Abstract
Migration of vascular smooth muscle cells (VSMCs) plays an important role in atherogenesis and restenosis after arterial interventions. The expression of matrix metalloproteinases (MMPs), particularly MMP-9, contributes to VSMC migration. This process requires degradation of basal laminae and other components of the arterial extracellular matrix. Peroxisome proliferator-activated receptors (PPARs), members of the nuclear receptor family, regulate gene expression after activation by various ligands. Recent studies have suggested opposing effects of PPAR gamma (PPARγ) activation on atherogenesis. The present study tested the hypotheses that human VSMCs express PPAR alpha (PPARα) and PPARγ and that PPAR agonists in VSMCs modulate MMP-9 expression and activity, as well as VSMC migration. Human VSMCs expressed PPARα and PPARγ mRNA and protein. Treatment of VSMCs with the PPARγ ligands troglitazone and the naturally occurring 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) decreased phorbol 12-myristate 13-acetate–induced MMP-9 mRNA and protein levels, as well as MMP-9 gelatinolytic activity in the supernatants in a concentration-dependent manner. Six different PPARα activators lacked such effects. Addition of prostaglandin F2α, known to limit PPARγ activity, diminished the MMP-9 inhibition seen with either troglitazone or 15d-PGJ2, further implicating PPARγ in these effects. Finally, troglitazone and 15d-PGJ2 inhibited the platelet-derived growth factor-BB–induced migration of VSMCs in vitro in a concentration-dependent manner. PPARγ activation may regulate VSMC migration and expression and activity of MMP-9. Thus, PPARγ activation in VSMCs, via the antidiabetic agent troglitazone or naturally occurring ligands, may act to counterbalance other potentially proathero-sclerotic PPARγ effects.
Keywords: gene expression, vascular smooth muscle cell, migration, peroxisome proliferator-activated receptor gamma, troglitazone
Vascular smooth muscle cell (VSMC) migration likely contributes to both atherogenesis and restenosis, complicating interventional treatments of atherosclerosis. Migration of VSMCs into and within the intima requires the degradation of basal laminae and interstitial extracellular matrix, processes that likely involve matrix metalloproteinases (MMPs).1 Among these, MMP-9, also known as gelatinase B, appears particularly important in migration of VSMCs after arterial injury. In situ hybridization demonstrated induction of MMP-9 mRNA in VSMCs within the first days after balloon injury in rat carotid arteries.2 Furthermore, systemic administration of an MMP inhibitor reduced early migration into the intima by 97%.2 Overexpression of MMP-9 occurs in vulnerable regions of human atheroma3 and in atherectomy specimens retrieved from humans with unstable coronary syndromes.4 Several mediators present in human atheroma, such as interleukin (IL)-1,5 tumor necrosis factor-α, and CD40 ligand6 can induce MMP-9 expression in VSMCs. The complexity of governing the balance of MMP activity is underscored by the presence of a family of inhibitors of MMP activity, proteins known as tissue inhibitors of MMP (TIMPs). However, regulatory signals directly inhibiting MMP-9 gene expression in VSMCs remain poorly understood.
Troglitazone, a new antidiabetic agent, reduces arterial injury-induced intimal hyperplasia, as well as migration and proliferation of rat VSMCs.7 Troglitazone acts as a ligand for peroxisome proliferator-activated receptor gamma (PPARγ),8 one of three members (alpha, delta, and gamma) of a family of ligand-activated nuclear receptor transcription factors. With ligand binding, PPARs form heterodimers with the retinoic X receptor and bind to PPAR response elements in the promoter region of specific target genes, thus regulating their expression.9–12 PPARα appears to interact with various fatty acids and eicosanoid derivatives, whereas PPARγ ligands, in addition to troglitazone, include the naturally occurring prostaglandin D2 metabolite, 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2) 13,14
Macrophages in human atheroma express PPARγ.15,15a In vitro studies have revealed that PPARγ activation inhibits expression of proinflammatory genes as well as macrophage scavenger receptor A and inducible nitric oxide synthetase genes in cells of the monocyte lineage.16,17 PPARγ activation also decreases MMP-9 expression and gelatinolytic activity in these same cells.15 In contrast, other recent studies implicate PPARγ activation in promoting atherogenesis. Tontonoz et al18 and Nagy et al19 report that the scavenger receptor CD36 is a PPARγ-regulated gene, that oxidized LDL is a naturally occurring PPARγ ligand, and that PPARγ activation contributes to monocyte differentiation into foam cells. Given the role of VSMCs in atherosclerosis and restenosis, we hypothesized that PPARγ effects in VSMCs might oppose potentially proatherogenic actions induced by PPARγ activation in monocyte/macrophages. Specifically, we tested whether VSMCs express PPAR and if so, whether PPAR activation in these cells inhibits MMP-9 expression and activity. Furthermore, we investigated if PPAR ligands might inhibit human VSMC migration.
Materials and Methods
Cell Culture
Human VSMCs were isolated from the tunica media human saphenous veins and cultured in DMEM (BioWhittaker, Walkersville, Md) with 1% glutamine (Sigma Chemical Co, St. Louis, Mo), 1% penicillin-streptomycin (Sigma), and 10% FCS. The cells were identified by their typical growth pattern and by immunofluorescence with anti–α-actin monoclonal antibodies (>99% positive cells). These experiments used cells at passages two to five. Cells were cultured in serum-free medium supplemented with insulin and transferrin (IT-medium) for 24 hours and then stimulated with phorbol 12-myristate 13-acetate (PMA) (50 mg/L) in the presence or absence of different activators of PPARα (docosahexanoic acid, eicosapentaenoic acid, fenofibrate, gemfibrozil, clofibrate [all from Sigma], and WY 14643 [Biomol, Plymouth Meeting, Pa]), or of PPARγ (troglitazone [provided by Parke-Davis, Ann Arbor, Mich] and 15d-PGJ2 [Calbiochem, La Jolla, Calif]). Viability of the cells after stimulation was >95% as tested by trypan blue staining and a lactate dehydrogenase assay on cell supernatants (Sigma).
RNA Extraction and Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR)
Total RNA from VSMCs was isolated by the single-step guanidinium thiocyanate-phenol-chloroform method using RNAzol (Tel-Test, Friendswood, Tex). Two micrograms of total RNA was reverse-transcribed into cDNA with 1U/mL reverse transcriptase (Superscript, Gibco-BRL, Gaithersburg, Md) at 37°C for 1 hour in standard buffer. For the amplification of PPARα and PPARγ cDNA, the following oligonucleotide primers were designed: for PPARα from nucleotide +200 to +476 (a 276-bp fragment): sense primer 5′-AGATTTCGCAATCCATCGGC-3′; antisense primer 5′-GCGTGGACTCCGTAATGATA-3′; for PPARγ from nucleotide +235 to +708 (a 473-bp fragment): sense primer 5′-TCTCTCCGTAATGGAAGACC-3′; antisense primer 5′-CCCCTACAGAGTATTACG-3′. For the amplification of GAPDH cDNA, 2 oligonucleotide primers were used (a 452-bp fragment): sense primer 5′-ACCACAGTCCATGCCATCAC-3′; antisense primer 5′-TCCACCACCCTGTTGCTGTA-3′. The PCR reaction was carried out in a standard buffer (Gibco-BRL) with 200 ng of each primer (IDT, Coralville, Iowa), 33 mmol/L MgCl2, and 0.5 U Taq polymerase (Gibco-BRL) for 30 cycles. PCR products (10 mL/25 mL) were analyzed on a 2% agarose gel.
Northern Blot Analysis
Total RNA (5 μg) was used for standard Northern blotting. After electrophoresis, RNA was transferred to a nylon membrane (ICN, Irvine, Calif) in 20× SSC by using capillary blotting overnight. Blots were UV-cross-linked, prehybridized (50% formamide, 5× Denhardt's solution, 5× SSC, 0.5% SDS, and 20 mmol/L salmon sperm DNA), and hybridized in the same buffer with a radiolabeled (α-32P dCTP) probe for human MMP-9. The probe was generated by RT-PCR from RNA of PMA-treated U937 cells using sense primer 5′-GGCGCTCATGTACCCTATGT-3′and antisense primer 5′-TCAAAGACCGAGTCCAGCTT-3′ (a 468-bp fragment) (IDT). The membranes were washed at 60°C in 1% SDS/2× SSC and autoradiographed with Kodak X-OMAT film at −70°C with an intensifying screen.
Preparation of Nuclear and Cytosolic Extracts and Western Blot Analysis
For Western blot analysis, positive controls were generated by transiently transfecting a PPARα (pCMX-PPARα) or PPARγ expression construct (pCMX-PPARγ)20 (both generous gifts from Dr Bruce Spiegelman, Dana Farber Cancer Institute, Boston, Mass) into human skin fibroblasts using lipofectamine (Gibco-BRL) according to the manufacturer's protocol. Nuclear and cytosolic extracts of VSMCs were prepared separately. Cells were lysed in 10 mmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.5% NP-40. Nuclei were pelleted at 13 000g for 5 minutes, and the resulting supernatant was used as the cytosol fraction. Nuclei were lysed in 20 mmol/L HEPES (pH 7.9), 1.5 mmol/L MgCl2, 420 mmol/L NaCl, and 0.2 mmol/L EDTA. After centrifugation at 13 000g for 5 minutes, the supernatant was diluted in equal volume of 20 mmol/L HEPES (pH 7.9), 100 mmol/L KCl, 0.2 mmol/L EDTA, and 20% glycerol and used as nuclear extract. Protein concentration of nuclear and cytosolic extracts was determined colorimetrically (Pierce, Rockford, Ill). Processed samples were applied to 10% SDS gels and transferred to nitrocellulose membranes (Millipore, Bedford, Mass) using semidry blotting, as described previously.6 Membranes were blocked overnight in TBSTween with 5% nonfat dry milk and incubated with goat anti-human PPARα or goat anti-human PPARγ antibodies (mAbs) (Santa Cruz, San Diego, Calif) for 1 hour. After washing, membranes were stained with horseradish peroxidase–conjugated rabbit anti-goat mAbs. Antigen detection was performed with a chemiluminescent detection system (NEN, Boston, Mass). Similar methods were used to perform Western blot analysis on MMP-9 or MMP-2 in VSMC supernatants using the respective anti-human mAbs (Oncogene Research, Cambridge, Mass). For the analysis of TIMP-1 and TIMP-2 in VSMC supernatants, we used anti-human TIMP-1 and anti-human TIMP-2 mAbs (Oncogene Research).
Substrate Gel Zymography
Human VSMCs were stimulated for 24 hours with PMA (50 mg/L) in the presence or absence of different PPARα or PPARγ activators. Culture supernatants were concentrated (10×), and the gelatinolytic activity of secreted MMP-9 was analyzed by zymography on gelatin-containing polyacrylamide gels.6 After washing in 2.5% Triton X-100, gels were incubated overnight at 37°C in 50 mmol/L Tris-HCl (pH 7.4), containing CaCl2 and 0.05% Brij 35. Gels were stained in 0.1% Brilliant Blue G–Colloidal (Sigma), 10% acetic acid, and 40% methanol for 2 hours and destained in 10% acetic acid and 40% methanol. Proteins having gelatinolytic activity were visualized as clear zones in a blue gel. Densitometric analysis was performed using NIH Image 1.6 software program, and the results were normalized to the band of constitutively expressed MMP-2.
In some experiments, VSMCs were stimulated with PMA, troglitazone, or 15d-PGJ2 and prostaglandin F2α (PGF2α), an agent known to inhibit PPARγ activation.21
Migration Assay
Migration of VSMCs was investigated through the use of a standard in vitro wound assay. VSMCs were grown in 6-well plates to confluence, and after 24 hours of culture in IT-medium, a reusable template was used to create a standard wound (≈30 mm). Cells were then stimulated with platelet-derived growth factor (PDGF)-BB (50 μg/L) in the presence or absence of troglitazone or 15d-PGJ2, and wound closure rates followed. A reference point was created on the bottom of the plate in the field of the wound using direct microscopic visualization. This procedure permitted photographing the identical spot each time. The remaining cell-free area was determined via microphotography performed immediately after injury as well as 6 hours after stimulation. Differences were analyzed using NIH Image 1.6 software program, and the results were expressed as percent of migration compared with cultures stimulated with PDGF lacking any PPARγ activators.
Statistical Analysis
Results of the experimental studies are reported as mean±SEM. Differences were analyzed by paired Student t test. A P value <0.05 in the 2-tailed test was regarded as significant.
Results
Human VSMCs Express PPARα and PPARγ mRNA and Protein
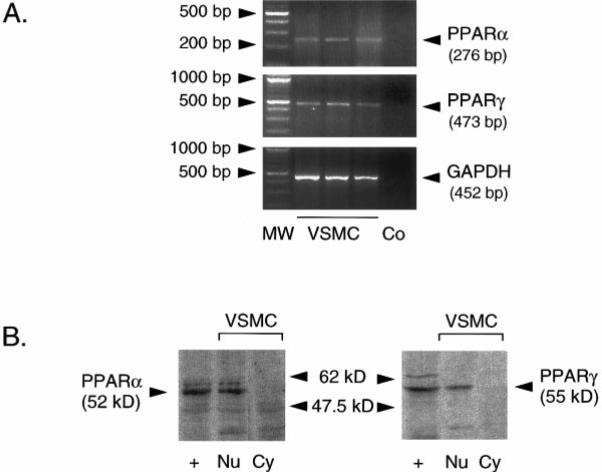
Cultured human VSMCs express PPARα and PPARγ mRNA as detected by RT-PCR products of the predicted size (Figure 1A). Western blot analysis revealed PPARα and PPARγ protein expression in the nuclear fraction, whereas neither protein was detected in the cytosol fraction (Figure 1B). The identity of the bands was confirmed by its apparent molecular weight and its comigration with the signal from nuclei of PPARα- or PPARγ-transfected fibroblasts. Nuclei from untransfected fibroblasts exhibit no similar signal (data not shown).
Figure 1.
Human VSMCs express PPARα and PPARγ mRNA and protein. A, RT-PCR of total RNA from 3 different VSMC preparations with PPARα-, PPARγ-, and GAPDH-specific primers. A 100-bp ladder (MW) and the control consisting of RT-PCR reactions lacking reverse transcriptase (Co) are also shown. B, Western blot analysis of PPARα and PPARγ protein expression in nuclear extracts (Nu) of human VSMCs reveals immunore-active protein of the appropriate size. Neither PPARα nor PPARγ is detected in the cytosolic fraction (Cy). Immunoreactive proteins comigrate with a band observed with nuclear extracts of PPARα- or PPARγ-transfected human skin fibro-blasts (+). Three independent experiments showed similar results.
PPARγ but not PPAR α Activators Decrease Both Secreted MMP-9 Protein Levels and Gelatinolytic Activity in VSMCs
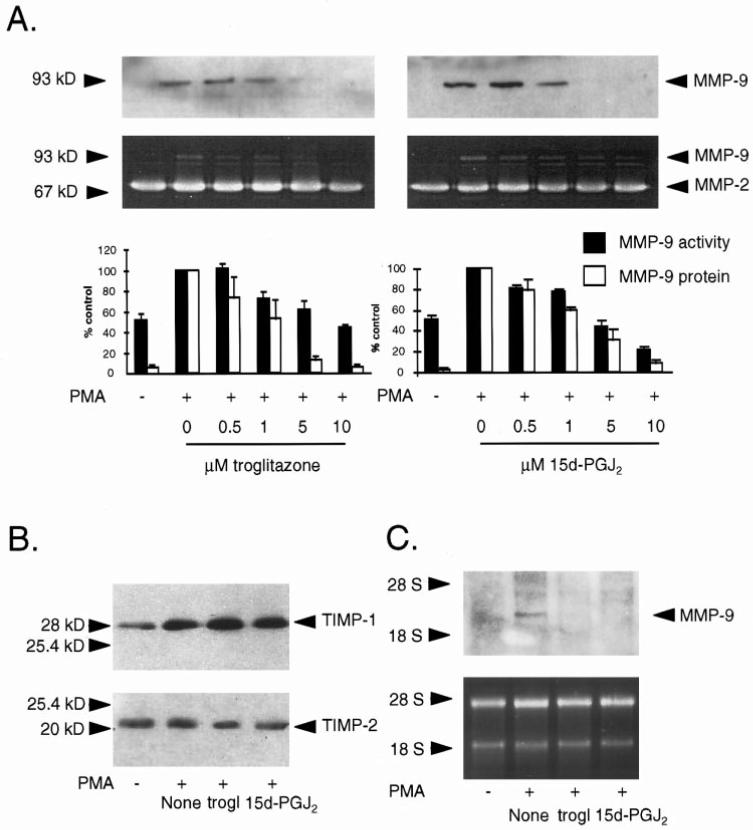
To investigate whether PPAR activation in VSMCs regulates MMP-9 protein expression and gelatinolytic activity, we stimulated human VSMCs with PMA in the presence or absence of PPARα or PPARγ activators and performed Western blot analysis as well as gelatin substrate zymography on supernatants. As previously reported, quiescent VSMCs secreted MMP-9 at very low levels,6 and stimulation with PMA markedly augmented MMP-9 protein levels in the supernatant. None of the PPARα activators used affected MMP-9 protein expression or gelatinolytic activity (Figure 2A). In contrast, treatment of PMA-stimulated VSMCs with the PPARγ activators troglitazone or 15d-PGJ2 decreased MMP-9 protein levels in a concentration-dependent manner (Figure 3A, upper panels). Moreover, gelatin zymography of the supernatants from VSMCs so treated revealed a decrease of MMP-9 gelatinolytic activity (Figure 3A, middle panels), with a maximal reduction to 44±3% at 10 μmol/L troglita-zone (P<0.01; n=3) and 21±3% at 10 μmol/L 15d-PGJ2 (P<0.01; n=3) compared with PMA-stimulated VSMCs (Figure 3A, lower panels).
Figure 2.
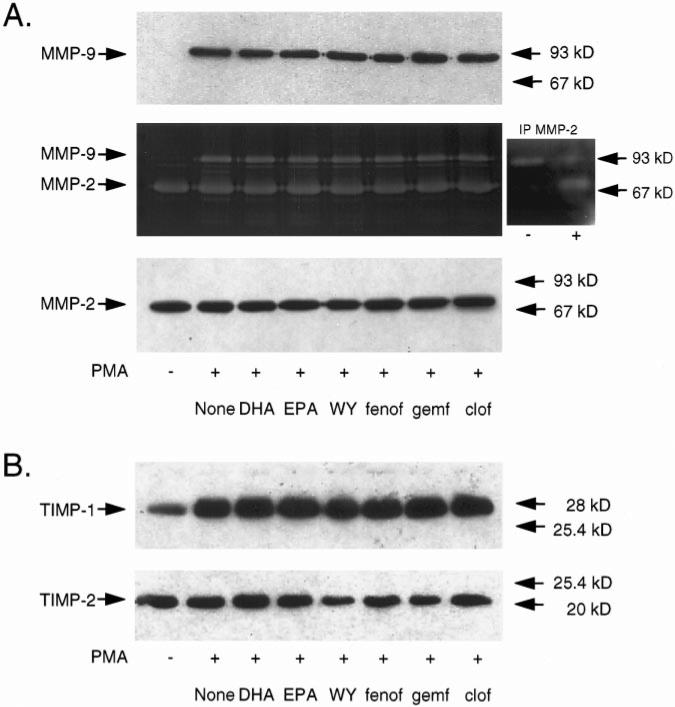
A, PPARα activators do not affect PMA-induced MMP-9 expression and gelatinolytic activity in VSMCs. VSMCs were cultured for 24 hours in serum-free medium and then stimulated with PMA (50 mg/L) in the presence or absence of 10 μmol/L docosahexanoic acid (DHA), 10 μmol/L eicosapentaenoic acid (EPA), 100 μmol/L WY 14643 (WY), 100 μmol/L fenofibrate (fenof), 100 μmol/L gemfibrozil (gemf), or 100 μmol/L clofibrate (clof). Culture supernatants were collected after 24 hours and used for MMP-9 Western blot analysis (upper panel) or gelatin zymography (middle panel). Western blot analysis of MMP-2 expression is also shown (lower panel). The identity of the 72-kDa band as MMP-2 in the zymography was confirmed by depletion of MMP-2 from VSMC supernatant with anti–MMP-2 mAbs (insert: −), whereas the pellet continued to show MMP-2 at 72 kDa (insert: +). B, PPARα activators do not affect TIMP-1 or TIMP-2 expression in VSMCs. Cells were treated as described above, and Western blot analysis for TIMP-1 and TIMP-2 in VSMC supernatants was performed.
Figure 3.
PPARγ activators troglitazone and 15d-PGJ2 inhibit MMP-9 expression and gelatinolytic activity in human VSMCs in a concentration-dependent manner. A, Western blot analysis (upper panels) and gelatin zymography (middle panels) on supernatants from human VSMCs after treatment with PMA (50 mg/L) in the presence or absence of troglitazone (trogl) or 15d-PGJ2 at different concentrations. Lower panel, Densitometric analysis of the MMP-9 gelatinolytic activity in zymograms (solid bars) or MMP-9 protein levels in Western blots (open bars) are shown as percent of PMA-stimulated cells. Results are presented as mean±SEM (n=3). B, PPARγ activators troglitazone (trogl) or 15d-PGJ2 (both 10 μmol/L) do not affect the expression of TIMP-1 and TIMP-2 in VSMC supernatants as shown by Western blot analysis. C, Northern blot analysis (upper panel) of total RNA of human VSMCs treated with PMA (50 mg/L) in the presence or absence of troglitazone (trogl) or 15d-PGJ2 (both 10 μmol/L). Ethidium bromide staining of the gel shows similar loading in all lanes (lower panel). Similar results were seen in 3 independent experiments.
Neither PPARα activators nor PPARγ agonists affected the constitutively expressed 72-kDa gelatinase (MMP-2), as shown by zymography (Figures 2A and 3) and Western blot (Figure 2A, lower panel) analysis. To confirm the identity of the lytic band at 72 kDa in the zymography as being MMP-2, we performed immunoprecipitation and immunodepletion experiments on supernatants from PMA-treated VSMCs. As shown in the insert in Figure 2A, the band corresponding to MMP-2 could be depleted from the supernatant with anti– MMP-2 antibodies, whereas the pellet continues to show a 72-kDa signal. Treatment of VSMCs with either PPARα or PPARγ activators in the absence of PMA did not change MMP-9 protein levels or gelatinolytic activity in supernatants (data not shown). Determination of lactate dehydrogenase in the supernatants revealed no significant differences between the samples, indicating that the effects observed did not result from cell death (data not shown).
Western blot analysis for TIMP-1 and TIMP-2 on super-natants from unstimulated (data not shown) or PMA- stimulated VSMCs (Figures 2B and 3B) revealed no changes after treatment with PPARα or PPARγ activators.
PPARγ Activation Decreases MMP-9 mRNA in VSMCs
Northern blot analysis of PMA-stimulated VSMCs treated with or without PPARγ activators for 18 hours demonstrated a marked reduction of MMP-9 mRNA (size, 2.3 kb) levels by either troglitazone or 15d-PGJ2 (Figure 3C, upper panel). Ethidium bromide staining of the gels showed equivalent loading in each lane (Figure 3C, lower panel).
Inactivation of PPARγ Through PGF2α Diminishes the Inhibition of MMP-9 Expression by Troglitazone and 15d-PGJ2
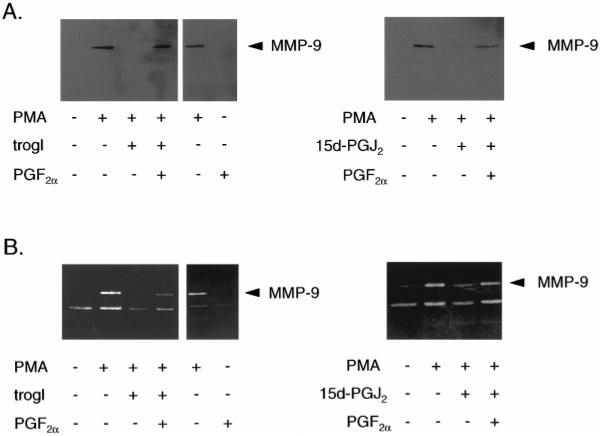
To determine whether PPARγ mediates the effects of troglitazone and 15d-PGJ2, we performed similar experiments in the presence of PGF2α, an agent known to inactivate PPARγ by causing its phosphorylation.21 Addition of PGF2α (200 nmol/L) diminished the inhibitory effect of troglitazone or 15d-PGJ2 on PMA-induced MMP-9 expression (Figure 4A) and gelatinolytic activity (Figure 4B). PGF2α alone had no effect on MMP-9 protein expression or gelatinolytic activity.
Figure 4.
Inhibition of PPARγ through PGF2α diminishes the inhibitory effect of troglitazone and 15d-PGJ2 on MMP-9 expression and gelatinolytic activity in VSMCs. Western blot (A) and gelatin zymography (B) on supernatants of human VSMCs after treatment for 24 hours with PMA (50 mg/L), troglitazone (10 μmol/L) (trogl), or 15d-PGJ2 (10 μmol/L) in the presence or absence of 200 nmol/L/L PGF2α. PGF2α alone had no effect on MMP-9 gelatinolytic activity. Similar results were seen in 3 independent experiments.
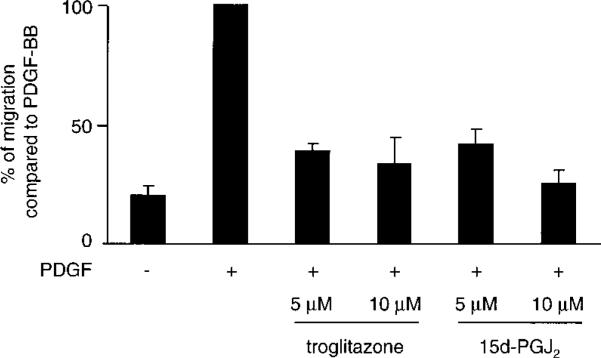
Troglitazone and 15d-PGJ2 Inhibit PDGF-BB–Induced Migration of VSMCs
To explore whether PPARγ activation directly affects PDGFBB–induced VSMC migration, we performed an in vitro wound closure assay. Treatment of human VSMCs with troglitazone significantly decreased the PDGF-BB–induced migration to 39±3% at 5 μmol/L and to 34±12% at 10 μmol/L (P<0.05), respectively (Figure 5). 15d-PGJ2 reduced the migration significantly to 42±7% at 5 μmol/L and to 25±6% at 10 μmol/L 15d-PGJ2 (P<0.05), respectively (Figure 5). Medium with solvent alone had no influence on VSMC migration (data not shown).
Figure 5.
PPARγ activators inhibit the PDGF-BB–induced migration of human VSMCs. Confluent human VSMCs were cultured in serum-free medium for 24 hours, wounded with a cell scraper, and then stimulated with PDGF-BB (50 mg/L) in the presence or absence of troglitazone or 15d-PGJ2 at concentrations indicated. Results (n=3) were expressed as percent of migration compared with cells stimulated with PDGF-BB alone. Results were replicated in 3 experiments.
Discussion
The present study reports expression of both PPARα and PPARγ by human VSMCs. Although PPARα activation had no effect on MMP-9 expression, stimulation of PPARγ inhibited MMP-9 mRNA and protein expression, gelatinolytic activity, and PDGF-BB–induced migration of human VSMCs. Neither PPARα nor PPARγ activators appear to influence TIMP expression.
The absence of an effect of PPARα agonists on MMP-9 expression or enzymatic activity could be explained by either low PPARα levels in these cells or a lack of transcriptional regulation by PPARα on this gene. Our data showing approximately similar levels of PPARα and PPARγ in VSMCs, within the limitations of RT-PCR and Western blotting techniques, argue against low PPARα levels as an explanation for these findings. Furthermore, while this article was in review, Staels et al20 also found that PPARα and PPARγ were present in VSMCs, at a modestly higher level of PPARα than PPARγ under the conditions of their experiments. These investigators also reported that PPARα, but not PPARγ, agonists inhibited the IL-1–induced production of IL-6 and prostaglandin and the expression of the cyclooxygenase-2 gene. As such, although PPARα appears to be functionally active in VSMCs, our work suggests that it is not involved in MMP-9 regulation.
In contrast, PPARγ activators do inhibit MMP-9 expression and gelatinolytic activity in stimulated VSMCs. Our data suggest PPARγ activation as the mechanism for the effects of these activators. Troglitazone is a synthetic compound known to have a high affinity for PPARγ as evidenced by ligand binding assays.8 15d-PGJ2 also has significant binding capacity to PPARγ, although it may also have some activity on other PPAR isoforms.13,22 However, because none of 6 established PPARα activators22,22a demonstrated any effect on MMP-9, PPARα activation by 15d-PGJ2 would seem to be an unlikely explanation for our findings. Furthermore, the ability to reverse the effects of troglitazone and 15d-PGJ2 on MMP-9 by PGF2α costimulation also supports that these agonists are acting through PPARγ; PGF2α has been shown to induce inhibitory phosphorylation of PPARγ by activation of mitogen-activated protein kinase.21
The mechanism through which PPARγ activators inhibit MMP-9 expression is likely to be transcriptional, although posttranscriptional modulation cannot be ruled out. Certainly, nuclear hormone receptors, including PPARs, negatively regulate expression of other genes. The transcriptional suppression of MMP-9 could be due to a negative PPAR-response element, as previously described for the apolipoprotein A-1 gene23 and for thyroid hormone regulation of thyroid-stimulating hormone.24 Alternatively, inhibitory effects might occur independent of a PPAR binding site, through competitive binding and “squelching” of transcriptional coactivators by liganded PPAR. This has been suggested as the mechanism of negative cross talk between other nuclear receptors and activator protein-125 as well as other transcription factors.26,27
The expression of PPARs in VSMCs, and its regulation of MMP-9 expression and activity, and VSMC migration have potentially important implications for atherosclerosis. Matrix degradation by MMPs and medial VSMC movement into the intima occur early during intimal hyperplasia in injured rat arteries.28 Similar processes are very likely to contribute to human atherogenesis. Interestingly, troglitazone inhibits intimal thickening after arterial injury in rats,7 a process that might be influenced by the MMP-9 effects described in the present study.
Beyond the part of PPARγ in VSMC biology is the question of its role in the pathology of the arterial wall. Several lines of evidence would suggest an antiatherosclerotic effect of PPARγ activation: inhibition of MMP-9 in VSMCs and monocyte/macrophages, decreased expression of cytokines and inducible nitric oxide synthetase,16,17 and decreased LDL oxidation in response to troglitazone.29 Furthermore, preliminary findings of decreased intimal and medial carotid thickening in diabetic patients treated with troglita-zone also suggest that PPARγ activation might limit atherogenesis.30
In contrast, Tontonoz et al18 and Nagy et al19 recently reported that oxidized LDL increases scavenger receptor (CD36) expression via direct PPARγ binding and transcriptional activation. They found, as did we, high levels of PPARγ in lesional macrophages, although our data also suggested inhibition of macrophage MMP-9 expression via PPARγ, which might limit atherosclerosis. The evidence to date in monocyte/macrophages might suggest complex regulatory effects of PPARγ, with mediation of macrophage development and oxidized LDL signaling on one hand and decreased MMP-9 and inflammatory cytokine production on the other. Reconciling these findings with the overall benefits seen with troglitazone in the clinical setting31,32 might suggest PPARγ effects in other arterial wall cells that limit the atherogenic response. The data in the present study suggest that PPARγ activation in human VSMCs might provide one such counterbalancing antiatherogenic mechanism.
Acknowledgments
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft to Dr Nikolaus Marx (MA 2047/1-1), the NIH to Dr Mitchell Lazar (P01 DK49210 and RO1 DK49780), and the NIH/NHLBI to Dr Peter Libby (HL48743) and to Dr Jorge Plutzky (HL03107). The Vascular Medicine and Atherosclerosis Unit of Brigham and Women's Hospital has received unrestricted grants from Parke-Davis. Drs Plutzky and Libby are also consultants and speakers for Parke-Davis. We thank Dr Marisia Muszynski (Brigham and Women's Hospital) for her skillful assistance.
References
- 1.Galis Z, Muszynski M, Sukhova G, Simon-Morrissey E, Unemori E, Lark M, Amento E, Libby P. Cytokine-stimulated human vascular smooth muscle cells synthesize a complement of enzymes required for extra-cellular matrix degradation. Circ Res. 1994;75:181–189. doi: 10.1161/01.res.75.1.181. [DOI] [PubMed] [Google Scholar]
- 2.Bendeck M, Zempo N, Clowes A, Galardy R, Reidy M. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 3.Galis Z, Sukhova G, Lark M, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92 kD gelatinase in human coronary atherosclerotic lesions. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 5.Fabunmi RP, Baker AH, Murray EJ, Booth RF, Newby AC. Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors or metalloproteinases-1, -2, and -3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315:335–342. doi: 10.1042/bj3150335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönbeck U, Mach F, Sukhova G, Murphy C, Bonnefoy J-Y, Fabunmi R, Libby P. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes. Circ Res. 1997;81:448–454. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 7.Law R, Meehan W, Xi X, Graf K, Wuthrich D, Coats W, Faxon D, Hsueh W. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897–1905. doi: 10.1172/JCI118991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Qi C, Korenberg J, Chen X, Noya D, Rao M, Reddy J. Structural organization of mouse peroxisome proliferator-activated receptor γ (mPPARγ) gene: alternative promotor use and different splicing yield two mPPARγ isoforms. Proc Natl Acad Sci U S A. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoonjans K, Peinado-Onsurbe J, Lefebvre A, Deeb S, Staels B, Auwerx J. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 11.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr Opin Lipidol. 1997;8:159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Fajas L, Auboeuff D, Raspe E, Schoonjans K, Lefebvre A-M, Saladin R, Najib J, Laville M, Fruchart J-C, Deeb S, Vidal-Puig A, Flier J, Briggs M, Staels B, Vidal H, Auwerx J. The organization, promotor analysis, and expression of the human PPARγ gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 14.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 15.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARγ: differentiation-dependent PPARγ expression and reduction of MMP-9 activity through PPARγ activation in mononuclear phagocytes. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Ricote M, Huang J, Fajas L, Li A, Welch J, Najib J, Witztum JL, Auwerx J, Palinski W, Glass CK. Expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) in human atherosclerosis and regulation in macrophages by colony stimulating factors and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1998;95:7614–7619. doi: 10.1073/pnas.95.13.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 17.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 18.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 19.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 20.Staels B, Koenig W, Habib A, Merval R, Lebret M, Pineda Torra I, Delerive P, Fadel A, Chinetti G, Fruchart J-C, Najib J, Maclouf J, Tedgui A. Activation of human aortic smooth muscle cells is inhibited by PPARα but not by PPARγ activators. Nature. 1998;393:790–793. doi: 10.1038/31701. [DOI] [PubMed] [Google Scholar]
- 21.Reginato MJ, Krakow SL, Bailey ST, Lazar MA. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma. J Biol Chem. 1998;273:1855–1858. doi: 10.1074/jbc.273.4.1855. [DOI] [PubMed] [Google Scholar]
- 22.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with the peroxisome proliferator-activated receptor alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Keller H, Dreyer C, Medin J, Mahfoudi A, Ozato K, Wahli W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc Natl Acad Sci U S A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu-Dac N, Schoonjans K, Laine B, Fruchart J, Auwerx J, Staels B. Negative regulation of the human apolipoprotein A-I promoter by fibrates can be attenuated by the interaction of the peroxisome proliferator-activated receptor with its response element. J Biol Chem. 1994;269:31012–31018. [PubMed] [Google Scholar]
- 24.Rentoumis A, Chatterjee V, Madison L, Datta S, Gallagher G, Degroot L, Jameson J. Negative and positive transcriptional regulation by thyroid hormone receptor isoforms. Mol Endocrinol. 1990;4:1522–1531. doi: 10.1210/mend-4-10-1522. [DOI] [PubMed] [Google Scholar]
- 25.Pfahl M. Nuclear receptor/AP-1 interaction. Endocr Rev. 1993;14:651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz E, Reginato M, Shao D, Krakow S, Lazar M. Retinoic acid blocks adipogenesis by inhibiting C/EBPbeta-mediated transcription. Mol Cell Biol. 1997;17:1552–1561. doi: 10.1128/mcb.17.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S, Heyman R, Rose D, Glass C, Rosenfeld M. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 28.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury, I: smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 29.Cominacini L, Garbin U, Fratta-Pasini A, Campagnola M, Davoli A, Foot E, Sighieri G, Sironi A, Lo Cascio V, Ferranini E. Troglitazone reduces LDL oxidation and lowers plasma E-selectin concentrations in NIDDM patients. Diabetes. 1998;47:130–133. doi: 10.2337/diab.47.1.130. [DOI] [PubMed] [Google Scholar]
- 30.Minamikawa J, Yamauchi M, Innoue D, Koshiyama H. Another potential use of troglitazone in non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:1041–1042. doi: 10.1210/jcem.83.3.4668-1. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz S, Raskin P, Fonseca V, Graveline J. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. N Engl J Med. 1998;338:861–866. doi: 10.1056/NEJM199803263381302. [DOI] [PubMed] [Google Scholar]
- 32.Inzuchi S, Maggs D, Spollet G, Page S, Rife F, Walton V, Shulman G. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]