Abstract
An 8-month-old golden retriever/standard poodle mixed breed dog was diagnosed with cricopharyngeal dysphagia and possibly asynchrony. After failing standard unilateral cricopharyngeal myectomy, the dog underwent a second surgery to completely resect the contralateral musculature resulting in immediate and complete resolution of clinical signs. Bilateral myectomy can be considered for dogs with cricopharyngeal dysphagia that fail unilateral myectomy.
Résumé
Traitement réussi de la dysphagie cricopharyngienne avec une myectomie bilatérale chez un chien. Un chien de race croisée Golden retriever/Caniche standard âgé de 8 mois a été diagnostiqué avec de la dysphagie cricopharyngienne et une asynchronie possible. Après un échec de la myectomie cricophryngienne unilatérale standard, le chien a subi une deuxième chirurgie pour réséquer complètement la musculature controlatérale, ce qui a produit une résolution immédiate et complète des signes cliniques. La myectomie bilatérale peut être considérée pour les chiens atteint de dysphagie cricopharyngienne pour lesquels la myectomie unilatérale est un échec.
(Traduit par Isabelle Vallières)
Introduction
Cricopharyngeal dysphagia is a rare swallowing disorder (1,2). Although the causes are usually unknown, most cases are thought to be a congenital neuromuscular disorder that results in an inability to transport a normally propelled pharyngeal bolus through the upper esophageal sphincter (1–4). Manifestations include repeated swallowing attempts, gagging, retching, regurgitation, and aspiration (2–5). The disorder has been further categorized as cricopharyngeal achalasia or cricopharyngeal asynchrony. Achalasia is failure of the upper esophageal sphincter to open during swallowing while asynchrony is lack of coordination between pharyngeal contraction and upper esophageal sphincter relaxation (1–4). Clinically, the processes are indistinguishable, and most veterinary reports do not differentiate between asynchrony and achalasia (1,5–8). This report highlights the difficulties in accurately distinguishing achalasia from asynchrony and questions whether the distinction is necessary. Most importantly, we describe a case of cricopharyngeal dysphagia in a young dog that was successfully managed only after bilateral cricopharyngeal myectomy was performed.
Case description
An 8-month-old, 16.5-kg, castrated male golden retriever/standard poodle mixed breed dog was evaluated at the Michigan State University Veterinary Teaching Hospital (MSU-VTH) for difficulty swallowing food and water since 5 wk of age. The dog made repeated swallowing attempts, but often gagged, retched, and then expelled both food and liquid. Multiple feeding strategies, including upright feedings, had been attempted with minimal success. At the time of evaluation, owners were feeding 1 to 2 moistened kibbles at a time, with an entire feeding lasting approximately 45 min. This was repeated several times daily. In addition to dysphagia, clinical signs included failure to gain weight, coughing, bloating, intermittent nasal discharge with epistaxis, and progressive lethargy. Empiric therapy consisting of antacids, antiemetics, antibiotics, and pro-motility agents had been used without clinical improvement.
On physical examination, the dog was bright and alert and had a rectal temperature of 39°C [reference interval (RI): 37.5°C to 39°C]. Cardiothoracic auscultation revealed harsh, increased cranioventral bronchovesicular sounds. Coughing was elicited with tracheal palpation near the thoracic inlet. The dog was in poor body condition (BCS 2/9). The dog’s cranial abdomen was mildly distended but non-painful, attributed to gastric gas accumulation. Cranial nerve examination and oral cavity evaluation were normal.
Initial diagnostics included a complete blood cell count, serum biochemical profile, thoracic radiographs, and contrast fluoroscopic evaluation of swallowing. Hematologic evaluation revealed mild non-regenerative anemia (hematocrit = 36.0%, RI: 41.0% to 55.0%) and a leukocytosis characterized by segmented neutrophilia (neutrophil count = 17.4 × 109/L, RI: 4.00 to 8.20 × 109/L), lymphocytosis (lymphocyte count = 4.7 × 109/L, RI: 0.8 to 3.6 × 109/L), and monocytosis (monocyte count = 1.7 × 109/L, RI: 0.0 to 0.9 × 109/L). Serum biochemical analysis revealed hypoalbuminemia (23 g/L, RI: 28 to 40 g/L). A serum thyroid profile (MSU Diagnostic Center for Population and Animal Health, Lansing, Michigan, USA) was normal. Testing for the presence of acetylcholine receptor autoantibodies (Comparative Neuromuscular Laboratory, UC San Diego School of Medicine, La Jolla, California, USA) was not consistent with acquired myasthenia gravis. Thoracic radiographs revealed likely aspiration pneumonia and aerophagia: severe alveolar pattern in the right cranial lung lobe, severe interstitial pattern in the right middle and left cranial lung lobes, and a diffuse bronchial pattern. The stomach was moderately gas-distended.
Contrast digital fluoroscopic analysis of swallowing was performed initially with the dog standing using liquid barium sulfate (Liquid Polibar; E-Z-EM Canada, Lake Success, New York, USA) mixed with soft food and kibble. The study was repeated in right lateral recumbency so that a higher frame rate (1/30 s) could be achieved with less patient motion (Figure 1). Normal stripping motion and bolus formation were seen in the oropharynx. The pharyngeal muscles would contract in response to a bolus; however, upper esophageal sphincter relaxation was highly variable, occurring more frequently in lateral recumbency. Successful swallows were observed every 12 to 13 pharyngeal contractions in standing position versus every 5 to 7 contractions in lateral recumbency. The upper esophageal sphincter relaxation was delayed in successful swallows, occurring 0.37 to 0.5 s after epiglottic closure. Food boluses would remain in the pharynx for extended periods of time. Intermittently, small amounts of kibble would pass through the upper esophageal sphincter when it opened. Once food entered the cervical esophagus, motility varied from normal to delayed. Mild laryngotracheal aspiration was also observed. The radiographic diagnoses were marked cricopharyngeal dysphagia, probable asynchrony rather than achalasia, and possible decreased cervical esophageal motility.
Figure 1.
Images from right laterally recumbent contrast digital fluoroscopic examination. A — Pre-pharyngeal contraction, seen as large ventrodorsal diameter of the pharynx, denoted by white line. White arrow depicts residual barium sulfate suspension in the contracted upper esophageal sphincter canal. Soft food admixed with barium suspension is present in the caudal laryngopharynx as well as within cranial esophagus (off edge of image) from previous swallowing attempt. B — Late pharyngeal contraction phase, during which the food and barium lie against a closed upper esophageal sphincter (white arrow). Closed pharyngeal lumen is denoted by black arrow. Food and barium are retained in the cranial esophagus.
Long-term medical management options, including gastrostomy tube placement, were discussed with the owners. Surgical intervention consisting of a cricopharyngeal myectomy was discussed as a last resort due to the guarded prognosis for asynchrony, questionable efficacy, and potential for worsening of clinical disease as speculated by some authors (1–4). While awaiting therapeutic decisions, the dog was discharged with doxycycline (Mutual Pharmaceutical Co, Philadelphia, Pennsylvania, USA), 4.5 mg/kg body weight (BW), PO, q12h for 3 wk, for treatment of aspiration pneumonia. Within days, the dog had marked improvement in lethargy and coughing, but the dysphagia was unchanged. Due to the severity of clinical signs and unwillingness to manage a feeding tube long-term, the owners elected surgical intervention.
Two weeks after initial evaluation, the dog returned to MSU-VTH for surgery. The dog was premedicated with methadone (Bioniche Pharma USA, Lake Forest, Illinois, USA), 0.5 mg/kg BW, IM, and acepromazine (Aceproject; Butler Animal Health Supply, Dublin, Ohio, USA) 0.03 mg/kg BW, IM. Anesthesia was induced with propofol (PropoFlo; Abbott Laboratories, North Chicago, Illinois, USA), 6 mg/kg BW, IV, and maintained via endotracheal intubation with isoflurane (IsoFlo; Abbott Laboratories) in 100% oxygen. Prior to intubation, pharyngeal and laryngeal examination was normal. Cefazolin (Hospira, Lake Forest, Illinois, USA), 22 mg/kg BW, IV, was administered after induction. Cricopharyngeal and partial thyropharyngeal myectomy via a left-lateral approach were performed as described elsewhere (5,7,9). The dog was positioned in right lateral recumbency, and the site was prepared in a routine manner. A 10-cm incision was made beginning at the level of the cranial larynx and extended caudally, ventral to the jugular vein. The platysma and paroditoauricularis muscles were incised. A 14 French red rubber catheter was placed (PO) in the esophagus to aid in palpation of the esophagus and its surrounding musculature. The larynx and esophagus were rotated 90° longitudinally to expose the dorsal aspect. The cricopharyngeal and thyropharyngeal muscles were identified. As the muscle fibers of the cricopharyngeus pass dorsally, they blend with the muscularis of the esophagus (10,11). For this reason, an approximately 2-cm section of the cricopharyngeal muscle, the caudal portion of the thyropharyngeal muscle and the esophageal muscularis were sharply dissected from the esophageal submucosa using tenotomy scissors, encompassing most of the left side and across the dorsal midline (Figure 2). The muscles appeared normal on gross inspection. Inadvertently, a small puncture into the esophageal lumen was created during dissection. The defect was closed using a single interrupted suture of 4-0 polydioxanone (PDS II; Ethicon, Somerville, New Jersey, USA). The resected tissue was submitted for histopathologic examination. The deep and superficial subcutaneous tissues were closed separately with 4-0 poliglecaprone 25 (Monocryl; Ethicon) in a simple continuous pattern, and the skin was closed with 4-0 poliglecaprone 25 in a simple continuous intradermal pattern. Recovery from surgery and anesthesia was uneventful, and the patient was discharged 1 d after surgery and prescribed tramadol (Amneal Pharmaceuticals, Glasgow, Kentucky, USA), 3 mg/kg BW, PO, q8h for 3 d.
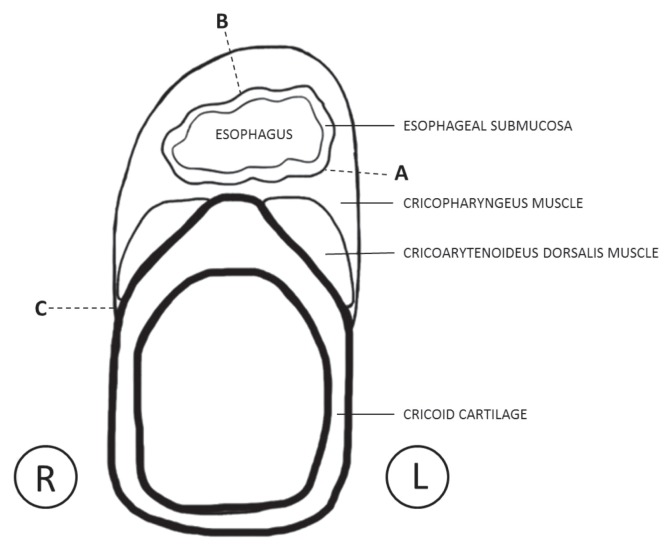
Figure 2.
Line drawing depicting a transverse section of the esophagus and larynx taken through the level of the cricoid cartilage. The region between dotted lines A and B represents the tissue removed in the first surgery. The region between dotted lines B and C represents the tissue removed in the second surgery. Sectioning of the muscles at dotted line C denotes the area of the cricopharyngeal muscle that was thickened and vascular upon sectioning.
Histopathology of the cricopharyngeal and thyropharyngeal muscles (Figure 3) was characterized by mild multifocal myofiber degeneration and atrophy. Low numbers of macrophages and eosinophils were present amongst myofibers. Low numbers of eosinophils accompanied by occasional mast cells were scattered throughout the perimysial connective tissue, often surrounding blood vessels. Rare attempts of myofiber regeneration (single amphophilic myofibers with plump nuclei) were also seen. Based on the mild eosinophilic infiltrate, serologic evaluations (IgG and IgM) for toxoplasmosis and neosporosis were performed, and results were negative.
Figure 3.
Photomicrograph of a section of the cricopharyngeal muscle. A — There is mild variation of the myofiber size, characterized by the presence of scattered single or small contiguous groups of mildly to moderately smaller and angular myofibers and scattered slightly enlarged myofibers. Some myofibers (< 10% of the total myofibers) have paracentral nuclei. The perimysium and endomysium are expanded by loose connective tissue. H&E stain; Bar = 200 μm. B — One myofiber near the center of the image is necrotic, with fragmentation of the sarcoplasm and infiltration by low number of macrophages and eosinophils. Low number of eosinophils are also scattered throughout the adjacent perimysial connective tissue. H&E stain; Bar = 50 μm.
There was no improvement following surgery. In fact, owners reported slight worsening of dysphagia. One week later, the dog returned for further evaluation. The dog made repeated swallowing attempts and often coughed during visual observation. Contrast fluoroscopy was repeated with similar findings to the previous study. In addition to marked cricopharyngeal dysphagia, moderate laryngotracheal aspiration and nasopharyngeal reflux were noted. An additional region of alveolar infiltrate was now present in the left cranial lung lobe.
The owners still opposed feeding tube placement and were considering euthanasia. Given the lack of alternative options, the decision was made to repeat the surgical procedure on the contralateral side. Two weeks after the initial surgery, the dog returned for right-sided cricopharyngeal and thyropharyngeal myectomy. Anesthesia protocol and antibiotic therapy were identical to the first surgery. The same approach used previously was performed on the right side. However, on this side, the caudal 10 mm of the cricopharyngeal muscle appeared slightly thickened and more vascular at its origin on the lateral aspect of the cricoid. Upon resection of this muscle from its origin with tenotomy scissors, the muscle felt coarse and granular and hemorrhaged more than the left side had previously. The entire remaining cricopharyngeus muscle, caudal thyropharyngeus muscle, and esophageal muscularis were excised (Figure 2), but were not submitted for histopathologic examination due to owner financial constraints. The deep and superficial subcutaneous tissues and skin were closed as previously described. Recovery from surgery and anesthesia was again uneventful, with similar post-operative pain management.
Several hours following surgery, the dog was offered a small meatball of canned food which was immediately consumed without any observable dysphagia. The dog was discharged from the hospital the day of surgery with instructions to offer several small moistened kibbles frequently each day. Over several days, feeding volumes were gradually increased until caloric requirements were met. The dog was reported to have a ravenous appetite and no difficulty swallowing food or water. The dog was transitioned to dry kibble which was also well-tolerated without any observed gagging, repeated swallowing attempts, nasal reflux, or coughing. Within 10 days, the dog was eating 2 cups of dry kibble twice daily without any observable dysphagia. The only owner concern at this time was increased aerophagia resulting in frequent belching and flatulence. Feedings were given over several minutes as the dog would otherwise attempt to consume all kibble within seconds, which worsened the aerophagia.
Two weeks after the second surgery, the dog was bright and alert, had no dysphagia, and had gained 2 kg. Flatulence and belching were occasionally noted during visual observation. Other physical examination findings remained normal. A third contrast fluoroscopic evaluation was performed (Figure 4). Prior to contrast administration, the cranial thoracic esophagus, stomach, and small intestines were moderately gas dilated. The oropharyngeal phase was normal. The region of the previous cricopharyngeal sphincter remained mildly, but persistently open throughout the study. Pharyngeal contraction propelled all boluses through the opening into the esophagus, and esophageal motility was normal. Aerophagia occurred during food consumption. The findings were the same with kibble and soft food.
Figure 4.
Images from standing contrast digital fluoroscopic examination after bilateral cricopharyngeal and thyropharyngeal myectomy. A — Prepharyngeal contraction, similar to Figure 1, with open pharynx depicted by the black line. Black arrow shows a persistently, mildly open upper esophageal sphincter, even in absence of a food bolus; soft food admixed with barium suspension is seen just cranioventral to the sphincter. Black circles superimposed on the cranial esophagus are Lucite bars present on a restraint apparatus made for standing esophagram. B — Early pharyngeal contraction, during which a soft food and barium suspension bolus (white arrow) may be seen traveling through an open esophageal sphincter. Closed pharynx is depicted by the black arrow. Black circles representing Lucite bars are seen over the cranial esophagus.
Eight weeks after the second surgery, the dog was in good body condition (BCS 4/9) and had gained an additional 3.5 kg. Physical examination was normal. The owners reported that flatulence and belching had significantly reduced in frequency and severity. In a follow-up conversation 1 year after the second surgery, the owners reported the dog was clinically normal with no belching or flatulence.
Discussion
Although structural abnormalities such as traumatic injury, congenital malformations, strictures, and neoplasia can affect swallowing, cricopharyngeal dysphagia is usually a functional abnormality (2–4). It is most common in young dogs and is due to abnormalities in the upper esophageal sphincter, which is composed of the caudal pharyngeal constrictors (the cricopharyngeus and thyropharyngeus muscles) (1,2,4,11). Cranial nerve X is intimately involved in this swallowing phase, and cricopharyngeal dysphagia can be induced by transecting the pharyngeal branch (1,12).
There are no reported veterinary cases in which bilateral approaches were used to completely resect the cricopharyngeus muscle and caudal portion of the thyropharyngeus muscle from one side of the cricoid and thyroid wing to the other. Previously described techniques have included cricopharyngeal myotomy, cricopharyngeal myectomy, cricopharyngeal and thyropharyngeal myotomy, and cricopharyngeal and thyropharyngeal myectomy (5–7,13–15). These surgeries have been performed either via a ventral midline approach, in which the larynx and upper esophageal unit is rotated to expose the relevant muscles, or through a lateral approach, similar to that used when performing a cricoarytenoid laryngoplasty (5–7,9,13–15). Overall success rates are variable and inconsistent, with persistent or recurrent dysphagia and aspiration pneumonia being the most common complications in about 50% of cases (15). The widely varied outcomes may reflect the surgical technique or underlying pathogenesis of the condition.
One of the more challenging technical aspects of cricopharyngeal myectomy is ensuring the muscle is completely transected, as the muscle fibers blend with those of the esophagus, and it can be difficult to distinguish between cricopharyngeal muscle fibers, blended muscle fibers, and muscularis fibers, until the submucosa is visualized (Figure 5) (10,11). The dog in this case improved dramatically only after a far more aggressive resection was performed. The bilateral procedures included complete resection of the cricopharyngeus muscle and underlying esophageal muscularis, and the caudal portion of the thyropharyngeus. In the authors’opinion, the accurate identification and meticulous dissection of both of the cricoid insertions of the cricopharyngeus and removal of the esophageal muscularis are critical.
Figure 5.
Histopathological cross-sectional image at the level of the cricoid from a necropsied normal dog, H&E stain. The esophageal submucosa (E) is surrounded by its muscularis (M). Note how the muscle fibers of the cricopharyngeus (CP) blend with the muscularis of the esophagus as they course dorsally over the esophagus (B), effectively becoming a single layer of muscle fibers.
Bilateral aggressive surgical intervention was deemed appropriate for the dog in this report given the absence of other identifiable neuromuscular disease, as owners were unwilling to manage a feeding tube. The reason bilateral resection was necessary is unclear as the first myectomy was aggressive, and both surgeries were performed by the same experienced surgeon. Given the abnormal findings at the time of the second surgery, it is possible that disease was more severe or even confined to one side. But given that pathology was seen on the left-sided biopsy, it is also possible that the overall severity of this dog’s condition necessitated such a radical resection. One limitation in our report is the absence of histopathology for the right-sided cricopharyngeal muscle.
Regardless of disease localization and severity, it is clear from this case and previous reports that arbitrary unilateral myectomy is not sufficient to resolve all cases of cricopharyngeal dysphagia (7). Interestingly, botulinum toxin injection into both sides of the cricopharyngeus muscles has successfully alleviated cricopharyngeal dysphagia associated with a variety of neuromuscular diseases in humans, including conditions associated with concurrent pharyngo-laryngeal weakness (16–18). If a positive response to botulinum toxin does predict surgical success (3,19,20), surgical intervention could have a larger role in the management of canine cricopharyngeal dysphagia than previously speculated. Given that bilateral botulinum toxin injection is employed in these studies, it is possible that bilateral surgical myotomy/myectomy would be required in some cases to mimic the success seen with chemical myotomy. Perhaps a more radical bilateral procedure, as performed in the dog in our report, would have led to higher surgical success rates in other veterinary reports (7). This hypothesis requires additional study.
The dog reported herein demonstrated features of both asynchrony and achalasia, dependent upon body position during fluoroscopic examination. Although swallowing has been studied extensively in the dog (12,21,22), there is no formal grading or classification scheme that differentiates achalasia from asynchrony. Perceived need to differentiate has evolved as veterinary authors speculate achalasia is readily treated with unilateral cricopharyngeal myotomy or myectomy (1,2,4), while this surgery is speculated to worsen dysphagia and aspiration in animals with cricopharyngeal asynchrony and is generally contraindicated (2–4). Unfortunately, most veterinary reports do not differentiate between these two dysphagias. In fact, some reports define both asynchrony and achalasia, and then state both would be included under cricopharyngeal achalasia (1–6). Other reports only use the term achalasia, but it is clear from case descriptions that animals with asynchrony are included (21,22). The lack of clarity in the veterinary literature has led to much confusion surrounding diagnosis, treatment, and surgical outcome of cricopharyngeal dysphagia. Studies on surgical intervention for cricopharyngeal dysphagia in limited numbers of dogs have reported spectacularly discordant success rates (complete and long-term resolution) ranging from 7% to 100% (5,7). Veterinary authors speculate that the low success rate in one report is due to inclusion of animals with asynchrony or concurrent neuromuscular disease such as myasthenia gravis or laryngeal paralysis (3,7). Currently, most authors recommend gastrostomy tube feeding for management of cricopharyngeal asynchrony, reserving surgical intervention only for cases of confirmed cricopharyngeal achalasia (2–4). However, there is no published evidence to support this practice.
One possibility that should be considered is asynchrony and achalasia are not necessarily exclusive conditions. This distinction does not exist in human reports, and differentiation in veterinary medicine is somewhat arbitrary. They might represent various stages of the same disease, especially when diagnosed in young dogs without other neuromuscular disease. From a functional standpoint, it appears intuitive that animals with asynchrony and achalasia would benefit from cricopharyngeal myectomy as long as pharyngeal function and esophageal motility are normal. There are no studies describing surgical intervention for cases of canine cricopharyngeal asynchrony with normal pharyngeal function, although cases were likely included in the previously mentioned reports on cricopharyngeal achalasia (5–7).
Although most cases of cricopharyngeal dysphagia in young dogs are likely an idiopathic congenital neuromuscular disorder, other differentials should be considered. Hypothyroidism (23), myasthenia gravis (24), and myositis (25) can cause or mimic cricopharyngeal dysphagia. Testing for inflammatory or systemic neuromuscular diseases should therefore be a part of the diagnostic work-up in dogs with cricopharyngeal dysphagia (3,4). This is especially important when considering surgical intervention. Electrodiagnostics could have differentiated between neuropathic and myopathic disease and given the pattern of muscle or nerve involvement. This information is also lacking in most previously reported cases of cricopharyngeal dysphagia (5–7,14), and reported results have ranged from normal to findings consistent with denervation (7). Given the life-long history of dysphagia and negative diagnostic test results, an idiopathic congenital neuromuscular disorder was considered most likely in the dog in our report. In summary, even when using contrast digital fluoroscopy, accurate sub-characterization of cricopharyngeal dysphagia is challenging. However, the distinction between achalasia and asynchrony may be unnecessary. Bilateral aggressive cricopharyngeal and thyropharyngeal myectomy should be considered for cases of cricopharyngeal dysphagia in which a unilateral myectomy was ineffective and other identifiable neuromuscular diseases have been excluded.
Acknowledgments
The authors acknowledge Dr. Ingeborg Langohr and Dr. Dalen Agnew for providing histopathological figures and interpretations.
Footnotes
Supported in part by the Michigan State University College of Veterinary Medicine Trinket Fund.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Elliot RC. An anatomical and clinical review of cricopharyngeal achalasia in the dog. J S Afr Vet Assoc. 2010;81:75–79. doi: 10.4102/jsava.v81i2.108. [DOI] [PubMed] [Google Scholar]
- 2.Jergens AE. Diseases of the esophagus. In: Ettinger SJ, editor. Textbook of Veterinary Internal Medicine. 7th ed. Vol. 2. St. Louis, Missouri: Elsevier Saunders; 2010. pp. 1487–1499. [Google Scholar]
- 3.Marks SL. Revisiting treatment of dysphagia. Am Coll Vet Intern Med Forum, Montreal. 2009:559–560. [Google Scholar]
- 4.Shelton GD. Orophayngeal dysphagias. In: Bonagura JB, Twedt DC, editors. Kirk’s Current Veterinary Therapy. 14th ed. St. Louis, Missouri: Elsevier Saunders; 2009. pp. 479–482. [Google Scholar]
- 5.Niles JD, Williams JM, Sullivan M, Crowsley FE. Resolution of dysphagia following cricopharyngeal myectomy in six young dogs. J Small Anim Pract. 2001;42:32–35. doi: 10.1111/j.1748-5827.2001.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 6.Pfeifer RM. Cricopharyngeal achalasia in a dog. Can Vet J. 2003;44:993–995. [PMC free article] [PubMed] [Google Scholar]
- 7.Warnock JJ, Marks SL, Pollard R, Kyles KE, Davidson A. Surgical management of cricopharyngeal dysphagia in dogs: 14 cases (1989–2001) J Am Vet Med Assoc. 2003;223:1462–1468. doi: 10.2460/javma.2003.223.1462. [DOI] [PubMed] [Google Scholar]
- 8.Watrous BJ. Clinical presentation and diagnosis of dysphagia. Vet Clin North Am Small Anim Pract. 1983;13:438–458. doi: 10.1016/s0195-5616(83)50052-1. [DOI] [PubMed] [Google Scholar]
- 9.Kyles AE. Esophagus. In: Tobias KM, Johnston SA, editors. Veterinary Surgery, Small Animal. 1st ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 1461–1483. [Google Scholar]
- 10.Dyce KM. The muscles of the pharynx and palate of the dog. Anat Rec. 1957;127:497–508. doi: 10.1002/ar.1091270302. [DOI] [PubMed] [Google Scholar]
- 11.Hermanson JW. The muscular system. In: Evans HE, De Lahunta A, editors. Miller’s Anatomy of the Dog. 4th ed. St. Louis, Missouri: Elsevier Saunders; 2013. pp. 185–276. [Google Scholar]
- 12.Suter PF, Watrous BJ. Oropharyngeal dysphagias in the dog: A cinefluorographic analysis of experimentally induced and spontaneously occurring swallowing disorders. Vet Radiol. 1980;21:24–30. [Google Scholar]
- 13.Rosin E. Cricopharyngeal dysphagia. In: Bojrab MJ, editor. Current Techniques in Small Animal Surgery. 4th ed. Baltimore, Maryland: Williams & Wilkins; 1998. pp. 145–147. [Google Scholar]
- 14.Sokolovsky V. Cricopharyngeal achalasia in a dog. J Am Vet Med Assoc. 1967;150:281–285. [PubMed] [Google Scholar]
- 15.Papazoglou LG, Mann FA, Warnock JJ, et al. Cricopharyngeal dysphagia in dogs: The lateral approach for surgical management. Comp Cont Educ Pract Vet. 2006;28:696–704. [Google Scholar]
- 16.Restivo DA, Marchese Ragona R, Staffieri A, De Grandis D. Successful botulinum toxin treatment of dysphagia in oculopharyngeal muscular dystrophy. Gastroenterology. 2000;119:1416. doi: 10.1053/gast.2000.20113. [DOI] [PubMed] [Google Scholar]
- 17.Restivo DA, Palmeri A, Marchese-Ragona R. Botulinum toxin for cricopharyngeal dysfunction in Parkinson’s disease. N Eng J Med. 2002;346:1174–1175. doi: 10.1056/NEJM200204113461517. [DOI] [PubMed] [Google Scholar]
- 18.Woisard-Bassols V, Alshehri S, Simonetta-Moreau M. The effects of botulinum toxin injections into the cricopharyngeus muscle of patients with cricopharyngeus dysfunction associated with pharyngo-laryngeal weakness. Eur Arch Otorhinolaryngol. 2013;270:805–815. doi: 10.1007/s00405-012-2114-4. [DOI] [PubMed] [Google Scholar]
- 19.Moerman MBJ. Cricopharyngeal botox injection: Indications and technique. Curr Opin Otolaryngol Head Neck Surg. 2006;14:431–436. doi: 10.1097/MOO.0b013e328010b85b. [DOI] [PubMed] [Google Scholar]
- 20.Zaninotto G, Marchese Ragona R, Briani C, et al. The role of botulinum toxin injection and upper esophageal sphincter myotomy in treating oropharyngeal dysphagia. J Gastrointest Surg. 2004;8:997–1006. doi: 10.1016/j.gassur.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Pollard RE, Marks SL, Davidson A, Hornof WJ. Quantitative video-fluoroscopic evaluation of pharyngeal function in the dog. Vet Radiol Ultrasound. 2000;41:409–412. doi: 10.1111/j.1740-8261.2000.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 22.Watrous BJ, Suter PF. Normal swallowing in the dog: A cineradiographic study. Vet Radiol. 1979;20:99–109. [Google Scholar]
- 23.Bruchim Y, Kushnir A, Shamir MH. L-thyroxine responsive cricopharyngeal achalasia associated with hypothyroidism in a dog. J Small Anim Pract. 2005;46:553–554. doi: 10.1111/j.1748-5827.2005.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 24.Shelton GD, Willard MD, Cardinet GH, Lindstrom J. Acquired myasthenia gravis: Selective involvement of esophageal, pharyngeal, and facial muscles. J Vet Intern Med. 1990;4:281–284. doi: 10.1111/j.1939-1676.1990.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 25.Ryckman LR, Krahwinkel DJ, Sims MH, Donnell RL, Moore PF, Shelton GD. Dysphagia as the primary clinical abnormality in two dogs with inflammatory myopathy. J Am Vet Med Assoc. 2005;226:1519–1523. doi: 10.2460/javma.2005.226.1519. [DOI] [PubMed] [Google Scholar]