Abstract
Activation of T lymphocytes and their ensuing elaboration of proinflammatory cytokines, such as interferon (IFN)-γ, represent a critical step in atherogenesis and arteriosclerosis. IFNγ pathways also appear integral to the development of transplantation-associated arteriosclerosis (Tx-AA), limiting long-term cardiac allograft survival. Although disruption of these IFNγ signaling pathways limits atherosclerosis and Tx-AA in animals, little is known about inhibitory regulation of proinflammatory cytokine production in humans. The present study investigated whether activators of peroxisome proliferator–activated receptor (PPAR) α and PPARγ, with their known antiinflammatory effects, might regulate the expression of proinflammatory cytokines in human CD4-positive T cells. Isolated human CD4-positive T cells express PPARα and PPARγ mRNA and protein. Activation of CD4-positive T cells by anti-CD3 monoclonal antibodies significantly increased IFNγ protein secretion from 0 to 504±168 pg/mL, as determined by ELISA. Pretreatment of cells with well-established PPARα (WY14643 or fenofibrate) or PPARγ (BRL49653/rosiglitazone or pioglitazone) activators reduced anti-CD3–induced IFNγ secretion in a concentration-dependent manner. PPAR activators also inhibited TNFα and interleukin-2 protein expression. In addition, PPAR activators markedly reduced cytokine mRNA expression in these cells. Such antiinflammatory actions were also evident in cell-cell interactions with medium conditioned by PPAR activator–treated T cells attenuating human monocyte CD64 expression and human endothelial cell major histocompatibility complex class II induction. Thus, activation of PPARα and PPARγ in human CD4-positive T cells limits the expression of proinflammatory cytokines, such as IFNγ, yielding potential therapeutic benefits in pathological processes, such as atherosclerosis and Tx-AA.
Keywords: atherosclerosis, fibrates, thiazolidinediones, peroxisome proliferator–activated receptors, T cells
The activation of T lymphocytes contributes importantly to atherogenesis.1,2 In human atheroma, CD4-positive cells, the major T-cell population, appear to promote atherosclerosis through their elaboration of proinflammatory cytokines, such as interferon (IFN) γ, tumor necrosis factors (TNFs), and interleukin (IL)-2.1,3,4 These cytokines contribute to plaque development through their activation of endothelial cells (ECs) and modulation of macrophage and vascular smooth muscle cell responses.5,6 Indeed, patients with atherosclerosis and acute coronary syndromes exhibit T-cell activation and increased IFNγ serum levels.7,8 In apoE-deficient mice, interruption of the IFNγ signaling pathway reduces the extent of atherosclerotic lesions.9 Similar proinflammatory effects of T-lymphocyte–derived cytokines participate in transplantation-associated arteriosclerosis (Tx-AA), a disease accounting for most cardiac transplantation failures.10 In various animal models of transplantation, decreased or absent IFNγ production limited subsequent allograft vasculopathy.11 Despite this large body of data implicating IFNγ in atherosclerosis and Tx-AA, pathways that might limit inflammatory cytokine production by human lymphocytes remain largely unexplored in the context of vascular disease.
Recent work from several groups implicates the nuclear receptors peroxisome proliferator–activated receptor (PPAR) α and PPARγ as antiinflammatory mediators in atheroma-associated cells.12–17 PPARs, like other nuclear receptors, regulate gene expression through their actions as transcription factors in response to specific ligands.18 PPARα activators include lipid-lowering fibric acid derivatives, such as fenofibrate or WY14643, and certain polyunsaturated fatty acids.19 PPARγ ligands include the thiazolidinedione (TZD) class of insulin sensitizers, such as rosiglitazone (previously known as BRL49653 [BRL]) and pioglitazone,20 as well as natural ligands, such as the prostaglandin D2 derivative 15-deoxy-Δ-12,14-prostaglandin J2 (15d-PGJ2)21 and oxidized linoleic acid (9- or 13-HODE).22 In vitro experiments demonstrate that PPARα and PPARγ activators decrease inflammatory proteins, such as adhesion molecules, cytokines, and chemokines, in monocytes/macrophages, ECs, and vascular smooth muscle cells.23 Moreover, recent in vivo studies suggest that PPAR activators can limit experimental atherosclerosis in animal models.24,25 In human trials, preliminary clinical data in diabetic patients suggest that TZD treatment can decrease carotid intimal-medial thickness,26 and recent studies with PPARα-activating fibric acids have also demonstrated decreased atherosclerosis among treated patients.27 Interestingly, fenofibrate treatment in patients with coronary heart disease reduced IFNγ plasma levels through an as-yet-undefined mechanism.28
Given the role of T-lymphocyte inflammatory cytokine production in atherosclerosis and evidence of PPARs as antiinflammatory mediators, we hypothesized that human T lymphocytes express PPARα and PPARγ and that stimulation of these cells by PPAR activators in clinical use would limit inflammatory cytokine expression. Indeed, concurrent work suggests that PPARγ ligands may influence T-cell activation and proliferation,29,30 although those studies did not address PPARα in T-cell cytokine responses or PPAR regulation of T-cell IFNγ production.
Materials and Methods
Cell Culture
Human CD4-positive T cells were isolated from freshly drawn blood of healthy volunteers by Ficoll-Histopaque (Sigma Chemical Co) gradient centrifugation to obtain mononuclear cells and subsequent negative selection of CD4-positive T cells by magnetic bead separation (Miltenyi Biotech), as described by the manufacturer’s protocol. The purity of CD4-positive T cells was >97%, as determined by flow cytometry. Human ECs and monocytes were isolated as previously described.31,32
Reverse Transcriptase–Polymerase Chain Reaction
Total RNA from freshly prepared CD4-positive T cells was isolated for reverse transcriptase (RT)–polymerase chain reaction (PCR) with amplification of PPARα, PPARγ, and GAPDH cDNA as described previously.15
Preparation of Nuclear and Cytosolic Extracts and Western Blot Analysis
For Western blotting, nuclear and cytosolic extracts of 107 cells were prepared as previously described.15
Stimulation Assays, ELISA, and Cell Viability Studies
Human CD4-positive T cells (1×106 cells/mL) were pretreated with PPARα activators (WY14643 or fenofibrate) or PPARγ activators (BRL or pioglitazone) for 2 hours before stimulation with immobilized anti-CD3 antibody (R&D Systems) for 48 hours or with phorbol 12-myristate 13-acetate (PMA, 10 ng/mL)/ionomycin (0.5 μ mol/L) for 6 hours, according to previously published time courses for these stimuli.33,34 Cells were then harvested, and IFNγ, TNFα, and IL-2 ELISAs (R&D Systems) were performed on cell-free supernatants, as recommended by the manufacturer. In some experiments, cells were stimulated with PPAR activators for 24 hours, and the release of IL-4, a typical TH2-cytokine, was measured by ELISA (R&D Systems).
Cell viability was assessed by standard trypan blue exclusion, as described previously.
Northern Blot Analysis
For Northern blot experiments, cells were pretreated with PPAR activators and then stimulated with anti-CD3 antibodies for 24 hours or with PMA/ionomycin for 2 hours. Five micrograms of total RNA was used in standard Northern blot analysis by using cDNA probes against IFNγ, TNFα, or IL-2 or against the housekeeping genes B41 or GAPDH.
Flow Cytometry
Immunofluorescence staining and flow cytometry were performed as previously described.35 Human CD4-positive T cells were incubated with an equal volume of PBS containing saturating concentrations (10 mg/L) of FITC-conjugated anti-CD3 antibodies and PE-conjugated anti-CD4 antibodies for 30 minutes at room temperature. To examine the influence of PPAR activators on the proinflammatory activity of T-cell supernatants toward other vascular cells, freshly isolated human monocytes or human ECs were incubated with supernatants (50% original monocyte or EC media and 50% conditioned media from T cells) derived from T cells after CD3 activation in the absence or presence of WY14643 or BRL (Figures 5A and 6A, right panels). In some experiments, cells were first treated with conditioned media from anti-CD3–activated T cells (Figures 5B and 6B, right panels) or IFNγ (Figures 5C and 6C, right panels), and then PPAR activators at similar concentrations were directly added to monocytes or ECs. After 18 hours (in monocyte experiments) or 72 hours (in EC experiments), cells were harvested for the investigation of monocyte CD64 or endothelial major histocompatibility complex (MHC) class II (MHC II) expression on the cell surface, respectively. After washing, monocytes were stained with FITC-conjugated anti-CD64 antibodies, and ECs were stained with FITC-conjugated anti–MHC II antibodies. Finally, T cells, monocytes, or ECs were washed three times and stored in 1% paraformaldehyde (Sigma) at 4°C until flow cytometric analysis was performed within 24 hours.
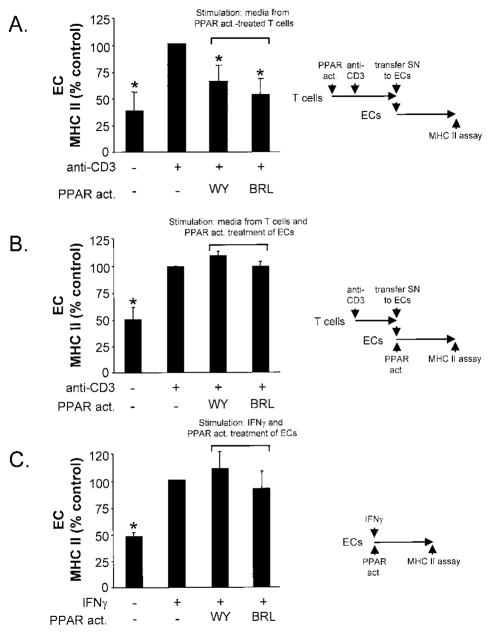
Figure 5.
PPAR activators reduce proinflammatory activity of T-cell supernatants on human monocytes in the absence of any direct effects on monocyte CD64 response. A, Freshly isolated human monocytes were incubated for 18 hours with conditioned media from CD4-positive T cells stimulated with anti-CD3 monoclonal antibodies in the presence or absence of PPAR agonists, and mean fluorescence intensity of monocyte CD64 expression was measured by flow cytometry (right). Results are expressed as percentage of control (monocytes incubated with supernatants from activated T cells). Bars represent mean±SEM (n=4). *P<0.05; **P<0.01. B, Human monocytes were incubated with conditioned media from CD3-activated T cells to induce CD64 expression and then directly stimulated with PPAR activators for 18 hours before CD64 expression was assessed by flow cytometry (right). Results are expressed as percentage of control (monocytes incubated with supernatants from activated T cells). Bars represent mean±SEM (n=4). No significant difference was seen, except for comparison with unstimulated cells. **P<0.01. C, Human monocytes were incubated with IFNγ (200 U/L) in the presence or absence of PPAR activators. After 18 hours, CD64 expression was measured by flow cytometry (right). Results are expressed as percentage of control (monocytes stimulated with IFNγ). Bars represent mean±SEM (n=5). No significant difference was seen, except for comparison with unstimulated cells. **P<0.01.
Figure 6.
PPAR activators reduce proinflammatory activity of T-cell supernatants on human ECs. A, Human ECs were incubated for 72 hours with conditioned media from CD4-positive T cells stimulated with or without PPAR activators, and endothelial MHC II surface expression was measured by flow cytometry (mean fluorescence intensity) (right). Results are expressed as percentage of ECs incubated with supernatants from activated T cells. Bars represent mean±SEM (n=3). *P<0.05. B, Human ECs were incubated with conditioned media from CD3-activated T cells to induce MHC II expression and then directly stimulated with PPAR activators for 72 hours before MHC II surface expression was assessed by flow cytometry (right). Results are expressed as percentage of control (ECs incubated with supernatants from activated T cells). Bars represent mean±SEM (n<4). No significant difference was seen, except for comparison with unstimulated cells. **P<0.01. C, Human ECs were incubated with IFNγ (1000 U/L) in the presence or absence of PPAR activators. After 72 hours, MHC II expression was measured by flow cytometry (right). Results are expressed as percentage of control (ECs stimulated with IFNγ). Bars represent mean±SEM (n=4). No significant difference was seen, except for comparison with unstimulated cells. *P<0.05.
Statistical Analysis
Results of the experimental studies are reported as mean±SEM. Differences were analyzed by one-way ANOVA, followed by the appropriate post hoc test. A value of P<0.05 was regarded as significant.
Results
Human CD4-Positive T Cells Express PPARα and PPARγ mRNA and Protein
Isolated human CD4-positive T cells express PPARα and PPARγ mRNA as determined by RT-PCR (Figure 1A). Western blot analysis revealed PPARα as well as PPARγ protein expression in the nuclear fraction but not in the cytosol of isolated CD4-positive human T cells (Figure 1B). Induction of IFNγ expression by stimulation with anti-CD3 antibodies and/or treatment with PPAR activators did not affect PPAR expression in these cells (data not shown).
Figure 1.

CD4-positive human T cells express PPARα and PPARγ. A, RT-PCR reaction of PPARα and PPARγ RNA in freshly isolated human CD4-positive T cells (T4) reveals a cDNA fragment of the expected size. Also shown are a DNA ladder (MW), RT-PCR product from macrophage RNA as a positive control (MØ), and a negative control consisting of RT-PCR reactions lacking RT (Co). B, Western blot analysis on nucleic (Nucl) and cytosolic (Cyto) fractions of human CD4-positive T cells with use of an anti-human PPARα antibody or an anti-human PPARγ antibody.
PPAR Activators Inhibit IFNγ Expression in Human CD4-Positive T Cells
Unstimulated human CD4-positive T cells did not secrete IFNγ, as determined by ELISA of cell-free supernatants. As expected, incubation of cells with immobilized anti-CD3 antibodies significantly increased IFNγ protein secretion from 0 to 504±168 pg/mL (P<0.01, n=6). Two-hour pretreatment with PPARα activators, either WY14643 or fenofibrate, inhibited this increase in a concentration-dependent manner. IFNγ production was not detected at WY14643 (250 μ mol/L) and was reduced by fenofibrate to 13±5% of the level elaborated by untreated control cells (P<0.01 for both compared with CD3-activated cells without agonist, n=4) (Figure 2A). Similarly, pretreatment of CD4-positive T cells with two different PPARγ-activating TZDs also reduced anti-CD3–induced IFNγ release in a concentration-dependent manner, with a maximal reduction to 52±9% at 10 μ mol/L BRL and to 28±8% at 10 μ mol/L pioglitazone (P<0.01 for both compared with CD3-activated cells, n=6) (Figure 2B). None of the PPAR activators that were used affected cell viability (by trypan blue exclusion) or cell surface CD3 expression, as determined by flow cytometry (Table).
Figure 2.
PPAR activators inhibit IFNγ expression in human CD4-positive T cells. A, Isolated CD4-positive T cells were pretreated with PPARα activators (WY14643 or fenofibrate) 2 hours before stimulation with anti-CD3 antibodies. After 48 hours, cytokine protein content in cell-free supernatants was measured by ELISA. Results are expressed as percentage of CD3-activated cells (% control). Bars represent mean±SEM (n=6). **P<0.01. B, Isolated CD4-positive T cells were pretreated with PPARγ activators (BRL or pioglitazone) 2 hours before stimulation with anti-CD3 antibodies. After 48 hours, cytokine protein content in cell-free supernatants was measured by ELISA. Results are expressed as percentage of CD3-activated cells (% control). Bars represent mean±SEM (n=6). *P<0.05; **P<0.01.
Table.
Effects of PPAR Activators
| Effects of PPARα Activators on Human CD4-Positive T Cells
|
|||
|---|---|---|---|
| CD3 Activated | CD3-Activated + 250 μ mol/L WY14643 | CD3-Activated + 100 μ mol/L Fenofibrate | |
| Cell viability, % | >90 | >90 | >90 |
| CD3 expression* (mean±SEM; n=5) | 32.0±3.9 | 29.4±1.0 | 31.2±1.5 |
| Effects of PPARγ Activators on Human CD4-Positive T Cells
|
|||
|---|---|---|---|
| CD3 Activated | CD3-Activated + 10 μ mol/L BRL49653 | CD3-Activated + 10 μ mol/L Pioglitazone | |
| Cell viability, % | >90 | >90 | >90 |
| CD3 expression* (mean±SEM; n=6) | 40.3±3.7 | 37.1±3.8 | 39.0±3.8 |
As determined by flow cytometry.
PPAR Activators Reduce the Expression of Other Proinflammatory Cytokines in CD4-Positive Human T Cells
To examine whether the effects of PPAR activators extended beyond IFNγ to other inflammatory cytokines, we performed similar experiments measuring TNFα and IL-2 protein expression of human CD4-positive T cells. Pre-treatment of cells with PPARα-activating WY14643 reduced anti-CD3–induced TNFα and IL-2 secretion in a concentration-dependent manner, with maximal inhibition to 7±4% of TNFα production at 250 μ mol/L WY14643, and abrogated IL-2 expression under similar conditions (P<0.01 for both compared with anti-CD3–activated cells, n=3) (Figure 3A). PPARγ-activating BRL had similar concentration-dependent, albeit less complete, effects; anti-CD3–induced TNFα and IL-2 protein expression decreased to 64±7% and 34±7%, respectively, at 10 μ mol/L BRL (P<0.01 for both compared with CD3-activated cells, n=3) (Figure 3B). To exclude the possibility that these results stemmed from a shift of T cells toward a TH2 response, we measured IL-4 in supernatants on stimulation with PMA/ionomycin or PPAR activators. PMA/ionomycin treatment induced IL-4 protein secretion from 5±4 to 205±42 pg/mL (P<0.01, n=3), whereas none of the PPARα or PPARγ activators had a similar effect (Figure 3C).
Figure 3.
PPAR activators reduce TNFα and IL-2 protein secretion from CD4-positive human T cells. A and B, Isolated CD4-positive T cells were pretreated with PPARα-activating WY14643 (A) or PPARγ-activating BRL 2 hours before stimulation with anti-CD3 antibodies (B). After 48 hours, cytokine protein content in cell-free supernatants was measured by ELISA. Results are expressed as percentage of CD3-activated cells (% control). Bars represent mean±SEM (n=3). *P<0.05; **P<0.01. C, PPAR activators do not induce expression of IL-4 in CD4-positive human T cells. Isolated CD4-positive T cells were treated with PPARα activators (250 μ mol/L WY14643 or 100 μ mol/L fenofibrate) or PPARγ activators (10 μ mol/L BRL or 10 μ mol/L pioglita-zone) for 24 hours before IL-4 protein content in cell-free supernatant was measured by ELISA. PMA/ionomycin (100 ng/mL/1 μ mol/L)–treated cells served as a positive control. Results are expressed as percentage of PMA/ionomycin-activated cells (% control). Bars represent mean±SEM (n=3). **P<0.01 compared with unstimulated cells. D, PPAR activators inhibit PMA/ionomycin-induced IFNγ expression. Isolated CD4-positive T cells were stimulated and treated with PMA/ ionomycin (10 ng/mL/0.5 μ mol/L) in the absence or presence of the PPARα activator WY14643 or the PPARγ activator BRL. After 6 hours, cytokine protein contents in cell-free supernatants were measured by ELISA. Results are expressed as percentage of PMA/ionomycin-treated cells (% control). Bars represent mean±SEM (n=3). *P<0.05; **P<0.01.
PPAR Activators Inhibit PMA/Ionomycin-Induced Proinflammatory Cytokine Expression in Human CD4-Positive T Cells
To investigate whether the effects of PPAR activators on T4 cell–derived IFNγ expression depended on the stimulus used, we used PMA/ionomycin to induce IFNγ release. PMA/ ionomycin treatment of human CD4-positive T cells stimulated more IFNγ protein expression than did CD3 activation, increasing IFNγ protein content in the supernatant to 4971±1596 pg/mL. Pretreatment of the cells with the PPARα activator WY14643 (250 μ mol/L) reduced IFNγ release to 36±10% (P<0.01 compared with PMA/ionomycin-stimulated cells, n=3), whereas pretreatment with the PPARγ activator BRL decreased IFNγ-protein secretion to 71±3% (P<0.05 compared with PMA/ionomycin-stimulated cells, n=3) (Figure 3D).
PPAR Activation Reduces Cytokine mRNA Expression in Human T4 Cells
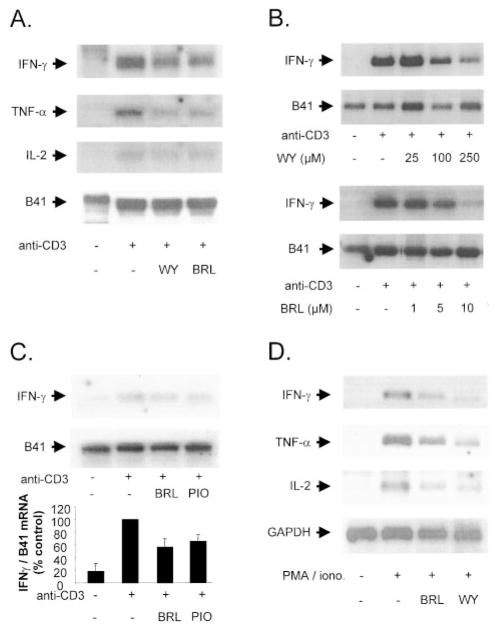
To examine whether the decrease in proinflammatory cytokine expression by PPAR activators resulted from reduced cytokine mRNA expression, we pretreated CD4-positive T cells with PPARα or PPARγ activators and performed Northern blot analysis after 24-hour stimulation with anti-CD3 antibodies. PPARα-activating WY14643 or PPARγ-activating BRL markedly reduced anti-CD3–induced IFNγ, TNFα, and IL-2 mRNA content but did not affect mRNA levels of the constitutively expressed gene B41 (Figure 4A). The inhibition of anti-CD3–induced IFNγ mRNA expression by WY14643 or BRL was concentration dependent, as shown in Figure 4B. In contrast to the results on protein expression, BRL or pioglitazone produced similar inhibition of IFNγ mRNA expression, as determined by densitometry of three different Northern blots (Figure 4C). In addition, the effects observed were not dependent on the stimulus used, as shown by similar WY14643 and BRL effects on PMA/ionomycin-induced IFNγ, TNFα, and IL-2 mRNA (Figure 4D).
Figure 4.
PPAR activators inhibit proinflammatory cytokine mRNA expression in human CD4-positive cells. A, Representative Northern blot analysis for cytokine expression of human CD4-positive T cells pretreated with WY14643 (250 μ mol/L) or BRL (10 μ mol/L) before 24-hour stimulation with anti-CD3 antibodies. Three independent experiments yielded similar results. B, Representative Northern blot analysis for cytokine expression of human CD4-positive T cells pretreated with WY14643 (top) or BRL (bottom) at concentrations indicated before incubation with anti-CD3 antibodies for 24 hours. Three independent experiments yielded similar results. C, Representative Northern blot analysis for cytokine expression of human CD4-positive T cells pretreated with BRL or pioglitazone (both at 10 μ mol/L) before incubation with anti-CD3 antibodies for 24 hours (top). At the bottom is a densitometric analysis of IFNγ mRNA expression normalized to housekeeping gene B41 of 3 independent experiments. D, Representative Northern blot analysis for cytokine mRNA expression of human CD4-positive T cells pretreated with WY14643 (250 μ mol/L) or BRL (10 μ mol/L) before 2-hour stimulation with PMA/ionomycin. Three independent experiments yielded similar results.
PPAR Activators Reduce Proinflammatory Function of T Lymphocytes on Human Monocytes and ECs
To examine the potential functional effects of PPAR-mediated reduced T-cell cytokine expression, we incubated supernatants from stimulated CD4-positive T cells with human monocytes or ECs and measured monocyte CD64 or endothelial MHC II surface expression by flow cytometry. CD64, the high-affinity receptor for IgG involved in phagocytosis and antigen capture, an IFNγ-regulated gene in human monocytes, indicates IFNγ activity on monocytes in vitro and in vivo.36 In addition, IFNγ potently stimulates MHC II expression on ECs and acts synergistically with TNFα. Incubation of freshly isolated human monocytes with supernatants from CD3-activated T cells significantly increased monocyte CD64 cell surface expression by ≈2-fold. Supernatants taken from activated CD4-positive T cells after WY14643 or BRL treatment reduced this increase significantly to 61±8% or 72±4%, respectively (P<0.01 or P<0.05, respectively, compared with monocytes incubated with supernatant from CD3-activated T cells; n=4) (Figure 5A), consistent with reduced cytokine content in the media (data not shown). To exclude the possibility that the results observed resulted from direct effects of residual PPAR agonist in T-cell supernatants, we stimulated human monocytes with conditioned media from CD4-positive cells to induce CD64 expression and then added WY14643 or BRL directly to the cells. None of the PPAR activators used had direct significant effects on monocyte CD64 expression (Figure 5B). Consistent with this finding, PPAR activators did not affect IFNγ-induced CD64 expression in human monocytes (Figure 5C).
Mean fluorescence intensity of MHC II expression in human ECs incubated with supernatants from unstimulated CD4-positive T cells was 10±4 (arbitrary units). Incubation of ECs with supernatants taken from CD3-activated T cells significantly increased MHC II cell surface expression to 51±13 (P<0.05, n=5). Medium conditioned by activated CD4-positive T cells after WY14643 or BRL treatment showed significantly reduced MHC II expression (64±17% or 53±14%, respectively; P<0.05 compared with ECs incubated with supernatant from CD3-activated T cells; n=5) (Figure 6A). Neither WY14643 nor BRL directly affected T-cell media– or IFNγ-induced endothelial MHC II expression (Figures 6B and 6C).
Discussion
The present study reports PPARα and PPARγ expression in human CD4-positive T cells with evidence of inhibition of inflammatory cytokine production by PPARα-activating fibric acid derivatives or PPARγ-activating TZDs in these cells. These results have potential physiological significance, given our finding that monocytes and ECs demonstrate reduced responses toward the proinflammatory effects of activated T cells treated with these same PPAR activators.
Although PPAR expression was initially considered to be restricted to tissues like liver and fat, recent work has demonstrated PPARα and PPARγ expression in vascular cells, such as monocytes/macrophages, ECs, and smooth muscle cells.23 Recent studies also documented PPARγ expression in murine and human T lymphocytes.29,30 Previous work has not addressed PPARα expression by lymphocytes. The decrease in IFNγ expression described in the present study likely occurred through the activation of PPARα and PPARγ by their respective agonists, given that such concentrations are similar to those found in the plasma of patients treated with these agonists.37 However, the results shown in the present study do not conclusively establish that the effects were due to specific receptor activation. Interestingly, recent work has revealed that some effects of TZDs could occur independent of the presence of PPARγ, at least in cells of the monocytic lineage.38 Although monocyte/macrophage responses differ in substantive ways from T-cell responses, particularly in terms of cytokine induction, the intriguing possibility that some of the effects observed in the present study might be PPARγ independent cannot be excluded. Regardless, these data reveal novel effects of antidiabetic TZDs on T lymphocytes and their interaction with vascular cells, with potential clinical relevance for patients. Interestingly, pioglitazone, despite a lower binding affinity to PPARγ, was more potent than BRL in inhibiting IFNγ protein production. Our results with PPARα agonists suggest that this might be due to a combined PPARγ and PPARγ effect of pioglitazone, given that this agent (in contrast to BRL) can also activate PPARα.39 The lack of a difference between pioglitazone and BRL on mRNA expression and the mild suppression of cytokine mRNA compared with protein levels suggest that posttranscriptional modification may also play a role. In this regard, recent work has shown that TZDs inhibit the initiation of translation independent of PPARγ,40 and similar mechanisms may be at work in our findings.
The effects of PPARα and PPARγ activators on human T cells extend to inhibition of other proinflammatory cytokines, including TNFα and IL-2, implicating PPARs as a potential nodal point for the regulation of T-cell–modulated inflammatory responses. In addition, the results obtained do not derive from a shift of T cells toward a TH2 response, because none of the PPAR activators used increased the levels of IL-4, a classic TH2 cytokine, in CD4-positive T cells.
Prior reports demonstrating the effects of PPARγ agonists on lymphocytes varied from ours in design and results in important ways. These studies used PPAR agonists at higher concentrations (TZDs at 20 to 40 μ mol/L), which are thought unlikely to prevail in vivo, or the studies used T-cell lines rather than primary isolates.30 Clark et al30 found reduced IL-2 secretion from murine T-cell clones after treatment with the PPARγ activator ciglitazone (20 to 40 μ mol/L) and the putative PPARγ agonist, but they did not examine the effect on IFNγ and TNFα. These higher concentrations raise the potential for pleiotropic effects, toxicity, and increased cross-reaction with other nuclear receptors. Yang et al29 showed that the PPARγ activators troglitazone and 15d-PGJ2 decrease IL-2 production in human T cells, whereas the PPARα activator WY14643 had no effects on phytohemagglutinin/ PMA-induced IL-2 release. Beyond issues specific to each agonist, eg, the potential antioxidant properties of troglita-zone or the low concentrations used for WY14643, relevant experimental differences include the use of mixed T-lymphocyte populations as opposed to selected CD4-positive cells, the nature of the stimuli used to induce IL-2 expression, and the differing protocols for the addition of agonists (concurrent addition versus pretreatment). Harris and Phipps41 recently found PPARγ expression in a transgenic lymphocyte mouse cell line (D011.10) and induction of apoptosis by troglitazone and 15d-PGJ2 at high concentrations (10 to 100 μ mol/L).
We find that stimulation of isolated CD4-positive human T cells, when stimulated with canonical PPARα and PPARγ agonists at clinically relevant concentrations, demonstrates decreased IFNγ, TNFα, and IL-2 production, with no effect on viability. Such findings likely have relevance to the function of T lymphocytes in atherosclerosis and Tx-AA. In human atheroma, activated CD4-positive T cells release inflammatory cytokines such as IFNγ, TNFα, and IL-2, presumably promoting lesion progression through the activation of other vascular cells in a paracrine fashion.4 In ECs, these T-cell–derived cytokines induce the expression of leukocyte-recruiting chemokines, such as monocyte chemoattractant protein-1 or interferon-inducible protein of 10 kDa,42 and the expression of adhesion molecules. Such actions may contribute to an ongoing cycle of inflammatory cell recruitment, attachment, and migration into the vessel wall, along with further cellular activation. Similar inflammatory effects contribute to Tx-AA, a condition in which IFNγ-induced MHC class II expression on the surface of donor ECs triggers host T-cell activation.10 A reduction of IFNγ release with inhibition of endothelial MHC class II expression, as shown in the present study, raises the possibility that PPAR agonists might modulate allograft vasculopathy.
In monocytes/macrophages, IFNγ stimulates the secretion of cytokines,6 whereas in smooth muscle cells, IFNγ inhibits proliferation and extracellular matrix synthesis.5 This mechanism might destabilize the protective fibrous cap of the lesion and, thus, contribute to plaque rupture with its sequelae, such as unstable angina or acute myocardial infarction. Interestingly, patients with unstable angina show increased IFNγ production by CD4-positive cells,8 bolstering the hypothesis that T-cell activation contributes to the acute coronary syndromes. In contrast, Tx-AA is characterized by smooth muscle cell proliferation, which is thought to be driven in part by cytokine and cytokine-induced growth factors. PPARγ agonists may oppose this response. The antiinflammatory effects of PPAR agonists on T lymphocytes presented in the present study or their reported effects on other gene targets in mononuclear or vascular wall cells might contribute to decreased cardiovascular events or Tx-AA in patients. Although it remains impossible to establish that the clinical effects of these agents occur through PPAR activation, noteworthy recent clinical trials of fibrates have shown decreases in atherosclerosis27 and cardiovascular events.43 PPARγ agonists have shown benefits in surrogate cardiovascular end points, such as carotid intimal-medial thickness and restenosis in humans.44 With increasing evidence of inflammatory pathways not only in atherosclerosis but also in the development of diabetes itself,45 the results reported in the present study suggest that PPAR modulation of inflammatory pathways in T cells may offer clinical benefits in pathological processes, such as atherosclerosis and TX-AA, and is certainly worthy of study in future clinical trials with PPAR agonists.
Acknowledgments
This work was supported by grants of the Else-Kröner-Fresenius-Stiftung and the Deutsche Forschungsgemeinschaft (MA 2047/2-1 and MA 2047/2-2) to Dr Marx, the American Diabetes Association Research Award to Dr Plutzky, and the National Heart, Lung, and Blood Institute (HL34636 and HL43364) to Dr Libby. We thank Dr R. Mitchell for critical reading of the manuscript and Helga Bach for excellent technical assistance.
References
- 1.Hansson GK, Jonasson L, Lojsthed B, Stemme S, Kocher O, Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988;72:135–141. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 3.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Frostegard J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 5.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon γ inhibits both proliferation and expression of differentiation-specific α-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. doi: 10.1161/01.cir.91.11.2844. [DOI] [PubMed] [Google Scholar]
- 7.Serneri GG, Prisco D, Martini F, Gori AM, Brunelli T, Poggesi L, Rostagno C, Gensini GF, Abbate R. Acute T-cell activation is detectable in unstable angina. Circulation. 1997;95:1806–1812. doi: 10.1161/01.cir.95.7.1806. [DOI] [PubMed] [Google Scholar]
- 8.Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, Weyand CM. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Pablo AM, Jiang X, Wang N, Tall AR, Schindler C. IFN-γ potentiates atherosclerosis in apoE knock-out mice. J Clin Invest. 1997;99:2752–2761. doi: 10.1172/JCI119465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomon RN, Hughes CC, Schoen FJ, Payne DD, Pober JS, Libby P. Human coronary transplantation-associated arteriosclerosis: evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991;138:791–798. [PMC free article] [PubMed] [Google Scholar]
- 11.Nagano H, Mitchell RN, Taylor MK, Hasegawa S, Tilney NL, Libby P. Interferon-γ deficiency prevents coronary arteriosclerosis but not myocardial rejection in transplanted mouse hearts. J Clin Invest. 1997;100:550–557. doi: 10.1172/JCI119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 13.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 14.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARγ: differentiation-dependent peroxisomal proliferator-activated receptor γ (PPARγ) expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marx N, Schönbeck U, Lazar MA, Libby P, Plutzky J. Peroxisome proliferator-activated receptor γ activators inhibit gene expression and migration in human vascular smooth muscle cells. Circ Res. 1998;83:1097–1103. doi: 10.1161/01.res.83.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marx N, Sukhova G, Collins T, Libby P, Plutzky J. PPARα activators inhibit cytokine-induced vascular cell adhesion molecule 1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staels B, Koenig W, Habib A, Merval R, Lebret M, Torra IP, Delerive P, Fadel A, Chinetti G, Fruchart JC, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 18.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol. 1997;8:159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for PPAR γ. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 21.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 22.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 23.Marx N, Libby P, Plutzky J. Peroxisome proliferator-activated receptors (PPARs) and their role in the vessel wall: possible mediators of cardiovascular risk? J Cardiovasc Risk. 2001;8:203–210. doi: 10.1177/174182670100800404. [DOI] [PubMed] [Google Scholar]
- 24.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor γ ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Ishibashi S, Perrey S, Osuga J, Gotoda T, Kitamine T, Tamura Y, Okazaki H, Yahagi N, Iizuka Y, Shionoiri F, Ohashi K, Harada K, Shimano H, Nagai R, Yamada N. Troglitazone inhibits atherosclerosis in apolipoprotein E-knock-out mice: pleiotropic effects on CD36 expression and HDL. Arterioscler Thromb Vasc Biol. 2001;21:372–377. doi: 10.1161/01.atv.21.3.372. [DOI] [PubMed] [Google Scholar]
- 26.Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83:1818–1820. doi: 10.1210/jcem.83.5.4932. [DOI] [PubMed] [Google Scholar]
- 27.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 28.Madej A, Okopien B, Kowalski J, Zielinski M, Wysocki J, Szygula B, Kalina Z, Herman ZS. Effects of fenofibrate on plasma cytokine concentrations in patients with atherosclerosis and hyperlipoproteinemia IIb. Int J Clin Pharmacol Ther. 1998;36:345–349. [PubMed] [Google Scholar]
- 29.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by PPARγ agonists: PPARγ co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 30.Clark RB, Bishop Bailey D, Estrada Hernandez T, Hla T, Puddington L, Padula SJ. The nuclear receptor PPAR γ and immunoregulation: PPAR γ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1364–1371. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 31.Marx N, Mackman N, Schönbeck U, Yilmaz N, Hombach V, Libby P, Plutzky J. PPARα activators inhibit tissue factor expression and activity in human monocytes. Circulation. 2001;103:213–219. doi: 10.1161/01.cir.103.2.213. [DOI] [PubMed] [Google Scholar]
- 32.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 33.Penix L, Weaver WM, Pang Y, Young HA, Wilson CB. Two essential regulatory elements in the human interferon γ promoter confer activation specific expression in T cells. J Exp Med. 1993;178:1483–1496. doi: 10.1084/jem.178.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada Y, Watanabe S, Yssel H, Arai K. Factors affecting the cytokine production of human T cells stimulated by different modes of activation. J Allergy Clin Immunol. 1996;98:S161–S173. doi: 10.1016/s0091-6749(96)70063-5. [DOI] [PubMed] [Google Scholar]
- 35.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schomig A. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 36.Liuzzo G, Vallejo AB, Kopecky SL, Frye RL, Holmes DR, Goronzy JJ, Weyand CM. Molecular fingerprints of interferon-γ signaling in unstable angina. Circulation. 2001;103:1509–1514. doi: 10.1161/01.cir.103.11.1509. [DOI] [PubMed] [Google Scholar]
- 37.Weil A, Caldwell J, Strolin-Benedetti M. The metabolism and disposition of 14C-fenofibrate in human volunteers. Drug Metab Dispos Biol Fate Chem. 1990;18:115–120. [PubMed] [Google Scholar]
- 38.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 39.Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, Sawada H. Activation of human PPAR subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 40.Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213–6218. [PubMed] [Google Scholar]
- 41.Harris SG, Phipps RP. PPAR-γ activation in naive mouse T cells induces cell death. Ann N Y Acad Sci. 2000;905:297–300. doi: 10.1111/j.1749-6632.2000.tb06565.x. [DOI] [PubMed] [Google Scholar]
- 42.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 43.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G, Wilt TJ, Wittes J. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol: Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 44.Murakami T, Mizuno S, Ohsato K, Moriuchi I, Arai Y, Nio Y, Kaku B, Takahash Y, Ohnaka M. Effects of troglitazone on frequency of coronary vasospastic-induced angina pectoris in patients with diabetes mellitus. Am J Cardiol. 1999;84:92–94. doi: 10.1016/s0002-9149(99)00199-x. [DOI] [PubMed] [Google Scholar]
- 45.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]