Background: SLO3 and CATSPER are two sperm-specific ion channels.
Results: SLO3 K+ channels control Ca2+ entry through CATSPER channels.
Conclusion: SLO3 control of CATSPER channel activity involves an intermediary step in which SLO3-dependent hyperpolarization may elicit internal alkalization via a voltage-dependent mechanism.
Significance: Understanding the control of Ca2+ entry in sperm is crucial to understanding fertility; this study also reveals an unusual role for a K+ channel.
Keywords: Calcium, Calcium Channel, pH Regulation, Potassium Channel, Sperm, Spermatozoa
Abstract
Here we show how a sperm-specific potassium channel (SLO3) controls Ca2+ entry into sperm through a sperm-specific Ca2+ channel, CATSPER, in a totally unanticipated manner. The genetic deletion of either of those channels confers male infertility in mice. During sperm capacitation SLO3 hyperpolarizes the sperm, whereas CATSPER allows Ca2+ entry. These two channels may be functionally connected, but it had not been demonstrated that SLO3-dependent hyperpolarization is required for Ca2+ entry through CATSPER channels, nor has a functional mechanism linking the two channels been shown. In this study we show that Ca2+ entry through CATSPER channels is deficient in Slo3 mutant sperm lacking hyperpolarization; we also present evidence supporting the hypothesis that SLO3 channels activate CATSPER channels indirectly by promoting a rise in intracellular pH through a voltage-dependent mechanism. This mechanism may work through a Na+/H+ exchanger (sNHE) and/or a bicarbonate transporter, which utilizes the inward driving force of the Na+ gradient, rendering it intrinsically voltage-dependent. In addition, the sperm-specific Na+/H+ exchanger (sNHE) possess a putative voltage sensor that might be activated by membrane hyperpolarization, thus increasing the voltage sensitivity of internal alkalization.
Introduction
To fertilize the egg, the sperm must undergo a process known as capacitation that takes place in the female genital tract but can also be accomplished in vitro by incubating the sperm in defined conditions (1, 2). Capacitation involves a complex series of molecular events that include: protein tyrosine phosphorylation (3), an increase in intracellular pH (pHi) (4–6), an increase in K+ permeability (7–11) a hyperpolarization of the plasma membrane (7–11), and an increase in intracellular Ca2+ (12–14). As a result of capacitation, sperm acquire a special form of motility known as hyperactivation and the ability to undergo a regulated acrosome reaction. Two major ion channels become active in mouse sperm during capacitation, the SLO3 K+ channel and the CATSPER Ca2+ channel. Knock-out mutations of either of these channel genes confers male infertility, indicating that both of these channels play a vital role in sperm physiology (15–22). It has been proposed that the elevation of Ca2+ necessary to hyperactivate the sperm is primarily driven by the concerted interplay of these two ion channel types (16, 18, 22). However, the functional relationship between the two channels has not yet been demonstrated. Both of these channels are activated by intracellular alkalization, and both are voltage-activated and open upon depolarization (15, 20). Although voltage-sensitive, sperm-specific SLO3 channels can contribute to the resting potential (9) SLO3 channels have relatively high conductance (15), and the opening of only a relatively small number of SLO3 channels at resting potential in response to intracellular alkalization is sufficient to hyperpolarize the sperm membrane (9). We and others have shown that activation of the SLO3 K+ channel is the principal mechanism whereby the sperm plasma membrane hyperpolarizes during capacitation (16–18). Supporting this are experiments showing that sperm from the knock-out strain of Slo3 (Slo3−/−) lack the hyperpolarization that occurs during capacitation and remain depolarized during the process (17, 18). The events that lead to the activation of SLO3 channels and membrane hyperpolarization are complex and varied, but generally, external conditions favorable for capacitation promote internal alkalization (5, 6). Indeed, we have found that sperm encountering an external alkaline environment alone is sufficient to activate SLO3 channels (9), probably by an increase in pHi.
The mechanisms that govern pHi in sperm are complex and might differ between species. In sea urchin sperm it has been proposed that membrane hyperpolarization raises intracellular pH, probably through a Na+/H+ exchange (NHE)2 mechanism (23–25). In mammalian sperm pHi is known to be an important factor regulating initial sperm motility (26–29), capacitation (4–6), and hyperactivation (30–32). Both the acid load during sperm storage and the subsequent alkaline shift after release are required for fertility. During their transit through the female genital tract sperm encounter increasing external pH and higher concentrations of HCO3−, and both HCO3− and H+ transporters are considered to play a key role in capacitation and sperm hyperactivation (33). Several types of these transporters have been described in mammalian sperm including the Na+/H+ exchanger (34–37), a Na+-Cl−/HCO3− transporter (4), and a Na+/HCO3− transporter (38), all of which have been proposed to participate in sperm alkalization during capacitation, and all of which use the energy stored in the Na+ gradient. Transport mechanisms that depend on the stored energy of the Na+ ion gradient rather than ATP are intrinsically voltage-dependent because they rely on the inwardly directed driving force of Na+, which increases with hyperpolarization. In addition, in mouse sperm, a different form of NHE has been described (sperm-specific Na+/H+ exchanger (sNHE)) that possess a putative voltage sensor domain that could possibly promote activation of Na+/H+ exchange upon membrane hyperpolarization (35–37).
It has also been proposed that linear increases observed in pHi following increases in extracellular pH might be due to a membrane proton permeability present in several mammalian species (29, 39). In human sperm the proton channel Hv1 has been proposed to play a key role in sperm alkalinization (40). This channel is activated by the combination of the pH gradient and membrane depolarization. In addition, it is proposed that Hv1 may also be activated by the removal of zinc during sperm passage through the female genital tract and by encountering anandamide during sperm penetration through the cumulus oophorus (40, 41). In contrast, in mouse sperm the Hv1 channel may not play as significant a role in the control of intracellular pH. Based on these facts it was proposed that human and mouse sperm control their intracellular pH in different ways (40). However, human sperm also have sNHE that can contribute to sperm alkalinization as well (34). This could be a significant factor as sperm exit the cauda epididymis, where [Na+] is <25 mm and pH is <7, into the higher [Na+] and slightly more alkaline conditions present in most of the female reproductive tract (42). As sperm enter this new environment, the driving force of the Na+ gradient increases, which may facilitate proton efflux through sNHE, and hence pHi alkalinization. Thus, it is possible that human sperm may utilize two mechanisms for proton efflux, one of which is shared with mouse. Details of the relative importance of Hv1 and sNHE in human sperm are yet to be established.
In this study we propose a model by which changes in pHi produced by different mechanisms may integrate the activity of the two sperm-specific ion channels, SLO3 and CATSPER, to produce an influx of Ca2+ ions. The process may unfold when sperm encounter HCO3− and a more alkaline environment in the female genital tract, both of which may initiate an increase in pHi. This may then be responsible for the initial activation of SLO3 channels leading to hyperpolarization, which in turn leads to a further rise in pHi via the effect of hyperpolarization on one of the voltage-sensitive mechanisms described above. The increasing alkalinity may then be sufficient to activate CATSPER channels, allowing Ca2+ influx either incrementally at hyperpolarized voltages or in a bolus upon depolarization. Depolarization can occur experimentally by the application of high extracellular K+ or by a more physiological source such as zona pellucida (ZP) (43). The data we present in this study are consistent with this model. We show that Slo3−/− mutant sperm, which lack capacitation-associated hyperpolarization, are also deficient in Ca2+ entry through CATSPER channels. Furthermore, we show that the mutant phenotype of deficient Ca2+ entry can be rescued in Slo3−/− mutant sperm by two different strategies: 1) artificial hyperpolarization using the K+-specific ionophore valinomycin, and 2) increasing extracellular pH, which results in an increase in intracellular pH. A plausible hypothesis then is that hyperpolarization per se, which increases the inward driving force on Na+, is contributing to intracellular alkalization, possibly by augmenting the efficiency of H+ export by NHE and/or bicarbonate transport. Supporting this model are experiments we present showing that the treatment of sperm with valinomycin to achieve hyperpolarization in itself elevates internal pH.
EXPERIMENTAL PROCEDURES
Reagents
Valinomycin (catalog number V0627) and dimethyl sulfoxide (DMSO) (catalog number D8418) were purchased from Sigma. Ionomycin (catalog number 407950) was obtained from Calbiochem (Darmstadt, Germany). Fluo-4 (catalog number F-14201), Pluronic F-127 (catalog number P3000MP), and 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF; catalog number B-1151) were purchased from Life Technologies.
Animals
All procedures described herein were reviewed and approved by the Animals Studies Committee of Washington University (St. Louis, MO) and were performed in accord with the National Institutes of Health Guiding Principles of the care and use of laboratory animals. Slo3 knock-out mice were synthesized by removal of the first two coding exons of the Kcnu1 gene (TG0050 TIGM). This removed the initiation codon and DNA sequence encoding the first and partial second membrane-spanning domains (17). CatSper1 knock-out mice were obtained from The Jackson Laboratory. CatSper knock-out mice were synthesized by replacing the second exon encoding the first putative transmembrane domain, with an IRES-LacZ sequence followed by a neomycin resistance gene in 129S4/SvJae-derived J1 ES cells (19).
Sperm Preparation
Caudal epididymal sperm were collected from ∼90-day-old C57BL/6 male breeders from wild-type, Slo3−/− mutant, and CatSper1−/− mutant mice. Minced cauda epididymis from each animal were placed in HS medium (in mm): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgSO4, 20 HEPES, 5 glucose, 10 lactic acid, 1 Na+-pyruvate in pH 7.4 or as indicated. The swim-up procedure was employed to separate the motile fraction of the sperm sample. To capacitate the sperm, we supplemented HS medium with 5 mg/ml BSA and 15 mm NaHCO3 and incubated for 60 min at 37 °C. For some experiments, we used solubilized ZP from homogenized ovaries of 60-day-old virgin females obtained according to Ref. 44.
Ca2+ Imaging
Motile cells were incubated with 2 μm Fluo-4 AM and 0.05% Pluronic F-127 in HS non-capacitated medium (lacking BSA or NaHCO3) for 30 min at 37 °C. Immediately after, sperm were washed once at 1,500 rpm during 5 min and were resuspended in HS non-capacitated media. Once loaded, sperm were attached to laminin (1 mg/ml)-coated coverslips and incubated 15 min to permit attachment. For capacitation, attached sperm were incubated in complete media (5 mg/ml BSA and 15 mm NaHCO3) for 60 min. Non-capacitated sperm were incubated with 15 mm NaHCO3 for 10 min before the recordings (45). Sperm were incubated with HS alone for control experiments or with 1 μm valinomycin for 15 min. A local perfusion device with an estimate exchange time of <0.5 s applied various test solutions, and recordings were started at least 2 min prior to the addition of 50 mm KCl, solubilized ZP, or changes in external pH. Ionomycin (5 μm) was added at the end of the recordings as a control stimulus. A DG4 combination light source/excitation filter wheel switcher (Sutter Instruments, Novato, CA) with a 175-W Xenon lamp was used to generate the excitation at 488 nm. A 63× oil objective on an inverted microscope (Zeiss Axiovert 200) was used for imaging. Emissions bandwith (515–565 nm) were band-pass-filtered (Lambda 10-2 emission filter wheel switcher; Sutter Instruments, Novato, CA), and images were collected with a Cascade 512B CCD camera (Photometrics, Tucson, AZ) (30 ms every 2 s). Online control and data collection were done using SlideBook 5.0 software (Intelligent Imaging Innovations, Boulder, CO). Images were analyzed using ImageJ software (version 1.48, National Institute of Health) and Origin 6 (MicroCal Software, Northampton MA). The Fluo-4 [Ca2+]i changes are presented as (F − F0)/F0 after background subtraction.
All the imaging experiments were done at room temperature. Motile sperm that were attached to the coverslip mainly by the head were used for the analysis. Images were obtained from the head region of the sperm. It has been previously shown (46) that although CATSPER channels are located in the principal piece of the flagellum, calcium influx through CATSPER channels propagates from the sperm tail to the head within seconds. Our results shown in Fig. 1 confirm these findings and show a similar [Ca2+]i increase in the flagellum and the head of an individual sperm upon alkaline depolarization. Both regions of the sperm responded in a similar way, but with a larger and delayed response in the head. Because Ca2+ responses are larger in the head, and laminin principally attached sperm at the head while the flagellum remained motile, we conducted our Ca2+ measurements in sperm heads. Cells with peak [Ca2+]i changes of >10% relative to the ionomycin changes were counted as responsive.
FIGURE 1.
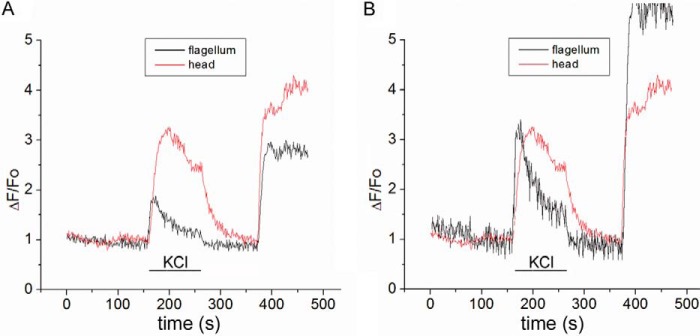
Simultaneous recordings of [Ca2+]i changes in the head and in the flagellum in a single mouse spermatozoa in response to 50 mm external KCl (pH 8.5) followed by 5 μm ionomycin. A, simultaneous recording shows fluorescent responses in the flagellum and the head, when stimulated with 50 mm KCl, pH 8.5; however, the response is larger and slightly more sustained in the head. B, fluorescent responses in the flagellum and the head of sperm were normalized to better illustrate a tail-to-head propagation of the response stimulated by 50 mm KCl; note that, as reported previously (46), the onset of the fluorescent change in the flagellum (black trace) slightly precedes the onset of the change in the head (red trace). A similar delay was not observed when the Ca2+ ionophore ionomycin was used to trigger [Ca2+]i at the end of the experiments. Similar results were reported by Xia et al. (46).
pH Imaging
Motile cells were loaded with 0.5 μm BCECF-AM (BCECF with acetoxymethyl ester) in non-capacitated medium during 10 min at 37 °C. Cells were washed once at 1,500 rpm during 5 min and resuspended in non-capacitated media without BCECF. For the recordings, cells were attached to laminin (1 mg/ml)-coated coverslips and were excited with a stroboscopic LED-based fluorescence illumination system as described previously (47) with 1-ms light excitation pulses, recording one image every 2 s. Fluorescence was captured with CoolSNAP (Photometrics). Images were obtained from the sperm head and analyzed using ImageJ software version 1.48v (National Institutes of Health) and Origin 6 (MicroCal Software). The BCECF pHi changes are presented as (F − F0)/F0 after background subtraction.
RESULTS
Increases in Internal Ca2+ Can Be Evoked by Alkaline Depolarization in Both Wild-type and Slo3−/− Mutant Sperm
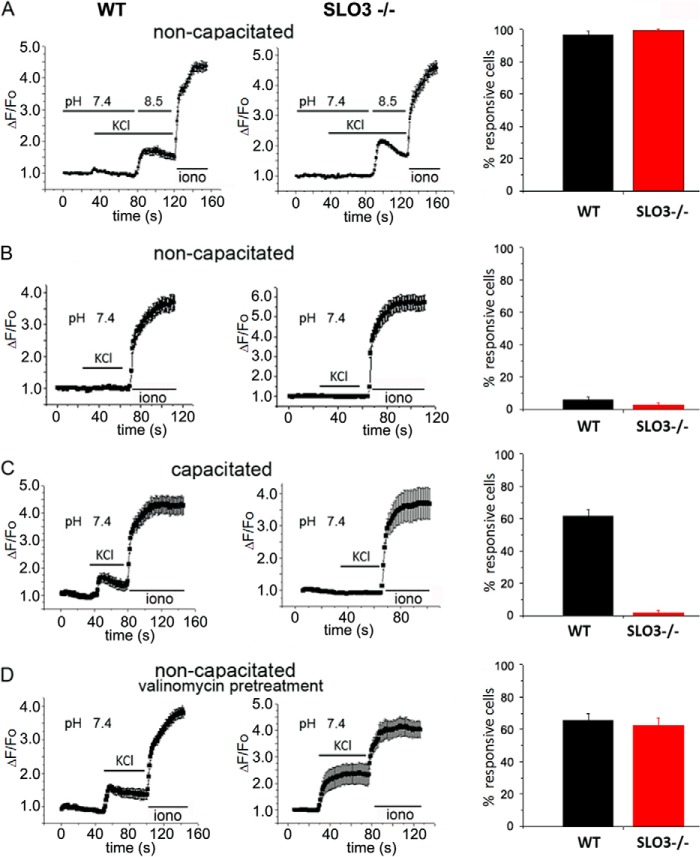
Previous studies have shown that alkaline depolarization (high KCl at pH 8.5) triggers an increase in intracellular Ca2+ in non-capacitated sperm (45). CATSPER channels are opened by both depolarization and intracellular alkaline pHi (20), and it has been shown that depolarization in an extracellular alkaline medium produces intracellular calcium ([Ca2+]i) increases through CATSPER channels (21, 46). This is apparently due to the combination of depolarization and the increase in pHi that results from a change in extracellular pH (29). This increase in [Ca2+]i triggered by external alkaline depolarization is absent in sperm from CatSper−/− mutant knock-out mice (21, 46). To determine whether this internal rise in Ca2+ in response to alkaline depolarization also occurred in Slo3 knock-out mice (Slo3−/−), we undertook a series of similar experiments in both Slo3−/− mice and wild-type mice as a control. In these experiments a similar increase in [Ca2+]i was found in virtually 100% of sperm from both Slo3−/− mice and wild-type mice (Fig. 2A). Thus, in alkaline depolarizing conditions, there seems to be no dependence of CATSPER channels on the activity of SLO3 channels. This indicates that either CATSPER channels are not dependent on the activity of SLO3 channels or the conditions of alkaline depolarization bypass the need for SLO3 channels. The following experiments suggest that this latter interpretation is correct and that the activity of SLO3 channels is essential for the internal rise of intracellular Ca2+ during sperm capacitation.
FIGURE 2.
Intracellular calcium changes in response to KCl in WT and mutant Slo3−/− sperm. A and B, alkaline depolarization produces an increase in [Ca2+]i in both WT and Slo3−/− mutant sperm in non-capacitated conditions, whereas depolarization at neutral pH does not induce a significant response. A, alkaline depolarization induced [Ca2+]i increases in 96.6 ± 1.9% of WT sperm tested (85/88 sperm cells, 4 mice) and in 99 ± 1% of Slo3−/− sperm (95/96 sperm cells, 3 mice). Traces are mean Ca2+ responses to KCl application (pH = 8.5) (n = 21 representative WT cells; n = 27 representative Slo3−/− sperm cells; error bars = S.E.) Ionomycin (iono, 5 μm) was added at the end of the recordings as a control stimulus. Cells with peak [Ca2+]i changes of >10% relative to the ionomycin changes were counted as responsive. B, the application of 50 mm KCl (pH 7.4) in non-capacitated sperm only induces [Ca2+]i increases in 6 ± 1.8% (10/168 sperm cells, 6 mice) of WT non-capacitated sperm cells and in 2.8 ± 1.4% (4/143 sperm cells, 8 mice) of Slo3−/− non-capacitated sperm. Traces of mean Ca2+ responses to KCl application are shown for n = 12 representative WT cells and n = 10 representative Slo3−/− sperm cells. C, depolarization at pH 7.4 induces a significant [Ca2+]i increase in WT but not in Slo3−/− mutant sperm in capacitated conditions. After capacitation, the application of 50 mm KCl at pH 7.4 induced [Ca2+]i increases in 61.3 ± 4.46% of WT sperm (73/119 sperm cells, 7 mice), whereas only 1.72 ± 1.2% (2/116 sperm cells, 6 mice) of the Slo3−/− sperm are able to respond. Mean traces of Ca2+ responses to KCl application are shown for n = 14 representative WT sperm cells and n = 7 representative Slo3−/− mutant sperm. D, valinomycin pretreatment rescues the Ca2+ responses in Slo3−/− sperm. Mean Ca2+ responses of WT and Slo3−/− mutant non-capacitated sperm pretreated with 1 μm valinomycin and subsequently exposed to 50 mm KCl (pH 7.4) are shown. This treatment induces [Ca2+]i increases in both WT non-capacitated sperm cells, where 65.5 ± 4.5% of the cells responded (74/113 sperm cells, 7 mice), and Slo3−/− mutant sperm cells, where 62.5 ± 4.42% of the sperm cells responded (74/120 sperm cells, 5 mice). Mean [Ca2+]i traces are shown for n = 22 representative WT sperm and n = 5 representative Slo3−/− mutant sperm.
Increases in [Ca2+]i Depend on SLO3 Channel Activity after Capacitation
It has previously been shown that alkaline depolarization triggers Ca2+ entry through CATSPER channels (19, 21), and it was proposed that alkaline depolarization causes an increase in pHi (29, 48). This experiment is reproduced in Fig. 2A. To show the dependence of the rise in internal [Ca2+] on high external pH, similar experiments were undertaken with high extracellular K+ applied at neutral pH. In these experiments a rise in [Ca2+]i was not seen in most (>94%) sperm from either wild-type or Slo3−/− mice (Fig. 2B). As in Fig. 2A, these experiments were undertaken in non-capacitated sperm where internal pH is reported to be low (∼6.5 (4, 5)).
After being subjected to capacitating conditions, however, it is known that internal pH becomes more alkaline in wild-type sperm (4–6). Hence, after being subject to capacitating conditions, sperm from both wild-type and Slo3−/− mice were again treated with high extracellular K+ applied this time at neutral pH. In these experiments (Fig. 2C) wild-type and Slo3−/− mutant sperm reacted differently; ∼60% of wild-type sperm reacted with a robust increase in [Ca2+]i, whereas only about 2% of Slo3−/− mutant sperm responded. Additionally, these few mutant respondents showed a [Ca2+]i increase, which was about half that of wild type. (Actual values and statistics for these and other experiments can be found in the figure legends, and Table 1 provides additional information.) The fact that only ∼60% and not 100% of wild-type sperm reacted with an increase in [Ca2+]i in response to high external KCl or ZP may be related to the fact that, in a population of sperm subjected to capacitated conditions, not all the sperm achieve a capacitated state (10, 49).
TABLE 1.
Percentage of responsive cells and response amplitudes
NC: non-capacitated, Cap: capacitated, valino: valinomycin. Data given are mean ± S.E.
| % of responsive cells | Response amplitudea | |
|---|---|---|
| Wild-type NC-K8.6 | 96.6 ± 1.9 (85/88) (4 mice) | 26.2 ± 1.2 |
| Wild-type NC-KCl | 5.95 ± 1.8 (10/168) (6 mice) | 14.5 ± 0.96 |
| Wild-type Cap-KCl | 61.3 ± 4.5 (73/119) (7 mice) | 29.9 ± 1.76 |
| Wild-type NC-valino-KCl | 65.5 ± 4.5 (74/133) (7 mice) | 32.8 ± 2.15 |
| Wild-type NC-ZP | 4.6 ± 2.2 (4/87) (4 mice) | 15.9 ± 2.9 |
| Wild-type Cap-ZP | 66.7 ± 5.7 (46/69) (4 mice) | 32.8 ± 3.3 |
| Wild-type NC-valino-ZP | 56 ± 5.9 (40/72) (3 mice) | 47.4 ± 4.7 |
| Slo3−/− NC-K8.6 | 99 ± 1 (95/96) (3 mice) | 35.5 ± 1.3 |
| Slo3−/− NC-KCl | 2.8 ± 1.4 (4/143) (8 mice) | 13.95 ± 1.3 |
| Slo3−/− Cap-KCl | 1.72 ± 1.2 (2/116) (6 mice) | 15.7 ± 3.2 |
| Slo3−/− NC-valino-KCl | 62.5 ± 4.4 (74/120) (5 mice) | 47.2 ± 2.7 |
| Slo3−/− NC-ZP | 3.2 ± 1.8 (3/95) (5 mice) | 11.7 ± 1.65 |
| Slo3−/− Cap-ZP | 15.8 ± 3.4 (20/118) (5 mice) | 25 ± 3.1 |
| Slo3−/− NC-valino-ZP | 81 ± 3.6 (98/121) (4 mice) | 38.6 ± 2.39 |
a Response amplitude is [Ca2+]i increase relative to the ionomycin-induced [Ca2+]i increase.
Thus, SLO3 channels appear to play a key role in the activation of CATSPER channels. As a control to verify that Ca2+ entry in capacitated wild-type sperm subjected to KCl depolarization at neutral pH was through CATSPER channels, we repeated the same experiment as shown in Fig. 2C with sperm from the CatSper knock-out mutant (CatSper−/−) and observed a negative result (Fig. 3B). Thus, there is a failure of Ca2+ entry in both Slo3−/− mutant sperm (Fig. 2C, right) and CatSper−/− mutant sperm (Fig. 3B) stimulated in the same way. This result suggests a functional relationship between the SLO3 and CATSPER ion channels. In Fig. 2C (right) there appears to be a failure of SLO3 to facilitate Ca2+ entry through CATSPER channels, whereas in Fig. 3B the CATSPER channel is simply not present.
FIGURE 3.

[Ca2+]i increases evoked by application of 50 mm KCl in capacitated WT sperm, or in non-capacitated sperm pretreated with valinomycin, are due to Ca2+ influx through CATSPER channels. A, WT capacitated positive control from Fig. 2C. iono, ionomycin. B, capacitated CatSper−/− cells show no Ca2+ response. Mean [Ca2+]i traces of n = 5 representative capacitated CatSper−/− sperm. As the figure indicates, no [Ca2+]i increases were observed in n = 119 CatSper−/− cells tested (0/119 sperm cells, 3 mice). C, WT non-capacitated positive control treated with 1 μm valinomycin from Fig. 1D. D, non-capacitated CatSper−/− mutant cells treated with valinomycin show no Ca2+ response. Mean [Ca2+]i traces of n = 5 representative CatSper−/− sperm pretreated with 1 μm valinomycin are shown. A tiny fraction (3.2% of CatSper−/− sperm = 3/63 sperm cells, 3 mice) showed an unexplained non-representative significant increase in [Ca2+]i.
One explanation for the result in Fig. 2C could be that Slo3−/− sperm fail to undergo internal alkalization when subjected to capacitating conditions. However, we (9, 11, 17) and other laboratories (16, 18) have shown that a primary role of SLO3 channels is to evoke sperm membrane hyperpolarization when exposed to capacitating conditions. Thus, membrane hyperpolarization could be a factor that itself substitutes for an increase in intracellular pH, or SLO3-dependent membrane hyperpolarization could be involved in the mechanism by which internal alkalization occurs during capacitation. To test whether membrane hyperpolarization is an essential step, we conducted experiments to evoke membrane hyperpolarization in a manner that bypasses the activation of SLO3 channels. These experiments are shown in Fig. 2D where membrane hyperpolarization was achieved by pretreatment of both non-capacitated wild-type and Slo3−/− sperm with the K+ ionophore valinomycin, which is known to hyperpolarize the sperm cell membrane. The results of these experiments (Fig. 2D) were similar to those in Fig. 2A, where an increase in [Ca2+]i was found in ∼60% of sperm from both Slo3−/− mice and wild-type mice. The similarity of responses in Fig. 2A, where elevated pH appears to be a key factor, and Fig. 2D, where a period of hyperpolarization appears to be a relevant factor, suggests that either elevated pH or membrane hyperpolarization can substitute for one another; on the other hand membrane hyperpolarization may be a functional step required for the intracellular rise in pH. We show in a following section that this latter suggestion appears to be correct.
As a control to substantiate that Ca2+ entry in these experiments is through CATSPER channels, we showed that CatSper−/− mutant sperm cannot be rescued by membrane hyperpolarization achieved by pretreatment with the K+ ionophore valinomycin (Fig. 3D). This failure of hyperpolarization to rescue sperm lacking the CATSPER channel suggests that hyperpolarization acts by facilitating the activity of CATSPER channels.
An Increase in [Ca2+]i in Response to ZP Application Is Also Deficient in Slo3−/− Mutant Sperm
The ZP is a glycoprotein membrane surrounding the plasma membrane of an oocyte. It has been shown that ZP, as well as high external KCl, can trigger sperm membrane depolarization (43). It has also been shown that ZP triggers calcium influx through CATSPER channels (50). Thus, to investigate whether ZP would have a different effect on Slo3−/− mutant sperm relative to wild-type sperm with regard to Ca2+ entry, we undertook similar experiments with ZP as we had done with high external KCl (51). First we applied ZP at neutral pH to both non-capacitated wild-type and Slo3−/− mutant sperm as had been done with KCl in Fig. 2B. The results of these experiments (Fig. 4A) were identical to those in Fig. 2B; a rise in [Ca2+]i was not seen in sperm from either wild-type or Slo3−/− mice (Fig. 4A). However, after being subjected to capacitating conditions, sperm from wild-type and Slo3−/− mice reacted differently with ∼70% of wild-type sperm responding with a robust increase in [Ca2+]i, whereas only ∼15% of Slo3−/− mutant sperm responded (Fig. 4B). Also, in the small number of Slo3−/− mutant capacitated sperm that did respond, the amplitudes of the responses were significantly smaller than in wild-type capacitated sperm (Table 1). This result paralleled that of Fig. 2C where KCl was the depolarizing factor rather than ZP. Because Slo3−/− mutant sperm lack the hyperpolarization induced by capacitating conditions, again membrane hyperpolarization may be an essential step necessary for ZP-induced Ca2+ entry as was shown for KCl-induced Ca2+ entry in Fig. 2D. Thus, as in Fig. 2D, we conducted experiments to evoke membrane hyperpolarization in a manner that bypasses the activation of SLO3 channels. Again, membrane hyperpolarization was achieved by pretreatment of both wild-type and Slo3−/− mutant sperm with the K+ ionophore valinomycin (Fig. 4C); sperm so treated where then subjected to ZP stimulation. In these experiments a similar robust increase in internal [Ca2+] was seen in sperm from both Slo3−/− mice and wild-type mice.
FIGURE 4.
Intracellular Ca2+ increases in response to ZP. A, the application of ZP does not induce [Ca2+]i increases in either WT or Slo3−/− non-capacitated sperm. 95.4 ± 2.2% of WT sperm (83/87 sperm cells, 4 mice), and 96.8 ± 1.8% of Slo3−/− mutant sperm (92/95 sperm cells, 5 mice), did not show a significant [Ca2+]i increase in response to ZP. The mean [Ca2+]i traces are from n = 17 representative WT cells (left panel) and n = 18 representative Slo3−/− sperm cells (right panel). iono, ionomycin. Error bars = S.E. B, ZP induces an increase in [Ca2+]i in WT capacitated sperm but not in Slo3−/− mutant capacitated sperm. ZP triggers an increase in [Ca2+]i in 66.7 ± 5.7% (46/69 sperm cells, 4 mice) of WT sperm, whereas only 15.8 ± 3.4% (20/118 sperm cells, 5 mice) of capacitated Slo3−/− mutant sperm respond. The mean [Ca2+]i traces are from n = 7 representative WT cells and n = 9 representative Slo3−/− cells. Error bars = S.E. C, pretreatment with the K+ ionophore valinomycin rescues the Ca2+ responses. The application of 50 mm KCl (pH 7.4) in non-capacitated WT sperm pretreated with 1 μm valinomycin induces a [Ca2+]i increase in 56 ± 5.9% (40/72 sperm cells, 3 mice) and also induces an increase in [Ca2+]i in 81 ± 3.6% of the Slo3−/− mutant cells (98/121 sperm cells, 4 mice). Mean [Ca2+]i traces are shown for n = 13 representative WT sperm cells and n = 27 representative Slo3−/− sperm cells. Error bars = S.E.
Both Fig. 2 and Fig. 4 suggest the same conclusion; a period of hyperpolarization prior to depolarization is important for Ca2+ ion entry through CATSPER channels. The hyperpolarization can arise either from artificially applied conditions or from a physiological source; the application of the K+ ionophore, valinomycin, to impose hyperpolarization is equally effective as when hyperpolarization results from the activation of SLO3 channels during capacitation. This result presents a conundrum because the evidence suggests that Ca2+ is entering through CATSPER channels, and there is no evidence that hyperpolarization directly activates or facilitates CATSPER channels. On the other hand hyperpolarization may be acting indirectly to enable CATSPER channel activity, possibly by facilitating intracellular alkalinization. This is a plausible hypothesis because sodium-hydrogen exchangers (Na+/H+ exchangers), which regulate intracellular pH, are ATP-independent and rely on inwardly directed Na+ ion electrochemical driving force to transport protons out of the cell. Hence, the system is inherently voltage-dependent and facilitated by hyperpolarization, which increases the driving force on Na+ ion.
One way to test whether the increased inward driving force on Na+ ion is a key factor that comes into play during membrane hyperpolarization is to eliminate it. We previously showed (9, 52) that sperm undergo membrane hyperpolarization when external Na+ ion is reduced (and replaced with choline). In this condition sperm membrane hyperpolarization occurs while simultaneously eliminating the inward driving force on Na+. Thus, we undertook the experiments to test the effectiveness of reducing external Na+ in evoking a rise in [Ca2+]i (Fig. 5). As we had done in Fig. 2D where we hyperpolarized the sperm membrane with valinomycin, we then depolarized the cells by applying high external K+. However, unlike the results when cells were hyperpolarized with valinomycin, cell depolarization with high K+ did not result in any detectable rise in [Ca2+]i in any of the cells tested (n = 30 for wild-type sperm, n = 27 for Slo3−/− mutant sperm). This result suggested that the inward driving force of Na+ could be a key factor during hyperpolarization and adds weight to the hypothesis that sperm membrane hyperpolarization may facilitate intracellular alkalization, which in turn would facilitate the activation of CATSPER channels. Finally, we also show that the treatment of sperm with valinomycin to achieve hyperpolarization in itself elevates intracellular pH (Fig. 6). Hyperpolarization with valinomycin, unlike hyperpolarization by lowering external Na+, leaves the driving force of Na+ undiminished, and we previously showed that it effectively promotes Ca2+ entry (Figs. 2D and 4C). It follows that if hyperpolarization promotes Ca2+ entry through CATSPER channels and the mechanism by which this occurs is through the increased Na+ driving force facilitating Na+/H+ exchange and/or bicarbonate transport, then valinomycin should promote internal alkalinity. Fig. 6 shows that this appears to be the case.
FIGURE 5.
Lowering external Na+ abolishes [Ca2+]i increases in response to KCl depolarization, in both wild-type and Slo3−/− mutant sperm. Mean [Ca2+]i responses to 50 mm KCl application in 1 mm external Na+ from n = 6 representative wild-type sperm and from n = 5 Slo3−/− mutant sperm are shown. Of the cells tested, n = 30 wild-type cells (2 mice) and n = 27 Slo3−/− mutant cells (2 mice), only a single sperm cell from genotype each showed a detectable Ca2+ response; however, the response was very small (<10% of the ionomycin (iono) response).
FIGURE 6.

pHi increase in response to the addition of the K+ ionophore valinomycin. Results of two separate experiments from different mice are shown. A, mean representative pHi traces of n = 6 wild-type sperm in the presence of 1 μm valinomycin and 10 mm NH4Cl. B, mean representative traces of n = 4 wild-type sperm. 10/20 sperm tested responded to the application of valinomycin with an increase in fluorescence.
DISCUSSION
There are at least two examples in the literature where pHi may be regulated by K+ channels. In sea urchin sperm, membrane hyperpolarization raises intracellular pH, probably through a voltage-sensitive NHE mechanism (23–25). In this instance, as in the example we are now reporting, the K+ channel type involved appears to have acquired unusual properties as a result of evolutionary pressure unique to reproductive physiology. On the other hand, an example of K+ channel involvement in the control of pH in somatic rather than reproductive physiology employs K+ channel types found in many somatic tissues (53). In this example where hydrochloric acid is produced in parietal cells of the gastric glands, a proton pump couples the outward movement of H+ to the inward movement of K+. To maintain the activity of the pump, K+ is recirculated over the apical membrane via KCNQ1/KCNE2 K+ channels. In our current study we show a significant role for both hyperpolarization and internal alkalization on Ca2+ entry into sperm and suggest a mechanism of interplay between the two physiological processes. Interestingly, a similar role of hyperpolarization in Ca2+ influx shown here for mouse sperm has also been reported for sea urchin sperm (25); in addition, the importance of internal alkalization on Ca2+ entry was also reported for sea urchin sperm (54).
It has been previously shown by our group and others that SLO3 K+ channels are key players in sperm physiology. The SLO3 knock-out mice are male infertile and show deficits both in hyperactivated motility and in the acrosome reaction (17, 18), both of which require Ca2+ entry. Slo3−/− mutant sperm fail to hyperpolarize during the capacitation process (17, 18). It has been suggested that hyperpolarization is probably necessary for the activation of CATSPER channels and that these two channels work together during capacitation to confer sperm hyperactivated motility (16), but the functional relationship between SLO3 and CATSPER channels has not been shown until now. In this study we established that the hyperpolarization produced by SLO3 channel activation during capacitation is crucial for Ca2+ influx through CATSPER channels. Our results indicate that the influx of Ca2+ through CATSPER channels into the cell requires a period of membrane hyperpolarization prior to membrane depolarization induced by high external KCl or ZP. However, CATSPER channels are Ca2+-permeable channels weakly activated by depolarization and strongly activated by intracellular alkalization and do not show voltage-dependent inactivation (20). Thus, the requirement for hyperpolarization prior to CATSPER channel activation is not obvious, nor is the relationship between SLO3 channel activation and the resulting hyperpolarization, and the activation of CATSPER channels and Ca2+ influx is straightforward. It was previously suggested that hyperpolarization might increase Ca2+ influx through CATSPER channels due to an increase in Ca2+ driving force (16, 18, 22). However, this appears to be irrelevant in the current experiments because we are testing Ca2+ entry upon depolarization.
In experiments to reveal the role of SLO3 channels in activating CATSPER channels, we show that the Ca2+ increase that fails to occur in capacitated Slo3−/− mutant sperm in response to high KCl can be restored by two different experimental treatments: 1) by increasing extracellular pH, and 2) by hyperpolarizing the sperm plasma membrane with the K+ ionophore valinomycin. It was previously shown in wild-type sperm that the application of high external KCl in the presence of high external pH can trigger Ca2+ responses through CATSPER channels, possibly by an increase in pHi (21, 48, 55). Our results showing that Ca2+ responses can also be obtained in Slo3−/− sperm under these conditions suggest that external alkalinity can bypass the need for hyperpolarization by increasing pHi.
On the other hand hyperpolarization of the sperm plasma membrane by a K+ ionophore (valinomycin) can also rescue the Ca2+ responses in Slo3−/− sperm. One possible conclusion from these experiments is that the necessary period of hyperpolarization prior to depolarization is indirectly rather than directly affecting CATSPER channels. Taking all these results into consideration, a plausible unifying principle underlying these phenomena could be that the high external pH and/or high concentrations of HCO3− that sperm encounter in the female genital tract could trigger an initial change in pHi and activation of SLO3 channels; the resulting membrane hyperpolarization, which increases the Na+ inward driving force, may then, in turn, further increase pHi by increasing the activity of the Na+/H+ exchanger or other Na+-dependent mechanisms controlling intracellular pH. This can result in a positive feedback loop as the increase in pHi further activates SLO3 channels, leading to further hyperpolarization. Supporting this model are several mechanisms present in sperm that may confer voltage dependence to sperm intracellular alkalization. An sNHE was identified in mice that contains a putative voltage sensor domain that theoretically could confer direct voltage sensitivity to proton export (35–37). sNHE null mice are infertile with a severe motility defect that can partially be rescued by the application of NH4Cl, which restores intracellular alkalinity (37). The Na+ dependence of sNHE and the HCO3− transport systems, which are powered by the energy stored in the inward Na+ ion gradient, furthermore suggests indirect mechanisms by which internal alkalization can be voltage-sensitive (4, 33, 38).
Our results showing that the application of valinomycin induces a pHi increase in 50% of wild-type sperm tested supports the hypothesis that intracellular pHi is voltage-dependent. This further supports the model that SLO3-elicited hyperpolarization and the elevation of pHi might work hand-in-hand with one factor augmenting the other in a positive feedback loop. The control of pHi and activation of CATSPER channels might be different in human sperm where the proton channel Hv1 has been proposed to be responsible for human sperm intracellular alkalinization (40). A H+ current carried by Hv1 was characterized using the whole-cell patch clamp technique and has been proposed as the major mechanism to produce intracellular alkalization in human sperm. This channel is activated in sperm by membrane depolarization and by alkaline extracellular pH and micromolar concentrations of anandamide (40). Although these currents are prominent in human sperm, their physiological relevance is not completely established yet. The Hv1 currents are activated when sperm membrane potential is set to values close to or greater than 0 mV, and it is not yet known whether sperm can generate these potentials under physiological conditions. It has been proposed that an as yet unidentified ligand-gated cation channel may provide such depolarization (41). Whether such a mechanism for depolarization exists in human sperm remains to be explored. It was recently shown by our group that the human sperm membrane potential does not depolarize during capacitation, as might be required for activation of Hv1, but instead hyperpolarizes (56). Nevertheless, the augmentation of Hv1 channel activity by anandamide and alkaline extracellular pH may be key factors in the physiological relevance of this channel and require further study. Hence, despite elegant patch clamp studies clearly suggesting the ability of HV1 to function as an acid extrusion mechanism in human sperm, it is too early to say the extent to which mouse and human sperm differ with regard to the control of intracellular pH. It is worth noting that human sperm, like murine sperm, also have an sNHE transporter that, like murine sNHE, also contains a putative voltage sensor (34, 37, 57). Because the patch clamp technique cannot detect transmembrane transport mechanisms, such as non-electrogenic transporters, it is conceivable that other mechanisms similar to those in mouse (like the Na+/H+ exchanger) contribute to human sperm acid extrusion, pHi regulation, and CATSPER activation in human sperm. Finally, the principal K+ channel active in human sperm is a subject of debate, with one group presenting evidence that the Ca2+-dependent SLO1 channel is the principle player in human sperm (58), whereas a second group, writing in the same journal shortly afterward, presented evidence that it is the sperm-specific SLO3 channel, as in mouse sperm (59). Because of the fundamental differences between these two channels, verification that it is SLO1, SLO3, or both is necessary to better understand the physiology of human sperm.
In summary, we have shown that SLO3 channels are essential for Ca2+ entry through CATSPER channels during the capacitation process and that the mechanism of CATSPER activation appears to be indirect and may involve a voltage-dependent change in intracellular pH. Conceivably, because SLO3 channels are themselves activated by alkaline pHi, they may go hand-in-hand with the sNHE to increase pHi functioning together as a positive feedback system. Significantly, both of these factors (an intracellular rise in pH and membrane hyperpolarization) are known intracellular changes that occur in sperm during capacitation (4–11). Our earlier findings, that sperm encountering an alkaline external environment, as exists in the female genital tract, can activate SLO3 channels (9) may have revealed the trigger for this positive feedback cascade.
Acknowledgments
We thank Drs. Paul Schlesinger and Robert Wilkinson for the generous loan of laboratory equipment, Dr. Richard Stewart for technical support, and Dr. Paul Schlesinger for valuable intellectual input.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HD069631 (to C. M. S.) and R01HD038082 (to Pablo Visconti subcontract to A. D.). This work was also supported by Consejo Nacional de Ciencia y Tecnología (CONACyT-México) Grant 128566 (to A. D. and C. L. T.); Dirección General de Asuntos del Personal Académico/Universidad Nacional Autónoma de México (DGAPA/UNAM) Grants IN225406 (to A.D.) and IN202212-3 (to C. L. T.); and a grant from the Alexander von Humboldt Foundation (to C. L. T.).
- NHE
- Na+/H+ exchange
- sNHE
- sperm-specific Na+/H+ exchanger
- BCECF
- 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein
- ZP
- zona pellucida
- HS
- high salt.
REFERENCES
- 1. Chang M. C. (1951) Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 168, 697–698 [DOI] [PubMed] [Google Scholar]
- 2. Austin C. R. (1951) Observations on the penetration of the sperm in the mammalian egg. Aust. J. Sci. Res. B 4, 581–596 [DOI] [PubMed] [Google Scholar]
- 3. Visconti P. E., Moore G. D., Bailey J. L., Leclerc P., Connors S. A., Pan D., Olds-Clarke P., Kopf G. S. (1995) Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 121, 1139–1150 [DOI] [PubMed] [Google Scholar]
- 4. Zeng Y., Oberdorf J. A., Florman H. M. (1996) pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev. Biol. 173, 510–520 [DOI] [PubMed] [Google Scholar]
- 5. Vredenburgh-Wilberg W. L., Parrish J. J. (1995) Intracellular pH of bovine sperm increases during capacitation. Mol. Reprod. Dev. 40, 490–502 [DOI] [PubMed] [Google Scholar]
- 6. Breitbart H. (2003) Signaling pathways in sperm capacitation and acrosome reaction. Cell. Mol. Biol. 49, 321–327 [PubMed] [Google Scholar]
- 7. Zeng Y., Clark E. N., Florman H. M. (1995) Sperm membrane potential: hyperpolarization during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev. Biol. 171, 554–563 [DOI] [PubMed] [Google Scholar]
- 8. Santi C. M., Orta G., Salkoff L., Visconti P. E., Darszon A., Treviño C. L. (2013) K+ and Cl− channels and transporters in sperm function. Curr. Top. Dev. Biol. 102, 385–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chávez J. C., de la Vega-Beltrán J. L., Escoffier J., Visconti P. E., Treviño C. L., Darszon A., Salkoff L., Santi C. M. (2013) Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PLoS One 8, e60578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arnoult C., Kazam I. G., Visconti P. E., Kopf G. S., Villaz M., Florman H. M. (1999) Control of the low voltage-activated calcium channel of mouse sperm by egg ZP3 and by membrane hyperpolarization during capacitation. Proc. Natl. Acad. Sci. U.S.A. 96, 6757–6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muñoz-Garay C., De la Vega-Beltrán J. L., Delgado R., Labarca P., Felix R., Darszon A. (2001) Inwardly rectifying K+ channels in spermatogenic cells: functional expression and implication in sperm capacitation. De.v Biol. 234, 261–274 [DOI] [PubMed] [Google Scholar]
- 12. DasGupta S., Mills C. L., Fraser L. R. (1993) Ca2+-related changes in the capacitation state of human spermatozoa assessed by a chlortetracycline fluorescence assay. J. Reprod. Fertil. 99, 135–143 [DOI] [PubMed] [Google Scholar]
- 13. Baldi E., Casano R., Falsetti C., Krausz C., Maggi M., Forti G. (1991) Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl. 12, 323–330 [PubMed] [Google Scholar]
- 14. Suarez S. S., Varosi S. M., Dai X. (1993) Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc. Natl. Acad. Sci. U.S.A. 90, 4660–4664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schreiber M., Wei A., Yuan A., Gaut J., Saito M., Salkoff L. (1998) Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem 273, 3509–3516 [DOI] [PubMed] [Google Scholar]
- 16. Navarro B., Kirichok Y., Clapham D. E. (2007) KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. U.S.A. 104, 7688–7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Santi C. M., Martínez-López P., de la Vega-Beltrán J. L., Butler A., Alisio A., Darszon A., Salkoff L. (2010) The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584, 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zeng X. H., Yang C., Kim S. T., Lingle C. J., Xia X. M. (2011) Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. U.S.A. 108, 5879–5884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ren D., Navarro B., Perez G., Jackson A. C., Hsu S., Shi Q., Tilly J. L., Clapham D. E. (2001) A sperm ion channel required for sperm motility and male fertility. Nature 413, 603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kirichok Y., Navarro B., Clapham D. E. (2006) Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature 439, 737–740 [DOI] [PubMed] [Google Scholar]
- 21. Carlson A. E., Westenbroek R. E., Quill T., Ren D., Clapham D. E., Hille B., Garbers D. L., Babcock D. F. (2003) CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. U.S.A. 100, 14864–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeng X. H., Navarro B., Xia X. M., Clapham D. E., Lingle C. J. (2013) Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. J. Gen. Physiol. 142, 305–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee H. C., Garbers D. L. (1986) Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J. Biol. Chem. 261, 16026–16032 [PubMed] [Google Scholar]
- 24. González-Martínez M., Darszon A. (1987) A fast transient hyperpolarization occurs during the sea urchin sperm acrosome reaction induced by egg jelly. FEBS Lett. 218, 247–250 [DOI] [PubMed] [Google Scholar]
- 25. González-Martínez M. T., Guerrero A., Morales E., de De La Torre L., Darszon A. (1992) A depolarization can trigger Ca2+ uptake and the acrosome reaction when preceded by a hyperpolarization in L. pictus sea urchin sperm. Dev. Biol. 150, 193–202 [DOI] [PubMed] [Google Scholar]
- 26. Acott T. S., Carr D. W. (1984) Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol. Reprod. 30, 926–935 [DOI] [PubMed] [Google Scholar]
- 27. Carr D. W., Acott T. S. (1989) Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol. Reprod. 41, 907–920 [DOI] [PubMed] [Google Scholar]
- 28. Giroux-Widemann V., Jouannet P., Pignot-Paintrand I., Feneux D. (1991) Effects of pH on the reactivation of human spermatozoa demembranated with Triton X-100. Mol. Reprod. Dev. 29, 157–162 [DOI] [PubMed] [Google Scholar]
- 29. Hamamah S., Gatti J. L. (1998) Role of the ionic environment and internal pH on sperm activity. Hum. Reprod. 13, Suppl. 4, 20–30 [DOI] [PubMed] [Google Scholar]
- 30. Ho H. C., Granish K. A., Suarez S. S. (2002) Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev. Biol. 250, 208–217 [DOI] [PubMed] [Google Scholar]
- 31. Marquez B., Suarez S. S. (2007) Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol. Reprod. 76, 660–665 [DOI] [PubMed] [Google Scholar]
- 32. Suarez S. S. (2008) Control of hyperactivation in sperm. Hum. Reprod. Update 14, 647–657 [DOI] [PubMed] [Google Scholar]
- 33. Nishigaki T., José O., González-Cota A. L., Romero F., Treviño C. L., Darszon A. (2014) Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 450, 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garcia M. A., Meizel S. (1999) Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol. Reprod. Dev. 52, 189–195 [DOI] [PubMed] [Google Scholar]
- 35. Woo A. L., James P. F., Lingrel J. B. (2002) Roles of the Na,K-ATPase α4 isoform and the Na+/H+ exchanger in sperm motility. Mol. Reprod. Dev. 62, 348–356 [DOI] [PubMed] [Google Scholar]
- 36. Liu T., Huang J. C., Zuo W. L., Lu C. L., Chen M., Zhang X. S., Li Y. C., Cai H., Zhou W. L., Hu Z. Y., Gao F., Liu Y. X. (2010) A novel testis-specific Na+/H+ exchanger is involved in sperm motility and fertility. Front. Biosci. (Elite Ed.) 2, 566–581 [DOI] [PubMed] [Google Scholar]
- 37. Wang D., King S. M., Quill T. A., Doolittle L. K., Garbers D. L. (2003) A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat. Cell Biol. 5, 1117–1122 [DOI] [PubMed] [Google Scholar]
- 38. Demarco I. A., Espinosa F., Edwards J., Sosnik J., De La Vega-Beltran J. L., Hockensmith J. W., Kopf G. S., Darszon A., Visconti P. E. (2003) Involvement of a Na+/HCO3− cotransporter in mouse sperm capacitation. J. Biol. Chem. 278, 7001–7009 [DOI] [PubMed] [Google Scholar]
- 39. Gatti J. L., Chevrier C., Paquignon M., Dacheux J. L. (1993) External ionic conditions, internal pH and motility of ram and boar spermatozoa. J. Reprod. Fertil. 98, 439–449 [DOI] [PubMed] [Google Scholar]
- 40. Lishko P. V., Botchkina I. L., Fedorenko A., Kirichok Y. (2010) Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140, 327–337 [DOI] [PubMed] [Google Scholar]
- 41. Lishko P. V., Kirichok Y. (2010) The role of Hv1 and CatSper channels in sperm activation. J. Physiol. 588, 4667–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Florman HM D. T. (2006) Fertilization in Mammals. in Physiology of Reproduction (Neill J., ed) pp. 55–112, Elsevier, San Diego, CA [Google Scholar]
- 43. Arnoult C., Zeng Y., Florman H. M. (1996) ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J. Cell Biol. 134, 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ward C. R., Kopf G. S., Storey B. T. (1994) Solubilization and partial purification from mouse sperm membranes of the specific binding activity for 3-quinuclidinyl benzilate, a potent inhibitor of the zona pellucida-induced acrosome reaction. Mol. Reprod. Dev. 39, 423–432 [DOI] [PubMed] [Google Scholar]
- 45. Wennemuth G., Westenbroek R. E., Xu T., Hille B., Babcock D. F. (2000) CaV2.2 and CaV2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J. Biol. Chem. 275, 21210–21217 [DOI] [PubMed] [Google Scholar]
- 46. Xia J., Reigada D., Mitchell C. H., Ren D. (2007) CATSPER channel-mediated Ca2+ entry into mouse sperm triggers a tail-to-head propagation. Biol. Reprod. 77, 551–559 [DOI] [PubMed] [Google Scholar]
- 47. Nishigaki T., Wood C. D., Shiba K., Baba S. A., Darszon A. (2006) Stroboscopic illumination using light-emitting diodes reduces phototoxicity in fluorescence cell imaging. Biotechniques 41, 191–197 [DOI] [PubMed] [Google Scholar]
- 48. Babcock D. F., Pfeiffer D. R. (1987) Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. J. Biol. Chem. 262, 15041–15047 [PubMed] [Google Scholar]
- 49. Ward C. R., Storey B. T. (1984) Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 104, 287–296 [DOI] [PubMed] [Google Scholar]
- 50. Xia J., Ren D. (2009) Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol. Reprod. 80, 1092–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De La Vega-Beltran J. L., Sánchez-Cárdenas C., Krapf D., Hernandez-González E. O., Wertheimer E., Treviño C. L., Visconti P. E., Darszon A. (2012) Mouse sperm membrane potential hyperpolarization is necessary and sufficient to prepare sperm for the acrosome reaction. J. Biol. Chem. 287, 44384–44393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hernández-González E. O., Sosnik J., Edwards J., Acevedo J. J., Mendoza-Lujambio I., López-González I., Demarco I., Wertheimer E., Darszon A., Visconti P. E. (2006) Sodium and epithelial sodium channels participate in the regulation of the capacitation-associated hyperpolarization in mouse sperm. J. Biol. Chem. 281, 5623–5633 [DOI] [PubMed] [Google Scholar]
- 53. Waldegger S. (2003) Heartburn: cardiac potassium channels involved in parietal cell acid secretion. Pflugers Arch. 446, 143–147 [DOI] [PubMed] [Google Scholar]
- 54. García-Soto J., González-Martínez M., de De la Torre L., Darszon A. (1987) Internal pH can regulate Ca2+ uptake and the acrosome reaction in sea urchin sperm. Dev. Biol. 120, 112–120 [DOI] [PubMed] [Google Scholar]
- 55. Babcock D. F., Rufo G. A., Jr., Lardy H. A. (1983) Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc. Natl. Acad. Sci. U.S.A. 80, 1327–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. López-González I., Torres-Rodríguez P., Sánchez-Carranza O., Solís-López A., Santi C. M., Darszon A., Treviño C. L. (2014) Membrane hyperpolarization during human sperm capacitation. Mol. Hum. Reprod. 20, 619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Donowitz M., Ming Tse C., Fuster D. (2013) SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Aspects Med. 34, 236–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mannowetz N., Naidoo N. M., Choo S. A., Smith J. F., Lishko P. V. (2013) Slo1 is the principal potassium channel of human spermatozoa. Elife 2, e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brenker C., Zhou Y., Müller A., Echeverry F. A., Trötschel C., Poetsch A., Xia X. M., Bönigk W., Lingle C. J., Kaupp U. B., Strünker T. (2014) The Ca2+-activated K+ current of human sperm is mediated by Slo3. Elife 3, e01438. [DOI] [PMC free article] [PubMed] [Google Scholar]