Abstract
Background
Childhood adverse experiences are known to induce persistent changes in the hypothalamic–pituitary–adrenal (HPA) axis reactivity to stress. However, the mechanisms by which these experiences shape the neuroendocrine response to stress remain unclear.
Method
We tested whether bullying victimization influenced serotonin transporter gene (SERT) DNA methylation using a discordant monozygotic (MZ) twin design. A subsample of 28 MZ twin pairs discordant for bullying victimization, with data on cortisol and DNA methylation, were identified in the Environmental Risk (E-Risk) Longitudinal Twin Study, a nationally representative 1994–1995 cohort of families with twins.
Results
Bullied twins had higher SERT DNA methylation at the age of 10 years compared with their non-bullied MZ co-twins. This group difference cannot be attributed to the children’s genetic makeup or their shared familial environments because of the study design. Bullied twins also showed increasing methylation levels between the age of 5 years, prior to bullying victimization, and the age of 10 years whereas no such increase was detected in non-bullied twins across time. Moreover, children with higher SERT methylation levels had blunted cortisol responses to stress.
Conclusions
Our study extends findings drawn from animal models, supports the hypothesis that early-life stress modifies DNA methylation at a specific cytosine–phosphate–guanine (CpG) site in the SERT promoter and HPA functioning and suggests that these two systems may be functionally associated.
Keywords: Bullying, childhood victimization, cortisol, DNA methylation, hypothalamic–pituitary–adrenal axis, SERT
Introduction
Evidence from animal studies suggests that exposure to adverse environments early in life has long-term consequences on later behavioural and neurobiological functioning including hypothalamic–pituitary–adrenal (HPA) axis reactivity to stress (Suomi, 1997; Levine, 2005; Meaney & Szyf, 2005; Sanchez, 2006). In humans, childhood maltreatment has been associated with higher HPA axis activity (for a review, see Tarullo & Gunnar, 2006). There is also growing interest in understanding the origins of lower cortisol activity, another marker of disruption of the HPA axis (Heim et al. 2000; Gunnar & Vazquez, 2001; Fries et al. 2005). One prevailing hypothesis is that childhood maltreatment may induce stable changes in HPA axis activity and increase vulnerability to psychopathology (Susman, 2006; van Goozen et al. 2007; Yehuda et al. 2010). In addition to lower diurnal cortisol secretion (Cicchetti & Rogosch, 2001; Dozier et al. 2006; Bruce et al. 2009), accumulating research also indicates lower reactivity in relation to childhood adversity (Carpenter et al. 2007; Elzinga et al. 2008; Tyrka et al. 2008). Similarly, we reported blunted cortisol responses to stress in bullied twins in comparison with their non-bullied monozygotic (MZ) co-twins (Ouellet-Morin et al. 2011a). Examining discordant MZ twins reduces the possibility that differences between bullied and non-bullied children can be attributed to genetic variation or shared family environments, thus supporting the idea of an environmentally mediated effect of childhood victimization on cortisol reactivity. However, the mechanisms by which early adverse experiences shape the HPA axis remain unclear.
Research suggests that susceptibility to psychopathology partly arises from the interplay between childhood maltreatment and a polymorphism in the serotonin transporter gene (SERT) promoter (Canli & Lesch, 2007; Caspi et al. 2010; Uher & McGuffin, 2010). Notably, this polymorphism is also associated with cortisol responses to stress in newborns (Mueller et al. 2010) and female adolescents (Gotlib et al. 2008). This association was shown to be moderated by early adversity in adults (Alexander et al. 2009), although non-replication exists (Bouma et al. 2010). Because this polymorphism explains a relatively small proportion of SERT expression (<10%; Olsson et al. 2010), the investigation of other mechanisms affecting serotonergic neurotransmission, and potentially HPA axis reactivity, should be pursued. One possibility is that epigenetic alterations of SERT expression account for the environmentally mediated effect of childhood victimization on HPA axis reactivity. This hypothesis is consistent with accumulating evidence, mainly derived from animal studies, showing that epigenetic remodelling represents a mechanism by which adverse experiences disrupt reactivity to stress and health (Jirtle & Skinner, 2007; Mill & Petronis, 2007; Tsankova et al. 2007; Feinberg, 2008; Johnstone & Baylin, 2010; Meaney, 2010). Preliminary evidence of increased DNA methylation in the SERT promoter region of adults with a history of childhood abuse (Beach et al. 2010, 2011) supports the idea that SERT DNA methylation may link childhood victimization to HPA axis activity.
The objectives of the present study were to examine the impact of bullying victimization on SERT DNA methylation and to test whether DNA methylation was associated with lower cortisol responses to stress. Similar to maltreatment, bullying victimization has been associated with a wide range of mental health problems (Arseneault et al. 2010). Both types of victimization are characterized by intentional harm and involve repeated harmful actions between individuals where there is a power imbalance between the perpetrator and the victim whereby it is difficult for the victims to defend themselves (Arseneault et al. 2011). Specifically, we tested whether differences in SERT DNA methylation between bullied and non-bullied children were detectable in childhood using a longitudinal discordant MZ twin design to exert a strong control over genetic and environmental confounds (Rutter, 2009). Based on previous findings, we hypothesized higher SERT DNA methylation levels in bullied twins compared with their non-bullied MZ cotwins. Moreover, we examined SERT DNA methylation prior to victimization to rule out the possibility that bullied children already had higher DNA methylation levels. We then tested whether SERT DNA methylation was associated with lower cortisol responses to stress.
Method
Sample
Participants were recruited from the Environmental Risk (E-Risk) Longitudinal Twin Study, which tracks the development of a nationally representative birth cohort of 2232 British children (Moffitt & the E-Risk Study Team, 2002). The sample was drawn from a larger birth register of twins born in England and Wales in 1994–1995. The E-Risk sample was constructed in 1999–2000, when 1116 families with same-sex 5-year-old twins (93% of those eligible) participated in home-visit assessments. Follow-up home visits were conducted when the children were aged 7 (98% participation), 10 (96%) and 12 years (96%). Zygosity of the twins was determined with a standardized questionnaire which has been shown to have 95% accuracy (Price et al. 2000). Ambiguous cases were zygosity typed using DNA. Parents gave informed consent and children gave assent to participate in the study. Ethical approval was granted by the Joint South London and Maudsley and the Institute of Psychiatry National Health Service (NHS) Ethics Committee, UK.
From the E-Risk sample, we identified twin pairs eligible to participate in a substudy of cortisol if they met the following five criteria: (1) MZ twins; (2) one twin was bullied at least occasionally; (3) bullying was reported by both mothers and children at age 12 years; (4) bullying incidents involved harm, either psychological or physical; and (5) co-twins never experienced bullying victimization. From this substudy sample of 30 MZ twin pairs (Ouellet-Morin et al. 2011a), two pairs had missing data on SERT DNA methylation at the age of 10 years. The present study thus comprises 28 pairs of 12-year-old MZ twins discordant for bullying victimization with valid cortisol and DNA methylation data at the age of 10 years (42.9% males). Most twins were Caucasian (92.9%) and one in four families came from a low socio-economic background (25.0%). Children in this subsample had intelligence quotient (IQ) scores within the normal range when they were 5 years old (from 69 to 134; mean = 100.1, S.D. = 15.4). We previously showed that one-third of the variance in bullying victimization is due to unique environments or random experiences (Ball et al. 2008). These factors possibly explain why genetically identical individuals could be differently exposed to bullying. For example, British twins are routinely separated into different classrooms in secondary schools, which may randomly place them at distinct risk for bullying victimization. Bullied and non-bullied MZ twins were similar on pre-existing risk factors (birth weight, IQ, behavioural and emotional problems), child-specific family environments (lifetime maltreatment, maternal warmth), concomitant factors (body mass index, pubertal maturity, bullying perpetration) and Psychosocial Stress Test (PST)-related measures (perceived stress and increased negative affect; Ouellet-Morin et al. 2011a). E-Risk discordant bullied MZ twins did not differ from concordant bullied MZ twins on socio-economic status, IQ or birth complications. A subset of 22 twin pairs had valid DNA data at both the ages of 5 and 10 years.
Bullying victimization
We prospectively assessed bullying victimization for all E-Risk participants during the interviews conducted with mothers when the children were 7, 10 and 12 years old and with the children themselves at the age of 12 years. Before asking questions related to bullying victimization, we explained that ‘Someone is being bullied when another child: says mean and hurtful things, makes fun or calls a person mean and hurtful names; completely ignores or excludes someone from their group of friends; hits, kicks or shoves a person, or locks them in a room; tells lies or spreads rumours about them; and does other hurtful things like these. We call it bullying when these things happen often, and when it is difficult to make it stop. We do not call it bullying when it is done in a friendly or playful way’. We asked mothers whether each twin had been bullied by another child, responding ‘never’, ‘yes’ or ‘frequently ’. We further asked mothers who reported bullying victimization whether the twin suffered physical harm (e.g. bruise, cut) or psychological distress (e.g. bad dreams or school avoidance) as a consequence of bullying, responding ‘never’, ‘yes’ or ‘frequently ’. During private interviews, we asked children ‘Have you been bullied by another person? ’. A senior investigator further reviewed all descriptions of the bullying events recorded by the interviewers to confirm instances of bullying by looking for evidence of (1) repeated harmful actions, (2) between children, and (3) where there was a power imbalance between the bully and the victim. A test–retest reliability of 0.87 was noted for 30 parents randomly selected from the total E-Risk sample and who were interviewed 3–6 weeks apart. Our findings indicate that both mothers and children are valid and reliable informants of bullying victimization and that they tended to agree with one another (Shakoor et al. 2011). In this subsample, mothers reported that 19.6, 28.6 and 51.8% of children were victims of bullying at the ages of 7, 10 and 12 years, respectively, while 32.1% of twins reported experiences of bullying since the beginning of formal schooling.
Psychosocial Stress Test
When they were 12 years old, twins from the substudy sample were invited to our research laboratory early in the afternoon. At 1 h after arrival, each twin took part in an adapted version of the Trier Social Stress Test for children, which included a social stressor (speaking in front of judges) and a cognitive stressor (mental arithmetic; Buske-Kirschbaum et al. 1997). The cognitive task was first administered using the Children’s Paced Auditory Serial Addition Task (Dyche & Johnson, 1991), a serial-addition task used to assess sustained attention, rate of information processing and working memory. Children heard a random series of 61 numbers ranging from 1 to 9 and were instructed to add the numbers in pairs such that each number was added to the previous one. The time interval between each number was 2.4 s for the first series of numbers and 2.0 s for the second series. Before the task started, children were told to make as few mistakes as possible because they were in competition against their co-twin and the winner would get a prize. The research interviewer did not offer support and avoided eye contact to enhance the stressful aspect of the challenge. The public speaking task immediately followed. Children were told to stand and to recall their most unpleasant experience at school in front of an unknown and inexpressive judge and the interviewer. Children had 2 min to prepare in silence, standing in front of the camera, and were then asked to speak for 5 min. The PST lasted approximately 15 min. This stress paradigm was selected because a combination of public speaking and cognitive tasks has been shown to elicit reliable cortisol responses in laboratory settings (Buske-Kirschbaum et al. 1997; Dickerson & Kemeny, 2004). At the end, the interviewer told the twins that they did well and rewarded their efforts.
Cortisol
We collected five saliva samples to measure cortisol responses to the PST. Saliva was collected by asking children to use a straw to pass through 1 ml of saliva into the cryovials. The first samples were collected 20 and 2 min prior to the PST. A third sample was collected immediately at the end. A fourth sample and a fifth sample were collected 25 and 35 min after the start of the tasks. Twins were asked to refrain from doing any vigorous exercise in the morning, to eat a light lunch before midday, avoiding dairy products and red meat. Saliva samples were stored at −20 °C in a freezer.
After thawing, saliva samples were centrifuged at 3500 revolutions per min for 10 min, which resulted in a clear supernatant fraction of low viscosity. Saliva cortisol concentrations were determined using the ‘Immulite 1000’ model – Siemens’ Immunoassay System (www.diagnostics.siemens.com; Mondelli et al. 2010). The assay had an analytical sensitivity of 0.2 nmol/l and inter-/intra-assay precision of less than 10%. All samples from each twin pair were analysed together. Cortisol measures were skewed and normalized using a log10 transformation.
DNA methylation analysis
All DNA samples were extracted from buccal cells using an established method that yields high-molecular- weight genomic DNA (Freeman et al. 2003). All DNA samples were tested for degradation and purity using spectrophotometry and gel electrophoresis. No samples were excluded because of poor sample quality. Genomic DNA (375 ng) was treated with sodium bisulfite using the EZ-96 DNA Methylation Kit (Zymo Research, USA) following the manufacturer’s standard protocol. Bisulfite–PCR primers were designed using Sequenom EpiDesigner software (http://www.epidesigner.com). The SERT amplicon was amplified using standard Sequenom MassCLEAVE tagged primers (tags in lower case): 5′-aggaagagagTATTGTTAGGTTTTAGGAAGAAAGAGAGAG-3′ (forward) and 5′-cagtaatacgactcactatagggagaaggctAACCCTCACATAATCTAATCTCTAAATAACC-3′ (reverse) and encompassed 471 base pairs (NCBI build 36, chromosome 17: 25586879–25587349). Bisulfite–PCR amplification was conducted using Hot Star Taq DNA polymerase (Qiagen, UK) and cycling conditions of 45 cycles with an annealing temperature of 56 °C. All reactions were performed in duplicate and DNA methylation analysis was subsequently conducted using the Sequenom EpiTYPER system (Sequenom Inc., USA) as described previously (Coolen et al. 2007). The Sequenom EpiTYPER system is a highly reliable and quantitative technology for determining the density of methylated cytosines across specific genomic loci (Coolen et al. 2007). It uses base-specific cleavage followed by matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry in which the size ratio of the cleaved products provides quantitative methylation estimates for each cytosine–phosphate–guanine (CpG) unit, which contains either one or an aggregate of neighbouring CpG sites (see Supplementary Figs 1 and 2). Artificially methylated and unmethylated samples were included as positive and negative controls to ensure unambiguous PCR amplification of bisulfite-treated samples. All samples were processed blind to sample identification. Data generated from the EpiTYPER software were treated with stringent quality-control analysis where CpG units with low calling rates (<80%) were removed from analyses (none identified).
Statistical analyses
We conducted statistical analyses in four steps. First, we replicated previous findings indicating a blunted pattern of cortisol response to stress in bullied twins in comparison with their non-bullied co-twins using repeated-measures analysis of variance (ANOVA) in this subsample of 28 twin pairs. Second, we tested a promoter-wide difference in DNA methylation between bullied and non-bullied MZ twins using linear regressions. In the presence of a significant finding, we further explored site-specific differences. To control for non-independent observations and patterns of within-pair clustering due to shared genetic and environmental influences, linear regression analyses were adjusted with tests based on the sandwich or Huber/White variance estimator (Williams, 2000). Third, we investigated separately the bullied and non-bullied twins who had DNA at both the ages of 5 and 10 years (22 pairs) to test whether methylation levels changed over time using repeated-measures ANOVAs. Fourth, we explored the association between DNA methylation and cortisol responses to stress using Pearson correlation adjusted with the sandwich or Huber/White variance estimator. Cortisol responses to the PST were indexed using the standardized residuals (Z) of the area under the curve with respect to increase (AUCi; Pruessner et al. 2003), calculated using the five cortisol measures and controlling for dairy product consumption and histaminic medication.
Results
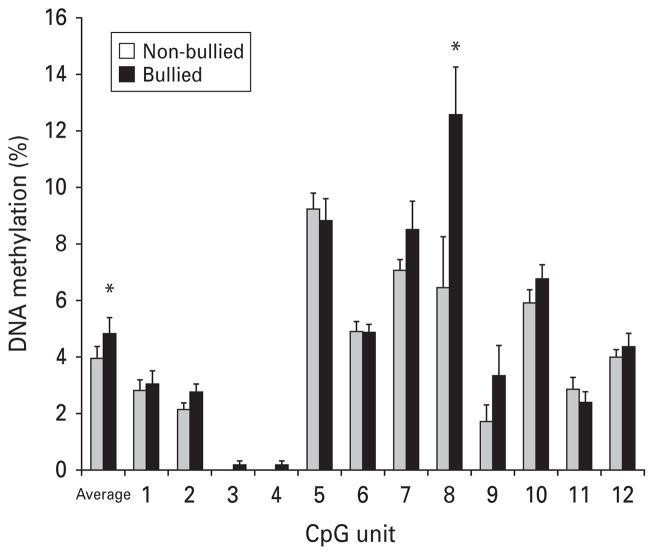
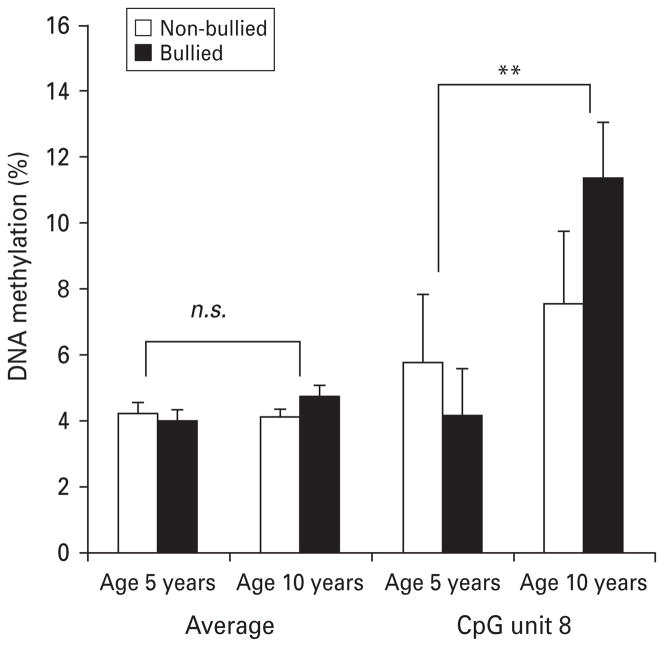
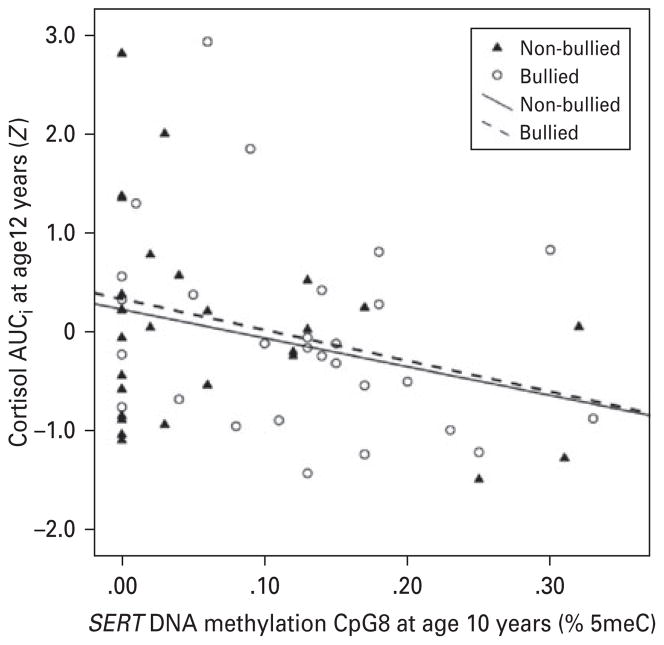
Similarly to our previous findings (Ouellet-Morin et al. 2011a), we observed distinct patterns of cortisol response to stress between bullied and non-bullied twins (time × bullying: F2.23,115.68 = 2.94, p = 0.05). While non-bullied twins showed the expected cortisol increase after the PST (F1.92,47.92 = 4.91, p = 0.01), bullied twins did not exhibit this increase (F2.55,63.75 = 0.56, p = 0.61). Fig. 1 shows that bullied twins had higher SERT DNA methylation at the age of 10 years, averaged across the 12 CpG units compared with their non-bullied MZ co-twins (t27 = 2.49, p = 0.02). Additional tests showed that this difference emerged primarily from a site-specific difference (CpG8 in our assay) (t27 = 2.39, p = 0.02). Notably, both groups had similar methylation levels prior to bullying victimization at the age of 5 years (across the promoter region: t21 = 0.56, p = 0.58; CpG8: t21 = 0.57, p = 0.58). Fig. 2 shows that while bullied twins had increased levels of SERT methylation from the ages of 5 to 10 years at CpG8 (F1,21 = 9.48, p = 0.006), their non-bullied co-twins did not show that increase (F1,21 = 0.32, p = 0.58). DNA methylation between the ages of 5 and 10 years remained unchanged across the promoter region for both bullied and non-bullied twins (F1,21 = 1.88, p = 0.18 and F1,21 = 0.10, p = 0.76, respectively; see Fig. 2). We previously showed that bullied and non-bullied twins did not differ on child-specific family environments (lifetime maltreatment, maternal warmth and lifetime stressful life events), individual risk factors prior (birth weight, IQ, externalizing and internalizing problems) and concomitant to bullying experiences (body mass index, pubertal maturity and bullying perpetration) or related to the PST (perceived stress and increase in negative affects; Ouellet-Morin et al. 2011a). Therefore, these factors cannot account for differences in DNA methylation between bullied and non-bullied twins. Furthermore, Fig. 3 shows that twins with higher methylation at CpG8 (age 10 years) exhibited lower cortisol responses to the PST at the age of 12 years (r = −0.28, p = 0.02).
Fig. 1.
Average and cytosine–phosphate–guanine (CpG ) unit serotonin transporter gene (SERT) DNA methylation in bullied and non-bullied monozygotic twins at 10 years of age (28 twin pairs). Values are means, with standard errors represented by vertical bars. * Mean value was significantly different from that for non-bullied twins (p < 0.05).
Fig. 2.
Average and cytosine–phosphate–guanine (CpG ) unit serotonin transporter gene (SERT) DNA methylation in bullied and non-bullied monozygotic twins from the age of 5 to 10 years (22 twin pairs). Values are means, with standard errors represented by vertical bars. ** Mean value was significantly different from that at age 5 years (p < 0.01).
Fig. 3.
Association between cytosine–phosphate–guanine (CpG) unit 8 serotonin transporter gene (SERT) DNA methylation and cortisol response to the Psychosocial Stress Test (56 twins). Standardized residuals (Z) were used to take into account the cortisol covariates. AUCi, Area under the curve with respect to increase; % 5meC, % 5-methylcytosine.
Discussion
We showed for the first time in humans that childhood victimization is associated with increased SERT DNA methylation in the absence of pre-existent differences between bullied and non-bullied children, providing longitudinal support that epigenetic processes are dynamic and responsive to early social environments. Our findings are consistent with higher levels of SERT methylation reported in adults exposed to harsh parenting, and physical and sexual abuses in childhood (Beach et al. 2010). Previous studies have also shown that SERT DNA methylation is associated with a life-time history of major depression (Philibert et al. 2008; Olsson et al. 2010), antisocial behaviour (Beach et al. 2011) and unresolved trauma, a known risk factor for post-traumatic stress disorder (PTSD) (Bakermans-Kranenburg & van IJzendoorn, 2009), suggesting the potential role of SERT methylation in psychopathology.
The present study extends current knowledge in three ways. First, the discordant MZ twin design makes it unlikely that group differences in DNA methylation at this locus are attributable to genetic or shared environmental factors such as parents’ psychopathology (Rakyan et al. 2011). This key feature of our study design further supports, in the absence of random assignment, that childhood victimization exerts an environmentally mediated effect on SERT DNA methylation. Second, our longitudinal design provides indirect evidence for directionality between victimization and methylation; only bullied twins showed increased DNA methylation from the age of 5 to 10 years, suggesting that it is not just a mere reflection of pre-existing differences. This result corroborates earlier findings showing that SERT DNA methylation is largely attributable to uniquely experienced environments (Wong et al. 2010) and substantiates the presumed impact of childhood victimization on higher SERT DNA methylation noted in retrospective studies of adults (Beach et al. 2010, 2011). Third, our findings suggest that the effect of early victimization on SERT DNA methylation can already be detected in childhood. This is consistent with the differential clustering patterns of DNA methylation reported across the genome between children raised in institutional care and controls (Naumova et al. 2011) and as a function of early maternal stress (Essex et al. 2011). Future studies examining DNA methylation prior to, concurrently and following naturally occurring adverse experiences could determine whether these changes are long lasting and are uniquely explained by early victimization.
Our findings suggest that SERT DNA methylation may be involved in the association between childhood victimization and cortisol responses to stress. Specifically, the increased SERT DNA methylation shown in bullied twins and lower cortisol responses reported in children with higher SERT methylation are in line with previous suggestions of an environmentally mediated effect of bullying victimization on HPA axis reactivity (Ouellet-Morin et al. 2011a). Two other investigations examined the association between early adversity, DNA methylation and the HPA axis in humans (Oberlander et al. 2008; Tyrka et al. 2012). The first study showed increased DNA methylation of the glucocorticoid receptor gene (GR) in infants prenatally exposed to maternal depressive/anxious moods which was, in turn, associated with higher cortisol reactivity. The second study also reported increased GR DNA methylation in adults with a history of childhood adversity and lower HPA reactivity (although not in the same CpG sites). Contrasting effects of adverse experiences on HPA axis functioning are thought to arise according to the nature, duration and timing of exposure (Miller et al. 2007; Lupien et al. 2009). Also consistent with a time-variant impact of adversity, maternal depressed/anxious moods during the 2nd trimester (but not in the 3rd) was associated with lower SERT DNA methylation (Devlin et al. 2010). Similar patterns of findings have been detected elsewhere in the epigenome as a function of famine exposure around the time of conception but not in late gestation (Heijmans et al. 2008; Tobi et al. 2009) and maternal stress in the year following birth but not during the preschool years (Essex et al. 2011). The timing of exposure to bullying victimization (middle childhood) may explain why we detected higher rather than lower SERT DNA methylation in bullied children. Altogether, these findings are consistent with the possibility that epigenetic processes affect HPA axis reactivity. Longitudinal research should investigate the possibility that heterogeneous epigenetic and HPA axis reactivity profiles emerge in children exposed to adversity taking place at distinct periods of development.
More generally, our findings are in line with a series of experiments conducted with rodents indicating that naturally occurring variation in maternal care mediated, independently from DNA sequence, epigenetic modifications in the hippocampus and resulted in long-lasting changes in HPA axis reactivity (Meaney & Szyf, 2005; McGowan & Szyf, 2010; Bagot & Meaney, 2010; Champagne, 2010). Specifically, rodents exposed to low maternal care (e.g. licking and grooming) were shown to have increased GR DNA methylation of the exon 17 promoter, lower hippocampal GR expression and higher HPA axis reactivity (Liu et al. 1997; Francis et al. 1999; Weaver et al. 2004). A similar finding in victims of suicide with a history of maltreatment supports the idea that analogous biological pathways may be present in humans (McGowan et al. 2009) and could jeopardize physical health (Filiberto et al. 2011). Additional findings suggest that early-life stress also alters DNA methylation of the arginine vasopressin gene (Murgatroyd et al. 2009) and the brain-derived neurotrophic factor gene (Roth et al. 2009). Past research thus suggests that early-life stress induces epigenetic remodelling of several genes directly or indirectly regulating the HPA axis. Genome-wide mapping of epigenetic alterations induced by early-life stress may help to uncover the complexity of the biological pathways underlying vulnerability to stress and psychopathology.
Our study raises the possibility that SERT methylation is involved in the recalibration of neuroendocrine stress reactivity following early adverse experiences and thus represents a molecular basis of vulnerability to stress and psychopathology. Lower cortisol responses to stress have recurrently been documented in individuals with externalizing problems (van Goozen et al. 2007; McCrory et al. 2010) or PTSD (Yehuda et al. 2010), especially in the context of childhood victimization (Meewisse et al. 2007; Ouellet-Morin et al. 2011b). The ‘attenuation hypothesis’ suggests that early adversity induces persistent cortisol elevation followed by the down-regulation of HPA axis reactivity (Susman, 2006). Lowering the set-points for initiating a stress response could be adaptive when exposed to uncontrollable and unpredictable harsh living circumstances although it may exert long-term constraints on neural circuits and brain structures regulating stress, emotion reactivity and social behaviour (Fairchild et al. 2008; Feder et al. 2009). It is not clear whether our findings are specific to bullying victimization or could be generalized to other forms of harmful experiences such as maltreatment by an adult. On the one hand, these experiences are both characterized by intentional harm and power imbalance (Arseneault et al. 2011). On the other hand, it is possible that these experiences have distinct effects on DNA methylation, especially if they occur at different times during development. More research is needed to identify which features of childhood victimization affect epigenetic regulation and later vulnerability to psychopathology.
We speculate that increased SERT DNA methylation following childhood victimization affects HPA axis reactivity over time through the disruption of serotonin (or 5-hydroxytryptamine; 5-HT) neurotransmission. This hypothesized ‘cascade’ effect of altered serotonin neurotransmission on HPA axis activity following early-life stress is consistent with the known facilitating and inhibiting effects of serotonin neurotransmission, tryptophan depletion and the serotonin transporter on HPA axis reactivity (Li et al. 1999; Lowry, 2002; Vielhaber et al. 2005). The proposed role of SERT expression on poor stress coping strategies is also suggested in studies conducted with infant rhesus macaques exposed to early-life stress (Miller et al. 2009; Kinnally et al. 2010a, 2010b). Evidence in humans also suggests that factors influencing serotonergic activity could modulate reactivity to stress through cortical–limbic regulation of emotions (Herman & Cullinan, 1997; Ochsner & Gross, 2005). For example, genetically based differences in SERT expression have been associated with increased amygdala activity to fearful stimuli (Hariri et al. 2002) and perturbed functional connectivity in the anterior cingulate cortex and the amygdala (Pezawas et al. 2005). It is also possible that persistent initial cortisol elevations triggered by childhood victimization affect SERT DNA methylation since glucocorticoids also regulate SERT expression (Lesch et al. 1996). Experiments conducted in rodents modelling the pharmacological use of glucocorticoids in premature babies support this possibility. Dexamethasone- and hydrocortisone-treated animals had lower SERT expression compared with controls, which could represent an adaptive mechanism that compensates for lower 5-HT levels (Vazquez et al. 2012). Childhood experiences triggering repeated and prolonged HPA axis activations, such as bullying victimization, may thus shape the 5-HT system in ways that it disrupts reactivity to stress and health. More research is needed to explore the biological pathways and temporal sequence of the complex bidirectional influences taking place between serotonergic pathways and the HPA axis during development.
The present findings provide support for the impact of childhood victimization on SERT DNA methylation and suggest an association between this epigenetic signal and cortisol reactivity; however, further tests are needed. First, the functional role of CpG8 methylation on SERT expression was not investigated. However, increased DNA methylation in the SERT promoter has been shown to decrease mRNA transcription (Philibert et al. 2008; Olsson et al. 2010). Second, we examined genomic DNA from buccal cells. Although buccal cells are a uniform cell population and of common embryonic origin with neuronal cells, it is not known whether the findings generalize to other tissues (Illingworth et al. 2008; Rakyan et al. 2008). Interestingly, a post-mortem investigation of 11 tissues (not buccal cells or blood) suggests, overall, a homogeneous pattern of DNA methylation across tissues (Byun et al. 2009). There are also preliminary indications of correlated epigenetic signals between blood and buccal cells for X-chromosome inactivation (Monteiro et al. 1998; Rosa et al. 2008) and in candidate genes such as the corticotropin-releasing hormone gene (CRH) (Talens et al. 2010). Third, we measured SERT DNA methylation from samples collected at the ages of 5 and 10 years while cortisol was assessed when twins were aged 12 years. Concurrent measures of SERT DNA methylation and HPA axis reactivity repeatedly collected over time would help to establish the directionality of this association. Finally, the study was conducted in a small sample and should be replicated in larger studies. The discordant MZ twin design, however, allowed for a strong control of genetic and shared environmental potential confounds hardly ever taken into account in human studies (Rutter, 2009).
Our findings show prospective evidence that bullying victimization is associated with increased SERT DNA methylation. Moreover, children with higher SERT methylation exhibited lower cortisol responses to stress. Our study extends findings drawn from animal models and raises the possibility that early experiences of victimization modify the neuroendocrine response to stress through the alteration of SERT DNA methylation. This epigenetic mechanism may serve as an interface between childhood victimization, later vulnerability to stress and psychopathology.
Supplementary Material
Acknowledgments
We are grateful to Avshalom Caspi and Terrie E. Moffitt for their helpful comments on the manuscript, to Michael Rutter and Robert Plomin, to Thomas Achenbach for kind permission to adapt the Child Behavior Checklist, to Irene Papadopoulos for technical assistance and to members of the E-Risk Study team for their dedication, hard work and insights. We also thank the families and the twins’ teachers for their participation.
The E-Risk Study is funded by the Medical Research Council (no. G9806489 and G1002190). Additional support was provided by the Jacobs Foundation, the British Academy, the Nuffield Foundation, the Economic and Social Research Council (no. RES-177-25-0013) and the National Institute of Child Health and Human Development (no. HD061298). I.O.-M. was supported by the Canadian Institutes of Health Research. A.D. and J.M. are supported by NARSAD Young Investigator Awards and C.C.Y.W. is supported by the Medical Research Council. L.A. is a British Academy Mid-Career Fellow.
Footnotes
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712002784.
Declaration of Interest
None.
References
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene–environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Bowes L, Shakoor S. Bullying victimization in youths and mental health problems: ‘Much ado about nothing’? Psychological Medicine. 2010;40:717–729. doi: 10.1017/S0033291709991383. [DOI] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Fisher HL, Polanczyk G, Moffitt TE, Caspi A. Childhood trauma and children’s emerging psychotic symptoms: a genetically sensitive longitudinal cohort study. American Journal of Psychiatry. 2011;168:65–72. doi: 10.1176/appi.ajp.2010.10040567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot RC, Meaney MJ. Epigenetics and the biological basis of gene x environment interactions. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:752–771. doi: 10.1016/j.jaac.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. The first 10,000 Adult Attachment Interviews: distributions of adult attachment representations in clinical and non-clinical groups. Attachment and Human Development. 2009;11:223–263. doi: 10.1080/14616730902814762. [DOI] [PubMed] [Google Scholar]
- Ball HA, Arseneault L, Taylor A, Maughan B, Caspi A, Moffitt TE. Genetic and environmental influences on victims, bullies and bully-victims in childhood. Journal of Child Psychology and Psychiatry. 2008;49:104–112. doi: 10.1111/j.1469-7610.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at SLC6A4 is linked to family history of child abuse: an examination of the Iowa Adoptee sample. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics. 2010;153B:710–713. doi: 10.1002/ajmg.b.31028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SR, Brody GH, Todorov AA, Gunter TD, Philibert RA. Methylation at 5HTT mediates the impact of child sex abuse on women’s antisocial behavior: an examination of the Iowa adoptee sample. Psychosomatic Medicine. 2011;73:83–87. doi: 10.1097/PSY.0b013e3181fdd074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma E, Riese H, Nederhof E, Ormel J, Oldehinkel A. No replication of genotype effect of 5-HTTLPR on cortisol response to social stress in larger adolescent sample. Biological Psychiatry. 2010;68:e33–e34. doi: 10.1016/j.biopsych.2010.04.041. [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, Levine S. Morning cortisol levels in preschool-aged foster children: differential effects of maltreatment type. Developmental Psychobiology. 2009;51:14–23. doi: 10.1002/dev.20333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic Medicine. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Byun HM, Siegmund KD, Pan F, Weisenberger DJ, Kanel G, Laird PW, Yang AS. Epigenetic profiling of somatic tissues from human autopsy specimens identifies tissue- and individual-specific DNA methylation patterns. Human Molecular Genetics. 2009;18:4808–4817. doi: 10.1093/hmg/ddp445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62:1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA. Epigenetic influence of social experiences across the lifespan. Developmental Psychobiology. 2010;52:299–311. doi: 10.1002/dev.20436. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopatholgy. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- Coolen MW, Statham AL, Gardiner-Garden M, Clark SJ. Genomic profiling of CpG methylation and allelic specificity using quantitative high-throughput mass spectrometry: critical evaluation and improvements. Nucleic Acids Research. 2007;35:e119. doi: 10.1093/nar/gkm662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dozier M, Manni M, Gordon MK, Peloso E, Gunnar MR, Stovall-McClough KC, Eldreth D, Levine S. Foster children’s diurnal production of cortisol: an exploratory study. Child Maltreatment. 2006;11:189–197. doi: 10.1177/1077559505285779. [DOI] [PubMed] [Google Scholar]
- Dyche G, Johnson D. Development and evaluation of CHIPASAT, an attentional test for children: II test–retest reliability and practice effect for a normal sample. Perceptual and Motor Skills. 1991;72:563–572. doi: 10.2466/pms.1991.72.2.563. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K, Tollenaar MS, Bakvis P, van Pelt J, Spinhoven P. Diminished cortisol responses to psychosocial stress associated with lifetime adverse events: a study among healthy young subjects. Psychoneuroendocrinology. 2008;33:227–237. doi: 10.1016/j.psyneuen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce TW, Hertzman C, Lam LL, Armstrong JM, Neumann SM, Kobor MS. Epigenetic vestiges of early developmental adversity: childhood stress exposure and DNA methylation in adolescence. Child Development. 2011 doi: 10.1111/j.14678624.2011.01641.x. Published online: 2 September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biological Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Reviews Neuroscience. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg AP. Epigenetics at the epicenter of modern medicine. Journal of the American Medical Association. 2008;299:1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Gagne LA, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6:566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Sciences. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Freeman B, Smith N, Curtis C, Huckett L, Mill J, Craig IW. DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behavior Genetics. 2003;33:67–72. doi: 10.1023/a:1021055617738. [DOI] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30:1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends in Neurosciences. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Illingworth R, Kerr A, Desousa D, Jorgensen H, Ellis P, Stalker J, Jackson D, Clee C, Plumb R, Rogers J, Humphray S, Cox T, Langford C, Bird A. A novel CpG island set identifies tissue-specific methylation at developmental gene loci. PLoS Biology. 2008;6:e22. doi: 10.1371/journal.pbio.0060022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nature Reviews Genetics. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SE, Baylin SB. Stress and the epigenetic landscape: a link to the pathobiology of human diseases ? Nature Reviews Genetics. 2010;11:806–812. doi: 10.1038/nrg2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Capitanio JP, Leibel R, Deng L, Leduc C, Haghighi F, Mann JJ. Epigenetic regulation of serotonin transporter expression and behavior in infant rhesus macaques. Genes, Brain and Behavior. 2010a;9:575–582. doi: 10.1111/j.1601-183X.2010.00588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnally EL, Tarara ER, Mason WA, Mendoza SP, Abel K, Lyons LA, Capitanio JP. Serotonin transporter expression is predicted by early life stress and is associated with disinhibited behavior in infant rhesus macaques. Genes, Brain and Behavior. 2010b;9:45–52. doi: 10.1111/j.1601-183X.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Li Q, Wichems C, Heils A, Van De Kar LD, Lesch KP, Murphy DL. Reduction of 5-hydroxytryptamine (5-HT)1A-mediated temperature and neuroendocrine responses and 5-HT1A binding sites in 5-HT transporter knockout mice. Journal of Pharmacology and Experimental Therapeutics. 1999;291:999–1007. [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic–pituitary–adrenal axis. Journal of Neuroendocrinology. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry. 2010;51:1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Reviews Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Szyf M. The epigenetics of social adversity in early life: implications for mental health outcomes. Neurobiology of Disease. 2010;39:66–72. doi: 10.1016/j.nbd.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neurosciences. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. British Journal of Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- Mill J, Petronis A. Molecular studies of major depressive disorder: the epigenetic perspective. Molecular Psychiatry. 2007;12:799–814. doi: 10.1038/sj.mp.4001992. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Miller JM, Kinnally EL, Ogden RT, Oquendo MA, Mann JJ, Parsey RV. Reported childhood abuse is associated with low serotonin transporter binding in vivo in major depressive disorder. Synapse. 2009;63:565–573. doi: 10.1002/syn.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE the E-Risk Study Team. Teen-aged mothers in contemporary Britain. Journal of Child Psychology and Psychiatry. 2002;43:727–742. doi: 10.1111/1469-7610.00082. [DOI] [PubMed] [Google Scholar]
- Mondelli V, Dazzan P, Hepgul N, Di Forti M, Aas M, D’Albenzio A, Di Nicola M, Fisher H, Handley R, Marques TR, Morgan C, Navari S, Taylor H, Papadopoulos A, Aitchison KJ, Murray RM, Pariante CM. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophrenia Research. 2010;116:234–242. doi: 10.1016/j.schres.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J, Derom C, Vlietinck R, Kohn N, Lesser M, Gregersen PK. Commitment to X inactivation precedes the twinning event in monochorionic MZ twins. American Journal of Human Genetics. 1998;63:339–346. doi: 10.1086/301978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Brocke B, Fries E, Lesch KP, Kirschbaum C. The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology. 2010;35:289–296. doi: 10.1016/j.psyneuen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nature Reviews Neuroscience. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Naumova OY, Lee M, Koposov R, Szyf M, Dozier M, Grigorenko EL. Differential patterns of whole-genome DNA methylation in institutionalized children and children raised by their biological parents. Development and Psychopathology. 2011;24:143–155. doi: 10.1017/S0954579411000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Foley DL, Parkinson-Bates M, Byrnes G, McKenzie M, Patton GC, Morley R, Anney RJ, Craig JM, Saffery R. Prospects for epigenetic research within cohort studies of psychological disorder: a pilot investigation of a peripheral cell marker of epigenetic risk for depression. Biological Psychology. 2010;83:159–165. doi: 10.1016/j.biopsycho.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I, Danese A, Bowes L, Shakoor S, Ambler A, Pariante C, Papadopoulos A, Caspi A, Moffitt TE, Arseneault L. A discordant MZ twin design shows blunted cortisol reactivity among bullied children. Journal of the American Academy of Child and Adolescent Psychiatry. 2011a;50:574–582. doi: 10.1016/j.jaac.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet-Morin I, Odgers C, Danese A, Bowes L, Shakoor S, Papadopoulos A, Caspi A, Moffitt TE, Arseneault L. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12 year-old children. Biological Psychiatry. 2011b;70:1016–1023. doi: 10.1016/j.biopsych.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics. 2008;147B:543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Balding DJ, Beck S. Epigenome-wide association studies for common human diseases. Nature Reviews Genetics. 2011;12:529–541. doi: 10.1038/nrg3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakyan VK, Down TA, Thorne NP, Flicek P, Kulesha E, Graf S, Tomazou EM, Backdahl L, Johnson N, Herberth M, Howe KL, Jackson DK, Miretti MM, Fiegler H, Marioni JC, Birney E, Hubbard TJ, Carter NP, Tavare S, Beck S. An integrated resource for genome-wide identification and analysis of human tissue-specific differentially methylated regions (tDMRs) Genome Research. 2008;18:1518–1529. doi: 10.1101/gr.077479.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A, Picchioni MM, Kalidindi S, Loat CS, Knight J, Toulopoulou T, Vonk R, van der Schot AC, Nolen W, Kahn RS, McGuffin P, Murray RM, Craig IW. Differential methylation of the X-chromosome is a possible source of discordance for bipolar disorder female monozygotic twins. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics. 2008;147B:459–462. doi: 10.1002/ajmg.b.30616. [DOI] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biological Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Epidemiological methods to tackle causal questions. International Journal of Epidemiology. 2009;38:3–6. doi: 10.1093/ije/dyn253. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Hormones and Behavior. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Shakoor S, Jaffee SR, Andreou P, Bowes L, Ambler AP, Caspi A, Moffitt TE, Arseneault L. Mothers and children as informants of bullying victimization: results from an epidemiological cohort of children. Journal of Abnormal Child Psychology. 2011;39:379–387. doi: 10.1007/s10802-010-9463-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. British Medical Bulletin. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Talens RP, Boomsma DI, Tobi EW, Kremer D, Jukema JW, Willemsen G, Putter H, Slagboom PE, Heijmans BT. Variation, patterns, and temporal stability of DNA methylation: considerations for epigenetic epidemiology. Federation of the American Societies for Experimental Biology. 2010;24:3135–3144. doi: 10.1096/fj.09-150490. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormone and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Human Molecular Genetics. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nature Reviews Neuroscience. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: preliminary findings in healthy adults. PloS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic–pituitary–adrenal function. Biological Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Molecular Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- Vazquez DM, Neal CR, Jr, Patel PD, Kaciroti N, Lopez JF. Regulation of corticoid and serotonin receptor brain system following early life exposure of glucocorticoids: long term implications for the neurobiology of mood. Psychoneuroendocrinology. 2012;37:421–437. doi: 10.1016/j.psyneuen.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhaber K, Riemann D, Feige B, Kuelz A, Kirschbaum C, Voderholzer U. Impact of experimentally induced serotonin deficiency by tryptophan depletion on saliva cortisol concentrations. Pharmacopsychiatry. 2005;38:87–94. doi: 10.1055/s-2005-837808. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, Moffitt TE, Mill J. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5:516–526. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Pratchett LC, Buxbaum J, Ising M, Holsboer F. Putative biological mechanisms for the association between early life adversity and the subsequent development of PTSD. Psychopharmacology. 2010;212:405–417. doi: 10.1007/s00213-010-1969-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.