Abstract
Peroxisome proliferator-activated receptor-γ (PPARγ), a member of the nuclear hormone receptor superfamily originally shown to play an important role in adipocyte differentiation and glucose homeostasis, is now known to regulate inflammatory responses. Given the importance of endothelial cell (EC)-derived chemokines in regulating leukocyte function and trafficking, we studied the effects of PPARγ ligands on the expression of chemokines induced in ECs by the Th1 cytokine IFN-γ. Treatment of ECs with PPARγ activators significantly inhibited IFN-γ-induced mRNA and protein expression of the CXC chemokines IFN-inducible protein of 10 kDa (IP-10), monokine induced by IFN-γ (Mig), and IFN-inducible T-cell α-chemoattractant (I-TAC), whereas expression of the CC chemokine monocyte chemoattractant protein-1 was not altered. PPARγ activators decreased IFN-inducible protein of 10 kDa promoter activity and inhibited protein binding to the two NF-κB sites but not to the IFN-stimulated response element ISRE site. Furthermore, PPARγ ligands inhibited the release of chemotactic activity for CXC chemokine receptor 3 (CXCR3)-transfected lymphocytes from IFN-γ-stimulated ECs. These data suggest that anti-diabetic PPARγ activators might attenuate the recruitment of activated T cells at sites of Th1-mediated inflammation.
Leukocyte recruitment to sites of inflammation is an essential component in the pathophysiology of disease. Chemokines are a superfamily of 8- to 10-kDa secreted basic chemotactic proteins that play an important role in this process (1). Chemokines secreted from activated endothelial cells (ECs)4 are uniquely positioned to play a central role in initiating leukocyte recruitment from the vascular space into tissues. IFN-γ is a critical mediator of Th1 immunity and is a potent activator of the endothelium. IFN-γ stimulates ECs to express specific adhesion molecules (e.g., ICAM-1) and chemokines that regulate the trafficking of lymphocytes and monocytes into sites of Th1-type inflammation. In particular, IFN-γ induces ECs to secrete three CXC chemokines, IFN-inducible protein of 10 kDa (IP-10), monokine induced by IFN-γ (Mig), and IFN-inducible T-cell α-chemoattractant (I-TAC), which are active on Th1 cells, and the CC chemokine monocyte chemoattractant protein-1 (MCP-1), which is active on monocytes (2). In addition, immunohistochemical analysis of human atherosclerotic lesions revealed IP-10, Mig, and I-TAC expression in ECs overlaying the plaque (2), suggesting that these chemokines likely play an important role in T cell recruitment to sites of inflammation.
Peroxisome proliferator-activated receptor-γ (PPARγ), as well as PPARα and PPARΔ, belong to the superfamily of nuclear receptor transcription factors that regulate gene expression in response to specific ligands. PPARγ activators include naturally occurring ligands, such as the prostaglandin D2 metabolite 15-deoxy−Δ12,14 prostaglandin J2 (15d-PGJ2) (3, 4), and syntheticligands, such as the antidiabetic thiazolidinedione agents troglitazone and rosiglitazone (BRL49653) (5, 6). In contrast, PPARα ligands include natural polyunsaturated fatty acids, eicosanoids, as well as synthetic fibric acid derivatives, such as fenofibrate and WY 14643 (7, 8). Although originally implicated in adipocyte differentiation and glucose homeostasis, PPARγ has recently been shown to regulate inflammatory responses. In monocytes and monocyte-derived macrophages, activation of PPARγ inhibits the expression of TNF-α, IL-1α, IL-6, inducible nitric oxide synthase, gelatinase B/matrix metalloproteinase-9, and scavenger receptor A (9–11). In a transformed colonic epithelial cell line, PPARγ activation inhibits the expression of IL-1-induced MCP-1 and IL-8 expression in vitro, and PPARγ ligands reduced the severity of colonic inflammation in a mouse model of colitis (12). PPARγ is also expressed in ECs, with data supporting PPARγ regulation of plasminogen activator inhibitor type-1, endothelin-1, and angiogenesis (13–15).
We hypothesized that PPARγ might be involved in the regulation of IFN-γ-induced chemokine expression in human ECs, and therefore we investigated the effect of naturally occurring and synthetic PPARγ activators on the expression of the CXC chemokines IP-10, Mig, and I-TAC and the CC chemokine MCP-1.
Materials and Methods
Cell culture
Human saphenous vein ECs were isolated from explants from unused portions of saphenous veins harvested at coronary artery bypass surgery (13). Cells were cultured in medium 199 (BioWhittaker, Walkersville, MD) containing 25 mmol/L HEPES, 1% (w/v) heparin, 50 μg/ml EC growth factor, 10 mM glutamine, 100 U/ml penicillin-streptomycin, and 5% FCS on low-pyrogen fibronectin (1.5 mg/cm2). ECs were >99% von Willebrand factor-positive as determined by flow cytometry and were used at passages 2–5 for all experiments. The human bladder cancer cell line ECV304 was obtained and cultured as described by American Type Culture Collection (ATCC, Manassas, VA), and the human CXC chemokine receptor 3 (CXCR3) 300-19 transfected cell line and parental untransfected lines (gifts from B. Moser, Theodor-Kocher Institute, Bern, Switzerland) were cultured as described (16).
RNA extraction and Northern blot analysis
Human ECs were treated in standard culture media as above for 12 h with IFN-γ (100 U/ml) in the absence or presence of different PPARγ activators (10 μ M 15d-PGJ2 (Calbiochem, La Jolla, CA), 10 μM troglitazone (gift from Parke-Davis, Morris Plains, NJ), 10 μM BRL49653 (rosiglitazone; gift from SmithKline Beecham, Philadelphia, PA)) or PPARα activators (30 μM docosahexaenoic acid (DHA), 30 μM eicosapentaenoic acid (EPA) (both from Sigma, St. Louis, MO), and 250 μM WY14643 (Biomol, Plymouth Meeting, PA)). Total RNA from 107 cells was isolated using RNAzol (Tel-Test, Friendswood, TX). Ten micrograms of total RNA was used for Northern blot analysis as described (17). Blots were hybridized sequentially with the following radiolabeled ([α-32P]deoxycytidine 5′-triphosphate) probes: a 1-kb PstI fragment from IP-10 cDNA (18), a 3-kb NotI fragment from human Mig cDNA (19) (gift from J. Farber, National Institutes Health, Bethesda, MD); a 300-bp BamHI/AvaI fragment from human I-TAC cDNA (20) (gift from K. Neote, Pfizer, Groton, CT); a 346-bp EcoRI fragment from human MCP-1 cDNA (ATCC); and a GAPDH cDNA as a control for RNA loading.
Transient transfection assay
ECV304 cells were transiently transfected with IP-10 promotor-luciferase constructs (21) and a CMV-β-galactosidase vector (pCMV-β-Gal, Clontech, Palo Alto, CA) using Lipofectamine according to the manufacturer’s protocol (Life Technologies, Gaithersburg, MD). Transfected cells were stimulated with IFN-γ (100 U/ml) in the presence or absence of 15d-PGJ2, troglitazone, or BRL49653 (all 10 μM). Cells were harvested after 16 h, and luciferase and β-galactosidase activity was measured using the Dual-Light assay (Tropix, Bedford, MA).
EMSA
Human ECs were stimulated for 12 h with IFN-γ (100 U/ml) and 15d-PGJ2 (10 μM) before the preparation of nuclear extracts. Standard EMSA was performed as described (22) using oligonucleotides for the IFN-stimulated response element (ISRE) site (5′-CGCTTTGGAAAGTGAAACCTAC CTC-3′), the κB1 site (5′-GCAACATGGGACTTCCCCAGGAAC-3′), and the κB2 site (5′-GAGCAGAGGGAAATTCCGTAACTT-3′) of the human IP-10 promoter.
In vitro chemotaxis assay
Sodium butyrate-treated CXCR3-transfected and -untransfected 300-19 cells (5 ×106 cells/ml) were placed in the top of a 48-well microchemotaxis chamber (Neuroprobe, Cabin John, MD) separated by a polycarbonate filter with 5-μm pores from 10-fold dilutions of conditioned media collected from untreated ECs, IFN-γ-treated ECs, IFN-γ - and 15d-PGJ2-treated ECs, and 15d-PGJ2-treated ECs or from IP-10 and I-TAC as positive controls. Cells were incubated at 37°C for 90 min in a 5% CO2 incubator, and migrated cells were stained with Diff-Quick and counted. Chemotaxis index (CI) is defined as the number of cells migrating in response to EC-conditioned medium divided by the number of cells migrating in response to medium alone.
ELISA
To determine the amount of secreted CXC chemokines in supernatants used for the chemotaxis assays, sandwich ELISAs for IP-10 (17), Mig (R&D Systems, Minneapolis, MN) and I-TAC were performed. mAbs were used for the capture Ab and rabbit polycloncal Abs were used for detection. For the I-TAC ELISA, mAb 8G4 and the polyclonal Ab were generously provided by K. Neote.
Assessment of total protein synthesis
To determine the effect of 15d-PGJ2 on the total protein synthesis in human ECs, cells were treated with IFN-γ in the absence or presence of 15d-PGJ2 in media containing radioactive-labeled methionine ([35S]-methionine, 0.2 μCi/ml). After 24 h, cells were harvested, and total protein synthesis in both lysates and supernatants was measured by counting radioactivity after cold trichloroacetic acid precipitation.
Statistical analysis
Differences were analyzed by one-way ANOVA and then by Fisher’s test. A p value of <0.05 was regarded as significant.
Results
PPARγ but not PPARα activators inhibit IFN-γ-induced CXC chemokine mRNA expression in human ECs
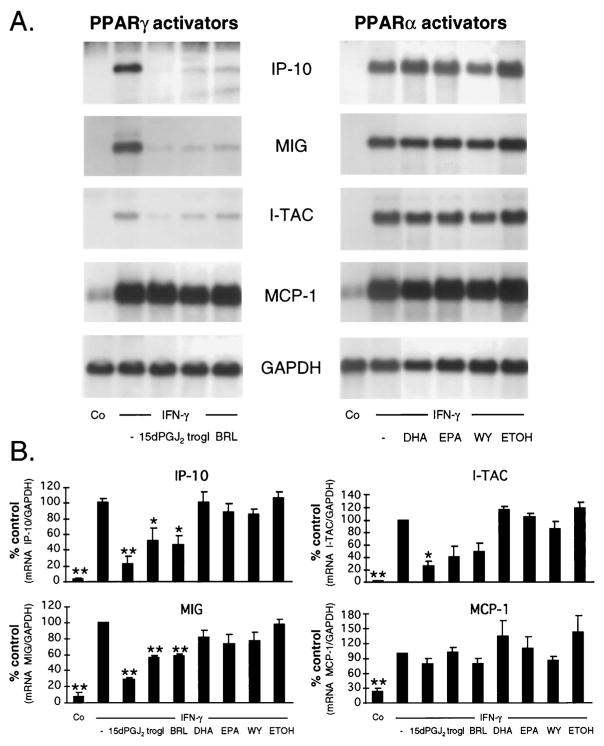
To investigate the effect of PPAR activators on endothelial chemokine mRNA expression, human ECs were stimulated for 12 h with IFN-γ (100 U/ml) in the absence or presence of different PPARγ (10 μM 15d-PGJ2, 10 μM troglitazone, 10 μM BRL49653) or PPARα activators (30 μM DHA, 30 μM EPA, 250 μM WY14643), and Northern blot analysis was performed. As expected, IFN-γ induced the expression of IP-10, Mig, I-TAC, and MCP-1 mRNA in human ECs. Treatment of ECs with three different PPARγ activators, added at the time of IFN-γ stimulation, significantly reduced IP-10, Mig, and I-TAC mRNA levels. In contrast, none of these PPARγ activators affected IFN-γ-induced MCP-1 expression (Fig. 1A, left panel). Densitometry analysis revealed 15d-PGJ2 as the strongest inhibitor of CXC chemokine mRNA expression in ECs, inhibiting IP-10, Mig, and I-TAC expression by 78, 73, and 75%, respectively (Fig. 1B). None of the different PPARα activators tested affected IFN-γ-induced chemokine mRNA expression significantly (Fig. 1A, right panel, and B). PPAR activators alone had no effect on chemokine expression (data not shown). Treatment of ECs with PPARγ activators did not affect cell viability or total protein synthesis (data not shown).
FIGURE 1.
PPARγ activators inhibit IFN-γ-induced CXC chemokine mRNA expression in human ECs. A, Representative Northern blot analysis of ECs treated for 12 h with IFN-γ (100 U/ml) and PPARγ activators (10 μM 15d-PGJ2, 10 μM troglitazone (trogl), 10 μM BRL49653 (BRL); left panel) or with PPARα activators (30 μM DHA, 30 μM EPA, 250 μM WY14643 (WY); right panel). Ethanol (ETOH) as a solvent for PPAR activators served as control. B, Densitometry analysis of three independent Northern blot experiments described in A. Results of chemokine expression were normalized to GAPDH and are expressed as percent of IFN-γ-stimulated cells (% control). Bars represent mean ± SEM. *, p < 0.05; **, p < 0.01; vs IFN-γ-stimulated cells.
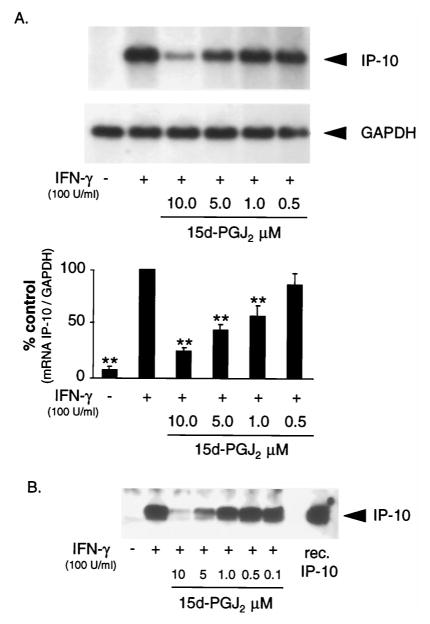
15d-PGJ2 reduces IP-10 mRNA and protein expression in a concentration-dependent manner
To further investigate the effect of PPARγ activators on IFN-γ-induced CXC chemokine expression, we asked whether 15d-PGJ2, the most efficacious inhibitor tested, blocked IP-10 mRNA accumulation and protein secretion in a concentration-dependent manner. ECs were costimulated with IFN-γ and different concentrations of 15d-PGJ2, and IP-10 mRNA accumulation was analyzed by Northern blot at 12 h. 15d-PGJ2 inhibited IP-10 mRNA expression in IFN-γ-stimulated ECs in a concentration-dependent manner with a maximal reduction to 24 ± 5% at 10 μM compared with IFN-γ-stimulated cells (p < 0.01; n = 3; Fig. 2A). Experiments in the presence of actinomycin D revealed that 15d-PGJ2 did not significantly reduce IP-10 mRNA half-life compared with control cells (9.9 ± 1.1 h in control cells vs 10.4 ± 1.8 h in 15d-PGJ2-stimulated cells; p = NS; n = 4; data not shown), indicating that the inhibitory effect of 15d-PGJ2 on IP-10 mRNA accumulation occurs at the transcriptional level rather than as a result of altered mRNA stability. Consistent with the data obtained by Northern blot analysis, treatment with 15d-PGJ2 also reduced secreted IP-10 protein levels in a concentration-dependent fashion (Fig. 2B).
FIGURE 2.
15d-PGJ2 inhibits IP-10 mRNA and protein expression in a concentration-dependent manner. A, Representative Northern blot analysis of human ECs stimulated for 12 h with IFN-γ (100 U/ml) and 15d-PGJ2 at concentrations shown. GAPDH expression demonstrates equal loading of intact RNA (upper panel). Lower panel shows densitometry analysis of three independent Northern blot experiments. Results of IP-10 expression were normalized to GAPDH and are expressed as percent of IFN-γ-stimulated cells (% control). Bars represent mean ± SEM. **, p < 0.01 vs IFN-γ-stimulated cells. B, Western blot analysis of secreted IP-10 protein from human ECs. Cells were stimulated for 24 h with IFN-γ (100 U/ml) and 15d-PGJ2 at concentrations indicated before supernatants were harvested for analysis. Recombinant IP-10 protein served as a positive control.
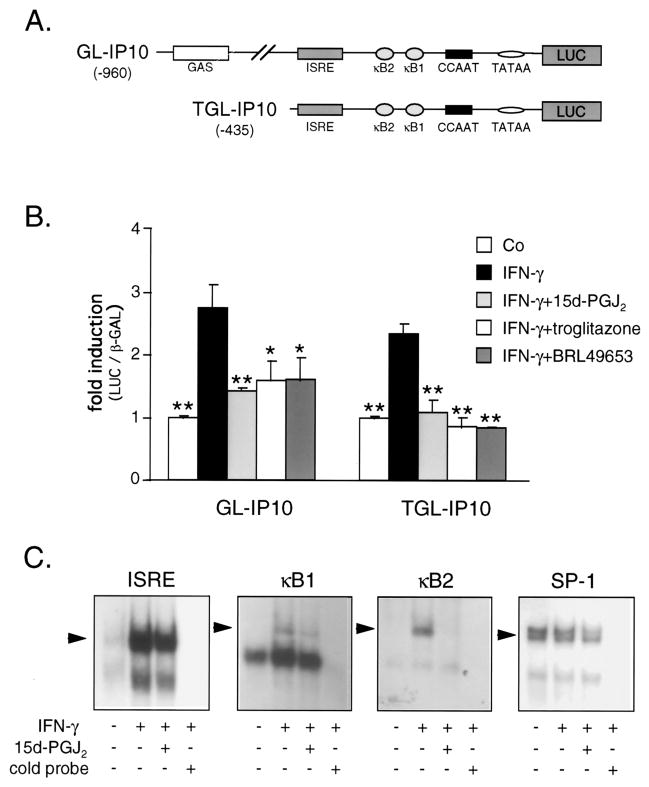
PPARγ activators inhibit IFN-γ-induced IP-10 promoter activity
To further investigate the effect of PPARγ activators on IP-10 transcription, we transiently transfected two well-characterized IP-10 promoter reporter luciferase constructs (Fig. 3A) into the human cell line ECV304. IFN-γ stimulation (16 h) of cells transfected with a 960-bp IP-10 promoter-reporter construct (GL-IP10) led to a 2.7 ± 0.4-fold increase in normalized promoter activity (luciferase/β-galactoside activity activity) similar to the effect seen in fibrosarcoma cells (21). Treatment with 15d-PGJ2, troglitazone, or BRL49653 significantly reduced this increase to 1.4 ± 0.1-fold (p < 0.01), 1.6 ± 0.3-fold (p < 0.05), and 1.6 ± 0.4-fold (p < 0.05, compared with IFN-γ-stimulated cells; n = 3), respectively. Transfection studies with a 435-bp IP-10 promoter-reporter deletion construct (TGL-IP10), lacking the gamma-activated sequence (GAS) site but containing the ISRE site and two NF-κB sites, revealed similar PPARγ activator responsiveness. Stimulation of TGL-IP10 transfected cells with IFN-γ-enhanced relative luciferase activity 2.3 ± 0.2-fold, similar to the effect seen in fibrosarcoma and astrocytoma cells (21, 23). Treatment with PPARγ activators inhibited this increase significantly: 15d-PGJ2 to 1.0 ± 0.3-fold, troglitazone to 0.9 ± 0.2-fold, and BRL49653 to 0.8 ± 0.1-fold (p < 0.01 for all, compared with IFN-γ-stimulated cells; n = 3; Fig. 3B).
FIGURE 3.
PPARγ activators inhibit IFN-γ-induced IP-10 promoter activity. A, Schematic of IP-10 promoter constructs used in transfection experiments. B, ECV304 cells were cotransfected with the two different IP-10 constructs shown in A and with a β-galactosidase expression construct (pCMV/β-GAL). Transfected cells were stimulated with agents indicated for 16 h, and assays were performed for luciferase and β-galactosidase activity. Results for each construct were normalized to β-galactosidase activity and expressed as fold induction compared with unstimulated cells. Bars represent mean ± SEM (n = 3), *, p < 0.05; **, p < 0.01; vs IFN-γ-stimulated cells. C, 15d-PGJ2 inhibits protein binding to the NF-κB sites but not to the ISRE site in the IP-10 promoter. EMSA of human ECs treated with IFN-γ (100 U/ml) and 15d-PGJ2 (10 μM) for 12 h. Specificity was determined by addition of 40 ng unlabeled ISRE, κB1, or κB2 oligonucleotide (cold probe). Specific complexes are indicated by arrowheads. Binding of nuclear proteins to a consensus SP-1 site served as a loading control. Three independent experiments yielded similar results.
15d-PGJ2 inhibits NF-κB activation in the IP-10 promoter
Because IFN-γ induction of IP-10 transcription requires cooperation of the ISRE site with at least one of the two NF-κB sites in the IP-10 promoter (24), we investigated whether PPARγ activators inhibit direct binding of transcription factors to the ISRE site or to the NF-κB sites. Treatment of human ECs with 15d-PGJ2 did not affect the amount of IFN-γ-induced DNA-protein complexes associated with the ISRE oligonucleotide (Fig. 3C, left panel), suggesting that PPARγ activators do not directly inhibit transcription factor binding to the ISRE site. In contrast, in experiments using the κB1 or κB2 oligonucleotides, 15d-PGJ2 markedly decreased the amount of shifted complexes induced by IFN-γ (Fig. 3C, middle panels). Therefore, PPARγ activators may directly inhibit NF-κB activation at both κB sites in the IP-10 promoter. The specificity of the detected κB complexes was determined by supershift analysis with anti-p50 Abs (data not shown). Protein binding to a consensus SP-1 site served as a loading control (Fig. 3C, right panel).
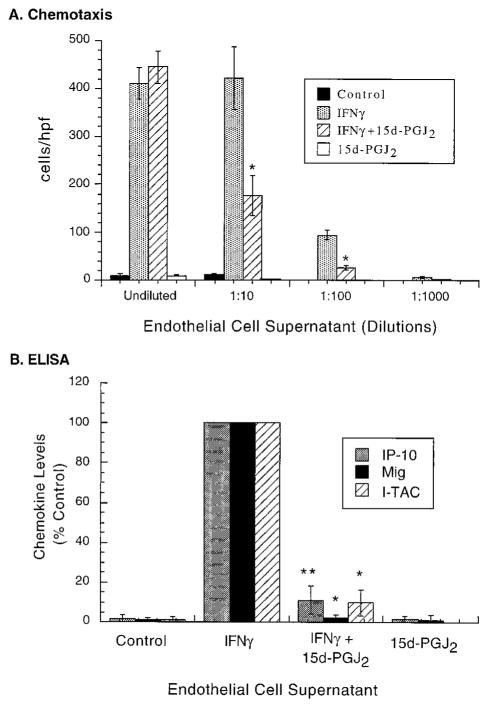
PPARγ activator 15d-PGJ2 inhibits CXCR3 chemotactic activity released from IFN-γ-stimulated ECs
To examine the potential functional relevance of inhibition by 15d- PGJ2 of IFN-γ-induced CXCR3 ligand expression in human ECs, we performed in vitro chemotaxis assay using CXCR3-transfected lymphocytes and supernatants from cultured ECs. Cell-free supernatants collected from IFN-γ-treated ECs contained substantial chemotactic activity for CXCR3 300-19 lymphocytes (CI = 65 at 1:10 dilution) compared with supernatants collected from untreated ECs (CI = 1 at all dilutions). ECs treated with 15d-PGJ2 (10 μM) and IFN-γ released significantly less CXCR3 chemotactic activity than IFN-γ-stimulated ECs. This was apparent at 1:10 and 1:100 dilutions of EC supernatant in which 15d-PGJ2 reduced the CXCR3 chemotactic activity found in IFN-γ-stimulated EC supernatants by 58 and 72%, respectively (p < 0.01; n = 3; Fig. 4). EC supernatants collected from all groups had no chemotactic activity toward untransfected 300-19 cells (data not shown). In concordance with the ability of 15d-PGJ2 to inhibit the release of IFN-γ-induced CXCR3 chemotactic activity from ECs, ELISAs specific for IP-10, Mig, and I-TAC revealed that 15d-PGJ2 reduced the amount of secreted CXCR3 ligands from IFN-γ-stimulated ECs by 89 ± 7% (p < 0.01; n = 5), 98 ± 2% (p < 0.05; n = 5), and 90 ± 6% (p < 0.05; n = 5), respectively (Fig. 4B). 15d-PGJ2 stimulation alone did not release CXCR3 chemotactic activity (CI = 1) or secretion of IP-10, Mig, and I-TAC protein from ECs. Interestingly, there was a hierarchy to the amounts of these chemokines secreted from IFN-γ-stimulated ECs: IP-10 was secreted to the highest levels (102 ± 18 ng/ml; n = 5) followed by Mig (10.6 ± 8.7 ng/ml; n = 5) and then I-TAC (1.7 ± 1.6 ng/ml; n = 5).
FIGURE 4.
15d-PGJ2 inhibits CXCR3 chemotactic activity released from IFN-γ-stimulated ECs. Supernatants of human ECs stimulated with IFN-γ (100 U/ml) for 48 h in the absence or presence of 15d-PGJ2 (10 μM) were tested for their chemotactic activity on CXCR3-transfected cells (A) and were analyzed for their levels of IP-10, Mig, and I-TAC protein (B). Controls included supernatants collected from untreated EC (Control) and 15d-PGJ2-treated (10 μM) ECs. A, EC supernatants were used in an in vitro chemotaxis assay at the indicated dilutions employing CXCR3-transfected 300-19 lymphocytes and a modified Boyden chamber. Data represent mean ± SEM of four fields counted of replicate wells and are presented as number of migrated cells per high-powered field (hpf). *, p < 0.01 vs IFN-γ-stimulated cells. Three independent sets of supernatants were each tested at dilutions indicated two to four times and yielded similar results. B, Levels of IP-10, Mig, and I-TAC protein in unconcentrated EC supernatants determined by specific ELISAs. Results are expressed as percent of IFN-γ-stimulated cells (% control). Bars represent mean ± SD of five independent experiments, each measured in triplicate. *, p < 0.05; **, p < 0.01; vs IFN-γ-stimulated cells.
Discussion
The present study demonstrates that PPARγ activators inhibit IFN-γ-induced expression of the CXC chemokines IP-10, Mig, and I-TAC in human ECs. The mechanism for this effect is likely to be through PPARγ activation, given prior evidence that troglitazone, BRL496553, and 15d-PGJ2 are all PPARγ activators with little activity for other PPAR forms. In addition, three PPARα activators, including the α-specific agonist WY14643, had no effect on IFN-γ-induced chemokine expression. Nevertheless, we find, as reported in other settings (9, 10), a greater biologic effect with 15d-PGJ2 compared with that of BRL49653, despite biochemical data demonstrating greater PPARγ receptor affinity for BRL49653 rather than 15d-PGJ2. This discrepancy has raised the intriguing possibility that 15d-PGJ2 might also act through other pathways (25), such as direct inhibition and modification of IκB kinase (26).
To further characterize the underlying mechanisms for the effects of PPARγ activators on CXC chemokine expression, we focused on the regulation of IP-10 expression. The inhibition of IP-10 mRNA expression occurs at a transcriptional level, because 15d-PGJ2 did not alter IP-10 mRNA half-life but did inhibit IFN-γ-induced IP-10 promoter activity. Interaction of PPARγ with the IP-10 promoter was independent of a GAS element, because PPARγ activators decreased the activity of both the longer GL-IP10 reporter construct (containing the GAS element) and the truncated TGL-IP10 construct (lacking the GAS site but containing an ISRE and two NF-κB sites). IFN-γ induction of IP-10 transcription requires cooperation of the ISRE site with at least one of the two NF-κB sites in the IP-10 promoter (27). Our EMSA results indicate that the inhibition of IFN-γ-induced IP-10 transcription by PPARγ activators occurs through an inhibition of NF-κB rather than ISRE activation. Our work extends the notion that PPARγ activators can inhibit NF-κB (12) by demonstrating that PPARγ inhibiton of IP-10 also involves NF-κB. This effect could result from direct interference with NF-κB binding to the IP-10 promoter, as postulated for the interaction of NF-κB with other nuclear receptors (e.g., the estrogen receptor (28)), or from reduced nuclear translocation of NF-κB after PPARγ activation by inhibition of IκB degradation as described in other settings of NF-κB inhibition (29).
Our data also suggest differential regulation of chemokine expression by PPARγ activators, given evidence for inhibition of the expression in human ECs of the CXC chemokines IP-10, Mig, and I-TAC, but no effect on the expression of the CC chemokine MCP-1. This difference in PPARγ regulation of CXC but not CC chemokine expression might result from their distinct transcriptional regulation by IFN-γ. Activation of IP-10 transcription by IFN-γ occurs through cooperation of the ISRE site with the NF-κB sites in the IP-10 promoter (27), whereas transcriptional induction of MCP-1 by IFN-γ occurs mainly through the GAS site (30). Interestingly, PPARγ activators reduce IL-1β-induced MCP-1 expression in a colon epithelial cell line (12), a mechanism thought to be mediated through an inhibition of NF-κB activation. It is noteworthy that the I-TAC promoter also contains an ISRE site and an NF-κB site (31). However, a tandem GAS-like element has been shown to be important for IFN-γ induction of Mig in some cells (32). Although the detailed mechanism by which PPARγ activators inhibit Mig induction remains to be determined, these data imply that there may be an unrecognized NF-κB site in the Mig promoter or that PPARγ activators affect Mig expression in a unique way.
Given the role of IFN-γ-induced chemokines in Th1-mediated inflammation, our study provides a novel mechanism for an anti-inflammatory effect of PPARγ in ECs, with potentially important implications for the treatment of diabetes given its associated risk for vascular diseases.
Acknowledgments
We thank Dr. Marisia Muszynski, Irina Chulsky, Elissa Simon-Morrisey, and Christy C. Ong (Brigham & Women’s Hospital) for their skillful assistance and Dr. Kuldeepe Neote (Pfizer) for the I-TAC reagents.
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft (MA 2047/1-1 and MA 2047/2-1) to N.M.; by a grant from the Swiss National Foundation to F.M., by National Institutes of Health Grant R01 CA69212 and a Charles E. Culpeper Foundation Medical Scholarship to A.D.L.; and by National Institutes of Health Grant HL03107, American Diabetes Association Research Award, and a Partners Nesson Award to J.P.; and National of Institutes of Health HL34636 to P.L.
Abbreviations used in this paper: EC, endothelial cell; IP-10, IFN-inducible protein of 10 kDa; Mig, monokine induced by IFN-γ; I-TAC, IFN-inducible T cell α-chemoattractant; MCP-1, monocyte chemoattractant protein-1; PPAR, peroxisome proliferator-activated receptor; 15d-PGJ2, 15-deoxy−Δ12,14 prostaglandin J2; CXCR3, CXC chemokine receptor 3; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ISRE, IFN-stimulated response element; CI, chemotaxis index; GAS, gamma-activated sequence; GL-IP10, IP-10 promoter-reporter construct; TGL-IPl0, truncated GL-IP10.
References
- 1.Luster AD. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;388:436. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 2.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. The interferon-γ inducible CXC chemokines IP-10, Mig, and I-TAC are differentially expressed by human atheroma-associated cells: implications for lymphocyte recruitment in atherogenesis. J Clin Invest. 1999;104:1041. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy−Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, Lenhard JM, Wilson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 6.Wilson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM. The structure-activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. J Med Chem. 1996;39:665. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 7.Forman BM, Chen J, Evans RM. Hypolipidemic drug, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and Δ. Proc Natl Acad Sci USA. 1997;94:4312. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchard JC. Mechanisms of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98:2088. doi: 10.1161/01.cir.98.19.2088. [DOI] [PubMed] [Google Scholar]
- 9.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 10.Ricote M, Li A, Willson T, Kelly C, Glass C. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 11.Marx N, Sukhova GK, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARγ: differentiation-dependent peroxisomal proliferator-activated receptor γ (PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su CG, Wen X, Bailey ST, Jiang W, Rangwala SM, Keilbaugh SA, Flanigan A, Murthy S, Lazar MA, Wu GD. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx N, Bourcier T, Sukkohova GK, Libby P, Plutzky J. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 14.Xin S, Yang S, Kowalski J, Gerritsen ME. Peroxisome proliferator activated receptor γ ligands are potent inhibitors of angiogenesis in vitro and in vivo. J Biol Chem. 1999;274:9116. doi: 10.1074/jbc.274.13.9116. [DOI] [PubMed] [Google Scholar]
- 15.Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, Maekawa H, Isoo N, Kimura S, Watanabe T. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARγ on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- 16.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewi I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and Mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549. [PubMed] [Google Scholar]
- 18.Luster AD, Unkeless JC, Ravetch JV. γ-Interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985;315:672. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- 19.Farber JM. HuMig: a new human member of the chemokine family of cytokines. Biochem Biophys Res Commun. 1993;192:223. doi: 10.1006/bbrc.1993.1403. [DOI] [PubMed] [Google Scholar]
- 20.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et al. Interferon-inducible T cell α chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. p48/STAT-1α-containing complexes play a predominant role in induction of IFN-γ-inducible protein, 10 kDa (IP-10) by IFN-γ alone or in synergy with TNF-α. J Immunol. 1998;161:4736. [PubMed] [Google Scholar]
- 22.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule 1 expression in human endothelial cells. Circulation. 1999;99:3125. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J Neurosci Res. 1998;54:169. doi: 10.1002/(SICI)1097-4547(19981015)54:2<169::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 24.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFNγ-and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677. [PubMed] [Google Scholar]
- 25.Thieringer R, Fenyk-Melody JE, Le Grand CB, Shelton BA, Detmers PA, Somers EP, Carbin L, Moller DE, Wright SD, Berger J. Activation of peroxisome proliferator-activated receptor γ does not inhibit IL-6 or TNF-α responses of macrophages to lipopolysaccharide in vitro or in vivo. J Immunol. 2000;164:1046. doi: 10.4049/jimmunol.164.2.1046. [DOI] [PubMed] [Google Scholar]
- 26.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 27.Ohmori Y, Hamilton TA. Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFNγ-and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J Biol Chem. 1993;268:6677. [PubMed] [Google Scholar]
- 28.Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17β-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-κB by the estrogen receptor. FEBS Lett. 1997;409:79. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 29.Mackman N. Protease inhibitors block lipopolysaccharide induction of tissue factor gene expression in human monocytic cells by preventing activation of c-Rel/p65 heterodimers. J Biol Chem. 1994;269:26363. [PubMed] [Google Scholar]
- 30.Valente AJ, Xie JF, Abramova MA, Wenzel UO, Abboud HE, Graves DT. A complex element regulates IFN-γ-stimulated monocyte chemoattractant protein-1 gene transcription. J Immunol. 1998;161:3719. [PubMed] [Google Scholar]
- 31.Rani MRS, Gauzzi C, Pellegrini S, Fish EN, Wei T, Ransohoff RM. Induction of β-R1/I-TAC by interferon-β requires catalytically active TYK2. J Biol Chem. 1999;274:1891. doi: 10.1074/jbc.274.4.1891. [DOI] [PubMed] [Google Scholar]
- 32.Wong P, Severns CW, Guyer NB, Wright TM. A unique palindromic element mediates γ interferon induction of Mig gene expression. Mol Cell Biol. 1994;14:914. doi: 10.1128/mcb.14.2.914. [DOI] [PMC free article] [PubMed] [Google Scholar]