Background: The p38 pathway is an evolutionarily conserved signaling pathway that responds to a variety of stresses.
Results: dIscU can be phosphorylated by dMK2, thereby impacting mitochondrial respiratory complex I activity.
Conclusion: Iron-sulfur cluster protein IscU phosphorylation by MK2 downstream of p38 signaling may regulate oxidative stress tolerance.
Significance: IscU is a novel substrate of MK2, mechanistically connecting the p38 pathway and mitochondria iron-sulfur clusters for the first time.
Keywords: Iron-Sulfur Protein, Mitochondrial Aconitase, Oxidative Stress, p38, p38 MAPK
Abstract
The p38 pathway is an evolutionarily conserved signaling pathway that responds to a variety of stresses. However, the underlying mechanisms are largely unknown. In the present study, we demonstrate that p38b is a major p38 MAPK involved in the regulation of oxidative stress tolerance in addition to p38a and p38c in Drosophila. We further show the importance of MK2 as a p38-activated downstream kinase in resistance to oxidative stresses. Furthermore, we identified the iron-sulfur cluster scaffold protein IscU as a new substrate of MK2 both in Drosophila cells and in mammalian cells. These results imply a new mechanistic connection between the p38 pathway and mitochondria iron-sulfur clusters.
Introduction
The p38-mitogen activated protein kinase (MAPK) pathway is an evolutionarily conserved signaling pathway that responds to a variety of stresses (1, 2). It can be activated by oxidative stresses and plays a pivotal role in oxidative stress tolerance in organisms ranging from yeasts to mammals. Recently, we generated mutants for all three p38 genes and reported that the p38 pathway-mediated stress response is part of the innate immunity of flies (3). However, the function of the p38 pathway in flies remains obscure, particularly the role of downstream kinases such as dMK2. In mammals, MAPK-activated protein kinase 2 (MK2)2 is known to regulate gene expression at the transcriptional and post-transcriptional level, control cytoskeletal architecture and cell cycle progression, and play a role in inflammation and cancer (4, 5). The Drosophila melanogaster genome encodes only one mammalian MK2 and MK3 ortholog, designated as MAPk-Ak2 (CG3086), or dMK2. Until now, little was known about the detailed function of dMK2 in Drosophila.
Iron-sulfur clusters are among the most ancient cofactors in nature. They are present in more than 200 different types of enzymes and proteins, promoting important roles in electron transport and oxidation-reduction reactions essential for numerous cellular processes, including ribosome biogenesis, purine catabolism, heme biosynthesis, DNA repair, iron metabolism, and so on (6). For example, after maturation, iron-sulfur clusters can be transferred into enzymes that are responsible for mitochondrial respiration and energy production, including aconitase, which is essential for the TCA cycle, and the mitochondrial respiratory complexes (complex I, II, and III), which are important components of the electron transport chain (7). Although the significance of iron-sulfur clusters has been recognized for more than 50 years, it was not until the late 1990s that it became clear that the assembly of iron-sulfur clusters is not spontaneous but a highly complex catalyzed process. Since then, more than 15 bacterial and 25 eukaryotic biogenesis components have been identified. Three types of iron-sulfur cluster biosynthetic machineries have been discovered in bacterial, archaeal and eukaryotic organelles: NIF (nitrogen fixation), ISC (iron-sulfur cluster), and SUF (sulfur utilization factor). Of these three, the ISC system is responsible for general iron-sulfur cluster biosynthesis in most organisms (6).
The ISC system contains several protein components, such as IscR, IscS, IscU, IscA, HscB, HscA, and Fdx. Among them, IscU is particularly important because it acts as a scaffold protein for iron-sulfur cluster biosynthesis and subsequent transfer of preformed clusters to the apo forms of acceptor proteins. IscU can facilitate the assembly of iron-sulfur clusters in both bacteria and eukaryotic mitochondria (8). The fundamental role of IscU has been demonstrated by knockdown and knock-out experiments in different species. There are two isoforms of IscU in humans (IscU1/2) and yeasts (ISU1/2) but only one in mice and Drosophila flies (CG9836). In yeasts, the double deletion of ISU1 and ISU2 has been shown to be lethal (9), and the complete loss of IscU results in early embryonic death in mice (10). In human cell lines, the knockdown of ISCU1/2 affects the activity of both aconitase and mitochondrial respiratory complex I (11). In Drosophila, however, the function of dIscU is completely unknown.
Here, we found that the Saccharomyces cerevisiae ISCU ortholog in D. melanogaster CG9836, dIscU, can be phosphorylated by dMK2, thereby impacting the mitochondrial respiratory complex I activity and regulating oxidative stress tolerance.
MATERIALS AND METHODS
Fly Husbandry and Fly Stocks
Flies were raised at 25 °C on standard yeast-cornmeal-agar medium (JazzMix, Fisher Scientific AS153). The following fly stocks were used: y1 w67c23 (Bloomington stock 6599); y1 w*; ry506 Sb1 P{ry[+t7.2] = [delta]2-3}99B/TM6 (Bloomington stock 3664); y1 w67c23 P{w[+mC] y[+mDint2] = EPgy2}EY11791 (Bloomington stock 20697); y1w67c23;P{SUPor-P}KG01476 ry506(Bloomington stock 13445); da-Gal4 (Bloomington stock 5460); y1 w67c23 dMK25B; y1 w67c23CG9836115; y1 w67c23 dMK25B-UAS-dMK2[w+]; y1 w67c23 dMK25B;dMK2[w+]8; y1 w67c23 dMK25B;dMK2[w+]9; y1 w67c23CG9836115;dIscU-S20A24; y1 w67c23CG9836115;dIscU-S20A25; y1 w67c23CG9836115;dIscU-S20A28; y1 w67c23CG9836115;dIscU-WT37; y1 w67c23CG9836115;dIscU-WT39; and y1 w67c23CG9836115;dIscU-WT40.
Reagents and Antibodies
Mouse anti-FLAG (F1804) (1:10000) was purchased from Sigma-Aldrich; rabbit anti-Mk2 and anti-phospho-MK2 (1:1000) were purchased from Cell Signaling; anti-phospho-mIscU (Ser-29) (1:200) antibody was generated by Abmart. All other antibodies (1:1000) were purchased from Santa Cruz Biotechnology. The expression plasmids of human MK2, human IscU2, human IscU2-S29A, Drosophila MK2, and Drosophila IscU were generated in pcDNA6 or pcDNA3 vectors. To generate GST fusion of MK2 and IscU, MK2 and IscU were expressed in pGEX vectors. Lentivirus vectors from Biosettia were used to express protein and shRNA in cultured cells. All mutant constructs of MK2 and IscU were created by PCR mutagenesis and verified by DNA sequencing.
Real-Time PCR (RT-PCR)
Total RNA was isolated from adult flies or mammalian cell using TRIzol reagent (Invitrogen catalogue number 15596-018). Complementary DNA was synthesized with oligo(dT) primers and the M-MLV reverse transcriptase RT-PCR system (Takara) and analyzed by PCR with gene-specific primers. Quantitative RT-PCR was then done on the BIO-RAD CFX 96TM real-time system. All assays were done in triplicate and normalized to rp49 levels. RT-PCR was done with the primers below: dMK2-forward, 5′-CTGCTACACTCCCTATTACGTG-3′, dMK2-reverse, 5′-ATGGCTAGGCCGTGGTTGCTG-3′; dIscU-forward, 5′-GTCCCTGGTGCGAAACTCCTCCC-3′; and dIscU-reverse, 5′-CCGGTGCCCACAGTGACATCCTT-3′.
Cell Culture, Transfection, and Lentivirus Infection
Mammalian Cells were cultured in DMEM (Invitrogen) containing 10% FBS (Invitrogen). The HeLa cell line was purchased from ATCC, and the MK2 KO and WT MEF lines were isolated and immortalized by the Han laboratory. Calcium phosphate precipitation or Lipofectamine 2000 was used for cell transfection. HEK293FT (Biosettia) was used to prepare the lentivirus, as described in lentivirus expression system (Biosettia). Drosophila S2 cells (ATCC) were cultured at 25 °C in SF900-II serum-free medium (Gibco). For transfection, S2 cells were incubated in Schneider's medium (Lonza) supplemented with 10% FBS, and transfections were performed by the calcium phosphate precipitation method. After 12 h, the medium was removed and replaced with SF900-II serum-free medium.
SDS-PAGE and Immunoblotting
Total cell lysates or immunoprecipitation samples were prepared with SDS-PAGE sample buffer on ice, boiled for 5 min, and separated by SDS-PAGE. Gels were then blotted, and blots were processed by standard methods using 5% skim milk in TBS consisting of 20 mm Tris-Cl (pH 7.5) and 154 mm NaCl with 0.1% Tween 20 for blocking and incubation steps. Primary antibodies were diluted to concentrations ranging from 1:1000 to 1:10,000 and incubated overnight at 4 °C. Blots were incubated with affinity-purified, HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (Pierce) diluted to 1:5000 and incubated 1 h at 22 °C. Molecular mass standards (10–250 kDa) were prestained with Precision Plus All Blue (Bio-Rad). Protein concentration was measured by the Pierce BCA protein assay kit (Thermo Scientific 23225).
In Vitro Kinase Assay
His-hsp27, GST-dMK2, GST-dMK2EE, GST-mIscU, GST-dIscU, GST-dIscU-S20A, GST-dIscU-S42A, GST-dIscU-T83A, and GST-dIscU-S104A were purified from Escherichia coli and subjected to a kinase assay in kinase buffer (25 mm Tris, pH 7.5, 10 mm MgCl2, 2 mm DTT, 5 mm glycerophosphate, 0.1 mm Na3VO4) at 30 °C for 30 min. To generate constitutively active dMK2, two point mutants were made: T178E and S228E.
Complex I Activity Assay
The fly mitochondrial fraction samples were prepared according to the manufacturer's protocol (MitoSciences MS141). To accurately assess enzyme activity in the linear range of measurements, mitochondrial protein from fly samples were loaded into each well for immunocapture of complex I. After washing, Complex I activity was measured by spectrophotometry at 340 nm (A340 nm), which reflects the oxidation of NADH to NAD+. About 200 6–7-day-old adult flies were used for each sample, and 300 μg of mitochondrial proteins from flies were used for each assay.
Aconitase Activity Assay
Following the homogenization of samples at 1%(w/v) in ice-cold 0.2 mm sodium citrate in 50 mm Tris-HCl, pH 7.4, for 15–20 s, total aconitase activity was measured using the Bioxytech Aconitase-340 kit (Oxis Research) by spectrophotometry reflecting aconitase-dependent NADPH generation. The mitochondrial fraction samples were prepared according to the kit protocol. About 200 6–7-day-old adult flies were used for each sample. 200 μg of total protein and 750 μg of mitochondrial proteins from fly samples were used for each assay.
Mass Spectrometry
Briefly, gel fragments were destained with 50% acetonitrile, 50 mm ammonium bicarbonate followed by incubation in 10 mm DTT at 56 °C for 30 min for cysteine reduction, and cysteine residues were then alkylated with 50 mm iodoacetamide for 30 min at room temperature in darkness. Trypsin was then added to protein at a protein:trypsin ratio of 50:1, and digestion was carried out at 37 °C for 12 h. Tryptic peptides were extracted from gels using 0.15% formic acid, 67% acetonitrile and dried prior to phosphopeptide enrichment. Phosphopeptide was enriched using immobilized metal ion affinity chromatography as described previously (12).
Isoelectric Focusing (IEF) and Immunoblotting
Proteins were precipitated with TCA/acetone, suspended in IEF sample buffer (7 m urea, 2 m thiourea, 2% CHAPS, 0.8% ampholytes, pH 3–10, 50 mm DTT, 4% glycerol), and subjected to isoelectric focusing using a mini gel format. After focusing, IEF gels were incubated in 12% TCA overnight at 4 °C, washed three times with water for 15 min, incubated in 7 m urea, 2 m thiourea, and 5 mm DTT for 10 min to renature the proteins, and then soaked in lower gel buffer (0.37 mm Tris, pH 8.8, 0.1% SDS) three times for 15 min. The focused proteins were then transferred onto a 0.45-mm PVDF membrane and immunoblotted with anti-GFP antibodies.
Oxidative Stress Assay
Paraquat or H2O2 was diluted to a suitable concentration in 5% sucrose. 750 μl of solution were pipetted on filter paper for each vial, and dead flies were counted three times a day until all the flies died. All the flies used for the assay were 3–5 days old, raised at 25 °C. 60 flies was used for each sample, and 20 flies were kept in each vial, and experiments were repeated three times.
RESULTS
dp38b and dMK2 Knock-out Flies Have Shorter Lifespan under Oxidative Stress
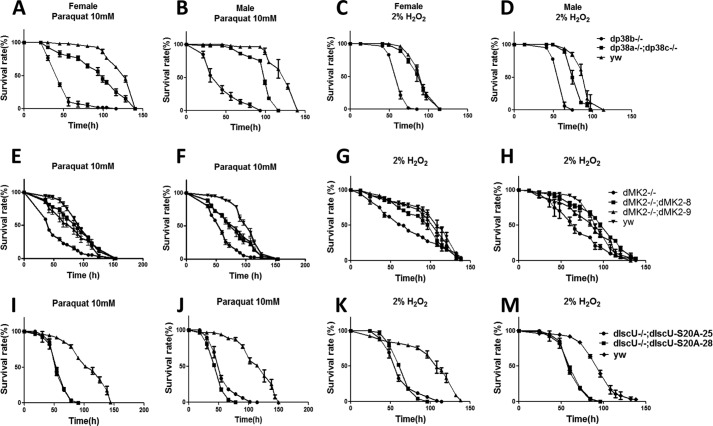
Based on previous work demonstrating the role of p38 signaling in oxidative stress responses (13), we tested the susceptibility of dp38b−/− mutant and dp38a−/−;dp38c−/− double mutant flies to paraquat or H2O2-induced oxidative stresses. dp38b−/− flies were much more sensitive to oxidative stress than dp38a−/−;dp38c−/− double mutants and yw control in female and male flies (Fig. 1, A–D), indicating that p38b is a major p38 MAPK involved in the regulation of oxidative stress tolerance. dMK2 is believed to play a role in regulating oxidative stress because of the upstream kinase p38. Thus, we wanted to evaluate whether the dMK2−/− flies are different from wild-type flies in their lifespan under oxidative stress. dMK2−/− flies were generated by P-element-mediated imprecise excisions (14) of a Drosophila line obtained from the Gene Disrupt Project database. In the dMK2−/− fly line we generated, exon 1 and exon 2 were deleted (Fig. 2A). Flies with single mutations of dMK2 did not have any visible morphological or developmental abnormalities. We then generated rescue fly lines of dMK2−/− flies by reintroducing dMK2 genomic fragment and named the two fly lines dMK2−/−;dMK28 and dMK2−/−;dMK29. Success of the rescue lines was confirmed by PCR (Fig. 2C). We treated these flies with paraquat or H2O2 and then determined their lifespan. Both female and male dMK2−/− flies were more sensitive to paraquat and H2O2 (Fig. 1, E–H) as compared with yw and dMK2 rescue flies, demonstrating that dMK2 contributes to oxidative stress tolerance.
FIGURE 1.
dIscU-S20A rescue flies, (dMK2−/− flies) have a shorter lifespan under oxidative stress like dp38b−/− flies. A and B, E and F, and I and J, different fly lines were exposed to 10 mm paraquat. C and D, G and H, and K and M, different fly lines were exposed to 2% H2O2. Data from two or three independent experiments are expressed as mean ± S.E.
FIGURE 2.
Generation of mutant and transgenic flies for dMK2 and dIscU. A, a fly line containing EY11791 excisions was established to characterize deficiencies in dMK2. The promoter region and 5′ portion of the dMK2 transcript, including the first two exons, were deleted in line dMK25B. B, a fly line containing KG01476 excisions was established to characterize deficiencies in CG9836. C, the mRNA expression in dMK2−/− and dMK2 rescue flies. D and E, the mRNA level of the selected transgenic flies for both dIscU-S20A and dIscU-s20E rescue flies. Error bars reflect S.E.
dMK2 Interacts with and Phosphorylates dIscU at Serine 20
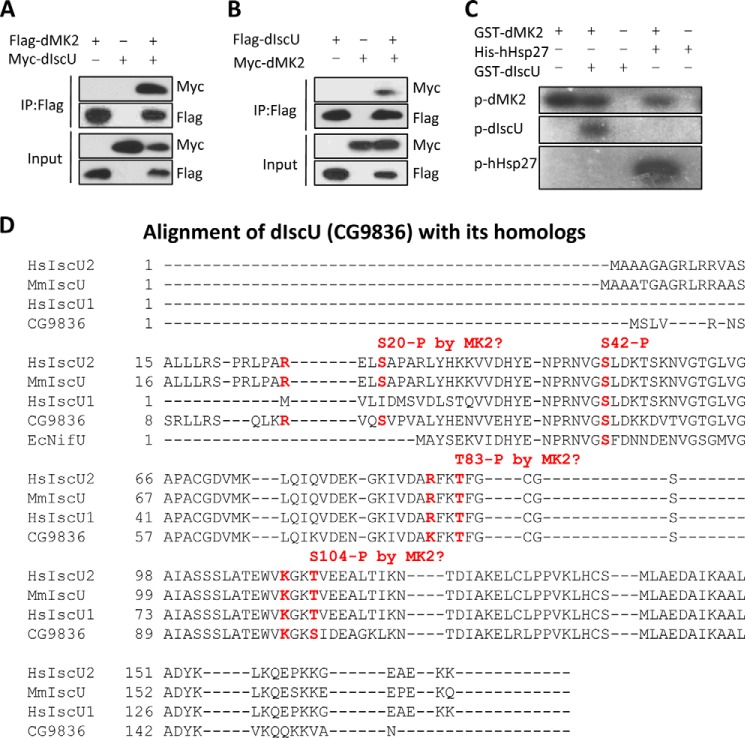
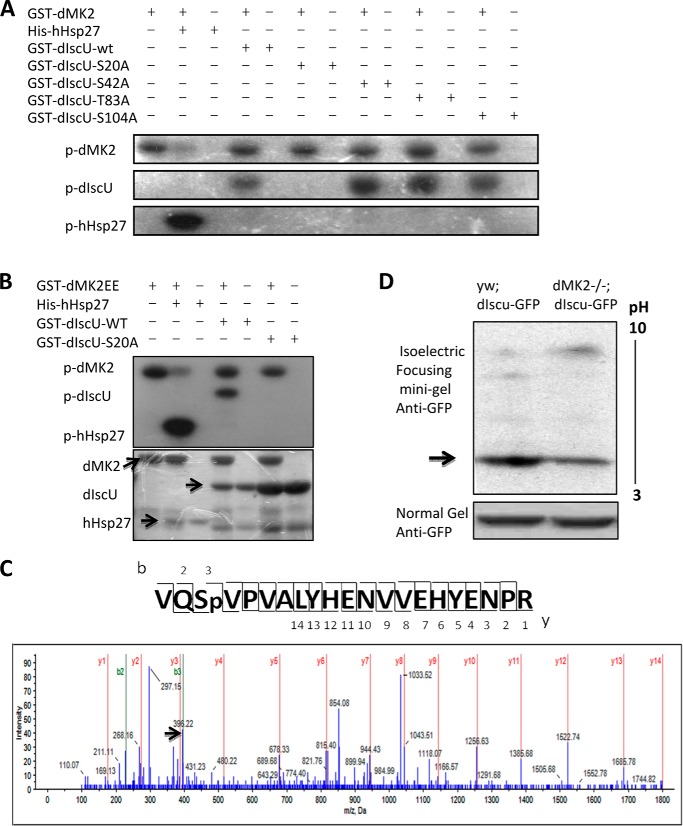
To explore unknown downstream genes of MK2, we searched available databases and found that in a genome-wide yeast two-hybrid screen (DroID – The Drosophila Interactions Database), the Drosophila CG9836 (dIscU) gene product was identified to interact with dMK2. To confirm the interaction between dMK2 and dIscU, we co-expressed dMK2 with dIscU in 293T cells and detected their association by co-immunoprecipitations (Fig. 3, A and B). Because MK2 is a protein kinase, we next determined whether dIscU is a substrate of dMK2. In vitro kinase assays were performed using the recombinant proteins expressed in E. coli. dMK2 phosphorylated dIscU but to a lesser extent than it phosphorylated human heat shock protein 27 (Hsp27), a known substrate of MK2 (Fig. 3C). To find the phosphorylation site(s) on dIscU, we analyzed the sequence of dIscU by aligning it with human and mouse IscU sequences (Fig. 3D). Three serine or threonine sites with features of consensus motif of MK2 phosphorylation site can be found in the sequences (15). We individually mutated each of the Thr or Ser residues (Ser-20, Thr-83, and Ser-104) as well as another conserved Ser residue (Ser-42) to alanine and determined whether any of these mutations prevented phosphorylation by dMK2 in in vitro kinase assays. Only the Ser-20 to Ala mutation completely abolished the phosphorylation (Fig. 4A), indicating that dIscU Ser-20 is the only site phosphorylated by dMK2. A similar result was obtained when the mutated constitutively active form of dMK2 (dMK2EE) was used in the kinase assay (Fig. 4B). The lower phosphorylation intensity of dIscU as compared with that of Hsp27 is most likely because there are multiple MK2 phosphorylation sites on Hsp27.
FIGURE 3.
dMK2 interacts with and phosphorylates dIscU. A and B, FLAG-dMK2 was coexpressed with Myc-dIscU, and FLAG-dIscU was coexpressed with Myc-dMK2 in 293T cells. FLAG-tagged protein was immunoprecipitated using FLAG M2 beads 36 h after transfection. The cell lysates and immunoprecipitates were analyzed by Western blot. C, GST-dIscU was incubated with or without GST-dMK2 in a kinase buffer containing [32P]ATP. Phosphorylation of dIscU was detected by autoradiography. D, alignment of dIscU (CG9836) with its homologs. Hs, Homo sapiens; Mm, Mus musculus; Ec, E. coli.
FIGURE 4.
dMK2 phosphorylates dIscU at serine 20. A, GST-dIscU, GST-dIscU-S20A, GST-dIscU-S42A, GST-dIscU-T83A, and GST-dIscU-S104A were incubated with or without GST-dMK2 in kinase buffer containing [32P]ATP. Phosphorylation of dIscU was detected by autoradiography. p indicates phospho form. B, GST-dIscU or GST-dIscU-S20A was incubated with or without GST-dMK2EE in a kinase buffer containing [32P]ATP. Phosphorylation of dIscU was analyzed by autoradiography and not detected in dIscU-S20A mutants. Proteins of dMK2EE, dIscU, and dIscU-S20A were determined by Coomassie Blue staining. C, FLAG-tagged-dIscU was overexpressed in Drosophila S2 cells, and FLAG-tagged protein was subsequently immunoprecipitated using FLAG M2 beads 4 days after transfection. The band corresponding to dIscU was excised from Coomassie Blue-stained gels and subjected to in-gel digestion for mass spectra. D, GFP-dIscU was overexpressed in background of both yw and dMK2−/− flies. Fly proteins were then precipitated with TCA/acetone, suspended in IEF sample buffer, and subjected to isoelectric focusing using a mini gel.
We next overexpressed FLAG-tagged-dIscU in Drosophila S2 cell line and then immunoprecipitated dIscU. The band corresponding to dIscU was excised from Coomassie Blue-stained gels and subjected to in-gel digestion for mass spectrometry. We identified several Ser and Thr sites on dIscU that can be phosphorylated, and Ser-20 was one of the most significant ones, indicating that dIscU Ser-20 can be phosphorylated in cultured S2 cell lines (Fig. 4C). To determine whether dMK2 mediates dIscU phosphorylation in vivo, we generated a UAS-dIscU-GFP transgenic fly and crossed it onto a dMK2−/− background. The whole body overexpression of dIscU was lethal, so we used the GMR-Gal4 driver to overexpress dIscU only in the eyes. After crossing with GMR-Gal4 flies, we used isoelectric focusing followed by Western blotting with anti-GFP antibodies to analyze the proteins from the heads of the flies. As shown in Fig. 4D, the negatively charged dIscU-GFP protein was decreased in dMK2−/− background flies as compared with yw background flies, suggesting reduced phosphorylation of dIscU in dMK2−/− flies. However, the negatively charged dIscU-GFP was still present to an extent, indicating that other kinase(s) either naturally phosphorylate dIscU or compensate dMK2 loss by phosphorylating dIscU. Taken together, our data indicated that dMK2 phosphorylates dIscU at serine 20 both in vitro and in vivo.
dIscU-S20A Rescue Flies Have a Shorter Lifespan under Oxidative Stress
To understand the function of dIscU phosphorylation, dIscU−/− flies were generated by P-element-mediated imprecise excisions (14) as previously done in the dMK2−/− flies. Because homozygous mutation of dIscU (Fig. 2B) was lethal, we inserted genomic fragments of dIscU-S20A or dIscU-S20E sequences into dIscU−/− flies to generate two transgenic rescue fly lines. Among them, dIscU-S20E; dIscU−/− flies lived only to the third instar larvae stage, whereas dIscU-S20A; dIscU−/− flies developed into adulthood without any visible morphological or developmental abnormalities (Table 1). We used RT-PCR to measure dIscU-S20A and dIscU-S20E expression in the different rescue fly lines and chose three dIscU-S20A and two dIscU-S20E rescue flies that had the same dIscU mRNA level as yw control flies (Fig. 2, D and E) for our next experiments.
TABLE 1.
dIscU transgenic flies
| Gene type | Number of lines | Status |
|---|---|---|
| dIscU−/−;dIscU-S20A | 10 | Normal |
| dIscU−/−;dIscU-S20E | 3 | Homozygous lethal after third instar larvae |
Next, we treated dIscU-S20A rescue flies with paraquat or H2O2 and determined their lifespan, as previously done with the dMK2−/− and dp38b−/− flies. As mentioned above, both lines of dIscU-S20A rescue flies had a shorter lifespan as compared with yw control flies (Fig. 1, I–M). These results indicated that the p38 pathway may regulate oxidative stress tolerance via dMK2 phosphorylation of dIscU at serine 20.
Phosphorylation of dIscU on Ser-20 by dMK2 Affects Complex I Activity but Not Aconitase Activity
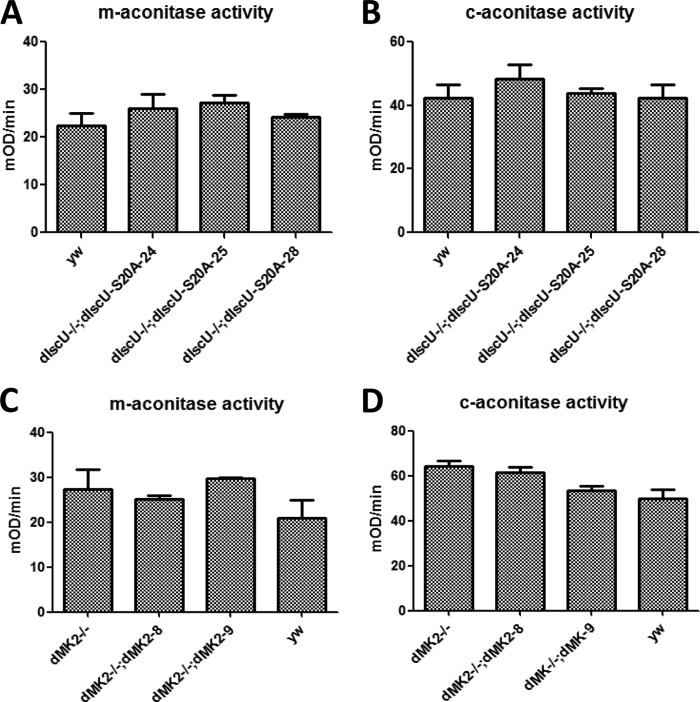
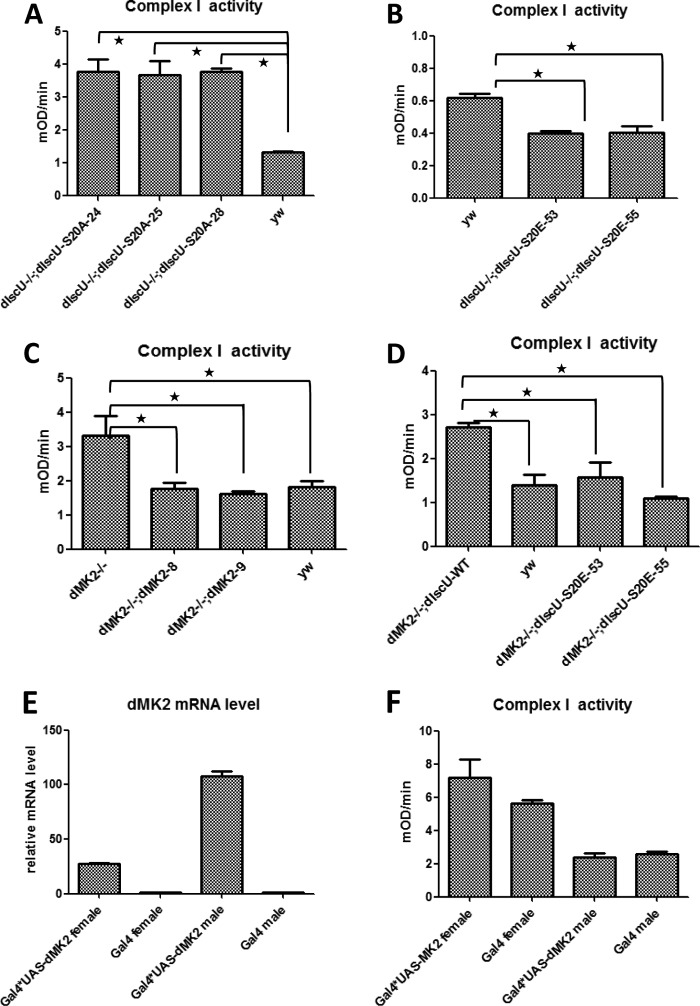
To investigate the mechanism of how dMK2 regulates dIscU by phosphorylation, we measured aconitase and mitochondrial respiratory complex I activity in dIscU rescue flies as aconitase activity and mitochondrial respiratory complex I activity have previously been used to study the function of IscU in mouse tissue and human cell lines (11, 16, 17). As shown in Fig. 5, A and B, dIscU-S20A rescue flies showed the same mitochondrial and cytoplasmic aconitase activities as yw flies, indicating that dIscU Ser-20 phosphorylation has no role in controlling aconitase activity. In contrast, dIscU-S20A rescue flies had much higher complex I activity in comparison with yw flies (Fig. 6A). We then used third instar larvae to compare the complex I activity between dIscU-S20E rescue and yw control flies because the dIscU-S20E mutation was adult lethal. As we expected, the complex I activity in dIscU-S20E rescue flies was much lower than in yw flies (Fig. 6B). It appears that phosphorylation of dIscU at Ser-20 might only affect some functions of dIscU.
FIGURE 5.
Both dIscU-S20A and dMK2−/− have no role in regulating aconitase activity neither in mitochondrial nor in cytosol. A and C, mitochondrial aconitase (m-aconitase) activity was measured by the Bioxytech Aconitase-340 kit (Oxis Research). 750 μg of mitochondrial protein were used. mOD, milli optical density. B and D, cytoplasmic aconitase (c-aconitase) activity was measured by the Bioxytech Aconitase-340 kit (Oxis Research). 200 μg of protein were used. Error bars reflect S.E.
FIGURE 6.
dMK2 regulates complex I activity just as dIscU-S20A mutant does. A–D, mitochondrial complex I activity was measured from different fly lines by complex I enzyme activity microplate assay kit (MitoSciences). mOD, milli optical density. E, UAS-dMK2 transgenic flies were crossed with da-Gal4 flies. The dMK2 mRNA level was then measured by RT-PCR. F, the complex I activity of dMK2-overexpressing flies was measured. Error bars reflect S.E.; star signifies p < 0.05.
We then measured aconitase and mitochondrial respiratory complex I activity in yw, dMK2−/−, and the two dMK2 rescue fly lines. As observed in the dIscU rescue flies, there was no significant difference in aconitase activity, both mitochondrial and cytoplasmic (Fig. 5, C and D), indicating that dMK2, like Ser-20 phosphorylation of dIscU, has no role in controlling aconitase activity. Again, as seen in the dIscU-S20A rescue flies, we observed significant difference in complex I activity between dMK2 normal (yw, dMK2−/−; dMK28, and dMK2−/−; dMK29) and dMK2−/− flies (Fig. 6C), suggesting that dMK2 negatively regulates complex I activity by phosphorylating dIscU Ser-20. To confirm our hypothesis, we introduced the dIscU-S20E mutation into dMK2−/− flies by crossing and then measured complex I activity. Again, as we expected, the up-regulated activity of complex I by dMK2−/− is prevented by dIscU-S20E (Fig. 6D). The close correlation between dMK2 deficiency and blocking of dIscU phosphorylation in their effects on aconitase and complex I activity strongly suggests that dMK2-mediated dIscU phosphorylation negatively affects complex I activity but has no effect on aconitase activity.
We also overexpressed dMK2 in flies by crossing UAS-dMK2 transgenic flies with da-Gal4 flies and subsequently measured the mitochondrial respiratory complex I activity. Although dMK2 mRNA level was much higher in Gal4*UAS-dMK2 flies (Fig. 6E), there were no significant differences between Gal4*UAS-dMK2 and control Gal4 lines in complex I activity (Fig. 6F). Thus, dIscU is most likely phosphorylated by low level basal activity of dMK2, and dMK2 overexpression appears to have no role in dIscU phosphorylation.
The Phosphorylation of IscU by MK2 Also Occurs in Mammalian Cells
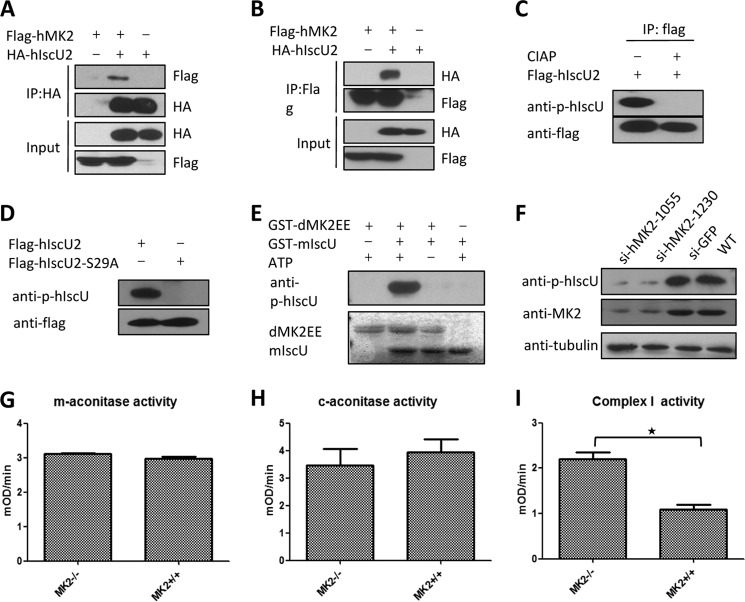
Because IscU is an evolutionarily conserved protein and the phosphorylation site Ser-20 on dIscU is conserved in mammals, we wanted to know whether the phosphorylation of IscU by MK2 also occurs in mammalian cells. There are two human IscU isoforms and one mouse isoform (Fig. 3D). hIscU2 contains the conserved Ser (Ser-29) residue, and it shares 96% sequence homology with mIscU and 69% with dIscU. We co-expressed hMK2 and hIscU2 in 293T cells and detected interaction between hMK2 and hIscU2 by co-immunoprecipitation (Fig. 7, A and B). To detect the phosphorylation of hIscU2 or mIscU, we generated an anti-Ser-29 phospho-hIscU2 antibody (Fig. 7, C and D). With this antibody, we detected Ser-29 phosphorylation of mIscU by dMK2EE in an in vitro kinase assay (Fig. 7E). Unfortunately, the phospho-hIscU2 antibody fail to detect endogenous mIscU in MEF cells, possibly due to a low level of phosphorylation. Because our phospho-hIscU2 antibody could detect endogenous proteins in HeLa cells, we used HeLa cells to determine whether MK2 could phosphorylate IscU in mammalian cells. We knocked down hMK2 in HeLa cells by shRNA and then evaluated the hIscU2 phosphorylation. As shown in Fig. 7F, phospho-hIscU2 was significantly decreased when hMK2 was knocked down. Thus, hMK2 is essential for the Ser-29 phosphorylation of hIscU2 in HeLa cells.
FIGURE 7.
The phosphorylation of IscU by MK2 also occurs in mammalian cells. A and B, FLAG-hMK2 was coexpressed with HA-hIscU2 in 293T cells. FLAG-hMK2 was immunoprecipitated using FLAG M2 beads 36 h after transfection, and HA-hIscU2 was immunoprecipitated using HA beads. The cell lysates and immunoprecipitates were analyzed by Western blot using antibodies against FLAG and HA. C, FLAG-hIscU2 was overexpressed in 293T cells and subsequently immunoprecipitated (IP) using FLAG M2 beads 36 h after transfection. The protein samples were treated with or without calf intestinal alkaline phosphatase (CIAP). The expression and phosphorylation levels of hIscU2 were determined by Western blot. D, FLAG-hIscU2 and FLAG-hIscU2-S29A were overexpressed in 293T cells, respectively. The expression and phosphorylation of hIscU2 were determined by Western blot. E, GST-mIscU was incubated with or without dMK2EE in kinase buffer. Phosphorylation of mIscU was detected by anti-phospho-mIscU antibody. Proteins of dMK2EE and mIscU were determined by Coomassie Blue staining. F, HeLa cells were infected with lentivirus-expressing shGFP or MK2 shRNAs for 48 h. Protein level of MK2 and phosphorylation level of hIscU2 were determined by Western blot. G, The mitochondrial aconitase (m-aconitase) activity was measured using MK2−/− and MK2+/+ MEF cells. mOD, milli optical density. H, the cytoplasmic aconitase (c-aconitase) activity was measured using MK2−/− and MK2+/+ MEF cells. I, the complex I activity was measured using MK2−/− and MK2+/+ MEF cells. Error bars reflect S.E.; star signifies p < 0.05.
Although IscU phosphorylation in MEF cells was undetectable by our anti-phospho-hIscU2 antibody, we still measured the activities of mitochondrial and cytoplasmic aconitase and complex I. As in the yw and dMK2−/− flies, we found that MK2 knock-out did not affect aconitase activity (Fig. 7, G and H) but increased complex I activity (Fig. 7I), suggesting conserved IscU phosphorylation by MK2.
DISCUSSION
The p38 signaling pathway plays an important role as a mediator of apoptosis in response to different stress stimuli, including oxidative stress (18–20). Here, we show that p38 −/− Drosophila flies were more sensitive to paraquat- and H2O2-induced oxidative stress, indicating the role of p38 signaling pathway in regulating oxidative stress tolerance. In our study, MK2, a protein kinase downstream of the p38 pathway, can phosphorylate iron-sulfur cluster protein IscU both in Drosophila cells and in mammalian cells. Furthermore, the dMK2−/− and the dIscU phosphorylation site mutation rescue flies were more sensitive to paraquat- and H2O2-induced oxidative stress. From these results, we believe that the p38 pathway regulates oxidative stress tolerance in Drosophila by MK2 phosphorylation of IscU.
Current knowledge of iron-sulfur clusters is mainly centered on the assembly mechanism, but the regulation mechanism is almost unknown. Recently, hIscU1/2, the key component of the ISC system, was shown to be a target of microRNA-210, which is highly induced during hypoxia. Under hypoxia, microRNA-210 represses the expression of IscU1/2 and affects mitochondrial metabolism by repressing aconitase and complex I activity (11, 16, 17). In our study, we observed that deletion of dIscU is embryonic lethal in Drosophila, and we also identified IscU as a new substrate of MK2 both in Drosophila cells and in mammalian cells. In vivo, MK2 deletion in Drosophila and mammalian cells and the mutation of dIscU phosphorylation site in Drosophila caused up-regulation of complex I activity, indicating that the phosphorylation of IscU by MK2 can affect the integrity of iron-sulfur clusters. These data strongly suggest that MK2-mediated IscU phosphorylation can negatively affect complex I activity, indicating that MK2 may be involved in the regulation of iron-sulfur cluster biology. We are not sure whether the increased complex I activity is directly responsible for reduced oxidative stress tolerance, and this needs to be investigated with further experiments.
In conclusion, we found that MK2 phosphorylates dIscU both in Drosophila cells and in mammalian cells. Knock-out of dMK2 in flies and cell lines had similar biological effects as dIscU-S20A mutation in flies, including higher complex I activity and no contribution to the regulation of aconitase activity. These results indicate that MK2-mediated IscU phosphorylation is a functional and conserved process. Furthermore, dIscU-S20A rescue flies have the same response to paraquat- and H2O2-induced oxidative stress as dMK2 and dp38 knock-out flies. This indicates the importance of dIscU phosphorylation by dMK2 during p38 signaling pathway in regulating oxidative stress tolerance. These data should serve as a foundation for further studies in the relationship between p38 signaling and iron-sulfur cluster biology.
Acknowledgments
We thank the website DroID – The Drosophila Interactions Database for providing the yeast two-hybrid information. We thank Biosettia for providing the HEK293FT cells.
This work was supported by National Science Foundation Grant 31171366, Marine Public Welfare Project 201005022, and Grant DY125-15-T-03 from the China Ocean Mineral Resources R&D Association.
This article was selected as a Paper of the Week.
- MK2
- MAPK-activated protein kinase 2
- Hsp27
- human heat shock protein 27
- ISC
- iron-sulfur-cluster
- RT-PCR
- real-time PCR
- MEF
- mouse embryonic fibroblast
- m
- mouse
- h
- human
- TCA
- tricarboxylic acid
- IEF
- isoelectric focusing.
REFERENCES
- 1. Ono K., Han J. (2000) The p38 signal transduction pathway: activation and function. Cell. Signal. 12, 1–13 [DOI] [PubMed] [Google Scholar]
- 2. Han J., Lee J. D., Bibbs L., Ulevitch R. J. (1994) A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265, 808–811 [DOI] [PubMed] [Google Scholar]
- 3. Chen J., Xie C., Tian L., Hong L., Wu X., Han J. (2010) Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc. Natl. Acad. Sci. U.S.A. 107, 20774–20779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaestel M. (2006) MAPKAP kinases — MKs — two's company, three's a crowd. Nat. Rev. Mol. Cell Biol. 7, 120–130 [DOI] [PubMed] [Google Scholar]
- 5. Ronkina N., Menon M. B., Schwermann J., Tiedje C., Hitti E., Kotlyarov A., Gaestel M. (2010) MAPKAP kinases MK2 and MK3 in inflammation: complex regulation of TNF biosynthesis via expression and phosphorylation of tristetraprolin. Biochem. Pharmacol. 80, 1915–1920 [DOI] [PubMed] [Google Scholar]
- 6. Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Structure, function, and formation of biological iron-sulfur clusters. Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 7. Lill R., Mühlenhoff U. (2008) Maturation of iron-sulfur proteins in eukaryotes: mechanisms, connected processes, and diseases. Annu. Rev. Biochem. 77, 669–700 [DOI] [PubMed] [Google Scholar]
- 8. Bandyopadhyay S., Chandramouli K., Johnson M. K. (2008) Iron-sulfur cluster biosynthesis. Biochem. Soc. Trans. 36, 1112–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schilke B., Voisine C., Beinert H., Craig E. (1999) Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 96, 10206–10211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nordin A., Larsson E., Thornell L. E., Holmberg M. (2011) Tissue-specific splicing of ISCU results in a skeletal muscle phenotype in myopathy with lactic acidosis, while complete loss of ISCU results in early embryonic death in mice. Hum. Genet. 129, 371–378 [DOI] [PubMed] [Google Scholar]
- 11. Chan S. Y., Zhang Y. Y., Hemann C., Mahoney C. E., Zweier J. L., Loscalzo J. (2009) MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 10, 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Adams R. H., Porras A., Alonso G., Jones M., Vintersten K., Panelli S., Valladares A., Perez L., Klein R., Nebreda A. R. (2000) Essential role of p38α MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6, 109–116 [PubMed] [Google Scholar]
- 13. Wu X., Tian L., Li J., Zhang Y., Han V., Li Y., Xu X., Li H., Chen X., Chen J., Jin W., Xie Y., Han J., Zhong C. Q. (2012) Investigation of receptor interacting protein (RIP3)-dependent protein phosphorylation by quantitative phosphoproteomics. Mol. Cell. Proteomics 11, 1640–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craig C. R., Fink J. L., Yagi Y., Ip Y. T., Cagan R. L. (2004) A Drosophila p38 orthologue is required for environmental stress responses. EMBO Rep. 5, 1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. (1988) A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stokoe D., Caudwell B., Cohen P. T., Cohen P. (1993) The substrate specificity and structure of mitogen-activated protein (MAP) kinase-activated protein kinase-2. Biochem. J. 296, 843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Favaro E., Ramachandran A., McCormick R., Gee H., Blancher C., Crosby M., Devlin C., Blick C., Buffa F., Li J. L., Vojnovic B., Pires das Neves R., Glazer P., Iborra F., Ivan M., Ragoussis J., Harris A. L. (2010) MicroRNA-210 regulates mitochondrial free radical response to hypoxia and Krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One 5, e10345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong W. H., Rouault T. A. (2006) Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron-sulfur cluster biogenesis and iron homeostasis. Cell Metab. 3, 199–210 [DOI] [PubMed] [Google Scholar]
- 19. Kurata S. (2000) Selective activation of p38 MAPK cascade and mitotic arrest caused by low level oxidative stress. J. Biol. Chem. 275, 23413–23416 [DOI] [PubMed] [Google Scholar]
- 20. Cai W., Rudolph J. L., Harrison S. M., Jin L., Frantz A. L., Harrison D. A., Andres D. A. (2011) An evolutionarily conserved Rit GTPase-p38 MAPK signaling pathway mediates oxidative stress resistance. Mol. Biol. Cell 22, 3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]