Abstract
Background and aims
A fraction of gastrointestinal stromal tumors (GIST) overexpress PDGFRA rather than KIT. Presently it is unknown if this reflects a complementary oncogenic potential of PDGFRA and KIT pathways, or heterogeneity in the cellular origin of GIST. Similarly unknown is the significance of activated Hedgehog (HH)/PATCHED1 (PTCH) signaling found in many GIST.
Methods
Mouse Ptch was conditionally inactivated using a Cre recombinase driven by the lysozyme M (LysM) promoter. Lineage tracing was done using R26R-LacZ reporter mice. Tumors were characterized by in situ hybridization, immunohistochemistry, Western blot and qRT-PCR. Cellular transformation was assessed by clonogenic assay.
Results
Inactivation of Ptch in LysM-expressing cells resulted in imatinib-responsive tumors of GIST-like localization and histology. In addition to activation of Hh signaling, the tumors showed overexpression and activation of Pdgfrα, but not of Kit. Lineage tracing revealed that LysM-expressing intestinal cells were Kit-negative. These cells were juxtapposed with Kit-positive interstitial cells of Cajal (ICC) and sometimes co-expressed Pdgfrα. In contrast to KIT, PDGFRA cooperated with HH signaling in cellular transformation.
Conclusions
Mutations in Ptch result in formation of Pdgfrα-positive Kit-negative GIST-like tumors in mice. These tumors may develop due to cooperativity between Hh and Pdgfrα pathways from Kit-negative cells in the intestine.
Keywords: GIST, mouse model, Hedgehog and Pdgfrα signaling
GIST are the most common mesenchymal tumors of the gastrointestinal tract1, with an annual frequency of 10 to 14.5 per million of the population2. They are most frequently found in the stomach or small intestine, but can arise anywhere in the gastrointestinal tract and occasionally in the omentum, peritoneum and mesentery1. Surgery is the standard care for primary disease, whereas the tyrosine kinase inhibitor imatinib is recommended as first-line treatment of unresectable and/or metastatic GISTs3. Since the introduction of imatinib the survival of GIST patients rose from 30% after 1 year to 50% after 5 years4. Nevertheless, primary resistance to imatinib occurs in 12-14% of patients, complete remissions are rare, and the majority of initially responsive patients eventually become resistant, with a median time to progression of 2 years5.
The responsiveness to imatinib reflects the activation of its targets KIT or PDGFRA, two closely related receptor tyrosine kinases with multiple roles in the regulation of cell survival, proliferation, and differentiation. Whereas wildtype KIT is activated by the stem cell factor SCF, PDGFRA can be activated by PDGFA, PDGFB, and PDGFC6, 7. In GIST, these kinases frequently show gain-of function mutations. Approximately 75-80% of GIST show KIT mutations and 5-10 % are driven by mutations in PDGFRA8. Further several percent of GIST are referred to as “wild-type”, as they show neither KIT nor PDGFRA mutations9-11.
Most GIST overexpress KIT protein, which has proven a reliable diagnostic tool to distinguish GIST from other tumors, e.g. leiomyosarcoma (LMS)3. However, lack of KIT protein expression has been reported in 4-6% of GIST. These cases frequently overexpress PDGFRA protein and are more likely to have PDGFRA mutations10, 12. It also has been reported that KIT-mutant, KIT-expressing GIST additionally express low levels of active PDGFRA and that, vice versa, PDGFRA-positive GIST showing PDGFRA mutations express low levels of active KIT13. This indicates that GIST can show activation of both pathways (either mutation- or ligand-dependent), which may replace each other in the course of tumor progression13.
These data brought about a discussion whether GIST subtypes may arise from different cells or from a common precursor. GIST associated with KIT mutations are thought to originate from KIT-expressing ICC14 in the muscularis propria associated with the myenteric plexus1. The origin of PDGFRA-mutant GIST is less clear and could be ascribed to ICC in which KIT expression has been turned down, or to different cells. In support of the ICC origin, Bardsley and colleagues reported PDGFRA-expressing ICC precursors with low KIT levels which differentiate into KIT-positive ICC cells and form subcutaneous tumors in nude mice15. However, unlike GIST, these tumors were resistant to imatinib and their significance to GIST formation in the intestine is unclear. In support of a separate cell of origin, KIT-negative but PDGFRA-positive fibroblast-like cells have been found to be associated with ICC and nerve bundles16-18.
Besides KIT or PDGFRA activation, many GIST display aberrant expression and activity of the HH signaling pathway19, previously implicated in several tumors, including basal cell carcinoma (BCC), the most common malignancy in humans. The most prevalent genetic event in BCC is the inactivation of the HH receptor and tumor suppressor gene PTCH. This results in the transcriptional activation of GLI1 and PTCH itself, which are established markers of abnormal HH signaling20. In BCC, aberrant HH signaling also induces expression and activation of PDGFRA21. As PDGFRA, HH, GLI1 and PTCH are overexpressed in a fraction of human GISTs19. Furthermore, the PTCH locus is lost in 30% - 50% of human GIST22, 23. Together with the observation that HH expression correlates with the grade of GIST risk category and size19, these findings suggest that HH signaling may play a role in the biology of this tumor entity. However, the cooperativity between the HH and PDGFR or KIT pathways in GIST formation has not been investigated.
While conditionally inactivating Ptch in the myeloid lineage of the mouse, we noticed GIST-like tumors developing in the gastrointestinal tract. Besides activation of the Hh pathway, these tumors overexpress Pdgfrα, but not Kit, and both pathways cooperate in cellular transformation. Lineage tracing showed that the Hh/Ptch-associated GIST-like tumors could arise from rare Pdgfrα-positive Kit-negative cells that lie closely apposed to the Kit-positive ICC in the muscular wall of the intestine. Together, these results may indicate a role of the HH pathway in the origin of PDGFRA-positive GIST.
Methods
Animals
All experiments using animals were performed in compliance with all legal and ethical requirements. Ptchflox/flox mice have loxP sites in Ptch introns 7 and 924. LysMcre mice express Cre recombinase under the control of the endogenous LysM-promoter. This Cre driver targets mouse cells of the myeloid lineage, due to their expression of lysozyme M25. R26R-LacZ and R26R-EYFP mice carry a Cre-inducible LacZ and YFP (yellow fluorescent protein) gene, respectively, under the control of the endogenous, ubiquitous Rosa26 promoter26, 27. Ptchflox/flox mice were on a mixed C57BL/6 × Balb/c and all other mice on a C57BL/6 background. Genotyping of Ptchflox/flox, LysMcre and R26R mice was performed as described26, 28, 29. All primers are listed in Supplementary Table 1.
Tumor specimens
The histology of all tumors detected in the gastrointestinal tract of Ptchflox/floxLysMcre+/− mice was investigated using hematoxylin and eosin (H&E)-stained sections. Gene expression was compared to LMS of Pten/p53 double-mutant mice (ref30 and Guijarro and Hernando, unpublished) and human GISTs or LMS. The human tumor samples were from the Department of Pathology at the Leiden University Medical Center. All human specimens were handled and coded according to the respective national ethical guidelines for secondary use of human tissue.
Imatinib treatment of mice
Ptchflox/floxLysMcre+/− mice were randomized into 2 groups. The daily oral treatment with 0.1g/kg Imatinib dissolved in 200 μl PBS was commenced when the mice were 8 weeks old and continued for 10 weeks. Vehicle treated mice served as controls. Following the completion of the treatment all mice were sacrificed and screened for tumors.
Adoptive transfer and repopulation assay
Bone Marrow (BM) was isolated from 8-week-old control and Ptchflox/floxLysMcre+/− mice and 2 × 106 cells were transplanted by tail vein injection into 9 weeks old Rag-2−/−γc−/− irradiated with 7 Gy. Reconstitution of the BM engraftment was analyzed by monitoring peripheral blood 6 weeks after transplantation by flow cytometry using anti-TCRβ-FITC, anti-CD3-PE-Cy5, anti-CD19-FITC and anti-B220-PE-Cy7 antibodies. Rag-2−/−γc−/− mice reconstituted with control or Ptch mutant BM cells were observed for 250 days.
Molecular analysis by quantitative RT-PCR, in situ hybridization, Western blot, Immunofluorescence, immunohistochemistry and LacZ staining were performed by standard procedures described in detail in the Supplementary Material and methods section.
Cellular transformation assay
To test for cooperation of HH/GLI and PDGFRA signaling in oncogenic transformation, 104 human non-tumorigenic HaCaT keratinocytes expressing GLI1 under the control of a doxycycline-inducible promotor31 were seeded as single cells in 0.4% select agar. Cells were either treated with 50ng/mL doxycycline (dox) (Sigma) to induce GLI1 expression, 50ng/mL recombinant PDGF-BB (R&D Systems) or with a combination of dox/PDGF-BB. To assay possible cooperation of HH/GLI with KIT signaling, the same GLI1-expressing HaCaT cells were stably transduced with the constitutively active D816V KIT mutant (GNNK+ splice form)32. Empty-vector transfections served as controls. 3D transformation assays were incubated for 4 weeks at 37°C with 5% CO2. Anchorage independent clonogenic growth was documented with a Cell^D Image capture system and quantified by automated colony counting using Colony Counter software (Microtec Nition)31.
Results
Gastrointestinal tumors in Ptchflox/floxLysMcre+/− mice
In a project intended to delete the Hh receptor Ptch in myeloid cells, we bred Ptchflox/flox mice to LysMcre+/− mice. The resulting Ptchflox/+LysMcre+/− mice were monitored for at least 398 days after birth and remained healthy without any macroscopic abnormalities (Table 1). Ptchflox/floxLysMcre+/− mice were obtained at the expected Mendelian ratio by backcrossing Ptchflox/+LysMcre+/− to Ptchflox/flox mice. The Ptch deletion efficiencies in cells of the myeloid lineage were 20% in Thy1.2+ cells, 18% in CD11c+ cells, 65% in CD11d+ cells, 52% in whole BM and 85% in peritoneal macrophages, i.e. similar to those reported for LysMcre-mediated recombination of other floxed genes25, 33. These deletions affected neither the peripheral blood nor the BM of Ptchflox/floxLysMcre+/− mice, as indicated by normal cell counts and cell morphology (data not shown).
Table 1. Absolute numbers and percentages of symptomatic and symptom-free animals used in this study.
| genotype | n | Age (days) range1; median |
manipulation | mice with GIST- like tumors |
mice with other abnormalities |
healthy |
|---|---|---|---|---|---|---|
| LysMcre +/− | 9 | 583-670; 604 | - | - | - | 9 |
| Ptch flox/flox | 17 | 96-524; 324 | - | - | - | 17 |
| Ptchflox/floxLysMcre+/− | 18 | 398-693; 640 | - | - | - | 18 |
| Ptchflox/floxLysMcre+/− | 34 | 116-555; 206 | - | 28 (82%) | 1 | 5 |
| Ptchflox/floxLysMcre+/− | 7 | 127 | 0.1g/kg/d imatinib orally starting at 8 weeks of age for 10 weeks |
7/7; tumor multiplicity2: 2.6±1.3 |
- | - |
| Ptchflox/floxLysMcre+/− | 6 | 127 | vehicle (200 μl PBS) orally starting at 8 weeks of age for 10 weeks |
6/6; tumor multiplicity2: 5.2±1.7 |
- | - |
| Rag−2−/−γc−/− | 5 | 253-323; 323 | transfer of Ptchflox/floxLysMcre+/− bone marrow at 8 weeks of age |
- | - | 5 |
| Rag-2−/− γ c−/− | 5 | 306-323; 323 | transfer of Ptchflox/flox bone marrow at 8 weeks of age |
- | - | 5 |
from birth on
mean ± SD
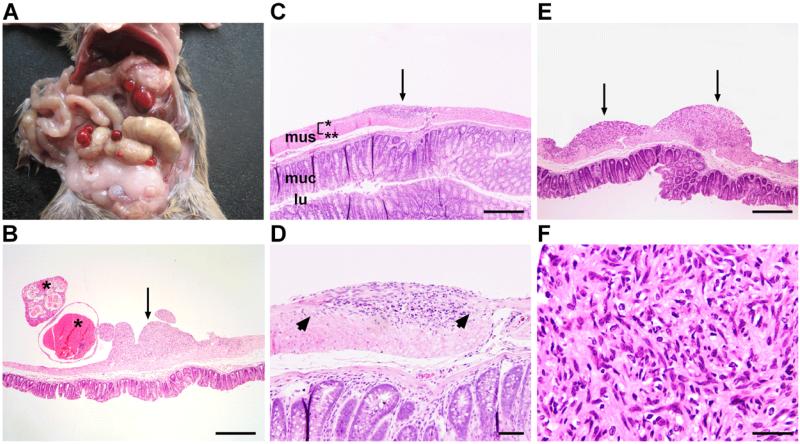
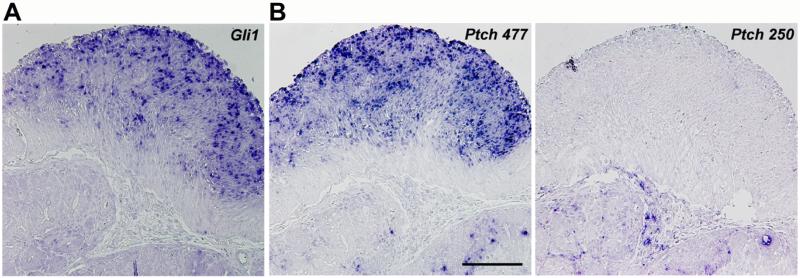
Despite Ptch being a tumor suppressor gene, we detected no malignancies of the hematopoietic system. Instead, at a median age of 206 days, 82% of Ptchflox/floxLysMcre+/− animals presented with - mostly multiple - tumors arising from the wall of the stomach or the intestine (Fig. 1A, 1B; Table 1). Larger tumors frequently displayed blood-filled cysts protruding into the peritoneal cavity (Fig. 1B), whereas smaller, i.e. most likely earlier lesions had a solid appearance (Fig. 1B-F). These lesions were localized between either the circular and longitudinal muscle layer or the longitudinal muscle layer and the serosa. Although the tumors with the former localization were located in close vicinity to the myenteric plexus, the plexus was frequently intact (Fig. 1D). Upon growth (Fig. 1E), the tumors acquired high cellularity, variably spindle-shaped cells, elongated nuclei, and pale eosinophilic cytoplasm (Fig. 1F). As expected and confirmed by in situ hybridization, the tumors overexpressed the Hh pathway target Gli1 when compared to normal intestine (Fig. 2A; Supplementary Figure 1A). They also overexpressed Gli2 and Gli3 (Supplementary Figure 1B), and mutant Ptch transcripts, in which exon 7 is spliced into exon 10 due to the Cre-mediated deletion of exon 8 and 9 of the Ptch gene, but not wt Ptch transcripts (Fig. 2B). This was consistent with an aberrant Hh pathway activation in tumor cells.
Figure 1. Ptch mutant mice develop gastrointestinal tumors.
(A) Gross appearance of tumors of Ptchflox/floxLysMcre+/− mice. (B) Tumors either have a solid (arrow) or cystic appearance (asterisks). The cystic appearance is associated with intratumoral bleeding. (C) Shows a precursor lesion (arrow). mus, muscularis (*: longitudinal muscle layer; **: circular muscle layer); muc, mucosa; lu, lumen of the intestine. (D) Arrows point to the intact myenteric plexus. (E) When precursor lesions become larger (arrows) they adopt a (F) GIST-like histology. Scale bars in μm: (B,C,E) 500; (D) 100; (F) 50.
Figure 2. Activation of Hh signaling in tumors of Ptch mutant mice.
(A) In situ hybridization of the Hh target genes Gli1 and (B) Ptch in tumors of Ptchflox/floxLysMcre+/− mice. The 477 bp Ptch probe identifies both wild type and mutant Ptch transcripts, in which exon 7 is spliced to exon 10 due to Cre-mediated deletion of exon 8 and 9. The 250 bp Ptch probe exclusively binds to wild type Ptch transcripts. The Figure demonstrates that the tumors overexpress exclusively mutant Ptch transcripts, since wild type Ptch transcripts were not detected. Scale bar in μm: 200.
Evidence for a cell of origin of the gastrointestinal tumors of Ptchflox/floxLysMcre+/− mice
The histology, the localization within the gut wall, and the development in the same location as do tumors in mice overexpressing a Kit K641E mutation34 were suggestive of GIST. However, this was inconsistent with the Ptch deletion in the myeloid lineage, as GIST are considered to arise from KIT- or PDGFRA-expressing cells of the smooth muscle layer of the GI tract. To resolve this discrepancy, we investigated if tumors in Ptchflox/floxLysMcre+/− mice were indeed derived from LysM-targeted cells.
To this end, we generated Ptchflox/floxLysMcre+/−R26R-LacZ+/− mice. In these mice, Cre-mediated recombination in LysM-expressing cells not only occurs at the Ptchflox locus, but also at the R26R-LacZ locus. The latter event labels all progeny of LysM-expressing cells by the permanent expression of LacZ. As demonstrated in Figure 3A, LacZ-positive cells localized to the tumor mass, confirming its origin from LysM-expressing cells (Fig. 3A). Secondly, we reconstituted irradiated Rag-2−/−γc−/− mice with BM cells from Ptchflox/floxLysMcre+/− mice. However, this did not result in tumor formation (Table 1). This suggests that GIST-like tumors were not derived from cells of the BM.
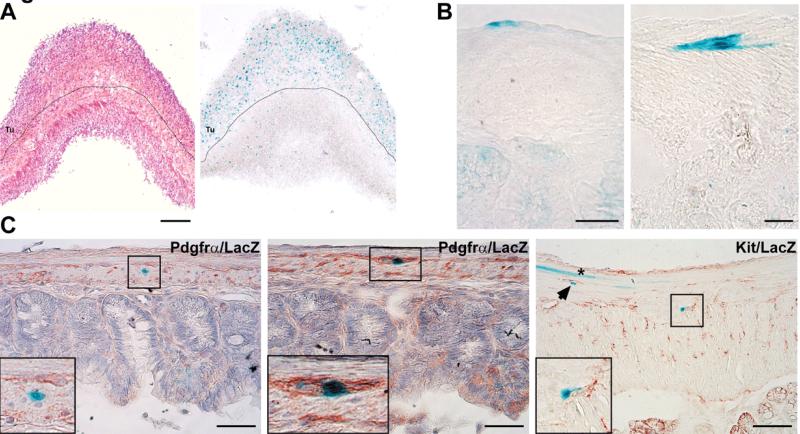
Figure 3. Lineage tracing identifies LysM-expressing cells in tumors of Ptch mutant mice and in the normal intestine, where they may co-express Pdgfra.
(A) The figure shows LacZ expression (blue color) in tumors of Ptchflox/floxLysMcre+/−R26R-LacZ+/− mice. Since LacZ expression in Ptchflox/floxLysMcre+/−R26R-LacZ+/− mice mark LysM-expressing cells, tumors are likely derived from LysM-positive cells. Dotted line marks the tumor (Tu)/normal muscle border. Left: H&E staining, Right: LacZ staining. (B) and (C) show LacZ-staining in normal intestine of LysMcre+/−R26R-LacZ+/− mice. Positive cells are detected between the longitudinal muscle layer and the serosa (B, left panel), the circular and longitudinal muscle layer (B, right panel) and within the muscle layer (C, left and right panels). Sections in (C) were co-stained with antibodies against Pdgfrα (left and middle panel) or Kit (right panel). Inset in the left panel shows a LacZ-positive, but Pdgfrα-negative cell. Inset in the middle panel shows a LacZ/Pdgfrα double-positive cell. Inset in the right panel shows a LacZ-positive cell juxtaposed to a Kit-positive cell (a similar cell is marked by an arrowhead). Please also note the LacZ-positive muscle fiber (asterisk, right panel) not juxtaposed to Kit-positive cells. Scale bars in μm: (A) 500; (B) 25; (C, left and middle panel) 50; (C, right panel) 25.
To investigate if LysM-expressing cells are present in the muscle layer of the normal intestine, we analyzed the expression of LacZ in the intestine of LysMcre+/−R26R-LacZ+/− mice. Specific LacZ staining was detected in rare cells localized between the longitudinal muscle layer and the serosa (Fig. 3B left panel), between the circular and the longitudinal muscle layers (Fig. 3B right panel) and within the muscle layers (Fig. 3C, left and right panel). Taken together, these results indicated that the tumors arose from a discrete population of LysMcre-expressing cells localized within the gut wall.
Both cells of origin and tumors express Pdgfrα, but not Kit
Considering the GIST-like appearance of the tumors and that GIST may originate from a KIT- and/or a PDGFRA-expressing cell of the intestine (see introduction), we investigated the expression status of Kit and Pdgfrα in LacZ-positive cells. The analysis revealed that approximately 20% of LacZ-positive cells in the muscle layer co-expressed Pdgfrα (Fig. 3C, left panel). In contrast, we did not detect any distinct LacZ-positive cells expressing Kit, but found that many LacZ-positive and Kit-positive cells were juxtapposed (Fig. 3C, right panel).
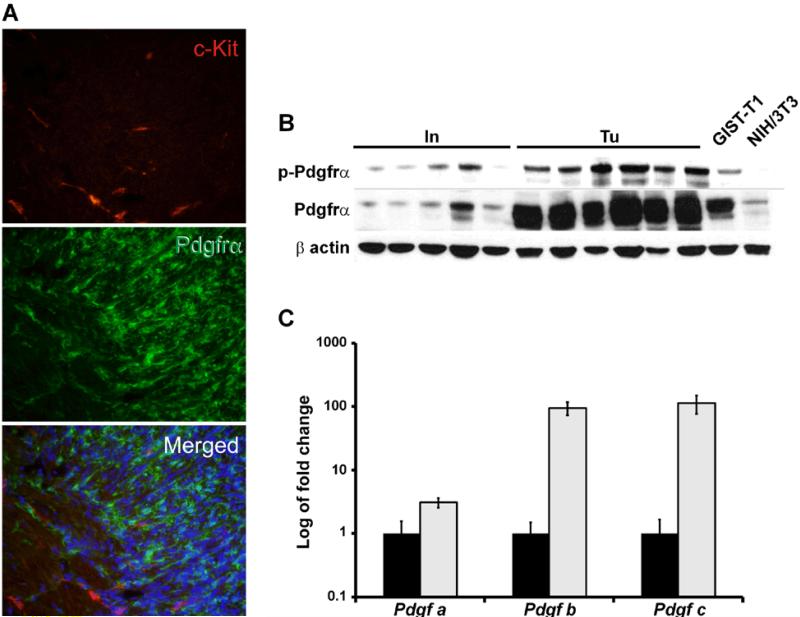
Similar relationships of Kit and Pdgfrα expression were found in tumors. Thus, all tumors were negative for Kit in immunofluorescence analysis (Fig. 4A; see Supplementary Fig. 2A and 2B for immunohistochemical and Western blot analysis, respectively), but highly expressed Pdgfrα (Fig. 4A and Supplementary Fig. 2C; lack of Pdgfrβ expression is shown in Supplementary Fig. 2D). In addition, Pdgfrα was substantially phosphorylated in tumors compared to normal intestine (Fig. 4B). This was consistent with the overexpression of the Pdgfrα ligands Pdgfa, −b and −c in tumor tissue (Fig. 4C). Taken together, some LysMcre-targeted cells in the gut wall and the GIST-like tumors found in the same location expressed activated Pdgfrα, but not Kit.
Figure 4. Tumors of Ptch mutant mice express Pdgfrα but not Kit.
(A) Immunofluorescence analysis of tumors of Ptchflox/floxLysMcre+/− mice using an anti-Pdgfrα (green) and anti-Kit antibody (red). No Pdgfrα/Kit double-positive cells were detected in the tumors. Scale bar 50 μm. (B) Western Blot analysis of tumors (Tu) and normal small intestine (In) of Ptchflox/floxLysMcre+/− mice using anti-Pdgfrα and anti-pPdgfrα (phosphorylated Pdgfrα) antibodies. GIST-T1 and NIH/3T3 cells were used as control samples. (C) qRT-PCR analysis of Pdgf-a, b and c in normal intestine and in tumors. Expression levels in tumors are shown in relation to normal intestine, which was set=1. (black bars: intestine; grey bars: tumors).
Differentiation from LMS and responsiveness to imatinib
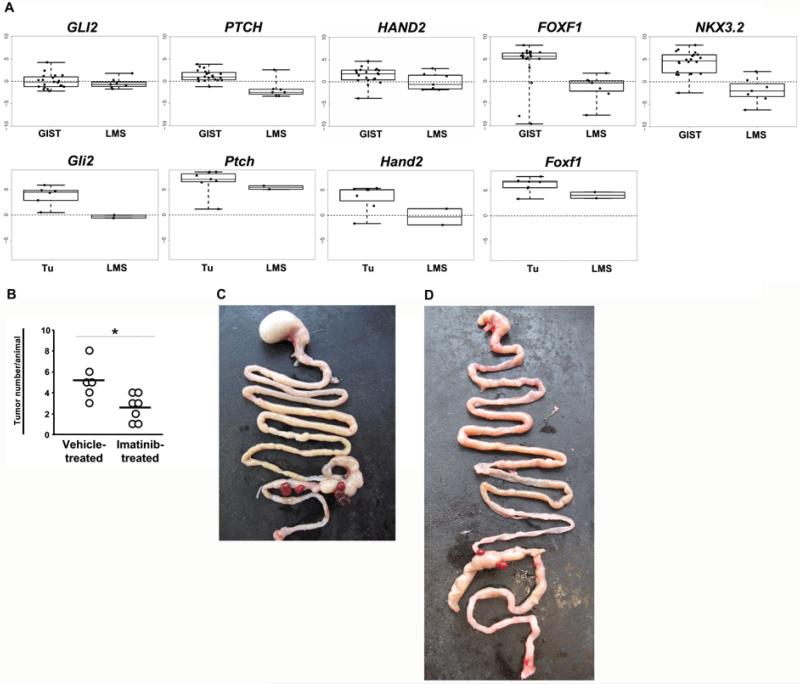
The intestinal tumors in our mice strikingly resembled GIST in humans. Since LMS is an important tumor entity in the differential diagnosis of GIST, we next investigated whether tumors of Ptchflox/floxLysMcre+/− mice were more related to GIST or to LMS. To this end we determined the expression of several Hh marker genes in tumors of Ptchflox/floxLysMcre+/− mice and in murine LMS derived from Pten/p53 double-mutant mice (reference30 and Guijarro and Hernando, unpublished). The data was compared to 20 human GIST and 7 human LMS samples. According to the GEO (gene expression omnibus) database (see 2 GIST and 6 LMS samples in the Dataset GDS1209), GIST should overexpress the HH targets PTCH, GLI2, FOXF1, HAND2 and NKX3.2 when compared to LMS. Indeed, the analysis showed overexpression of these Hh target genes in human GIST and in Ptch inactivation-induced GIST-like tumors when compared to human or murine LMS samples (Fig. 5A; the establishment of a murine qRT-PCR assay for Nkx3.2 was not possible in our hands). These results demonstrate that the tumors of Ptchflox/floxLysMcre+/− mice are indeed GIST-like rather than LMS-like, at least in terms of Hh pathway activity. In addition, they confirm the aberrant HH signaling reported previously in human GIST19. Furthermore, the murine GIST-like tumors do not overexpress markers of follicular dendritic tumors such as CD21, CD23, CD3535 or the mesothelioma-specific marker keratin 736 (data not shown). As many human GIST, the tumors do not express desmin (data not shown).
Figure 5. Differentiation of the tumors from LMS and responsiveness to imatinib.
(A) qRT-PCR analysis of Hh-target genes in 20 human GISTs compared to 7 human LMS samples (upper panel), and in tumors of Ptchflox/floxLysMcre+/− mice (Tu) compared to murine LMS derived from mice harboring a Pten/p53 mutation (lower panel). Expression levels in human and murine samples were normalized to the expression of β-actin and 18S rRNA, respectively. Data shows Box-Whisker-Plots of the relative gene expression. (B) Shown is the mean tumor number per animal for Ptchflox/floxLysMcre+/− mice after treatment with imatinib or with vehicle control (the asterisk indicates p<0.05 by unpaired t-test). Due to frequent clustering of tumor nodules (see Fig. 1A and 1E), each cluster was counted as one separate tumor. (C) shows the intestine of a vehicle-treated and (D) of an imatinib-treated animal.
An important characteristic of most human GIST is the responsiveness to imatinib, which inhibits the activity of both the KIT and PDGFR tyrosine kinases4. We tested the effect of imatinib on the growth of GIST-like tumors in symptom-free Ptchflox/floxLysMcre+/− mice. After 10 weeks of treatment, the animals were sacrificed and subjected to autopsy. All mice included in the analysis developed GIST-like tumors. However, whereas vehicle-treatment was associated with an average of 5.2±1.7 tumors, imatinib-treatment decreased the number to an average of 2.6±1.3 per animal (P<0.05 by t-test, Fig. 5B-D). This is consistent with the expression, and was likely mediated by the inhibition, of the imatinib target Pdgfrα in tumors of Ptchflox/floxLysMcre+/− mice.
HH signaling cooperates with PDGFRA signaling in cellular transformation
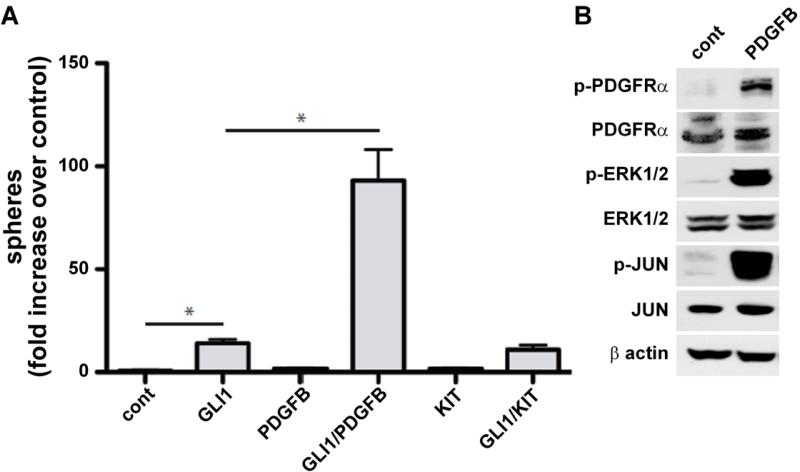
While the overexpression of the HH signaling pathway in the murine GIST-like tumors was genetically engineered, this pathway is overexpressed also in a substantial fraction of human GIST19. The significance of this overexpression to GIST biology is unknown. We investigated if HH and PDGFRA signaling pathways cooperate in oncogenic transformation by measuring the anchorage-independent clonal growth of non-tumorigenic human HaCaT keratinocytes. This model had been used previously to assay proteins modulating the oncogenic activity of GLI proteins37. Expression of GLI1 cooperated with the PDGFRA ligand PDGF-BB to induce cellular transformation, as evidenced by growth of 3-D cultures (Fig. 6A). As PDGFB induced activation of PDGFRA/ERK/JUN signaling (Fig. 6B), we conclude that PDGFRA signaling can cooperate with HH/GLI in oncogenic transformation. In addition, the cooperative transformation effect may be similar to that of HH and EGFR signaling, which necessitates activation of JUN37. In contrast, simultaneous expression of GLI1 and of the constitutively active KIT did not enhance transformation (Fig. 6B).
Figure 6. HH signaling cooperates with PDGFRA signaling in cellular transformation.
(A) Quantification of anchorage-independent clonogenic growth of human non-tumorigenic HaCaT keratinocytes after activation of either HH signaling (by overexpression of GLI1) or KIT signaling (overexpression of the constitutively active Kit D816V mutant) with or without stimulation of PDGFR signaling by PDGFB. Either signal alone has little or no activity towards cellular transformation, whereas GLI1 in combination with PDGFB treatment, but not in combination with KIT activation, leads to formation of clonogenic colonies (spheres) in 3D cultures (asterisks indicate p<0.03 by unpaired t-test). (B) PDGFRA signaling in HaCaT cells after incubation with PDGFB. Western blot analysis shows activation of PDGFRA/ERK/JUN signaling in response to PDGFB treatment.
Discussion
We show that mutations in Ptch in LysM-expressing cells result in GIST-like tumors in mice. These tumors seem to arise from Kit-negative cells of the intestine. Furthermore, the tumors express Pdgfrα, but not Kit. In addition, and as many human GISTs, the GIST-like tumors express markers of active Hh signaling.
The classification of the gastrointestinal tumors described in our work as GIST-like is based on several lines of complementary evidence. Firstly, these tumors exhibit a GIST-typical localization between either the circular and longitudinal muscle layer or the longitudinal muscle layer and the serosa of the gut wall. Secondly, although blood-filled cysts are rare in human GIST38, the histology and molecular features of these tumors resemble GIST rather than other tumors of the gut, including LMS, the most important differential diagnosis of GIST. Thirdly, they are responsive to the GIST drug imatinib. Taken together with the absence of Kit expression, these tumors appear to constitute a first mouse model of the PDGFRα-positive KIT-negative GIST subtype in humans. Our designation of these tumors as GIST-like instead of GIST takes into account their murine rather than human origin.
The LysMCre driver used in this study for Ptch inactivation mainly targets macrophages and granulocytes25. This, at a first glance, would imply the origin of the GIST-like tumors from myeloid cells which settled within the gut wall. However, the absence of tumor formation in irradiated Rag-2−/−γc−/− mice reconstituted with BM cells from Ptchflox/floxLysMcre+/− mice, while not formally excluding, does not support this possibility. It follows that the GIST-like tumors may rather arise from non-myeloid cells that permit activation of the LysM promoter.
The existence of such cells is indeed evidenced by our data. Most importantly, using reporter mice we have identified rare, LysM-expressing cells with the same localization as GIST-like tumors arising in consequence of a LysM-driven Ptch inactivation. Admittedly, this co-localization suggests rather than formally proves that the GIST-like tumors are derived from the LysM-expressing cells of the gut wall. However, we also note that approximately 20% of LysM-expressing cells are Pdgfrα-positive, as are the GIST-like tumors. Although this may be just another coincidence, it is tempting to speculate that the LysM-expressing Pdgfrα-positive cells may serve as an origin for the GIST-like tumors in Ptchflox/floxLysMcre+/− mice. Together with the juxtaposition to the Kit-positive ICC, these cells may represent a subset of the Pdgfrα-positive Kit-negative fibroblast-like cells as reported by others16-18. Alternatively, they could represent a subset of the reported Pdgfrα-expressing ICC precursors, in which Kit expression is downregulated15. The fact that KIT and PDGFRA activity probably can replace each other during GIST progression in humans13 may argue for this possibility.
On the other hand, mice expressing mutant PDGFRA under the control of the endogenous Pdgfrα-promoter do not develop GIST39. At the first glance, this rather argues against an origin of GIST from Pdgfrα-positive intestinal cells. However, whereas exclusive activation of Pdgfrα in Pdgfrα-expressing cells may not suffice for GIST formation, additional events may be required for the formation of these tumors. Indeed, in our mouse model, activated Pdgfrα most likely cooperates with Hh-signaling in tumor formation. This is suggested by the cooperative effect of PDGFRA and of the HH target GLI1 on cellular transformation. Admittedly, the aberrant activity of the Hh pathway in mouse GIST-like tumors results from the intended deletion of the Hh receptor and tumor suppressor Ptch. Notwithstanding the artificial nature of this manipulation, deletions of the PTCH locus or aberrant activity of the HH signaling pathway have been reported in up to 50% or 80% of GIST cases, respectively19, 22. It follows that the mutational or otherwise activation of the HH pathway may be a driving and early event in the formation of human GIST. The aberrant PDGFRA pathway activity in human GIST can be attributed to PDGFRA mutations or to an increased PDGF ligand expression13. The latter also can explain Pdgfrα pathway activity in the GIST-like tumors in the Ptchflox/floxLysMcre+/− model. Alternatively, and as shown for BCC21, aberrant HH signaling may induce the expression and activation of PDGFRA. Finally, active PDGFRA signaling (e.g. due to a PDGFRA mutation) may stimulate GLI activity, as recently shown for signaling by EGFR31.
Together, we here present a new model of GIST formation, which does not involve Kit activation, but probably an interaction between Hh- and Pdgfrα-signaling. Thus, it is reasonable to speculate that GIST patients with HH- and PDGFRA-pathway activation may benefit from combination treatment protocols that besides imatinib include an HH antagonist e.g. GDC-0449, which recently has been shown to exhibit antitumor activity in clinical studies40.
Supplementary Material
Grant support and acknowledgements
We thank Lars Rönnstrand, Experimental Clinical Chemistry, Dept. of Laboratory Medicine, Lund University, Sweden, for KIT expressing plasmids. Work of H.H. is supported by the Wilhelm-Sander-Stiftung (project 2003.112.3) and the Deutsche Krebshilfe (KoSAR, project 109837). Work of F.A. is supported by the Austrian Science Fund (FWF, project P20652), the Austrian Genome Project GEN-AU (MedSys) and the priority program “Biosciences and Health” of the University of Salzburg.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests
Contributions: P.P. designed and performed research, collected and analyzed data and wrote the paper. A.Z., W.A.v.D., J.H., A.B., W.G., T.B. F.N., A.U., K.D., R.D. C.D. designed and performed the research and collected data; M.V.G., E.H., J.W., T.T. contributed vital reagents. P.C.W.H., C.R.A., B.P.R. and W.S.S. contributed vital reagents, performed research, collected and analyzed data. L.W., F.A, G.R.v.d.B and H.H designed research, contributed vital reagents and analytical tools, collected and analyzed data or wrote the paper.
References
- 1.Graadt van Roggen JF, van Velthuysen ML, Hogendoorn PC. The histopathological differential diagnosis of gastrointestinal stromal tumours. J Clin Pathol. 2001;54:96–102. doi: 10.1136/jcp.54.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer. 2005;103:821–829. doi: 10.1002/cncr.20862. [DOI] [PubMed] [Google Scholar]
- 3.Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111–127. doi: 10.1007/s00428-010-0891-y. [DOI] [PubMed] [Google Scholar]
- 4.Sleijfer S, Wiemer E, Seynaeve C, et al. Improved insight into resistance mechanisms to imatinib in gastrointestinal stromal tumors: a basis for novel approaches and individualization of treatment. Oncologist. 2007;12:719–726. doi: 10.1634/theoncologist.12-6-719. [DOI] [PubMed] [Google Scholar]
- 5.Agaram NP, Besmer P, Wong GC, et al. Pathologic and molecular heterogeneity in imatinib-stable or imatinib-responsive gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:170–181. doi: 10.1158/1078-0432.CCR-06-1508. [DOI] [PubMed] [Google Scholar]
- 6.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones AV, Cross NC. Oncogenic derivatives of platelet-derived growth factor receptors. Cell Mol Life Sci. 2004;61:2912–2923. doi: 10.1007/s00018-004-4272-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin BP, Heinrich MC, Corless CL. Gastrointestinal stromal tumour. Lancet. 2007;369:1731–1741. doi: 10.1016/S0140-6736(07)60780-6. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889–894. doi: 10.1097/00000478-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Ostrowski J, Polkowski M, Paziewska A, et al. Functional features of gene expression profiles differentiating gastrointestinal stromal tumours according to KIT mutations and expression. BMC Cancer. 2009;9:413. doi: 10.1186/1471-2407-9-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossi G, Valli R, Bertolini F, et al. PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours of the gastrointestinal tract. Histopathology. 2005;46:522–531. doi: 10.1111/j.1365-2559.2005.02128.x. [DOI] [PubMed] [Google Scholar]
- 13.Negri T, Bozzi F, Conca E, et al. Oncogenic and ligand-dependent activation of KIT/PDGFRA in surgical samples of imatinib-treated gastrointestinal stromal tumours (GISTs) J Pathol. 2009;217:103–112. doi: 10.1002/path.2450. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 15.Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–952. doi: 10.1053/j.gastro.2010.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan F, Liu Y, Sun H, et al. Distribution and possible role of PDGF-AA and PDGFR-alpha in the gastrointestinal tract of adult guinea pigs. Virchows Arch. 2010;457:381–388. doi: 10.1007/s00428-010-0946-0. [DOI] [PubMed] [Google Scholar]
- 17.Iino S, Horiguchi K, Horiguchi S, et al. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 18.Iino S, Nojyo Y. Immunohistochemical demonstration of c-Kit-negative fibroblast-like cells in murine gastrointestinal musculature. Arch Histol Cytol. 2009;72:107–115. doi: 10.1679/aohc.72.107. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizaki A, Nakayama T, Naito S, et al. Expressions of sonic hedgehog, patched, smoothened and Gli-1 in human intestinal stromal tumors and their correlation with prognosis. World J Gastroenterol. 2006;12:5687–5691. doi: 10.3748/wjg.v12.i35.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wetmore C. Sonic hedgehog in normal and neoplastic proliferation: insight gained from human tumors and animal models. Curr Opin Genet Dev. 2003;13:34–42. doi: 10.1016/s0959-437x(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Aszterbaum M, Zhang X, et al. A role of PDGFRalpha in basal cell carcinoma proliferation. Proc Natl Acad Sci U S A. 2001;98:9255–9259. doi: 10.1073/pnas.151173398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meza-Zepeda LA, Kresse SH, Barragan-Polania AH, et al. Array comparative genomic hybridization reveals distinct DNA copy number differences between gastrointestinal stromal tumors and leiomyosarcomas. Cancer Res. 2006;66:8984–8993. doi: 10.1158/0008-5472.CAN-06-1972. [DOI] [PubMed] [Google Scholar]
- 23.Belinsky MG, Skorobogatko YV, Rink L, et al. High density DNA array analysis reveals distinct genomic profiles in a subset of gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2009;48:886–896. doi: 10.1002/gcc.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uhmann A, Dittmann K, Nitzki F, et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 25.Clausen BE, Burkhardt C, Reith W, et al. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8:265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 26.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 27.Sommer G, Agosti V, Ehlers I, et al. Gastrointestinal stromal tumors in a mouse model by targeted mutation of the Kit receptor tyrosine kinase. Proc Natl Acad Sci U S A. 2003;100:6706–6711. doi: 10.1073/pnas.1037763100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zibat A, Uhmann A, Nitzki F, et al. Time-point and dosage of gene inactivation determine the tumor spectrum in conditional Ptch knockouts. Carcinogenesis. 2009;30:918–926. doi: 10.1093/carcin/bgp068. [DOI] [PubMed] [Google Scholar]
- 29.Sinha P, Chi HH, Kim HR, et al. Mouse lysozyme-M knockout mice reveal how the self-determinant hierarchy shapes the T cell repertoire against this circulating self antigen in wild-type mice. J Immunol. 2004;173:1763–1771. doi: 10.4049/jimmunol.173.3.1763. [DOI] [PubMed] [Google Scholar]
- 30.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 31.Schnidar H, Eberl M, Klingler S, et al. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen M, Ronnstrand L, Sun J. The c-Kit/D816V mutation eliminates the differences in signal transduction and biological responses between two isoforms of c-Kit. Cell Signal. 2009;21:413–418. doi: 10.1016/j.cellsig.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 34.Rubin BP, Antonescu CR, Scott-Browne JP, et al. A knock-in mouse model of gastrointestinal stromal tumor harboring kit K641E. Cancer Res. 2005;65:6631–6639. doi: 10.1158/0008-5472.CAN-05-0891. [DOI] [PubMed] [Google Scholar]
- 35.Moz U, Pignatelli U, Forner P, et al. Follicular dendritic cell tumour of the cervical lymph node: case report and brief review of literature. Acta Otorhinolaryngol Ital. 2004;24:223–225. [PubMed] [Google Scholar]
- 36.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13:962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 37.Eberl M, Klingler S, Mangelberger D, et al. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4:218–233. doi: 10.1002/emmm.201100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cruz RJ, Jr., Vincenzi R, Ketzer BM, et al. Spontaneous intratumoral bleeding and rupture of giant gastric stromal tumor (> 30 cm) in a young patient. World J Surg Oncol. 2008;6:76. doi: 10.1186/1477-7819-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olson LE, Soriano P. Increased PDGFRalpha activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.