Background: Narcolepsy is caused by deficiency of neuropeptide orexin/hypocretin, of which downstream signaling pathways are unclear.
Results: Orexin activates the mTOR pathway in the mouse brain and multiple cell lines expressing OX1R or OX2R.
Conclusion: Orexin activates mTOR complex 1 (mTORC1) via calcium-stimulated lysosome v-ATPase pathway.
Significance: mTORC1 may play a key role in the functions of orexin in physiology and metabolism.
Keywords: Calcium, G Protein-coupled Receptor (GPCR), Lysosome, mTOR Complex (mTORC), Neuropeptide, Rag GTPase, Vacuolar ATPase, OX1R Or OX2R, Orexin
Abstract
The lack of the neuropeptide orexin, also known as hypocretin, results in narcolepsy, a chronic sleep disorder characterized by frequent sleep/cataplexy attacks and rapid eye movement sleep abnormalities. However, the downstream pathways of orexin signaling are not clearly understood. Here, we show that orexin activates the mTOR pathway, a central regulator of cell growth and metabolism, in the mouse brain and multiple recombinant cell lines that express the G protein-coupled receptors (GPCRs), orexin 1 receptor (OX1R) or orexin 2 receptor (OX2R). This orexin/GPCR-stimulated mTOR activation is sensitive to rapamycin, an inhibitor of mTOR complex 1 (mTORC1) but is independent of two well known mTORC1 activators, Erk and Akt. Rather, our studies indicate that orexin activates mTORC1 via extracellular calcium influx and the lysosome pathway involving v-ATPase and Rag GTPases. Moreover, a cytoplasmic calcium transient is sufficient to mimic orexin/GPCR signaling to mTORC1 activation in a v-ATPase-dependent manner. Together, our studies suggest that the mTORC1 pathway functions downstream of orexin/GPCR signaling, which plays a crucial role in many physiological and metabolic processes.
Introduction
Narcolepsy, a chronic sleep disorder that affects 1/600 to 1/2,000 individuals, is characterized by excessive daytime sleepiness and sleep attacks, as well as abnormal transition from wake to rapid eye movement sleep, manifested by cataplexy attacks, sleep paralysis, and hypnagogic hallucinations (1). Deficiencies in signaling by the neuropeptide orexin/hypocretin have been linked to narcolepsy in humans, dogs, and mice (2–4). Two orexin peptides, orexin-A and orexin-B, which are derived from the same prepropepetide, are produced exclusively by a small number (∼50,000) of neurons in the lateral hypothalamus of human brain (5, 6). The unexplained loss of these orexin neurons is the most common cause for human narcolepsy. The orexin neurons spread projections throughout the whole brain and regulate a variety of important physiological processes such as sleep/wake cycle, reproduction, brown fat and bone development, feeding, and energy metabolism (7–10).
At the cellular level, orexin-A and B exert their effects by binding and activating either of two related GPCRs,3 OX1R and OX2R (11). Whereas orexin-A binds to OX1R and OX2R non-selectively, orexin-B preferentially binds OX2R with much higher affinity (6, 12). A well studied cellular response to orexin is a dose-dependent transient increase in intracellular calcium concentration ([Ca2+]i). It is thought that this [Ca2+]i surge is a result of extracellular Ca2+ influx through TRPC3 and l-type Ca2+ channels following the activation of phospholipase C by orexin/GPCR signaling (12–15).
Furthermore, orexin has been shown to activate multiple protein kinases such as PKA, PKC, mitogen-activated protein kinase (MAPK)/Erk, and PDK1 in various cell contexts (16). Activation of a particular signaling pathway depends on the combination of the peptide (orexin-A or B), the receptor subtype (OX1R or OX2R), and the cellular context. Because it is difficult to obtain cell lines naturally expressing the orexin receptors, the majority of these studies use recombinant cell lines engineered to express either OX1R or OX2R (16). Despite the intense series of genetic and biochemical studies in the last 16 years, the downstream signaling pathways of orexin/GPCR are still not clearly understood. It is also uncertain which pathway plays a crucial role in mediating the many important functions of orexin in physiology and metabolism.
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase and a master regulator of cell growth and metabolism (17, 18). mTOR exists in two distinct complexes: complex 1 (mTORC1) and complex 2 (mTORC2) (17). mTORC1, which consists of mTOR, Raptor, mLST8, PRAS40, and DEPTOR, is much better studied than mTORC2 in terms of both upstream regulators and downstream substrates (17). mTORC1 regulates cellular growth by increasing anabolic processes such as the macromolecular synthesis and nutrient storage and suppressing catabolic processes such as autophagy. For example, mTORC1 enhances the efficiency of protein synthesis by phosphorylation of the eukaryotic translation initiation factor 4E (eIF4E) binding proteins (e.g. 4E-BP1) and the S6 kinases (e.g. S6K1) (19–21). Activation of S6Ks promotes mRNA translation through several substrates such as the ribosome protein S6 (21), eIF4B (20), and eukaryotic elongation factor 2 kinase (22).
mTORC1 can be activated in response to a wide range of upstream signals such as nutrient, oxygen, and energy availability (18, 23–26) as well as growth factors (e.g. IGF-1) signaling through the receptor tyrosine kinases (27). Two major downstream signaling pathways, phosphatidylinositol-3 kinase (PI3K)-Akt and Ras-Erk, are involved in the activation of mTORC1 (24, 28). The activity of mTORC1 is negatively controlled by the tumor suppressors tuberous sclerosis complex 1 (TSC1, also known as hamartin) and TSC2 (also known as tuberin) (29–31). TSC2 functions as a GTPase activation protein for the small GTPase Rheb, a direct activator for mTORC1. TSC2 can be phosphorylated by Akt (32), Erk (33, 34), and p90 RSK1 (35). Phosphorylation of TSC2 suppresses its ability to inhibit Rheb, allowing Rheb-GTP to accumulate and activate mTORC1 on the surface of lysosome (34, 35). Alternatively, Erk and RSK1 can phosphorylate the Raptor, which binds mTOR and recruits substrate to mTOR, to promote the mTORC1 activation (36, 37).
Amino acids can activate mTORC1 by signaling to the vacuolar proton (H+)-translocating ATPases (v-ATPase) from the lysosome lumen via an undefined “inside-out” mechanism (26). The primary function of v-ATPase is to hydrolyze ATP and pump H+ into the lysosome to maintain its acidic environment (38, 39). The v-ATPase physically interacts with Ragulator and controls the binding between the Ragulator and RAG complexes. As a result, v-ATPase regulates the guanine exchange factor activity of Ragulator to facilitate the exchange of GTP onto RagA and RagB GTPases (25, 26). Activated RAG complex (RagA/BGTP-RagC/DGDP) recruits mTORC1 to the lysosome surface for activation by Rheb (23, 40).
There are a few reports of GPCR signaling to mTORC1 activation in the literature. A GPCR taste receptor T1R1/T1R3 plays a critical role in amino acid-stimulated mTORC1 activation (24). It has been proposed that T1R1/T1R3 may function as a direct sensor for extracellular amino acids to rapidly and transiently activate Erk1/2 activity. Inhibition of Erk1/2 activation by U0126 diminishes the amino acids-stimulated mTORC1 activation (24). Similarly, the GPCR ligand prostaglandin F2α-stimulated mTORC1 activation is sensitive to U0126, suggesting that MAPK/Erk is an important signal transducer from GPCR signaling to mTORC1 (28).
Here, we show that the neuropeptide orexin can activate the mTORC1 pathway in the mouse brain and three recombinant cell lines expressing either OX1R or OX2R. Moreover, our studies suggest that the orexin-stimulated mTORC1 activation was independent of Erk and Akt in the hypothalamic N41 neuronal cell model. Rather, orexin/GPCR signaling activates mTORC1 via cytoplasmic calcium transient that triggers the lysosome v-ATPase pathway. These studies identified the mTORC1 pathway as a key component of the downstream signaling network of orexin/GPCR. The discovery of this orexin-mTORC1 signaling axis may have important mechanistic implications for the various functions of orexin in physiology and metabolism.
EXPERIMENTAL PROCEDURES
General Reagents and Antibodies
Orexin-A/B were obtained from Peptide Insitute, Inc. (Osaka, Japan), and IGF-1 was from ProSpec (Rehovot, Israel). MK-2206, rapamycin, and BI-D1870 were purchased from Selleck Chemicals (Houston, TX). AZD-6244, bafilomycin A1, and ionomycin were purchased from LC Laboratories (Woburn, MA). EBSS, Fura-2/AM, and Lipofectamine 2000 were from Invitrogen. EGTA, BAPTA/AM, CaCl2, W7, and thapsigargin were purchased from Sigma-Aldrich. Universal nuclease was purchased Thermo Scientific Pierce (Rockford, IL). Saliphenylhalamide (saliPhe) was a generous gift from Dr. Jef DeBrabander at University of Texas Southwestern Medical Center.
Anti-ribosomal S6 kinase (p70 S6K), anti-phospho-Thr-389 p70 S6K, anti-phospho-Ser-422 eIF4B, anti-phospho-Ser-235/236 S6 ribosomal protein, anti-ERK1/2, anti-phospho-Thr-202/Tyr-204 ERK1/2, anti-Akt, anti-phospho-Thr-308 Akt, anti-phospho-Ser-473 Akt, anti-RagC, anti-Raptor, and U0126 were all purchased from Cell Signaling Technologies (Danvers, MA).
Cell Lines and Tissue Culture
Stably transfected HEK-293T cell lines expressing the OX1R or OX2R were described previously by Sakurai et al. (6). The mouse embryonic hypothalamus N41 neuronal cell line (mHypoE-N41) was obtained from Cedarlane Laboratories, Ltd. (Burlington, NC), which were stably infected with retroviruses expressing OX1R (N41/OX1R) or OX2R (N41/OX2R). Mouse embryonic fibroblasts expressing OX1R (MEF/OX1R) was similarly generated. Transgenic cell lines were selected with puromycin (2 μg/ml) and cultured in growth media consisting of high glucose DMEM supplemented with 10% FBS (Sigma).
Serum Starvation and Stimulation of Cells
Cultured cells were plated on six-well dishes at 40–60% confluence. After 24 h, the cells were washed once with PBS and incubated in starvation medium (DMEM supplemented with 20 mm HEPES (pH 7.0)) for 24 h before IGF-1 (10 ng/ml), orexin-A/B (50 nm), ionomycin (1 μm), or thapsigargin (5 μm) treatment for 1 h in the serum-starved condition. All of the compounds such as rapamycin, EGTA, BAPTA/AM, saliPhe, and bafilomycin A1 as well as the various kinase inhibitors were added 10 min prior to addition of IGF-1 or orexin to the growth media.
Western Blot Analysis
Cells were rinsed once with ice-cold PBS and lysed in lysis buffer (20 mm HEPES (pH 7.4), 2 mm MgCl2, 1% SDS, and universal nuclease). After centrifugation at 13,000 rpm for 10 min, the soluble fractions of cell lysates were saved for further analysis. Equal amounts of protein samples were resolved by SDS-PAGE and transferred to PVDF membrane, and Western blotting was performed according to standard procedures using the corresponding antibodies.
RNAi in Mammalian Cells
The control GFP shRNA (shGFP) and the shRNAs targeting RagC (shRagC) were described previously (41, 42). The oligonucleotides of lentiviral shRNAs were obtained from Sigma and cloned into plasmid pLKO.1-Hygro. The shRNA target sequence is as follows: mRagC-1, TGGCCATTATCAAGCTGAATA; mRagC-2, GTGGATATGCAGTCTTATGAA; and hRagC-1, TGGCAATTATCAAGCTGAATA; hRagC-2, GTGGATATGCAATCTTATGAA.
The shRNA-encoding plasmids were cotransfected with the pCMV-dR8.2 dvpr envelope and pCMV-VSVG packaging plasmids into actively growing HEK-293TD cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Virus-containing supernatants were collected 48 h after transfection and passed through a 0.45-mm filter. Target cells in 10-cm culture dishes were infected in the presence of 8 μg/ml polybrene. After 24 h, cells were selected with hygromycin for 3 days and then plated on six-well dishes at 40–60% confluence. After 24 h, the cells were washed once with PBS and incubated in starvation medium for 24 h and then subjected to orexin-A (50 nm) or ionomycin (1 μm) treatment for 1 h in the serum-starved condition.
Cell Viability Assay
Cell viability was measured by trypan blue exclusion test. N41/OX1R and N41/OX2R cells were seeded in six-well plates at a density of 2.5 × 105 cells/ml and incubated overnight before serum starvation. Cells were serum starved in parallel incubated with the 50 nm orexin-A or orexin-B for 48 h. Cells were washed in PBS before staining with trypan blue dye at room temperature. The cell suspension was then applied to Bio-Rad TC20TM Automated Cell Counter.
Cytoplasmic Ca2+ Transient Assay
N41/OX1R and N41/OX2R cells were incubated in the loading buffer (20 mm HEPES-Hanks, pH 7.4, containing 10 mm glucose, 0.1% BSA, and 4 μm Fura-2/AM) for 1 h at 37 °C. Cytoplasmic calcium levels were monitored by Functional Drug Delivary System FDSS 7000EX (Hamamatsu Photonics). Intracellular calcium was assessed by the excitation ratio at 340 nm/380 nm with emission at 505 nm.
Animal Studies
C57BL/6J wild-type mice or CAG/orexin transgenic mice (CAG/orexin) mice (45) were housed, and all experiments were conducted under humidity- and temperature-controlled conditions (22–25 ± 1 °C) on a 12-h light/dark cycle. Animals were high fat diet-fed 12 weeks before sacrifice. At the indicated times, the animals were euthanized, and brain tissues were removed and frozen in liquid N2.
RESULTS
Orexin Activates mTORC1 in HEK293T Cells Expressing OX1R or OX2R
To investigate downstream signaling pathways of orexin, we used synthetic orexin peptides to treat human embryonic kidney (HEK)-293T cells that express either of two orexin receptors, OX1R and OX2R. These cell lines were previously used to biochemically purify orexin-A and B from rat brain extract as specific neuropeptide ligands for OX1R and OX2R (then as orphan receptors) (6). We showed that orexin-A/B treatment induced the rapid phosphorylation of eIF4B at serine 422, the S6 kinase p70S6K at Thr-389, and ribosomal protein S6 at Ser-235/236 and Ser-240/244 in the serum-starved 293T/OX1R and 293T/OX2R cells (Fig. 1A). All of these phosphorylation events are characteristic downstream markers for activation of the mTOR pathway. In contrast, the same orexin-A/B treatment could not induce any of these phosphorylation events in empty vector control HEK-293T cells (Fig. 1B). Furthermore, rapamycin, a potent inhibitor of mTORC1, abolished the orexinA/B-induced S6K and S6 phosphorylation in the 293T/OX1R and 293T/OX2R cells (Fig. 1, C and D), suggesting that orexin/GPCR signaling specifically activates the mTORC1 pathway in human HEK-293T cells expressing either OX1R or OX2R.
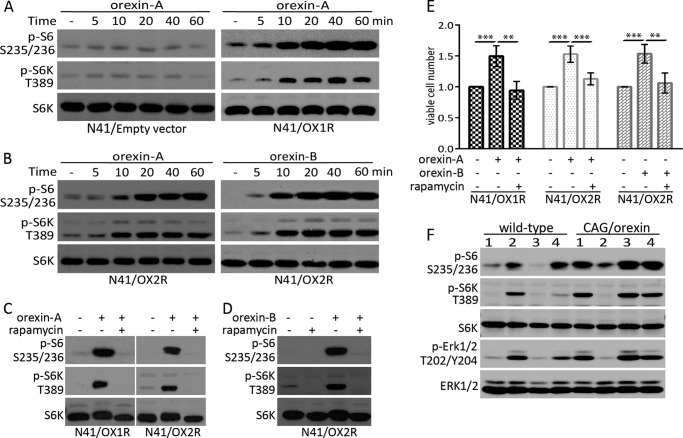
FIGURE 1.
Orexin activates mTORC1 in HEK-293T cells expressing OX1R or OX2R. A, 293T/OX1R and 293T/OX2R cells were serum-starved for 24 h followed by treatment with 50 nm orexin-A/B. B, serum-starved 293T/empty vector cells were stimulated with 50 nm orexin-A or orexin-B or 10 ng/ml IGF-1 for 1 h with or without 20 nm rapamycin. C, serum-starved 293T/OX1R and 293T/OX2R cells were stimulated with 50 nm orexin-A for 1 h with or without 20 nm rapamycin. D, serum-starved 293T/OX2R cells were stimulated with 50 nm orexin-B for 1 h in the absence or presence of 20 nm rapamycin. Activation of the mTOR pathway was measured by detecting phosphorylation of S6, S6K, and eIF4B at specific residues by Western blotting with corresponding phospho-specific antibodies.
Orexin Activates mTORC1 in Mouse Hypothalamic N41 Neuronal Cell Lines
Both orexins and orexin receptors are highly expressed in the brain, especially in the hypothalamus area (43). Therefore, we wanted to study the orexin signaling in a more natural context such as in a neuronal cell model. However, it was difficult to identify established neuronal cell lines that naturally express detectable levels of OX1R or OX2R. Thus, we engineered the mouse embryonic hypothalamus N41 cell line to stably express OX1R or OX2R using retroviruses as described previously (6, 44). Similarly, orexin-A or -B could rapidly activate the mTOR pathway as shown by both S6K and S6 phosphorylation in the serum-starved N41/OX1R and N41/OX2R neurons, but not in the serum-starved N41/empty vector neurons (Fig. 2, A and B). The orexin-A/B-induced mTORC1 activation could also be abolished by rapamycin treatment (Fig. 2, C and D). In accordance with that, mTORC1 is a central regulator of cell growth, we observed that orexin-A/B treatment could enhance the growth/survival of serum-starved N41/OX1R and N41/OX2R neurons in the serum-starved condition, and this effect of orexin-A/B was alleviated by rapamycin treatment (Fig. 2E). Taken together, these studies indicate that orexin/GPCR signaling can promote cell growth/survival through the activation of the mTORC1 pathway.
FIGURE 2.
Orexin activates mTORC1 in hypothalamic N41/OX1R and N41/OX2R neurons and mouse brain. A, serum-starved N41/empty vector and N41/OX1R cells were treated with 50 nm orexin-A. B, serum-starved N41/OX2R cells were stimulated with 50 nm orexin-A/B. C and D, serum-starved N41/OX1R or N41/OX2R cells were stimulated with 50 nm orexin-A (C) or orexin-B (D) for 1 h with or without 20 nm rapamycin. E, under serum starvation conditions, N41/OX1R and N41/OX2R neurons were untreated or treated with 50 nm orexin-A/B with or without rapamycin for 2 days. Data are presented as mean ± S.E. (n = 6); **, p < 0.01; ***, p < 0.001. F, C57BL/6J wild-type and CAG/orexin mice were fed a high fat diet for 12 weeks before sacrifice. Activity of the mTOR pathway was measured by detecting phosphorylation of S6, S6K by Western blotting.
Overexpression of Orexin Causes Hyperactivation of mTOR in the Mouse Brain
Previous studies showed that the CAG/orexin transgene expressed severalfold higher levels of orexin-A and B in the mouse brain and could rescue the narcolepsy and cataplexy phenotype of mice lacking the endogenous orexin-producing neurons (45). To study whether orexin activated the mTOR pathway in vivo, we examined the effect of orexin overexpression on the mTOR activity in whole brain extracts of wild-type and CAG/orexin mice fed on high fat diet (46). In three of four mice, we observed significantly higher levels of phosphorylations of S6K, S6, and Erk1/2 in the CAG/orexin brain extracts than wild-type brain extracts (Fig. 2F). This in vivo experiment suggests that overexpression of orexin can cause hyperactivation of the mTOR pathway in the mouse brain.
Orexin Activates mTORC1 Independent of Erk and Akt
As reported previously (47), we observed that orexin-A treatment induced the phosphorylation and activation of the MAPK kinase/MEK1/2, MAPK/Erk, and RSK in the N41/OX1R neurons (Fig. 3A). Pretreatment of N41/OX1R cells with the MEK1/2 inhibitor, U0126 or AZD6244, increased phosphorylation of MEK1/2, but inhibited the phosphorylation of the downstream substrates Erk1/2 and RSK (Fig. 3B). At 10 μm concentration, both U0126 and AZD6244 abolished the orexin-induced Erk1/2 and RSK phosphorylation (Fig. 3B). However, neither treatment had any noticeable effect on the orexin-induced mTORC1 activation as measured by the level of S6K and S6 phosphorylation (Fig. 3B). Similar results were obtained with the RSK inhibitor, BI-D1870, which enhanced phosphorylation of RSK, but did not affect the orexin-stimulated mTORC1 activation (Fig. 3C). These results suggest that the MAPK/Erk and RSK does not play a critical role in the orexin-induced mTORC1 activation.
FIGURE 3.
Orexin activates mTORC1 independent of Erk and Akt kinases. A, serum-starved N41/OX1R cells were treated with 10 ng/ml IGF-1 or 50 nm orexin-A. Phosphorylation of MEK1/2, RSK, and Erk1/2 were evaluated by Western blotting. B, serum-starved N41/OX1R cells were treated with 50 nm orexin-A for 1 h in the absence or presence of 5 or 10 μm U0126 or AZD6244. C, serum-starved N41/OX1R cells were treated with 50 nm orexin-A for 1 h with or without 1 or 10 μm BI-D1870. Phosphorylation of S6K and S6 as well as MEK1/2, RSK, and Erk1/2 were evaluated by Western blotting. D, serum-starved N41/OX1R cells were treated with 10 ng/ml IGF-1 or 50 nm orexin-A. E, serum-starved N41/OX1R cells were treated with 10 ng/ml IGF-1 or 50 nm orexin-A for 1 h with or without 5 μm MK-2206. Phosphorylation of S6, S6K, and Akt are evaluated by Western blotting. F, serum-starved N41/OX1R cells were treated with 50 nm orexin-A for 1 h in the absence or presence of 5 μm MK-2206 and AZD6244.
However, IGF-1, but not orexin-A, could induce rapid and robust activation of Akt kinase in the N41/OX1R neurons (Fig. 3D). Pretreatment of N41/OX1R cells with the Akt inhibitor, MK-2206, abolished the IGF-1-induced activation of Akt as well as the mTORC1-mediated S6K and S6 phosphorylation (Fig. 3E). In contrast, the same MK-2206 treatment had no effect on the orexin-induced S6K and S6 phosphorylation (Fig. 3E). Furthermore, inhibition of both Erk and Akt activation did not affect orexin-stimulated mTORC1 activation either (Fig. 3F). Therefore, we concluded that orexin could activate mTORC1 via an Erk- and Akt-independent pathway.
Orexin-induced mTORC1 Activation Is Dependent on Extracellular Calcium Influx
In the N41/OX1R and N41/OX2R neurons, orexin-A/B treatment could induce rapid increase in [Ca2+]i and mTORC1 activation in a corresponding dose-dependent manner (Fig. 4, A and B). To determine whether extracellular Ca2+ is essential for orexin signaling to mTORC1, we cultured N41/OX1R neurons in media that contained or lacked Ca2+ ion. The results showed that extracellular Ca2+ greatly enhanced both orexin-A and IGF-1-induced S6K and S6 phosphorylation (Fig. 4C). Addition of EGTA, an extracellular Ca2+ chelator, to the growth media diminished orexin-induced S6K and S6 phosphorylation, consistent with that Ca2+ influx from extracellular space is the primary response of orexin signaling (Fig. 4D). In contrast, EGTA partially reduced IGF-1-induced S6K and S6 phosphorylation (Fig. 4D). This is because IGF-1 could trigger both extracellular Ca2+ influx and release of Ca2+ from intracellular stores (48, 49). Accordingly, treatment of BAPTA/AM, a cell permeable Ca2+ chelator, abolished both orexin-A and IGF-1-induced S6K and S6 phosphorylation (Fig. 4E). A similar result was obtained with orexin-B treatment of N41/OX2R cells (Fig. 4F). Moreover, extracellular Ca2+ was essential for the orexin-induced Erk1/2 phosphorylation, but not the IGF-1-mediated activation of Akt (Fig. 4, C and D). Taken together, these results indicate that extracellular Ca2+ influx is required for orexin-induced mTORC1 activation.
FIGURE 4.
Orexin activates mTORC1 via extracellular calcium influx. A, N41/OX1R or N41/OX2R neurons were challenged with orexin-A or orexin-B for 1 h, respectively. Peak [Ca2+]i values are represented as percentages of the maximum response. Data are presented as mean ± S.E. (n = 4). B, serum-starved N41/OX1R or N41/OX2R cells were stimulated with orexin-A or orexin-B for 1 h. C, serum-starved N41/OX1R cells were placed in EBSS media with or without 2 mm CaCl2 5 min before IGF-1 or orexin-A treatment for 1 h. D and E, serum-starved N41/OX1R cells were pretreated with 2 mm EGTA (D) or 20 μm BAPTA/AM (E) before IGF-1 or orexin-A treatment for 1 h. F, serum-starved N41/OX2R cells were pretreated with 2 mm EGTA or 20 μm BAPTA/AM before orexin-B treatment for 1 h. Phosphorylations of Erk1/2, AKT, S6, and S6K at specific residues were evaluated by Western blotting.
Orexin Activates mTORC1 via the Lysosome v-ATPase Pathway
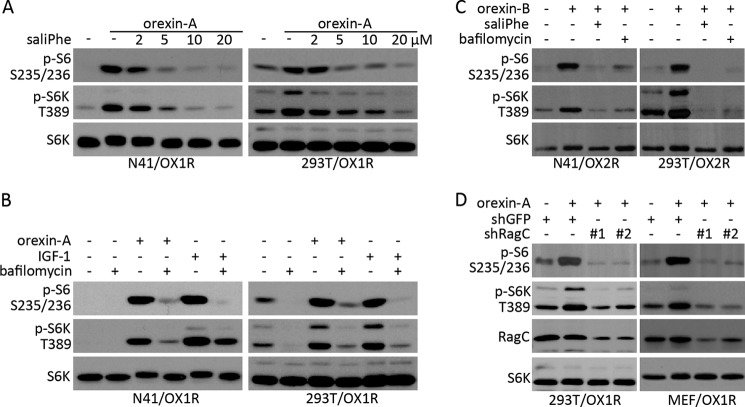
Both amino acids and growth factors-induced mTORC1 activation require the lysosome v-ATPase-Ragulator-RAG pathway (18, 25, 26, 40). To examine whether this lysosome pathway is also required for the orexin-induced mTORC1 activation, we treated N41/OX1R and 293T/OX1R cells with two specific v-ATPase inhibitors, saliPhe (50) and bafilomycin A1 (51). In both cell lines, saliPhe inhibited the orexin-A-induced S6K and S6 phosphorylation in a dose-dependent manner (Fig. 5A). Likewise, bafilomycin A1 blocked both orexin-A and IGF-1-stimulated mTORC1 activation (Fig. 5B). A similar phenomenon was observed with orexin-B treatment of the N41/OX2R and 293T/OX2R cells (Fig. 5C). Furthermore, small hairpin RNA (shRNA)-mediated knockdown of RagC GTPase reduced the orexin-induced S6K and S6 phosphorylation in the 293T/OX1R cells (Fig. 5D). Because we could not achieve efficient shRNA-mediated knockdown in the N41/OX1R neurons, we constructed MEFs expressing OX1R as a substitute for this experiment. In the MEF/OX1R cells, orexin-A also activated the mTORC1-mediated phosphorylation of S6K and S6, which was diminished by the shRNA-mediated knockdown of RagC expression (Fig. 5D). These results demonstrate that orexin/GPCR signaling stimulates mTORC1 activation via the lysosome v-ATPase-Rag GTPase pathway.
FIGURE 5.
Orexin activates mTORC1 via the lysosome v-ATPase pathway. A, serum-starved N41/OX1R and 293T/OX1R cells were treated with 50 nm orexin-A in the absence or presence of saliPhe. B, serum-starved N41/OX1R and 293T/OX1R cells were treated with orexin-A or IGF-1 for 1 h in the absence or presence of 10 μm bafilomycin A1. C, serum-starved N41/OX2R and 293T/OX2R cells were treated with 50 nm orexin-B in the absence or presence of saliPhe or bafilomycin A1. D, 293T/OX1R and MEF/OX1R cells were infected with control shGFP or shRagC lentivirus for 24 h. After hygromycin selection for 72 h, the shRNA knockdown cells were serum-starved for 24 h followed by orexin treatment for 1 h. Western blotting was performed to examine the levels of S6K, RagC proteins as well as the phosphorylation of S6 and S6K.
Intracellular Calcium Surge Stimulates mTORC1 Activation in a v-ATPase-dependent Manner
Because Ca2+ influx was essential for the orexin-induced mTORC1 activation (Fig. 3), we wanted to ask whether an intracellular Ca2+ surge was sufficient to mimic orexin signaling to mTORC1. To address this question, we incubated N41/OX1R cells with ionomycin or thapsigargin. Whereas ionomycin triggers extracellular Ca2+ influx, thapsigargin causes the release of Ca2+ from intracellular stores (52, 53). Our studies showed that both ionomycin and thapsigargin efficiently induced the phosphorylation of S6K and S6, which were abolished by BAPTA/AM treatment (Fig. 6, A and B). This intracellular calcium-stimulated S6K and S6 phosphorylation was also abolished by rapamycin treatment (Fig. 6C), suggesting that cytoplasmic Ca2+ increase is sufficient to mimic orexin signaling to mTORC1 activation in the N41/OX1R cells.
FIGURE 6.
Calcium activates mTORC1 via the lysosome v-ATPase pathway. A and B, serum-starved N41/OX1R cells were treated with 50 μm orexin-A or 1 μm ionomycin (A) or 5 μm thapsigargin (TG; B) for 1 h in the absence or presence of 20 μm BAPTA/AM. C, serum-starved N41/OX1R cells were treated with thapsigargin or ionomycin in the absence or presence of 20 nm rapamycin. D, serum-starved N41/OX1R cells were treated with 1 μm ionomycin for 1 h in the absence or presence of 5 μm or 10 μm bafilomycin A1. E, serum-starved N41/OX1R cells were treated with 5 μm thapsigargin for 1 h in the absence or presence of 10 μm saliPhe or bafilomycin A1. F, 293T/OX1R cells were infected with control shGFP or shRagC lentivirus for 24 h. After hygromycin selection for 72 h, the infected cells were serum-starved for 24 h followed by ionomycin treatment for 1 h. The levels of S6K, RagC, and Raptor and phosphorylations of S6 and S6K were detected by Western blotting.
In contrast, ionomycin, but not thapsigargin, resulted in the activation of Erk1/2 (Fig. 6, A and B). Moreover, orexin-induced phosphorylation of Erk1/2 was only inhibited by EGTA, an extracellular Ca2+ chelator, but not by BAPTA/AM, an extracellular Ca2+ chelator (Fig. 4, D and E). These results suggest that the event of Ca2+ influx through plasma membrane, but not the subsequent cytoplasmic Ca2+ surge, is responsible for orexin-stimulated activation of the MAPK/Erk pathway. Together with our finding that orexin does not activate Akt, these results further support our conclusion that orexin induces mTORC1 activation independent of Erk and Akt.
Furthermore, this intracellular calcium-stimulated mTORC1 activation was diminished not only by pharmacological inhibition of v-ATPase activity by saliPhe or bafilomycin A1 (Fig. 6, D and E), but also by the shRNA-mediated knockdown of RagC GTPase (Fig. 6F). Taken together, our studies suggest that cytoplasmic calcium transient is necessary and sufficient for orexin/GPCR signaling to stimulate mTORC1 activation through the lysosome v-ATPase-Ragulator-RAG pathway.
DISCUSSION
Although orexin and its receptors were discovered 16 years ago, their downstream signaling pathways have not been fully characterized. Here, we have discovered that orexin/GPCR signaling results in rapid and robust activation of the mTORC1 pathway in human HEK-293T cells, MEFs, and mouse hypothalamic N41 neurons that express either OX1R or OX2R. Accordingly, overexpression of orexin caused hyperactivation of the mTORC1 pathway in the CAG/orexin mouse brain. In contrast to previous reports of Erk-dependent mTORC1 activation in response to GPCR signaling (24, 28), we showed that neither of the two well known upstream mTORC1 activators, Erk and Akt, played a critical role in orexin/GPCR signaling to the mTOR pathway. Rather, the orexin-induced mTORC1 activation is dependent on cytoplasmic calcium transient that activates the lysosomal v-ATPase pathway through an unknown mechanism. This study uncovers a novel regulatory link that the mTORC1 pathway functions as a key component of orexin/GPCR signaling network.
Involvement of Calcium in mTORC1 Activation
It is known that intracellular Ca2+ transient is also necessary for the activation of mTORC1 in response to amino acids and growth factors (24, 52, 54, 55). However, the mechanism of calcium involvement in mTORC1 activation remains unclear. Previous studies proposed that mTORC1 formed a “signalosome” in the phosphatidylinositol 3-phophate-rich endosome structure (56). Amino acids could increase intracellular Ca2+ level to enhance Ca2+/calmodulin interaction with hVps34, thus activating hVps34 kinase activity and elevating the phosphatidylinositol 3-phophate level on the endosomes (54, 57). However, a recent study contradicted this idea by showing that hVps34 bound to calmodulin, but its activity was not suppressed by BAPTA, EGTA, or calmodulin-inhibitor W7 in vivo (58). Furthermore, the function of Vps34 in mTORC1 activation was not substantiated by genetic studies in Drosophila, although the amino acid-induced TORC1 activation is conserved from yeast to mammals (59). In our study, neither PI3K inhibitors nor calmodulin-inhibitor W7 had any effect on the orexin-stimulated mTORC1 activation (data not shown). Thus, more detailed studies are required to elucidate the specific role of calcium in mTORC1 activation in the future.
How Does Calcium Signal to Lysosome v-ATPase?
We have shown that intracellular calcium surge is sufficient to mimic orexin/GPCR signaling to activate mTORC1 through the lysosome v-ATPase pathway. The v-ATPases are highly conserved proton pumps consisting of a peripheral membrane sub-complex called V1, which contains the sites of ATP hydrolysis, and an integral membrane subcomplex called V0, which encompasses the proton pore and is attached to V1 (60). The ATPase activity of the v-ATPase and the associated rotation of its V0 section appear to be essential to relay the amino acids signal from the lysosome lumen to the Ragulator and Rag GTPase complex on the lysosome surface, but exactly how the v-ATPase functions to do so is unknown (26). A previous study reported that v-ATPases exhibited both Mg2+- and Ca2+-dependent ATPase activity (39, 61). Unlike the Mg2+-dependent v-ATPase activity, the Ca2+-dependent v-ATPase activity decays with time and is inhibited by ADP in vitro (61). Additionally, the proton pump activity is detected only in the presence of Mg2+, but not in the presence of Ca2+ (39). Thus, the differential regulation of v-ATPase by Mg2+ and Ca2+ may provide a potential mechanism for the cytoplasmic Ca2+ surge to directly regulate the functional state of v-ATPase and couple it to mTORC1 activation on the lysosome surface. There are more than 800 GPCRs encoded by the human genome, of which mainly the Gq-coupled GPCRs upon activation trigger intracellular calcium transient. It is plausible that activation of the mTORC1 pathway is a general cellular response to Gq-coupled GPCR signaling in a wide variety of physiological processes. Thus, it is important to investigate the detailed mechanism by which calcium activates lysosomal v-ATPase to stimulate mTORC1 activation in the future.
Does the mTORC1 Pathway Contribute to Functions of Orexin in Physiology and Metabolism?
Because mTORC1 is a central regulator of cell growth and metabolism, we postulate that the mTORC1 pathway may play a key role in mediating the functions of orexin in many physiological processes such as sleep/wake cycle, development, feeding behavior, and energy homeostasis.
Narcolepsy patients, who lack the orexin/GPCR signaling in the brain, tend to be overweight (62). Conversely, prolonged orexin overexpression in mice prevents the high fat diet-induced obesity and insulin resistance by enhancing the “satiety hormone” leptin signaling (46). It has been reported that mTORC1 signaling is required for the suppressed food intake and enhanced sympathetic activity induced by leptin (63, 64). Although the detailed mechanism remains unclear, our present results suggest that orexin/GPCR and leptin signaling converges on the mTORC1 pathway to negatively regulate food intake and metabolism. Consistent with this hypothesis, activated mTORC1 regulates energy homeostasis through multiple mechanisms. For example, mTORC1 positively regulates the activities of SREBP1 and PPARγ, the two transcription factors that control expression of proteins involved in lipid and cholesterol homeostasis (65, 66). Moreover, mTORC1 activates the transcription factor HIF1α to stimulate specific metabolic pathways, including glycolysis and the oxidative arm of the pentose phosphate pathway (65, 67–69). Therefore, the orexin-mTORC1 signaling axis may provide a plausible explanation for the metabolic phenotypes resulting from chronic gain or loss-of-function of orexin/GPCR signaling. Finally, a complete understanding of orexin/GPCR signaling network is essential to understanding the functions of orexin from cellular to organismal levels and for developing new therapeutic approaches to restore the balance of the orexin/GPCR signaling system disturbed in many disease states.
Acknowledgments
We thank Drs. Melanie Cobb and Benjamin Tu for helpful comments on this manuscript and Drs. Jef DeBrabander, Hidetoshi Kumagai, and Yohko Takata for useful reagents.
This work was supported in part by Welch Foundation Grants I-1608 (to Q. L.) and I-1800 (to Y. Y.), American Heart Association Grant 13GRNT16270022 (to Q. L.), Cancer Prevention and Research Institute of Texas Grant CPRIT-R1103 (to Y. Y.), as well as by Grants-in-aid for Scientific Research of the Japan Society for the Promotion of Science (JSPS KAKENHI) Grants 26253015 and 26220207 (to M. Y.) and Grants-in-aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology (MEXT KAKENHI) Grants 25460318 and 25126725 (to H. F.).
- GPCR
- G protein-coupled receptor
- OX1R
- orexin 1 receptor
- mTOR
- mammalian target of rapamycin
- mTORC1
- mTOR complex 1
- TSC
- tuberous sclerosis complex
- S6K
- S6 kinase
- saliPhe
- Saliphenylhalamide
- MEF
- mouse embryonic fibroblast
- CAG
- β-actin/cytomegalovirus hybrid promoter
- RAG
- Ras-related GTP-binding protein
- BAPTA
- 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid.
REFERENCES
- 1. Mignot E. (1998) Genetic and familial aspects of narcolepsy. Neurology 50, S16–S22 [DOI] [PubMed] [Google Scholar]
- 2. Chemelli R. M., Willie J. T., Sinton C. M., Elmquist J. K., Scammell T., Lee C., Richardson J. A., Williams S. C., Xiong Y., Kisanuki Y., Fitch T. E., Nakazato M., Hammer R. E., Saper C. B., Yanagisawa M. (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98, 437–451 [DOI] [PubMed] [Google Scholar]
- 3. Lin L., Faraco J., Li R., Kadotani H., Rogers W., Lin X., Qiu X., de Jong P. J., Nishino S., Mignot E. (1999) The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376 [DOI] [PubMed] [Google Scholar]
- 4. Mignot E., Lammers G. J., Ripley B., Okun M., Nevsimalova S., Overeem S., Vankova J., Black J., Harsh J., Bassetti C., Schrader H., Nishino S. (2002) The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch. Neurol. 59, 1553–1562 [DOI] [PubMed] [Google Scholar]
- 5. de Lecea L., Kilduff T. S., Peyron C., Gao X., Foye P. E., Danielson P. E., Fukuhara C., Battenberg E. L., Gautvik V. T., Bartlett F. S., 2nd, Frankel W. N., van den Pol A. N., Bloom F. E., Gautvik K. M., Sutcliffe J. G. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 95, 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richardson J. A., Kozlowski G. P., Wilson S., Arch J. R., Buckingham R. E., Haynes A. C., Carr S. A., Annan R. S., McNulty D. E., Liu W. S., Terrett J. A., Elshourbagy N. A., Bergsma D. J., Yanagisawa M. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585 [DOI] [PubMed] [Google Scholar]
- 7. Carter M. E., Schaich Borg J., de Lecea L. (2009) The brain hypocretins and their receptors: mediators of allostatic arousal. Curr. Opin. Pharmacol. 9, 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakurai T., Mieda M., Tsujino N. (2010) The orexin system: roles in sleep/wake regulation. Ann. N.Y. Acad. Sci. 1200, 149–161 [DOI] [PubMed] [Google Scholar]
- 9. Wei W., Motoike T., Krzeszinski J. Y., Jin Z., Xie X. J., Dechow P. C., Yanagisawa M., Wan Y. (2014) Orexin regulates bone remodeling via a dominant positive central action and a subordinate negative peripheral action. Cell Metab. 19, 927–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sellayah D., Bharaj P., Sikder D. (2011) Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 14, 478–490 [DOI] [PubMed] [Google Scholar]
- 11. Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richarson J. A., Kozlowski G. P., Wilson S., Arch J. R., Buckingham R. E., Haynes A. C., Carr S. A., Annan R. S., McNulty D. E., Liu W. S., Terrett J. A., Elshourbagy N. A., Bergsma D. J., Yanagisawa M. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 1 page following 696 [DOI] [PubMed] [Google Scholar]
- 12. Ammoun S., Holmqvist T., Shariatmadari R., Oonk H. B., Detheux M., Parmentier M., Akerman K. E., Kukkonen J. P. (2003) Distinct recognition of OX1 and OX2 receptors by orexin peptides. J. Pharmacol. Exp. Ther. 305, 507–514 [DOI] [PubMed] [Google Scholar]
- 13. Lund P. E., Shariatmadari R., Uustare A., Detheux M., Parmentier M., Kukkonen J. P., Akerman K. E. (2000) The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J. Biol. Chem. 275, 30806–30812 [DOI] [PubMed] [Google Scholar]
- 14. Xia J. X., Fan S. Y., Yan J., Chen F., Li Y., Yu Z. P., Hu Z. A. (2009) Orexin A-induced extracellular calcium influx in prefrontal cortex neurons involves L-type calcium channels. J. Physiol. Biochem. 65, 125–136 [DOI] [PubMed] [Google Scholar]
- 15. Peltonen H. M., Magga J. M., Bart G., Turunen P. M., Antikainen M. S., Kukkonen J. P., Akerman K. E. (2009) Involvement of TRPC3 channels in calcium oscillations mediated by OX(1) orexin receptors. Biochem. Biophys. Res. Commun. 385, 408–412 [DOI] [PubMed] [Google Scholar]
- 16. Kukkonen J. P. (2013) Physiology of the orexinergic/hypocretinergic system: a revisit in 2012. Am. J. Physiol. Cell Physiol. 304, C2–C32 [DOI] [PubMed] [Google Scholar]
- 17. Laplante M., Sabatini D. M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jewell J. L., Russell R. C., Guan K. L. (2013) Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 14, 133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schalm S. S., Fingar D. C., Sabatini D. M., Blenis J. (2003) TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr. Biol. 13, 797–806 [DOI] [PubMed] [Google Scholar]
- 20. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 21. Magnuson B., Ekim B., Fingar D. C. (2012) Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 441, 1–21 [DOI] [PubMed] [Google Scholar]
- 22. Browne G. J., Proud C. G. (2004) A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Mol. Cell. Biol. 24, 2986–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Demetriades C., Doumpas N., Teleman A. A. (2014) Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell 156, 786–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wauson E. M., Zaganjor E., Lee A. Y., Guerra M. L., Ghosh A. B., Bookout A. L., Chambers C. P., Jivan A., McGlynn K., Hutchison M. R., Deberardinis R. J., Cobb M. H. (2012) The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol. Cell 47, 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bar-Peled L., Schweitzer L. D., Zoncu R., Sabatini D. M. (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150, 1196–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zoncu R., Bar-Peled L., Efeyan A., Wang S., Sancak Y., Sabatini D. M. (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334, 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rommel C., Bodine S. C., Clarke B. A., Rossman R., Nunez L., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 3, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 28. Arvisais E. W., Romanelli A., Hou X., Davis J. S. (2006) AKT-independent phosphorylation of TSC2 and activation of mTOR and ribosomal protein S6 kinase signaling by prostaglandin F2α. J. Biol. Chem. 281, 26904–26913 [DOI] [PubMed] [Google Scholar]
- 29. Gao X., Zhang Y., Arrazola P., Hino O., Kobayashi T., Yeung R. S., Ru B., Pan D. (2002) Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 4, 699–704 [DOI] [PubMed] [Google Scholar]
- 30. Jaeschke A., Hartkamp J., Saitoh M., Roworth W., Nobukuni T., Hodges A., Sampson J., Thomas G., Lamb R. (2002) Tuberous sclerosis complex tumor suppressor-mediated S6 kinase inhibition by phosphatidylinositide-3-OH kinase is mTOR independent. J. Cell Biol. 159, 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tee A. R., Fingar D. C., Manning B. D., Kwiatkowski D. J., Cantley L. C., Blenis J. (2002) Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc. Natl. Acad. Sci. U.S.A. 99, 13571–13576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inoki K., Li Y., Zhu T., Wu J., Guan K. L. (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657 [DOI] [PubMed] [Google Scholar]
- 33. Ma L., Teruya-Feldstein J., Bonner P., Bernardi R., Franz D. N., Witte D., Cordon-Cardo C., Pandolfi P. P. (2007) Identification of S664 TSC2 phosphorylation as a marker for extracellular signal-regulated kinase mediated mTOR activation in tuberous sclerosis and human cancer. Cancer Res. 67, 7106–7112 [DOI] [PubMed] [Google Scholar]
- 34. Naegele S., Morley S. J. (2004) Molecular cross-talk between MEK1/2 and mTOR signaling during recovery of 293 cells from hypertonic stress. J. Biol. Chem. 279, 46023–46034 [DOI] [PubMed] [Google Scholar]
- 35. Roux P. P., Ballif B. A., Anjum R., Gygi S. P., Blenis J. (2004) Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U.S.A. 101, 13489–13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carrière A., Cargnello M., Julien L. A., Gao H., Bonneil E., Thibault P., Roux P. P. (2008) Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr. Biol. 18, 1269–1277 [DOI] [PubMed] [Google Scholar]
- 37. Carriere A., Romeo Y., Acosta-Jaquez H. A., Moreau J., Bonneil E., Thibault P., Fingar D. C., Roux P. P. (2011) ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J. Biol. Chem. 286, 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forster C., Kane P. M. (2000) Cytosolic Ca2+ homeostasis is a constitutive function of the V-ATPase in Saccharomyces cerevisiae. J. Biol. Chem. 275, 38245–38253 [DOI] [PubMed] [Google Scholar]
- 39. Crider B. P., Xie X. S. (2003) Characterization of the functional coupling of bovine brain vacuolar-type H+-translocating ATPase. Effect of divalent cations, phospholipids, and subunit H (SFD). J. Biol. Chem. 278, 44281–44288 [DOI] [PubMed] [Google Scholar]
- 40. Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S., Sabatini D. M. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 42. Sancak Y., Peterson T. R., Shaul Y. D., Lindquist R. A., Thoreen C. C., Bar-Peled L., Sabatini D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marcus J. N., Aschkenasi C. J., Lee C. E., Chemelli R. M., Saper C. B., Yanagisawa M., Elmquist J. K. (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25 [DOI] [PubMed] [Google Scholar]
- 44. Belsham D. D., Cai F., Cui H., Smukler S. R., Salapatek A. M., Shkreta L. (2004) Generation of a phenotypic array of hypothalamic neuronal cell models to study complex neuroendocrine disorders. Endocrinology 145, 393–400 [DOI] [PubMed] [Google Scholar]
- 45. Mieda M., Willie J. T., Hara J., Sinton C. M., Sakurai T., Yanagisawa M. (2004) Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc. Natl. Acad. Sci. U.S.A. 101, 4649–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Funato H., Tsai A. L., Willie J. T., Kisanuki Y., Williams S. C., Sakurai T., Yanagisawa M. (2009) Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 9, 64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ammoun S., Johansson L., Ekholm M. E., Holmqvist T., Danis A. S., Korhonen L., Sergeeva O. A., Haas H. L., Akerman K. E., Kukkonen J. P. (2006) OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol. Endocrinology 20, 80–99 [DOI] [PubMed] [Google Scholar]
- 48. Kojima I., Matsunaga H., Kurokawa K., Ogata E., Nishimoto I. (1988) Calcium influx: an intracellular message of the mitogenic action of insulin-like growth factor-I. J. Biol. Chem. 263, 16561–16567 [PubMed] [Google Scholar]
- 49. Poiraudeau S., Lieberherr M., Kergosie N., Corvol M. T. (1997) Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J. Cell. Biochem. 64, 414–422 [PubMed] [Google Scholar]
- 50. Lebreton S., Jaunbergs J., Roth M. G., Ferguson D. A., De Brabander J. K. (2008) Evaluating the potential of vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg. Med. Chem. Lett. 18, 5879–5883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu Y., Parmar A., Roux E., Balbis A., Dumas V., Chevalier S., Posner B. I. (2012) Epidermal growth factor-induced vacuolar (H+)-atpase assembly: a role in signaling via mTORC1 activation. J. Biol. Chem. 287, 26409–26422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Graves L. M., He Y., Lambert J., Hunter D., Li X., Earp H. S. (1997) An intracellular calcium signal activates p70 but not p90 ribosomal S6 kinase in liver epithelial cells. J. Biol. Chem. 272, 1920–1928 [DOI] [PubMed] [Google Scholar]
- 53. Conus N. M., Hemmings B. A., Pearson R. B. (1998) Differential regulation by calcium reveals distinct signaling requirements for the activation of Akt and p70S6k. J. Biol. Chem. 273, 4776–4782 [DOI] [PubMed] [Google Scholar]
- 54. Gulati P., Gaspers L. D., Dann S. G., Joaquin M., Nobukuni T., Natt F., Kozma S. C., Thomas A. P., Thomas G. (2008) Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 7, 456–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ito N., Ruegg U. T., Kudo A., Miyagoe-Suzuki Y., Takeda S. (2013) Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat. Med. 19, 101–106 [DOI] [PubMed] [Google Scholar]
- 56. Hannan K. M., Thomas G., Pearson R. B. (2003) Activation of S6K1 (p70 ribosomal protein S6 kinase 1) requires an initial calcium-dependent priming event involving formation of a high-molecular-mass signalling complex. Biochem. J. 370, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nobukuni T., Joaquin M., Roccio M., Dann S. G., Kim S. Y., Gulati P., Byfield M. P., Backer J. M., Natt F., Bos J. L., Zwartkruis F. J., Thomas G. (2005) Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc. Natl. Acad. Sci. U.S.A. 102, 14238–14243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yan Y., Flinn R. J., Wu H., Schnur R. S., Backer J. M. (2009) hVps15, but not Ca2+/CaM, is required for the activity and regulation of hVps34 in mammalian cells. Biochem. J. 417, 747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Juhász G., Hill J. H., Yan Y., Sass M., Baehrecke E. H., Backer J. M., Neufeld T. P. (2008) The class III PI(3)K Vps34 promotes autophagy and endocytosis but not TOR signaling in Drosophila. J. Cell Biol. 181, 655–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kane P. M. (2012) Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr. Protein Pept. Sci. 13, 117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gräf R., Harvey W. R., Wieczorek H. (1996) Purification and properties of a cytosolic V1-ATPase. J. Biol. Chem. 271, 20908–20913 [DOI] [PubMed] [Google Scholar]
- 62. Kok S. W., Overeem S., Visscher T. L., Lammers G. J., Seidell J. C., Pijl H., Meinders A. E. (2003) Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes. Res. 11, 1147–1154 [DOI] [PubMed] [Google Scholar]
- 63. Cota D., Proulx K., Smith K. A., Kozma S. C., Thomas G., Woods S. C., Seeley R. J. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 [DOI] [PubMed] [Google Scholar]
- 64. Harlan S. M., Guo D. F., Morgan D. A., Fernandes-Santos C., Rahmouni K. (2013) Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 17, 599–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Düvel K., Yecies J. L., Menon S., Raman P., Lipovsky A. I., Souza A. L., Triantafellow E., Ma Q., Gorski R., Cleaver S., Vander Heiden M. G., MacKeigan J. P., Finan P. M., Clish C. B., Murphy L. O., Manning B. D. (2010) Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Porstmann T., Santos C. R., Griffiths B., Cully M., Wu M., Leevers S., Griffiths J. R., Chung Y. L., Schulze A. (2008) SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Feng Y., Liu T., Li X. Q., Liu Y., Zhu X. Y., Jankovic J., Pan T. H., Wu Y. C. (2014) Neuroprotection by Orexin-A via HIF-1α induction in a cellular model of Parkinson's disease. Neurosci. Lett. 579, 35–40 [DOI] [PubMed] [Google Scholar]
- 68. Yuan L. B., Dong H. L., Zhang H. P., Zhao R. N., Gong G., Chen X. M., Zhang L. N., Xiong L. (2011) Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology 114, 340–354 [DOI] [PubMed] [Google Scholar]
- 69. Sikder D., Kodadek T. (2007) The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 21, 2995–3005 [DOI] [PMC free article] [PubMed] [Google Scholar]