Abstract
Low density lipoprotein (LDL) exists in various forms that possess unique characteristics, including particle content and metabolism. One circulating subfraction, electronegative LDL (LDL(−)), which is increased in familial hypercholesterolemia and diabetes, is implicated in accelerated atherosclerosis. Cellular responses to LDL(−) remain poorly described. Here we demonstrate that LDL(−) increases tumor necrosis factor α (TNFα)–induced inflammatory responses through NFκB and AP-1 activation with corresponding increases in vascular cell adhesion molecule-1 (VCAM1) expression. LDL receptor overexpression increased these effects. In contrast, exposing LDL(−) to the key lipolytic enzyme lipoprotein lipase (LPL) reversed these responses, inhibiting VCAM1 below levels seen with TNFα alone. LPL is known to act on lipoproteins to generate endogenous peroxisomal proliferator-activated receptor α (PPARα) ligand, thus limiting inflammation. These responses varied according to the lipoprotein substrate triglyceride content (very low density lipoprotein ≫ LDL > high density lipoprotein). The PPARα activation seen with LDL, however, was disproportionately high. We show here that MUT LDL activates PPARα to an extent proportional to its LDL(−) content. As compared with LDL(−) alone, LPL-treated LDL(−) increased PPARα activation 20-fold in either cell-based transfection or radioligand displacement assays. LPL-treated LDL(−) suppressed NFκB and AP-1 activation, increasing expression of the PPARα target gene IκBα, although only in the genetic presence of PPARα and with intact LPL hydrolysis. Mass spectrometry reveals that LPL-treatment of either LDL or LDL(−) releases hydroxy-octadecadienoic acids (HODEs), potent PPARα activators. These findings suggest LPL-mediated PPARα activation as an alternative catabolic pathway that may limit inflammatory responses to LDL(−).
Extensive data links low density lipoprotein (LDL)1 to atherosclerosis (1, 2). This occurs in part through the induction of early atherogenic inflammatory responses, including the expression of adhesion molecules like VCAM1 by endothelial cells (ECs) (3, 4). Consistent with this, increased dietary cholesterol rapidly induces atherosclerosis in animal models, with changes in VCAM1 expression seen within 2 weeks (3). LDL, a major carrier of cholesterol, circulates in several forms in vivo (5, 6). Most LDL pathogenicity becomes manifest after LDL oxidation (1). For example, oxidized LDL (oxLDL), but not native LDL, induces endothelial VCAM1 expression in the presence of TNFα (7).
More recently, a form of native LDL containing intermediately modified subfractions with higher electronegative charge, referred to as LDL(−), has been identified and characterized (8, 9). Several lines of evidence implicate LDL(−) as a particularly pro-atherogenic particle. Like oxLDL, LDL(−) has pro-inflammatory effects, e.g. inducing the chemokine monocyte chemotactic protein-1 and cytokine interleukin-8 (10), both of which are NFκB- and AP-1-regulated (11). LDL(−) is a significant component of more atherogenic small dense LDL subfractions (5, 6). LDL(−) also appears to be enriched in conditions characterized by accelerated atherosclerosis, namely familial hypercholesterolemia (10), diabetes (12), and hemodialysis (13).
Despite some similarities, native LDL, oxLDL, and LDL(−) have distinct characteristics that likely determine their biologic effects (14). Most fundamentally, these particles have unique compositional profiles. For example, LDL(−) contains fewer lipid peroxidation products than oxLDL, but more than native LDL (9, 14). These forms of LDL are also cleared from the circulation in different ways, potentially contributing to their unique roles in atherosclerosis. In contrast to both native LDL and LDL(−), which are taken up through the LDL receptor (LDLR) (15), oxLDL is removed after binding to scavenger or Fc receptors (1). Hydrolytic pathways for LDL particles also differ. For example, lipoprotein lipase (LPL), the predominant enzyme in triglyceride-rich lipoprotein (TRL) metabolism, can hydrolyze mildly oxidized LDL forms like LDL(−) (16), although this ability may be limited as suggested by the higher content of both triglycerides (8) and the LPL inhibitor Apo CIII (17) in LDL(−) as compared with native LDL fractions.
Recently, we reported LPL enzymatic action as a mechanism for generating endogenous peroxisome proliferator-activated receptor α (PPARα) ligands (18). This LPL/PPARα pathway replicated synthetic PPARα agonist effects, e.g. decreasing cytokine-induced VCAM1 expression (18) in vitro and in vivo. The PPARα activation through LPL varied depending on the lipoprotein substrate; it was greatest with VLDL, less with LDL, and minimal with HDL, a series corresponding to triglyceride content (18). Although this pattern and the absolute requirement for intact enzymatic catalysis for LPL-mediated PPARα activation suggests fatty acid release accounted for the responses seen, LPL-treated LDL activated PPARα to a disproportionate extent. This suggested LPL might release different PPARα mediators from LDL as compared with VLDL. If so, this indicates that the transcriptional responses to LDL might vary depending on LPL action, LDL particle composition, or its mechanism of uptake.
To pursue this hypothesis, we tested cellular responses, including PPARα activation, to different forms of native and oxidized LDL with and without LPL treatment. In the presence of TNFα, LDL(−) uptake by the LDLR induced VCAM1 through NFκB and AP-1 activation, a previously unreported pathogenic LDL(−) effect. In contrast, LPL treatment of LDL(−) reversed this response, decreasing VCAM1 expression in a PPARα-dependent manner. Further studies reveal that LPL hydrolysis of LDL(−) generated oxidized linoleic acid (hydroxy-octadecadienoic acid, or HODE) in concentrations likely to account for the PPARα activation and the subsequent anti-inflammatory effects.
EXPERIMENTAL PROCEDURES
Reagents
All reagents were purchased from Sigma-Aldrich unless otherwise indicated. All media were obtained from BioWhittaker (Walkersville, Maryland) and contained fungizone/penicillin/streptomycin. Human and murine TNFα were purchased from R&D Systems (Minneapolis, Minnesota). Fenofibric acid was a generous gift from Laboratories Fournier (Daix, France).
Cell Culture
Human ECs, isolated from a saphenous vein and cultured in M199 medium (supplemented with endothelial cell growth factor (ECGF) and 5% fetal calf serum) as before (19), were all passage 3 to 5. PPARα+/+ (129S3/SvImJ) mice were obtained from Jackson Laboratories (Bar Harbor, Maine). PPARα−/− mice were a generous gift from F. Gonzalez (National Institutes of Health) (20). Murine ECs from 1-month-old PPARα+/+ and PPARα−/− mouse hearts were isolated using double selection with intercellular adhesion molecule 2 (ICAM-2) and platelet endothelial cell adhesion molecule 1 (PECAM-1) antibodies (BD PharMingen) bound to Dynabeads (Dynal, Lake Success, New York) as before (21).
LDL Isolation
Lipoproteins were isolated using gradient ultracentrifugation of human plasma pooled from at least six healthy donors (9). Plasma of healthy donors was subjected to hemoglobin-mediated oxidation as described (13). The specific form of LDL that is formed by this type of oxidation is referred to as HBLDL. Anion exchange chromatography was utilized to purify the LDL(−) fraction and measure LDL(−) content in LDL modified during hemoglobin-mediated plasma oxidation (9).
RNA Analysis
Total cell RNA was isolated using RNeasy kit (Qiagen, Valencia, California), separated in 1% agarose gel, and transferred to Hydrobond membrane (Amersham Biosciences). Northern blotting was performed using cDNA probes obtained from American Type Culture Collection (Manassas, VA).
Western Blotting
Western blotting was performed on proteins extracted from cells harvested in phosphate-buffered saline lysis buffer containing freshly added 1 mM phenylmethylsulfonyl fluoride at 4 °C. Nuclear extracts were isolated as before (19). Protein concentration was determined using the bicinchoninic acid kit (Pierce). Electrophoresis was performed on 12% polyacrylamide gels under reducing conditions (β-mercaptoethanol). Proteins were transferred onto Immobilon-P membranes using semi-dry transfer (45min, 16V). Nonspecific binding sites were blocked for 1 h with 5% delipidated milk in TBST (20 mM Tris, 55 mM NaCl, and 0.1% Tween 20). Monoclonal antibody against PPARα was a gift from Dr. J. Woods (1:500, Merck), polyclonal antibody against IκBα (1:500) was from Santa Cruz Biotechnology (Santa Cruz, CA), and monoclonal antibody against glyceraldehyde-3-phosphate de-hydrogenase (GAPDH; 1:2000) was from Biodesign (Saco, Maine). Signals were visualized by chemiluminescence (PerkinElmer Life Sciences) after incubation with secondary horseradish peroxidase-conjugated antibody.
Electromobility Shift Assay (EMSA)
Nuclear extracts were analyzed by EMSA (19). Oligonucleotide sequences for NFκB, AP-1, and PPRE were purchased from Santa Cruz Biotechnology. Supershift was performed using antibodies for PPARα, PPARγ (Biomol, Plymouth Meeting, PA), p50, and p65 (Santa Cruz Biotechnology). Nonspecific binding was blocked with 0.5 μg/μl bovine serum albumin and 0.1 μg/μl poly(dI·dC).
Enzyme-linked Immunosorbent Assay (ELISA)
ELISA was performed in 96-well plates on confluent human umbilical vein endothelial cell (HUVEC) monolayers (19). Treated cells were kept on ice for 10 min, washed with cold phosphate-buffered saline, incubated with human VCAM1 monoclonal antibodies (gift from Dr. M. Gimbrone), and visualized using alkaline phosphate secondary antibody (450 nm).
Flow Cytometry
Flow cytometry was performed using confluent mouse ECs obtained from heart of PPARα+/+ and PPARα−/− mice. Cells were washed in phosphate-buffered saline, harvested by trypsinization, and incubated (1 h at 4 °C) with fluorescein isothiocyanate-conjugated anti-mouse VCAM1 antibody (BD PharMingen). The EC culture purity was examined using anti-mouse phosphatidylethanolamine-conjugated platelet endothelial cell adhesion molecule 1 (BD PharMingen). Subsequently, washed cells were analyzed in a BD Biosciences FACScan™ flow cytometer using CELLQuest™ software. At least 20,000 viable cells per condition were analyzed.
HPLC-MS
A stock standard solution containing hydroperoxy-octadecadienoic (HpODE) and hydroxy-octadecadienoic acids was prepared by diluting and combining solutions of standard mixtures obtained from Cayman Chemical (Ann Arbor, MI). Samples were extracted by the Folch method, reconstituted in isopropanol, and filtered (0.2-μm nylon). The samples and stock standard were serially diluted with 50:50 acetonitrile/H2O.
The separation was performed using a Waters 2690 LC with photodiode array (Waters 996) and time-of-flight mass spectrometry (TOF-MS) detection (Micromass LCT). The column used was a Luna C18 (2) 50 × 2 mm, 5 μm from Phenomenex (Torrance, CA). Mobile phases for the isocratic separation were 50% A, 4 mM ammonium acetate (Sigma-Aldrich) in H2O, and 50% B, acetonitrile (Sigma-Aldrich) flowing at 0.5 ml/min. The separation was performed at 30 °C with a total run time of 5 min. UV absorption was acquired from 200 – 400 nm. MS was performed using electrospray ionization operating in negative ionization mode. The ionization parameters were as follows: capillary voltage, 3200V; sample cone, 37V; extraction cone, 4V; desolvation temperature, 300 °C; source temperature, 120 °C; and ion scanning m/z range, 100–1000. Extracted ion chromatograms were constructed for HODE and HpODE using m/z values of 295 and 293, respectively. When the signal-to-noise ratio was sufficient, samples were quantified using an external calibration curve. When the signal-to-noise ratio was too low, only semi-quantitative estimates were made.
Transfection
Transfection was carried out in 24-well plates at 2.3 × 104 primary bovine aortic ECs (BAECs) (passage 3–6) per well using FuGENE (F. Hoffmann-La Roche). Cells were transfected 3 h after replating in Dulbecco’s modified Eagle’s medium containing 1% of de-lipidated fetal calf serum (for PPAR ligand binding domain (LBD) studies) or 1% Nutridoma SP (F. Hoffmann-La Roche) for VCAM1 promoter studies. Transfected cells were treated with the indicated compounds for at least 10 h. Constructs were generous gifts from T. Willson (GlaxoSmithKline; LBD/yeast Gal4), T. Collins (Children’s Hospital, Boston, MA; VCAM1 promoter), H. Hobbs (University of Texas Southwestern, LDLR expression vector), and D. Rader (University of Pennsylvania; LPL and LPL mutant expression vectors). The catalytically inactive LPL mutant has a two-base pair difference (AGC/GCC) that replaces the active site serine 132 with alanine (18). PCMX-β-galactosidase expression vector was used for transfection control. Luciferase (BC Pharmingen) and β-galactosidase activity utilizing chlorophenol red-β-D-galactopyranoside as substrate (F. Hoffmann-La Roche) were measured according to manufacturer’s protocols.
Scintillation Proximity Assay (SPA)
A scintillation proximity assay was carried out using human full-length cDNA for PPARα, PPARδ, and PPARγ2 that were subcloned into the pGEX-KT expression vector (22). The 3H2-labeled known synthetic PPAR agonists used were nTZD3 and nTZD4 with relative Kd values as follows: nTZD3, PPARγ 2.5 nM PPARα 5 nM; and nTZD4, PPARδ 1 nM (22). Results are expressed as percent inhibition with a calculated inhibitory constant (Kis).
RESULTS
LPL Decreases LDL(−)-mediated VCAM-1 Induction in a PPARα-dependent Manner
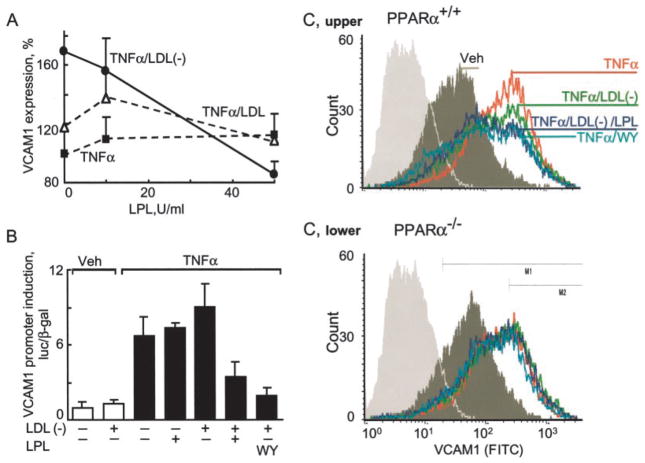
We compared the effect of native LDL and LDL(−) on TNFα-induced VCAM1 expression in human ECs. As expected, TNFα induced VCAM1 protein in ECs (8-fold, set as 100% induction) on ELISA (Fig. 1A). Although concomitant treatment with native LDL led to only a modest further increase in VCAM1 (20%), LDL(−) augmented VCAM1 levels by 70% relative to TNFα alone (14-fold as compared with basal untreated levels). The presence of LPL inhibited LDL(−)-mediated VCAM1 induction in a dose-dependent fashion (Fig. 1A). Similar effects were evident on Northern blotting (data not shown) and activation of the human VCAM1 promoter (Fig. 1B). Although LDL(−) treatment induced the VCAM1 promoter 120–180% in bovine aortic EC transfections, this same stimulation in the presence of LPL repressed this response by 60 – 80% as compared, in both cases, with TNFα alone (Fig. 1B). These effects of LPL/LDL(−) on TNFα-induced VCAM1 expression equaled those seen with synthetic PPARα ligands (Wy14163 or fenofibric acid, Fig. 1B).
Fig. 1. LPL treatment of LDL(−) opposes VCAM-1 induction in a PPARα-dependent manner.
A, confluent ECs were pretreated with the amount of LPL indicated and total LDL or LDL(−) (10 μg/ml, 3 h) and then stimulated with human TNFα (10 ng/ml, 12 h). VCAM1 expression, measured by ELISA, is shown as a percentage of VCAM1 induced by TNFα alone. Mean ± S.D. of triplicate experiments is shown. B, BAECs were transfected with a human VCAM1 promoter construct as described under “Experimental Procedures.” Cells were pretreated (4 h) with the synthetic PPARα ligand WY14647 (WY; 250 μM) or LPL (200 units/ml) in the presence and absence of LDL(−) (10 μg/ml) and then stimulated with human TNFα (10 ng/ml, 12 h). VCAM1 promoter activation is shown as a comparison between stimulated and non-stimulated cells. In all transfection experiments, luciferase activity was normalized to co-transfected β-galactosidase activity. Data represent mean ± S.D. of triplicate measurement of experiments performed three times using pooled LDL(−) from multiple normal donors. Veh, vehicle. C, fluorescence-activated cell sorter analysis of VCAM1 expression on ECs isolated from the hearts of PPARα+/+ and PPARα−/− mice. Confluent ECs were pretreated with WY14647 (WY; 250 μM), the indicated amounts of LPL and LDL or LDL(−) (10 μg/ml 3 h), and stimulated with murine TNFα (50 ng/ml, 12 h). FITC, fluorescein isothiocyanate.
To test whether treatment of LDL(−) reduced VCAM1 expression in a PPARα-dependent manner, VCAM1 responses were examined using FACS analysis of PPARα+/+ and PPARα−/− microvascular ECs (Fig. 1C). Both TNFα and TNFα/LDL(−) stimulation of PPARα+/+ ECs markedly increased VCAM1 content on the EC surface. LPL-treated LDL(−) repressed this TNFα induction in the presence, but not the genetic absence, of PPARα with effects replicating those seen with the synthetic PPARα agonist WY14643. These data suggest that LPL action on LDL(−) limits inflammation through a PPARα mechanism.
LPL Treatment of LDL(−) Decreases NFκB Binding
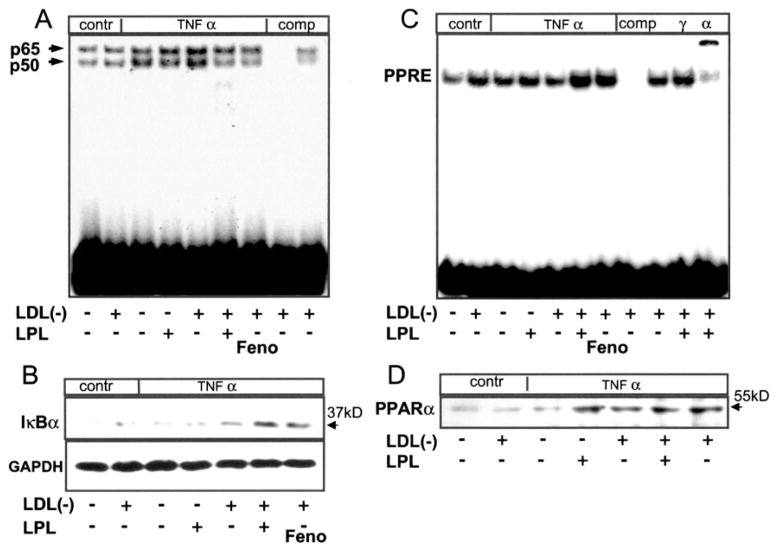
We and others have shown that PPARα ligands decrease cytokine-mediated VCAM1 expression through effects on NFκB signaling (19, 23). Mechanisms involved reportedly include direct PPARα interaction with p65 and PPARα-dependent expression of IκBα, sequestering the inactive p65/p50 complex in the cytoplasm (24). To explore how LPL negatively regulates LDL(−)/TNFα induction of the VCAM1 promoter, we performed an EMSA of EC nuclear extracts using NFκB and PPRE binding sites. As expected, TNFα induced NFκB binding, a response augmented in the presence of LDL(−) (Fig. 2A). In the presence of LPL, however, LDL(−) significantly decreased NFκB binding; Wy14163 had similar effects. This decrease in NFκB binding was paralleled by increased IκBα levels from the very same ECs (Fig. 2B). Similarly, the increase in AP-1 binding induced by LDL(−)/TNFα was decreased after LPL treatment (data not shown). In parallel with decreased NFκB binding, both LPL/LDL(−) and WY14643 markedly increased binding to a canonical PPRE (Fig. 2C). This response was much greater than that seen with LDL(−), TNFα, or their combination (Fig. 2C). PPRE binding involved PPARα as indicated by the supershift in the presence of PPARα but not the PPARγ antibody. These effects appear to be due to increases in PPARα activators and not PPARα itself, given the lack of significant differences in nuclear PPARα protein levels after treating cells with LDL(−), LPL, or their combination (Fig. 2D). Together, these data suggest that LPL treatment of LDL(−) exerts its effects through direct interaction of LDL-derived components with NFκB and AP-1 in a manner similar to that of synthetic PPARα ligands.
Fig. 2. LPL treated LDL(−) inhibits NFκB activation and increases IκBα expression while inducing PPRE binding.
Confluent human ECs were pretreated with LPL (200 units/ml, 4 h) in the presence or absence of LDL(−) (10 μg/ml) and then stimulated with human TNFα (10 ng/ml, 1.5 h). Fenofibric acid (Feno; 100 μM) pre-treatment was used for comparison. Nuclear extracts were analyzed by EMSA using 5 μg of nuclear extracts and 100 ng of radiolabeled NFκB (A), AP-1 (data not shown), and PPRE (C) sequences (Santa Cruz Biotechnology). Supershift analysis was performed to confirm the identity of PPARα and PPARγ as well as p65 and p50 (data not shown) using specific antibodies. Cytosolic fractions (25 μg protein) of the same cells were analyzed for IκBα expression (B), whereas nuclear fractions (25 μg protein) were studied for PPARα expression (D) using Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression confirmed equal loading. Data shown are representative of one experiment from three with similar results.
LPL Lipolysis of LDL(−) Generates PPARα Ligands
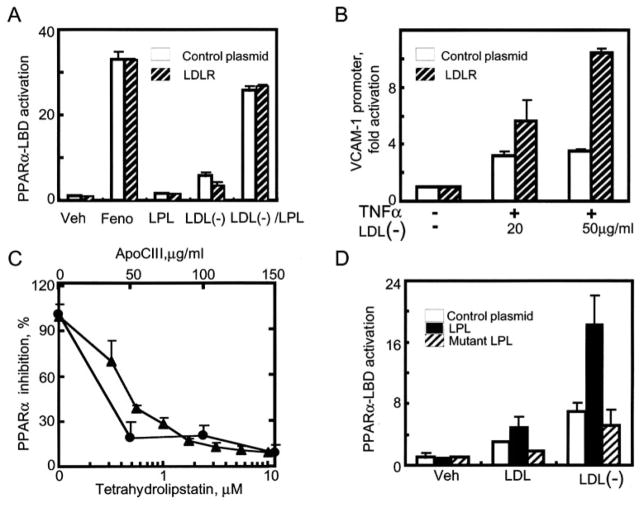
We further examined PPARα ligand generation as a result of LPL treatment of LDL(−). The yeast Gal4/PPAR LBD hybrid assay is classically used to screen for PPARα ligand formation (25). LDL(−) stimulation led to a modest 3-fold PPARα LBD activation; in the presence of LPL and LDL(−), PPARα activation increased 30-fold (Fig. 3A). In addition to its catalytic activity, LPL can also promote the uptake of lipoproteins like LDL through non-enzymatic bridging of lipoproteins to receptors like the LDLR (26). We investigated the contribution of LPL bridging to the effects reported above. Transient transfection of the LDLR into EC did not alter PPARα LBD activation by LDL(−)/LPL. In contrast, LDL(−) treatment of LDLR-transfected ECs markedly increased VCAM1 promoter activity (Fig. 3B).
Fig. 3. Divergent cellular responses to LDL(−) depend on mechanisms of lipoprotein uptake.
LPL-mediated hydrolysis of LDL(−) activates PPARα LBD independent of LDLR expression. BAECs were co-transfected with expression constructs for PPARα-LBD, the luciferase response pUASx4-TK-luc, and β-galactosidase. Cells were also transfected with either an LDLR overexpression vector (striped bar) or a control plasmid (PcDNA3.0, open bar) and then stimulated with LDL(−) (10 μg protein/ml), LPL (20 units/ml), or their combination (1% delipidated serum/Dulbecco’s modified Eagle’s medium, 18 h). The synthetic PPARα ligand fenofibric acid (Feno; 100 μM) served here and throughout as a positive control. Concentration-dependent LDL(−) induction of the human VCAM1 promoter is increased by LDLR overexpression. BAECs were transfected with the VCAM1 promoter as in Fig. 1B and the LDLR as in Fig. 3A. Before TNFα stimulation (10 ng/ml), cells were pretreated with LDL(−) in the amounts shown. C. LPL inhibitors decrease LPL/LDL(−)-mediated PPARα LBD in a dose-dependent manner. BAECs were co-transfected with the PPARα-LBD system as in panel A and treated with either natural (ApoCIII, circles) or synthetic (tetrahydrolipstatin, triangles) LPL inhibitors (15 min, 20 °C) as shown. Subsequently, LDL(−) (10 μg/ml) was added and cells were stimulated (18 h). Results are shown as percentage of inhibition compared with LDL(−)/LPL treatment, which led to 23 ± 1.8-fold PPARα LBD activation. Transfection of wild-type but not catalytically inactive mutant LPL increases PPARα LBD activation induced by LDL and LDL(−). BAECs were transfected with PPARα LBD, pUASx4-TK-luc, and β-galactosidase constructs as well as control plasmid (open bars), wild type LPL (black bars), and mutant LPL lacking hydrolytic activity (striped bars). Transfected cells were treated with either native LDL or LDL(−) (10 μg/ml, 18 h).
LPL/LDL(−) mediated PPARα-LBD activation required intact LPL catalysis, as evident by the concentration-dependent inhibition of PPARα responses in the presence of the synthetic lipase inhibitor tetrahydrolipstatin or the natural LPL inhibitor ApoCIII (Fig. 3C). Similar repression was seen with an antibody raised against LPL, but not an antibody to the LDLR receptor (data not shown). Furthermore, repeating these PPARα LBD experiments in bovine EC transfected with a catalytically inactive LPL point mutant failed to activate PPARα by LDL(−) above levels induced by LDL(−) alone (Fig. 3D).
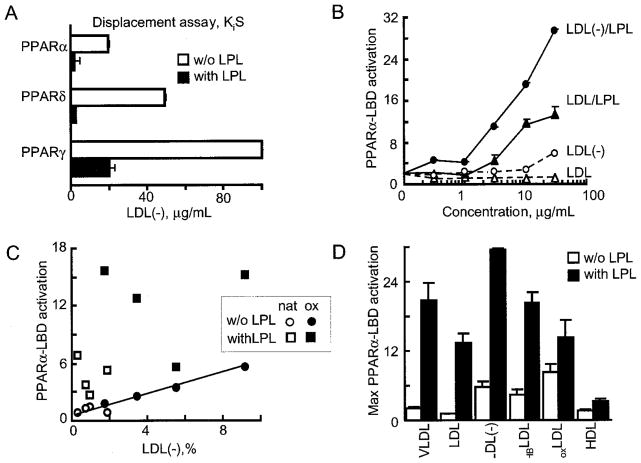
The generation of direct PPARα ligands by LPL treatment of LDL(−) was tested further in cell-free PPAR radioligand displacement assays. LPL treatment of LDL(−) decreased the EC50 of PPARα activation 20-fold as compared with non-treated LDL(−) (Fig. 4A). This LPL treatment may have produced greater amounts of the ligands already present in LDL(−), converted less potent ligands to more potent forms, or both. In contrast to our prior findings with native triglyceride-rich lipoprotein (18), LPL/LDL(−) did not preferentially activate any one PPAR isoform, at least in direct ligand displacement and LBD assays (Fig. 4A).
Fig. 4. LPL hydrolysis of LDL generates PPAR ligands in a manner dependent on oxidation.
A, lipolysis markedly increases the generation of PPAR ligands from LDL(−). The most potent response was seen with PPARα and PPARδ, with less displacement of PPARγ ligand. Direct radiolabeled ligand displacement (scintillation proximity assay) PPAR assays were performed using LDL(−) hydrolyzed with LPL (200 units/ml, 2 h, 37 °C under argon). Competition curves were generated across a range of LDL(−) concentrations (0.003–10 μg protein/ml) by incubating the reaction mixture with specific radiolabeled PPAR activators, i.e. 5 nM nTZD3 for GST-hPPARα or GST-hPPARγ, or 2.5 nM nTZD4 for GST-hPPARδ (22, 37). Data from duplicate determinations were plotted and Kis values (IC50) were obtained from the dose-response curves. The white bars indicate the higher concentration (Kis) of non-hydrolyzed LDL(−) required for PPAR ligand displacement; black bars reveal the more potent displacement for the same LDL(−) after LPL hydrolysis. B, concentration-dependent PPARα-LBD activation by LDL (circles) and LDL(−) (triangles) in the presence (solid line) or absence (dashed line) of LPL is shown. BAECs, transfected as in Fig. 3C, were stimulated with the concentration of lipoproteins shown after LPL (20 units/ml) treatment. C, LDL oxidation increases PPARα activation independent of LPL treatment. PPARα LBD activation was measured as in Fig. 3C. BAECs were treated with LDL (10 μg/ml; circles) or LDL/LPL (squares) obtained from four different healthy donors. The role of oxidation was assessed using native LDL (open circles) or the same LDL treated ex vivo to achieve higher LDL(−) content (black circles). The latter was produced by exposing plasma samples to hemoglobin (10 μM) under argon (13). The levels of LDL(−) in LDL obtained from native and oxidized plasma achieved were determined using HPLC and are shown on the x axis. The linear correlation between LDL(−) content and PPARα-LBD activation (p < 0.001, Pearson) is shown with the drawn line. No correlation was evident after LPL treatment of LDL. D, relative PPARα LBD activation by different lipoproteins in the absence (open bars) or presence of LPL (black bars) is shown. VLDL, LDL, LDL(−), and HDL were isolated from human blood, and the plateau of maximum PPARα activation was determined from concentration-dependent curves as shown in panel A. Responses were also compared with HBLDL, an LDL species enriched in LDL(−) generated in vitro by exposing plasma to hemoglobin (10 μM) under anaerobic conditions before lipoprotein isolation (13). oxLDL was obtained after exposing LDL to CuSO4 (10 μM, 24 h), which completely oxidizes polyunsaturated fatty acids (32).
We next sought to examine how these responses varied depending on the nature of LDL particles, especially in LDL with high LDL(−) content. In the presence or absence of LPL, LDL(−) had a much more potent concentration-dependent effect on PPARα-LBD activation (Fig. 4B). One fundamental characteristic of LDL(−) is its higher relative proportion of oxidized lipids as compared with native LDL (14). To examine the contribution of oxidation on PPARα responses, we utilized standard in vitro techniques to increase the proportion of LDL(−) present in human LDL samples, employing the mild oxidative stress method in which different plasma samples are exposed to hemoglobin under anaerobic conditions (13). The LDL isolated from such plasma has a higher LDL(−) content, although TG concentrations remain unchanged (13). Using the same total LDL (10 μg) for all treatments, we found that a direct linear relationship existed between PPARα-LBD activation and the proportion of LDL(−) in the absence of LPL. This was evident for both unoxidized (Fig. 4B, white circles) and oxidized (Fig. 4B, black circles) plasma samples (Pearson, p < 0.001, Fig. 4C). Thus, increased LDL(−) proportions substantially affect PPARα activation in the absence of LPL. In contrast, LPL treatment of these LDL(−) samples (Fig. 4C, squares) markedly increased PPARα LBD activation regardless of the LDL(−) content. Taken together, this suggests that degree of LDL oxidation and the extent of LPL-mediated hydrolysis are separate variables influencing PPARα activation. Of note, although LPL-treatment increased the PPARα activation seen with all lipoproteins tested, the greatest induction occurred with LDL(−) as a substrate. Although TG is the principal LPL substrate, the robust PPARα-activation seen with LPL treatment of LDL(−) as opposed to TG-rich VLDL suggests generation of a potent PPARα ligand.
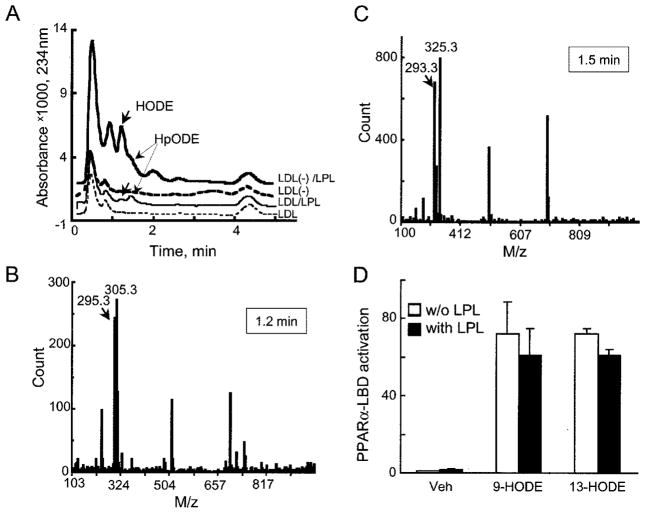
To identify this PPARα-activating component in LDL(−), hydrolytic products of LPL-treated LDL(−) were extracted and separated using HPLC-MS. The major lipophylic compounds in LDL(−), either with or without LPL treatment, were HpODE and HODE acids (Fig. 5A). Even though HODE was present at a negligible amount in untreated LDL(−), after LPL treatment HODE amounts were ~2.5 μg per 100 μg of LDL(−). Importantly, LPL treatment of native LDL also increased HODE and HpODE, albeit to a much smaller extent. The identity of compounds was confirmed using MS (HODE and HpODE, Fig. 5, B and C, respectively).
Fig. 5. LPL treatment of LDL(−) increases concentrations of oxidized linoleic acid products HpODE and HODE at levels sufficient to activate PPARα-LBD.
A, HPLC-UV chromatograms of lipophylic extracts obtained from LDL (thin line) and LDL(−) (bold line) before (dashed lines) and after (solid lines) LPL treatment (200 units/ml, 2 h, 37 °C) are shown. The release of HODE (bold arrows) and HpODE (thin arrows) were identified by mass spectrometric analysis as shown in panels B and C. The HODE and HpODE release concentrations are 1.2 μg per 100 μg of LDL(−). The analysis does not discriminate between 9- and 13-HODE isomers. B, mass spectrometry for [M–H]− ion for HODE (arrow) is shown at its respective retention time as indicated in the box. C, a similar mass spectrometric analysis is shown for the [M-H3O]− ion for HpODE. D, PPARα LBD activation of BAECs (as in Fig. 3A) stimulated with 1.2 μg of HODE isomer per well without (open bars) or with LPL (black bars) is shown. Similar PPAR activation is seen with either the 9- or the 13-HODE isoform. These responses equaled those seen with the synthetic PPARα activator WY14643 (100 μM, 68 ± 5-fold). Veh, vehicle.
Prior work established HODE as a PPARγ activator (27, 28), although its affinity may be relatively low (29). Recently, PPARα LBD activation by HODE has been reported (30). To evaluate the extent of PPARα activation by HODE and HpODE, cell-free displacement and cell-based LBD assays were performed. Two common forms of HODE, 9- and 13-HODE, were all effective PPARα ligands. The EC50 for 13-HODE equaled 10 μM, whereas the EC50 for 9-HODE was 2.6 μM in PPARα LBD displacement assays (data not shown). These results suggest that the combined action of hydrolysis and oxidation increases the proportion of HODE to levels sufficient for PPARα activation.
DISCUSSION
These studies provide evidence that LPL acts on LDL(−) to generate PPARα ligands, thus countering the endothelial adhesion molecule expression induced by LDL(−) alone. These data highlight the differing transcriptional responses to LDL that depend on the mechanism of lipoprotein uptake, namely through the LDLR as opposed to hydrolysis by LPL (Fig. 6). Enhanced LDL(−) uptake through LDLR overexpression further increases VCAM-1 expression and promoter activity through TNFα-mediated NFκB and AP-1 activation. In contrast, LPL hydrolysis of LDL(−) limits these responses by generating PPARα ligands, thereby increasing IκBα expression with subsequent NFκB inhibition. Interestingly, LPL hydrolysis of LDL(−) activates PPARα to a greater extent than either LDL or VLDL. These efficient PPARα responses were proportional to the level of LDL oxidation, particularly to the HODE content of the lipoprotein particles. Thus, both hydrolysis and oxidation appear to influence PPARα activation by LDL(−), suggesting that responses to this pro-inflammatory, pro-atherogenic particle may depend on its catabolism.
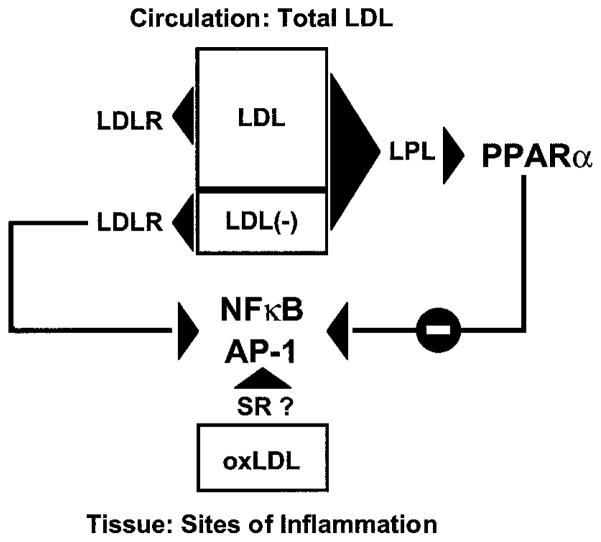
Fig. 6. Transcriptional responses to LDL depend on both the LDL species and its mechanism of uptake.
Different LDL species generate unique cellular responses depending on specific mechanisms of metabolism and uptake. Oxidized LDL, taken up by scavenger receptors (SR), promotes inflammatory responses through NFκB and AP-1 activation. Here we demonstrate that whereas LDL(−) uptake through the LDLR induces VCAM1 expression through activation of NFκB and AP-1, LDL(−) hydrolysis by LPL has the opposite effect, decreasing VCAM1 expression by generating PPARα ligands and suppressing NFκB and AP-1 responses (7, 38, 39). These LPL effects on LDL(−), which are independent of the LDLR, may be especially relevant given the presence of LDL(−) in the circulation.
LDL(−) is a unique lipoprotein (14). It is the only mildly oxidized LDL subfraction in the circulation that undergoes both LPL hydrolysis and LDLR uptake. One recent report suggests that mild oxidation increases LDL susceptibility to LPL-mediated hydrolysis (16), potentially amplifying the responses seen here with LDL(−). In contrast, oxLDL, which is more extensively oxidized, does not bind to LPL and is taken up via scavenger and Fc receptors in an LPL-independent manner (1, 16). Even though oxLDL contains several reported PPARα activators, including oxidized phospholipids (30, 31) and non-esterified fatty acids (such as HODE) (30), PPARα activation in vitro by oxLDL does not decrease inflammation as is characteristically seen with synthetic PPARα agonists. OxLDL reportedly increases VCAM1 expression in the presence of TNFα (7). This discrepancy may be due to high levels of other lipid peroxidation and decomposition products that may have potent inflammatory and cytotoxic effects, e.g. oxysterols, hydroxynonenal, or lysophosphatidylcholine (32). This last product is a known potent NFκB activator (33) that may overcome PPARα activation. Alternatively, responses may vary depending on the pathway of lipoprotein uptake. The effects of oxLDL on PPARα may depend on cytoplasmic phospholipase A2 action (30); this is not likely relevant to the results presented here, because LPL neither acts on nor binds to oxLDL (16). In our studies, LDLR overexpression significantly increased LDL(−) activation of the VCAM1 promoter but not PPARα LBD activation. In contrast, LDL(−) hydrolysis significantly decreased VCAM1 expression in a PPARα-dependent manner, with responses equivalent to synthetic PPARα agonists (Fig. 1). Inhibiting LPL hydrolytic activity through expression of a catalytically inactive LPL mutant, the presence of either natural (ApoCIII) or synthetic (tetrahydrolipstatin) LPL inhibitors, or co-stimulation with an LPL antibody prevented LDL(−)/LPL-mediated PPARα activation (Fig. 3). Together, these findings imply that intact LPL hydrolysis is required for PPARα ligand release from LDL(−) while also supporting the concept that differential lipoprotein catabolism and uptake can influence distal transcriptional responses.
Several prior studies have found that oxidation plays a role in PPAR ligand generation, with certain oxidized molecules, e.g. oxidized phospholipids and certain fatty acids, having greater effects than their native forms (27, 29–31). To examine this issue, the amount of LDL(−) in a given LDL sample was increased in a controlled fashion using hemoglobin plasma oxidation in vitro (HBLDL). Greater PPARα activation occurred with HBLDL stimulation than with native LDL, suggesting that oxidation changes the ligands present in these lipoproteins. In fact, the proportion of LDL(−) in these samples correlated with PPARα activation in a linear fashion (Fig. 4C). The oxidized molecules likely responsible for these effects have been identified as 9- and 13-HODE, both established PPARγ (27) and PPARα activators (30). Such HODEs are released during LDL hydrolysis of LDL(−) (Fig. 5A). In fact, the PPARα activation seen with LPL-treated LDL(−) equaled that seen after direct HODE stimulation. The larger proportion of HODE in LDL(−) as compared with native LDL may account for the greater PPARα activation seen either before or after LPL treatment. Moreover, hydrolysis of native LDL also increases its HODE content, potentially contributing to its LPL-mediated PPARα activation. The possibility that the HODEs liberated from LDL and LDL(−) undergo further changes intracellularly cannot be excluded. The greater PPARα activation seen after HODE treatment of cell-based LBD assays as compared with cell-free direct displacement assays supports such a notion. Although HODEs seem the most likely candidate for explaining the LDL(−) responses seen, PPARα activators other than HODEs may be generated by LPL or augmented in its presence.
Previously, the formation of the natural PPARα ligands HETE and LTB4 during acute inflammatory events was suggested as a feedback loop terminating inflammation and decreasing pro-inflammatory eicosanoids via β-oxidation (34). The data presented here suggest another endogenous mechanism through PPARα that may limit inflammatory responses, namely LPL catalysis of LDL(−). Increased concentrations of LDL(−) have been associated with pro-inflammatory conditions such as familial hyperlipidemia, diabetes, and hemodialysis, with levels up to 20% of total LDL found in some subjects (10, 13). Interestingly, many of these clinical conditions are also characterized by dysfunctional LPL (35, 36). One recent study found that only the LDL content of the LPL inhibitor apolipoprotein CIII independently predicted cardiovascular events, whereas total LDL concentration did not (35). Our results suggest that the combination of increased circulating pro-inflammatory LDL(−) and ineffective LPL action may be a particularly deleterious combination, with the responses to LDL(−) compounded by decreased PPARα ligand generation. Our prior observations suggested that LPL mediates PPARα ligand generation under physiologic conditions (18); these findings extend lipolytic PPAR activation to limiting pathologic responses through LDL(−). Such data further support the concept of circulating lipoproteins as a reservoir for PPAR ligands and lipolysis as a means of accessing them.
Acknowledgments
We thank A. Gomes, S. LaClair, and N. Sharma for excellent technical support and R. Tupy for excellent editorial help.
Footnotes
The abbreviations used are: LDL, low density lipoprotein; AP-1, adaptor protein 1; BAEC, bovine aortic endothelial cell; EC, endothelial cell; ELISA, enzyme-linked immunosorbent assay; EMSA, electromobility shift assay; HBLDL, LDL modified during hemoglobin-mediated plasma oxidation; HDL, high density lipoprotein; HODE, hydroxy-octadecadienoic acid; HpODE, hydroperoxy-octadecadienoic acid; HPLC, high pressure liquid chromatography; LBD, ligand binding domain; LDL(−), electronegative LDL; LDLR, LDR receptor; LPL, lipoprotein lipase; MS, mass spectrometry; NFκB, nuclear factor κB; oxLDL, oxidized LDL; PPAR, peroxisome proliferation-activated receptor; PPRE, peroxisome proliferator response element; TNFα, tumor necrosis factor α; VCAM1, vascular cell adhesion molecule-1; VLDL, very low density lipoprotein.
This work was supported in part by grants from the American Diabetes Association and the LeDucq Foundation (to J. P. and O. Z.) and National Institutes of Health Grant HL50350 (to A. S.).
References
- 1.Steinberg D. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastin Survival Study Group. Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3.Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Sevanian A, Hwang J, Hodis H, Cazzolato G, Avogaro P, Bittolo-Bon G. Arterioscler Thromb Vasc Biol. 1996;16:784–793. doi: 10.1161/01.atv.16.6.784. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Quesada J, Benitez S, Otal C, Franco M, Blanco-Vaca F, Ordonez-Llanos J. J Lipid Res. 2002;43:699–705. [PubMed] [Google Scholar]
- 7.Khan B, Parthasarathy S, Alexander R, Medford R. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cazzolato G, Avogaro P, Bittolo-Bon G. Free Radic Biol Med. 1991;11:247–253. doi: 10.1016/0891-5849(91)90120-r. [DOI] [PubMed] [Google Scholar]
- 9.Hodis H, Kramsch D, Avogaro P, Bittolo-Bon G, Cazzolato G, Hwang J, Peterson H, Sevanian A. J Lipid Res. 1994;35:669–677. [PubMed] [Google Scholar]
- 10.Sanchez-Quesada J, Camacho M, Anton R, Benitez S, Vila L, Ordonez-Llanos J. Atherosclerosis. 2003;166:261–270. doi: 10.1016/s0021-9150(02)00374-x. [DOI] [PubMed] [Google Scholar]
- 11.Roebuck K, Carpenter L, Lakshminarayanan V, Page S, Moy J, Thomas L. J Leukoc Biol. 1999;65:291–298. doi: 10.1002/jlb.65.3.291. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Quesada J, Perez A, Caixas A, Ordonmez-Llanos J, Carreras G, Payes A, Gonzalez-Sastre F, de Leiva A. Diabetologia. 1996;39:1469–1476. doi: 10.1007/s001250050600. [DOI] [PubMed] [Google Scholar]
- 13.Ziouzenkova O, Asatryan L, Akmal M, Tetta C, Wratten M, Loseto-Wich G, Jurgens G, Heinecke J, Sevanian A. J Biol Chem. 1999;274:18916–18924. doi: 10.1074/jbc.274.27.18916. [DOI] [PubMed] [Google Scholar]
- 14.Ziouzenkova O, Sevanian A. Blood Purif. 2000;18:169–176. doi: 10.1159/000014415. [DOI] [PubMed] [Google Scholar]
- 15.Avogaro P, Bon G, Cazzolato G. Arteriosclerosis. 1988;8:79–87. [PubMed] [Google Scholar]
- 16.Wang X, Greilberger J, Levak-Frank S, Zimmermann R, Zechner R, Jurgens G. Biochem J. 1999;343:347–353. [PMC free article] [PubMed] [Google Scholar]
- 17.Vedie B, Jeunemaitre X, Megnien JL, Myara I, Trebeden H, Simon A, Moatti N. Arterioscler Thromb Vasc Biol. 1998;18:1780–1789. doi: 10.1161/01.atv.18.11.1780. [DOI] [PubMed] [Google Scholar]
- 18.Ziouzenkova O, Perrey S, Asatryan L, Hwang J, MacNaul KL, Moller DE, Rader DJ, Sevanian A, Zechner R, Hoefler G, Plutzky J. Proc Natl Acad Sci U S A. 2003;100:2730–2735. doi: 10.1073/pnas.0538015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marx N, Sukhova G, Collins T, Libby P, Plutzky J. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Gonzalez F. Ann N Y Acad Sci. 1996;804:524–529. doi: 10.1111/j.1749-6632.1996.tb18642.x. [DOI] [PubMed] [Google Scholar]
- 21.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. J Immunol Methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 22.Berger JP, Petro AE, Macnaul KL, Kelly LJ, Zhang BB, Richards K, Elbrecht A, Johnson BA, Zhou G, Doebber TW, Biswas C, Parikh M, Sharma N, Tanen MR, Thompson GM, Ventre J, Adams AD, Mosley R, Surwit RS, Moller DE. Mol Endocrinol. 2003;17:662–676. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SM, Parhami F, Xi XP, Berliner JA, Hsueh WA, Law RE, Demer LL. Arterioscler Thromb Vasc Biol. 1999;19:2094–2104. doi: 10.1161/01.atv.19.9.2094. [DOI] [PubMed] [Google Scholar]
- 24.Delerive P, Gervois P, Fruchart JC, Staels B. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 25.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 26.Merkel M, Eckel RH, Goldberg IJ. J Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.r200015-jlr200. [DOI] [PubMed] [Google Scholar]
- 27.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 28.Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- 29.Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, Prestwich GD, Hilaire AS, Prescott SM, Zimmerman GA, McIntyre TM. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- 30.Delerive P, Furman C, Teissier E, Fruchart J, Duriez P, Staels B. FEBS Lett. 2000;471:34–38. doi: 10.1016/s0014-5793(00)01364-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Shi W, Tontonoz P, Wang S, Subbanagounder G, Hedrick CC, Hama S, Borromeo C, Evans RM, Berliner JA, Nagy L. Circ Res. 2000;87:516–521. doi: 10.1161/01.res.87.6.516. [DOI] [PubMed] [Google Scholar]
- 32.Esterbauer H, Gebicki J, Puhl H, Jurgens G. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 33.Sugiyama S, Kugiyama K, Ogata N, Doi H, Ota Y, Ohgushi M, Matsumura T, Oka H, Yasue H. Arterioscler Thromb Vasc Biol. 1998;18:568–576. doi: 10.1161/01.atv.18.4.568. [DOI] [PubMed] [Google Scholar]
- 34.Devchand P, Keller H, Peters J, Vazquez M, Gonzalez F, Wahli W. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Campos H, Moye LA, Sacks FM. Arterioscler Thromb Vasc Biol. 2003;23:853–858. doi: 10.1161/01.ATV.0000066131.01313.EB. [DOI] [PubMed] [Google Scholar]
- 36.Sacks F, Alaupovic P, Moye L, Cole T, Sussex B, Stampfer M, Pfeffer M, Braunwald E. Circulation. 2000;102:1886–1892. doi: 10.1161/01.cir.102.16.1886. [DOI] [PubMed] [Google Scholar]
- 37.Elbrecht A, Chen Y, Adams A, Berger J, Griffin P, Klatt T, Zhang B, Menke J, Zhou G, Smith RG, Moller DE. J Biol Chem. 1999;274:7913–7922. doi: 10.1074/jbc.274.12.7913. [DOI] [PubMed] [Google Scholar]
- 38.Li D, Chen H, Romeo F, Sawamura T, Saldeen T, Mehta JL. J Pharmacol Exp Ther. 2002;302:601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 39.Maziere C, Djavaheri-Mergny M, Frey-Fressart V, Delattre J, Maziere JC. FEBS Lett. 1997;409:351–356. doi: 10.1016/s0014-5793(97)00545-0. [DOI] [PubMed] [Google Scholar]