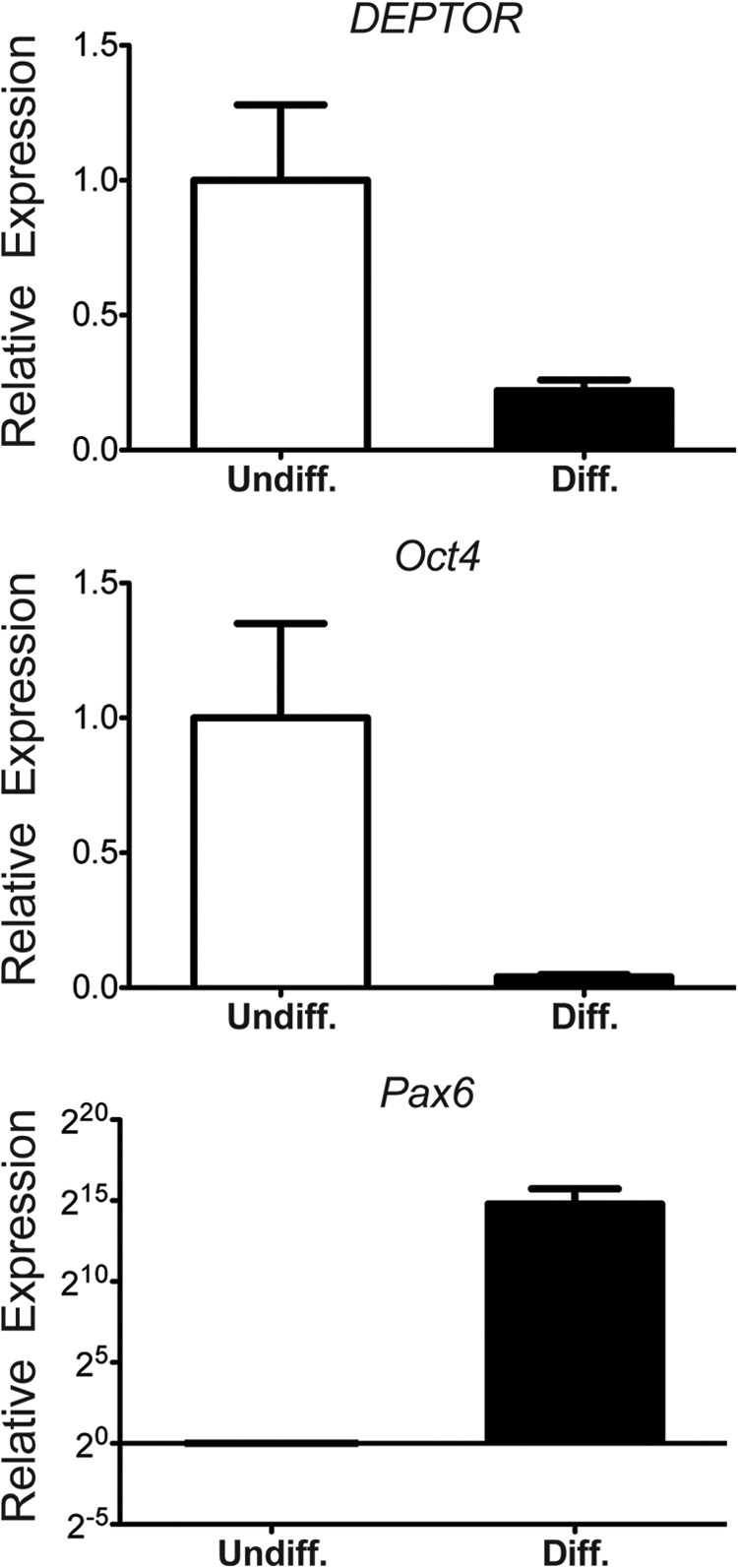
DEPTOR is down-regulated during hESCs differentiation. H1 hESCs were differentiated for 2 weeks, and DEPTOR, OCT4, and PAX6 expression was analyzed using qPCR. Data for three biological replicates are shown. Error bars, S.E.
DEPTOR Knockdown Is Sufficient to Promote Differentiation of mESCs
To test if DEPTOR played a role in the maintenance of mESC pluripotency, we reduced DEPTOR levels in the cells using shRNA. R1 mESCs were infected with lentivirus expressing either control shRNA constructs or shRNA targeting DEPTOR (Figs. 5 and 6). Following infection, drug selection was used to enrich for cells expressing shRNA. Knockdown of DEPTOR (shRNA2) was verified by qPCR and Western blot analysis (Fig. 5, A and B). DEPTOR knockdown cells displayed differentiation phenotypes in culture conditions (+LIF +2i) used to maintain pluripotent mESCs within 3–5 days of drug selection. This included a loss in the tightly packed refractile colony morphology of mESC colonies (Figs. 5C and 6B) (22, 23). qPCR analysis showed a reduction in expression of pluripotency markers Oct4, Nanog, and Zfp42 in cells with DEPTOR knockdown (Fig. 5A). These results suggest that DEPTOR is a key regulator of differentiation in mESCs. Interestingly, we observed that the differentiation phenotype was sensitive to the degree of DEPTOR knockdown. When a different shRNA sequence targeting DEPTOR (shRNA4) giving less than 80% knockdown was used, differentiation of mESCs was observed only in media lacking LIF but still containing two inhibitors involved in maintaining ESC fate (Fig. 6) (24). This suggests that mESCs are extremely sensitive to DEPTOR levels, and even small amounts of DEPTOR can promote the pluripotent phenotype. Fig. 6 also shows the effects of DEPTOR shRNA4 on expression of pluripotent markers (A), colony morphology (B), and induction of expression of the endodermal and ectodermal differentiation markers Hnf4A and Pax6, respectively (C).
FIGURE 5.
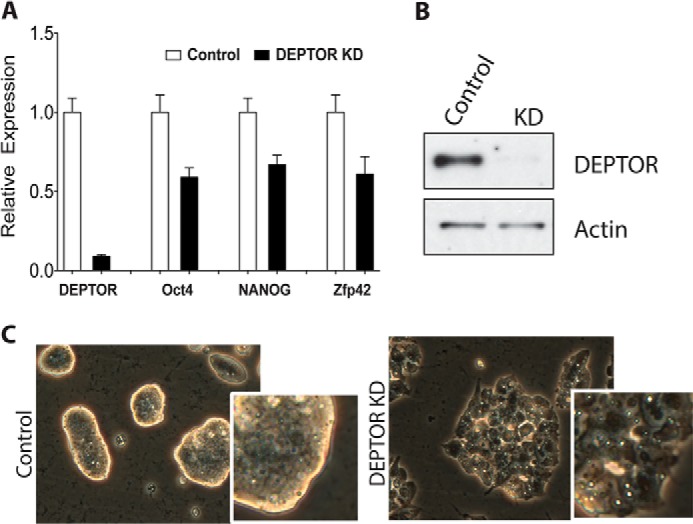
DEPTOR knockdown promotes mESCs differentiation. A, Deptor, Oct4, Nanog, and Zfp42 mRNA expression upon DEPTOR knockdown in R1 mESCs. B, representative blot of DEPTOR levels upon DEPTOR knockdown. C, representative phase-contrast image of R1 mESC morphological changes observed upon DEPTOR knockdown using shRNA2. The insets show higher magnification images of colony edges. Error bars, S.E.
FIGURE 6.
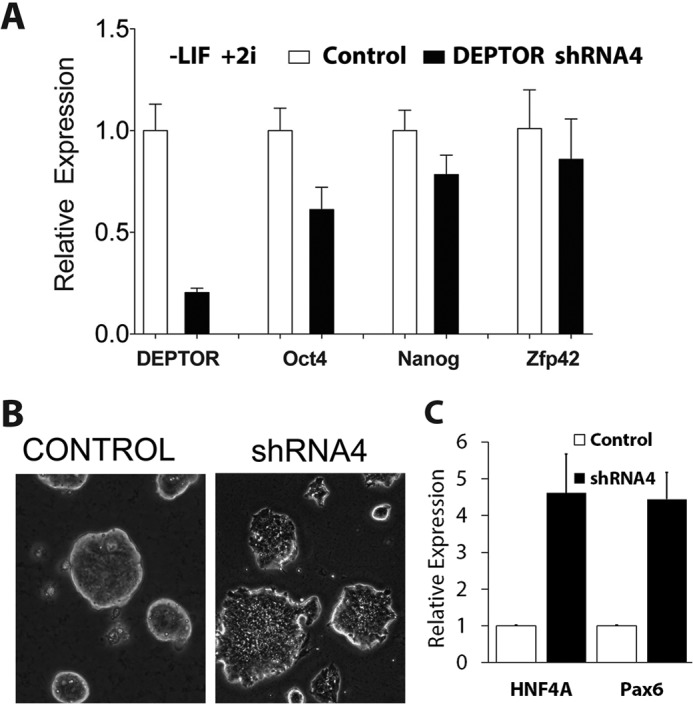
Validation of DEPTOR knockdown-mediated mESC differentiation. A, R1 mESCs were infected with control or DEPTOR shRNA4, and cells were selected using puromycin. Cells were then grown in the absence of LIF, mRNA was extracted, and qPCR was performed for Deptor, Oct4, Nanog, and Zfp42 as in Fig. 1. Actin was used as an internal control. B, morphological change of R1 mESCs upon DEPTOR knockdown in A was analyzed under phase contrast, and representative images are shown. C, Hnf4A and Pax6 mRNA expression mESCs after knockdown of DEPTOR using shRNA4. Error bars, S.E.
Whereas differentiation or knockdown of the transcription factor OCT4 resulted in reduced expression of DEPTOR and other pluripotent markers to similar degrees (Fig. 1, B and E), knockdown of DEPTOR had more modest effects on expression of OCT4 (Fig. 5A). This suggested that DEPTOR expression might be regulated by OCT4 directly. We searched for potential OCT4 binding sites in the promoter region of DEPTOR as well as in genes encoding other mTOR pathway regulators, TSC2 and PRAS40 (AKT1S1), using LASAGNA-Search (25). Upstream promoter regions of these genes were compared with the JASPAR (human) and TRANSFAC (mouse) public databases for predicted OCT4 binding motifs. Both human and the mouse DEPTOR promoters contain highly significant predicted OCT4 binding sites (human DEPTOR promoter, p value = 0.000575; mouse DEPTOR promoter, p value = 0.00035). The human promoter also has a predicted binding site for SOX2 (a pluripotency transcription factor) immediately adjacent to the OCT4 binding site. The same analysis of the PRAS40 and TSC2 promoters predicted no OCT4 binding sites. This result is consistent with our observation of changes in PRAS40 and TSC2 levels during differentiation of mESCs (Fig. 3B).
DISCUSSION
Our studies establish a novel role for DEPTOR as a stemness factor that promotes pluripotency of stem cells. DEPTOR is the only endogenous negative regulator of mTOR known to inhibit both mTORC1 and mTORC2 activities (10). DEPTOR acts as a tumor suppressor, and DEPTOR knockdown results in increased mTORC1/2 signaling. At high levels, DEPTOR facilitates increased mTORC2 activity by inhibiting negative feedback and promotes cell survival (10). Given its central role in coupling growth factor and nutrient signaling with metabolism and growth, the mTOR pathway has emerged as a critical regulator of stem cell maintenance. Activation of mTOR signaling through deletion of negative regulators, TSC1 and PTEN, or gain of function of mTOR activators, RHEB2 and AKT, in mouse hematopoietic stem cell populations results in hyperproliferation and decreased SC pools (3, 26, 27). Conversely, modest reduction in mTOR activity with rapamycin results in enhanced self-renewal of SC populations by preventing uncontrolled cell growth and exhaustion (2). Studies in Drosophila germ line and intestinal SCs have also demonstrated a role for TSC1/2 in mTOR regulation of SC maintenance (28, 29). We recently reported that the stemness factor USP9X is a negative regulator of mTOR and muscle stem cell differentiation (6). Together, these studies demonstrate the importance of inhibiting mTOR signaling to maintain the regenerative capacity of stem cells. Here we describe a novel and specific role for DEPTOR in this process.
Differentiation of mESCs toward a neuronal fate was accompanied by a dramatic decrease in DEPTOR (Fig. 1). No DEPTOR was observed in differentiated cells, as measured by immunofluorescence (Fig. 1D). Moreover, knockdown of OCT4 drastically reduced Deptor to levels comparable with those of the pluripotency markers Nanog and Zfp42 (Fig. 1E), indicating that it might have a critical role in SC maintenance. To investigate this, we knocked down DEPTOR levels in mESCs using shRNA. This was sufficient to cause these cells to differentiate (Figs. 5 and 6). DEPTOR knockdown with shRNA also caused reduction in pluripotency markers Oct4, Nanog, and Zfp42. Based on the knockdown data and bioinformatics analysis, Deptor plays a role downstream of OCT4. Recent work has shown that SOX2-induced suppression of mTOR is necessary for induced pluripotent stem cell generation (8), and although they did not look at DEPTOR, it is plausible that DEPTOR is the intermediate in the process based on our data. Overall, the Deptor-mTOR pathway very likely modulates pluripotency by regulating protein synthesis and autophagy by post-transcriptionally balancing the pluripotency network and repressing the differentiation network (9, 30).
In addition to mESCs, DEPTOR levels were dramatically decreased upon differentiation of human ESCs (Fig. 4). This suggests that the function of DEPTOR in stem cell maintenance is conserved. Given the decrease in DEPTOR levels with differentiation, it is not surprising that DEPTOR is absent in most adult tissues and in primary cell lines that have committed to a defined cell fate. These results indicate that DEPTOR is a novel stemness factor that predominantly functions in stem cells as an on/off switch to regulate mTOR signaling.
To further characterize the modulation of mTOR signaling during differentiation, we analyzed the phosphorylation of ribosomal protein S6. S6 phosphorylation increased 2–3-fold with differentiation in mESC, indicating a strong increase in mTORC1 activity (Fig. 3). This is consistent with what is seen during hESC differentiation (32). We did not observe any significant change in mTORC2 activity during differentiation (Fig. 3). Several groups have reported that constitutive activation of mTORC1 substrate S6K1 can reduce mTORC2 activity (33–37). Therefore, it is possible that we did not observe changes in mTORC2 activity in differentiated cells due to hyperactivation of mTORC1. However, we did observe that AKT levels increased upon differentiation and that a majority of AKT migrated slowly in SDS-PAGE, suggestive of posttranslational modification. AKT activity is known to be regulated by phosphorylation, ubiquitination, acetylation, and sumoylation (38, 39).
We also observed small but significant decreases in the levels of mTOR itself and several canonical mTOR-interacting proteins upon differentiation (Fig. 3). Decreased RICTOR and TSC2 levels with differentiation are consistent with a previous report that analyzed mTOR signaling during differentiation of hESCs (32). Reduction of TSC2 levels that we observed with differentiation of mESCs is consistent with the work done in Drosophila intestinal SCs, where knockdown of TSC2 caused an increased mTOR activity and loss of SC pools due to enhanced differentiation (28).
We also analyzed the levels of two other negative regulators of mTOR, PRAS40 and USP9X. PRAS40 regulates protein synthesis in myocytes and apoptosis during early embryoid body formation (40, 41). We found that PRAS40 levels decreased with differentiation of mESCs (Fig. 3). USP9X is a stemness gene that promotes self-renewal of neural progenitors (42). The decrease that we observed in USP9X levels upon differentiation of mESCs is in agreement with the inhibitory effect of USP9X on differentiation (Fig. 3). We note that the magnitudes of decrease in mTOR levels and in the levels of its interactors were similar (∼40% in all cases), suggesting that the stoichiometries of these components in mTOR complexes were not altered during differentiation. This is in contrast to the significant decrease in DEPTOR levels relative to other mTORC components.
The decrease in DEPTOR levels was comparable with that of pluripotency markers in the differentiated mESCs. This was also accompanied by increased mTORC1 activity. This indicates that DEPTOR-mediated regulation of mTOR signaling is critical to the maintenance of pluripotent stem cell populations, and disruption of this results in differentiation of stem cells. Recently, it was shown that inhibition of mTOR activity by resveratrol increased the efficiency of somatic cell reprogramming to form induced pluripotent stem cells (43). Resveratrol is known to cause inhibition of mTOR activity by promoting the association of DEPTOR with mTOR (44). This is consistent with a model where increased DEPTOR inhibition of mTOR facilitates the transition from non-stem cell to pluripotent states.
Complete mTOR inhibition in hESCs promotes increased differentiation toward mesodermal and endodermal lineages (9, 31). This is not surprising, given that loss of mTOR signaling in mESCs results in a loss of ESC viability (7). As discussed above, hyperactivation of mTOR can lead to proliferation and depletion of the stem cell pool. Based on these studies, it appears that a fine balance of mTOR activity is required in ESCs and that complete inhibition and/or overactivation of mTOR functions can cause reduced pluripotency in ESCs. Our data indicate that DEPTOR is a stemness factor that helps to maintain this balance of mTOR signaling in ESCs. High levels of DEPTOR in ESCs modulate mTOR activity to facilitate self-renewal. When conditions are favorable for differentiation, mTOR activity increases, accompanied by a reduction in DEPTOR levels. Because mTOR can promote down-regulation of DEPTOR, this results in a positive feedback loop that ensures continued mTOR signaling and thereby efficient differentiation of cells. Our study provides a novel connection between DEPTOR and mTOR regulation in stem cells that can be modulated to favor either increased regeneration through enhanced DEPTOR activity or differentiation through DEPTOR inhibition.
Regulation of stem cell pluripotency is critical for embryonic development and tissue homeostasis in the adult. The mTOR pathway, a central modulator of cell growth and proliferation in response to growth factor and nutrient signaling, is known to have a role in stem cell differentiation. We show that DEPTOR, an inhibitor of TORC1 activity, is highly expressed in embryonic stem cells and is significantly down-regulated as they differentiate. The expression pattern of DEPTOR during ESC differentiation is similar to that of the other known stemness genes Oct4, Nanog, and Zfp24. We demonstrate that DEPTOR knockdown is sufficient to promote differentiation of ESCs and that DEPTOR is a critical stemness factor regulating the role of mTOR in maintaining stem cell pluripotency.
Acknowledgment
We thank Henri Jasper for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant RL1 GM084432 (to R. E. H.). This work was also supported in part by California Institute for Regenerative Medicine Research Grant RB4-05785 (to D. A. L.).
- mTOR
- mammalian target of rapamycin
- mTORC
- mTOR complex
- SC
- stem cell
- mESC
- mouse embryonic stem cell
- hESC
- human embryonic stem cell
- qPCR
- quantitative PCR
- LIF
- leukemia inhibitory factor.
REFERENCES
- 1. Zoncu R., Efeyan A., Sabatini D. M. (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castilho R. M., Squarize C. H., Chodosh L. A., Williams B. O., Gutkind J. S. (2009) mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen C., Liu Y., Liu Y., Zheng P. (2009) mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal. 2, ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thompson B. J., Jankovic V., Gao J., Buonamici S., Vest A., Lee J. M., Zavadil J., Nimer S. D., Aifantis I. (2008) Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J. Exp. Med. 205, 1395–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J., Grindley J. C., Yin T., Jayasinghe S., He X. C., Ross J. T., Haug J. S., Rupp D., Porter-Westpfahl K. S., Wiedemann L. M., Wu H., Li L. (2006) PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature 441, 518–522 [DOI] [PubMed] [Google Scholar]
- 6. Agrawal P., Chen Y. T., Schilling B., Gibson B. W., Hughes R. E. (2012) Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR). J. Biol. Chem. 287, 21164–21175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murakami M., Ichisaka T., Maeda M., Oshiro N., Hara K., Edenhofer F., Kiyama H., Yonezawa K., Yamanaka S. (2004) mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol. Cell. Biol. 24, 6710–6718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang S., Xia P., Ye B., Huang G., Liu J., Fan Z. (2013) Transient activation of autophagy via Sox2-mediated suppression of mTOR is an important early step in reprogramming to pluripotency. Cell Stem Cell 13, 617–625 [DOI] [PubMed] [Google Scholar]
- 9. Zhou J., Su P., Wang L., Chen J., Zimmermann M., Genbacev O., Afonja O., Horne M. C., Tanaka T., Duan E., Fisher S. J., Liao J., Chen J., Wang F. (2009) mTOR supports long-term self-renewal and suppresses mesoderm and endoderm activities of human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 106, 7840–7845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S., Sabatini D. M. (2009) DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137, 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laplante M., Horvat S., Festuccia W. T., Birsoy K., Prevorsek Z., Efeyan A., Sabatini D. M. (2012) DEPTOR cell-autonomously promotes adipogenesis, and its expression is associated with obesity. Cell Metab. 16, 202–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Proud C. G. (2009) Dynamic balancing: DEPTOR tips the scales. J. Mol. Cell Biol. 1, 61–63 [DOI] [PubMed] [Google Scholar]
- 13. Kazi A. A., Hong-Brown L., Lang S. M., Lang C. H. (2011) Deptor knockdown enhances mTOR activity and protein synthesis in myocytes and ameliorates disuse muscle atrophy. Mol. Med. 17, 925–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Z., Zhong J., Gao D., Inuzuka H., Liu P., Wei W. (2012) DEPTOR ubiquitination and destruction by SCF(beta-TrCP). Am. J. Physiol. Endocrinol. Metab. 303, E163–E169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamba D. A., Gust J., Reh T. A. (2009) Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell 4, 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lamba D. A., Karl M. O., Ware C. B., Reh T. A. (2006) Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 103, 12769–12774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. La Torre A., Lamba D. A., Jayabalu A., Reh T. A. (2012) Production and transplantation of retinal cells from human and mouse embryonic stem cells. Methods Mol. Biol. 884, 229–246 [DOI] [PubMed] [Google Scholar]
- 18. Torres J., Prieto J., Durupt F. C., Broad S., Watt F. M. (2012) Efficient differentiation of embryonic stem cells into mesodermal precursors by BMP, retinoic acid and Notch signalling. PLoS One 7, e36405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loh Y. H., Wu Q., Chew J. L., Vega V. B., Zhang W., Chen X., Bourque G., George J., Leong B., Liu J., Wong K. Y., Sung K. W., Lee C. W., Zhao X. D., Chiu K. P., Lipovich L., Kuznetsov V. A., Robson P., Stanton L. W., Wei C. L., Ruan Y., Lim B., Ng H. H. (2006) The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 38, 431–440 [DOI] [PubMed] [Google Scholar]
- 20. Rosner M., Siegel N., Valli A., Fuchs C., Hengstschläger M. (2010) mTOR phosphorylated at S2448 binds to raptor and rictor. Amino Acids 38, 223–228 [DOI] [PubMed] [Google Scholar]
- 21. Soliman G. A., Acosta-Jaquez H. A., Dunlop E. A., Ekim B., Maj N. E., Tee A. R., Fingar D. C. (2010) mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J. Biol. Chem. 285, 7866–7879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davey R. E., Zandstra P. W. (2006) Spatial organization of embryonic stem cell responsiveness to autocrine gp130 ligands reveals an autoregulatory stem cell niche. Stem Cells 24, 2538–2548 [DOI] [PubMed] [Google Scholar]
- 23. Kelly K. F., Ng D. Y., Jayakumaran G., Wood G. A., Koide H., Doble B. W. (2011) β-Catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8, 214–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nichols J., Silva J., Roode M., Smith A. (2009) Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development 136, 3215–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee C., Huang C. H. (2013) LASAGNA-Search: an integrated web tool for transcription factor binding site search and visualization. BioTechniques 54, 141–153 [DOI] [PubMed] [Google Scholar]
- 26. Campbell T. B., Basu S., Hangoc G., Tao W., Broxmeyer H. E. (2009) Overexpression of Rheb2 enhances mouse hematopoietic progenitor cell growth while impairing stem cell repopulation. Blood 114, 3392–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalaitzidis D., Sykes S. M., Wang Z., Punt N., Tang Y., Ragu C., Sinha A. U., Lane S. W., Souza A. L., Clish C. B., Anastasiou D., Gilliland D. G., Scadden D. T., Guertin D. A., Armstrong S. A. (2012) mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell 11, 429–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapuria S., Karpac J., Biteau B., Hwangbo D., Jasper H. (2012) Notch-mediated suppression of TSC2 expression regulates cell differentiation in the Drosophila intestinal stem cell lineage. PLoS Genet. 8, e1003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun P., Quan Z., Zhang B., Wu T., Xi R. (2010) TSC1/2 tumour suppressor complex maintains Drosophila germline stem cells by preventing differentiation. Development 137, 2461–2469 [DOI] [PubMed] [Google Scholar]
- 30. Cho Y. H., Han K. M., Kim D., Lee J., Lee S. H., Choi K. W., Kim J., Han Y. M. (2014) Autophagy regulates homeostasis of pluripotency-associated proteins in hESCs. Stem Cells 32, 424–435 [DOI] [PubMed] [Google Scholar]
- 31. Lee K. W., Yook J. Y., Son M. Y., Kim M. J., Koo D. B., Han Y. M., Cho Y. S. (2010) Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev. 19, 557–568 [DOI] [PubMed] [Google Scholar]
- 32. Easley C. A., 4th, Ben-Yehudah A., Redinger C. J., Oliver S. L., Varum S. T., Eisinger V. M., Carlisle D. L., Donovan P. J., Schatten G. P. (2010) mTOR-mediated activation of p70 S6K induces differentiation of pluripotent human embryonic stem cells. Cell. Reprogram. 12, 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corradetti M. N., Guan K. L. (2006) Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25, 6347–6360 [DOI] [PubMed] [Google Scholar]
- 34. Dibble C. C., Asara J. M., Manning B. D. (2009) Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 29, 5657–5670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrington L. S., Findlay G. M., Lamb R. F. (2005) Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem. Sci. 30, 35–42 [DOI] [PubMed] [Google Scholar]
- 36. Julien L. A., Carriere A., Moreau J., Roux P. P. (2010) mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 30, 908–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manning B. D. (2004) Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 167, 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fan C. D., Lum M. A., Xu C., Black J. D., Wang X. (2013) Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the insulin-like growth factor-1 response. J. Biol. Chem. 288, 1674–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Manning B. D., Cantley L. C. (2007) AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fuchs C., Rosner M., Dolznig H., Mikula M., Kramer N., Hengstschläger M. (2012) Tuberin and PRAS40 are anti-apoptotic gatekeepers during early human amniotic fluid stem-cell differentiation. Hum. Mol. Genet. 21, 1049–1061 [DOI] [PubMed] [Google Scholar]
- 41. Kazi A. A., Lang C. H. (2010) PRAS40 regulates protein synthesis and cell cycle in C2C12 myoblasts. Mol. Med. 16, 359–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jolly L. A., Taylor V., Wood S. A. (2009) USP9X enhances the polarity and self-renewal of embryonic stem cell-derived neural progenitors. Mol. Biol. Cell 20, 2015–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen T., Shen L., Yu J., Wan H., Guo A., Chen J., Long Y., Zhao J., Pei G. (2011) Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell 10, 908–911 [DOI] [PubMed] [Google Scholar]
- 44. Liu M., Wilk S. A., Wang A., Zhou L., Wang R. H., Ogawa W., Deng C., Dong L. Q., Liu F. (2010) Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 285, 36387–36394 [DOI] [PMC free article] [PubMed] [Google Scholar]