Abstract
The effect of maternal antibodies (MatAb) on immunological priming by neonatal parenteral vaccination for bovine respiratory syncytial virus (BRSV) was addressed for the first time in experimental infection in 34 Holstein calves. Both vaccinated and control calves developed moderate to severe respiratory disease characteristic of acute BRSV infection. There were no differences in clinical signs, BRSV shed, arterial oxygen concentrations, or mortality between vaccinated and control calves after BRSV challenge approximately 11 wk after vaccination. There were no anamnestic antibody or cytokine responses in the vaccinates after challenge. Lung lesions were extensive in both groups, and although there was a statistically significant (P = 0.05) difference between groups, this difference was considered not biologically significant. These data indicate that stimulation of protective immune responses was inhibited by maternal antibodies when a combination modified-live BRSV vaccine was administered parenterally to young passively immune calves. Alternate routes of administration or different vaccine formulations should be used to successfully immunize young calves with good passive antibody transfer.
Résumé
Inhibition de l’amorçage pour les réponses immunitaires protectrices spécifiques pour le virus respiratoire syncytial bovin après la vaccination parentérale des veaux ayant une immunité passive. L’effet des anticorps maternels sur l’amorçage immunologique par une vaccination parentérale néonatale pour le virus respiratoire syncytial bovin (VRS) a été abordé pour la première fois dans une infection expérimentale chez 34 veaux Holstein. Les veaux vaccinés et témoins ont développé une maladie respiratoire de modérée à grave présentant les caractéristiques d’une infection aiguë au VRS. Il n’y avait pas de différences au niveau des signes cliniques, de l’excrétion du VRS, des concentrations d’oxygène artérielle ou de la mortalité entre les veaux vaccinés et témoins après un test de provocation de VRS, environ 11 semaines après le vaccin. Il n’y avait aucune réponse d’anticorps ou de cytokines anamnestiques chez les veaux vaccinés après le test de provocation. Les lésions aux poumons étaient importantes dans les deux groupes et, même s’il y avait une différence statistiquement significative (P = 0,05) entre ces groupes, cette différence n’était pas considérée significative sur le plan biologique. Ces données indiquent que la stimulation des réponses immunitaires protectrices a été inhibée par les anticorps maternels lors de l’administration parentérale d’une combinaison de vaccin à VRS vivant modifié aux jeunes veaux ayant une immunité passive. D’autres voies d’administration ou différentes formulations de vaccins devraient être utilisées pour immuniser avec succès les jeunes veaux ayant un bon transfert passif.
(Traduit par Isabelle Vallières)
Introduction
Maternal antibodies (MatAb) can have life-saving disease-sparing effects in a variety of neonatal infections (1). This has been demonstrated in epidemiological and laboratory studies of bovine respiratory syncytial virus (BRSV), the leading cause of viral pneumonia in calves (2–4). In order to protect calves from disease when their variable initial concentrations of MatAb decay to non-protective levels at different times (1–5), and to prime calves for protective active immune responses, there is increasing interest in vaccinating early in calfhood. Correspondent to the protective effects of MatAb are their inhibitory effects on vaccination (1). These effects have been widely documented in veterinary medicine following parenteral vaccination for infections as disparate as canine distemper virus and bovine viral diarrhea virus, but have been less clear in the case of BRSV (1). Mucosal delivery of vaccines is more likely to “override” passive immunization and prime the immune system in the passively immune young animal (6,7); however, because of differences in management and veterinarian and producer preference, there continues to be interest in and widespread use of parenteral vaccination of calves with MatAb (8,9).
There are few and conflicting data concerning the ability of parenteral BRSV vaccines to stimulate protective immune responses in calves, further adding to the confusion regarding the use and efficacy of these vaccines in young calves. Some of this is due to the inconsistency in outcome variables that are measured, such as only antibodies and other in vitro parameters in the absence of challenge (10,11), and, more importantly, variability in challenge models that have been used to assess vaccine efficacy, all of which produced only minimal or no disease (6,12,13), making it difficult to determine the robustness of induced responses. The purpose of this study was to investigate the immune stimulatory effects of parenteral vaccination with a typical combination modified-live viral vaccine containing BRSV in calves with moderate to high concentrations of MatAb against the virus, using a challenge model that mimics naturally occurring disease and has been employed to clearly demonstrate the efficacy of similar vaccines (14) in seronegative calves that are the usual candidates for licensing trials.
Materials and methods
Calves
Newborn Holstein calves were fed 2.1 L of a reconstituted commercial colostrum replacement product (Calf’s Choice Total; The Saskatoon Colostrum Company, Saskatoon, Saskatchewan) containing a total of 150 g of IgG that is BRSV antibody positive. The mean BRSV ELISA unit value in the reconstituted colostrum is 102 ELISA units compared to 100 units in the hyperimmune serum positive control similarly diluted. All calves were given 1.5 mL of tulathromycin (Draxxin; Pfizer Animal Health, Whitby, Ontario) subcutaneously, and 2 mL of a modified-live combination bovine coronavirus and bovine rotavirus vaccine (Calfguard; Pfizer Animal Health, Whitby, Ontario) intranasally, were tested for bovine diarrhea virus (15), and were reared as previously described (14).
Vaccines
A combination “5-way” vaccine (Vista 5 SQ; Merck Animal Health, Summit, New Jersey, USA) containing modified-live BRSV, bovine parainfluenza-3 virus (BPIV-3), bovine herpesvirus-1 (BHV-1), and bovine viral diarrhea virus BVDV) types 1 and 2, and a “3-way” (BHV-1, BVDV 1,2) control vaccine (Vista 3 SQ; Merck Animal Health), which did not contain BRSV, were obtained from the manufacturer. These vaccines are licensed for calves > 3 months of age, but the BRSV component has been previously shown (7) to stimulate disease-sparing responses when administered subcutaneously to 3- to 8-day-old calves at a minimum immunizing dose (approximately 1/100 commercial release dose).
BRSV challenge inoculum
The challenge inoculum consisted of lung wash obtained from a newborn calf infected with BRSV (Asquith strain; 17). The lung wash was confirmed negative for bacterial contamination, Mycoplasma sp., and BHV-1, BPIV-3, and bovine viral diarrhea viruses by use of standard diagnostic methods (16,17).
Clinical assessment
Calves were observed for clinical signs as previously described (Table 1, 16) post-vaccination, on days −1 and 0 prior to challenge, and on days 1 through 8 after challenge with the BRSV inoculum. Calves were euthanized on day 8 after challenge or earlier on the basis of predetermined criteria (16) if 2 clinical signs indicative of substantial respiratory tract disease were observed, including, signs of moderate depression, dull eyes, droopy ears, rough coat, gauntness, and moderate respiratory distress or dyspnea (> 100 breaths/min) were observed for 2 consecutive days. Calves were euthanized immediately if they were observed at any time with severe respiratory distress, for example, pronounced open-mouthed, labored breathing (as evidenced by an expiratory grunt), if they were severely depressed and recumbent with total reluctance to rise, or if they had a PaO2 < 45 mmHg. These criteria were consistent with Canadian Council of Animal Care guidelines and were approved by the Committee on Animal Care and Supply at the University of Saskatchewan.
Table 1.
Clinical scoring of calves after challenge
| Depression |
| 0 = normal, 1 = mild — moves slowly, head down; 2 = moderate — tends to lie down, staggers; 3 = severe — recumbent or stands with difficulty |
| Respiratory rate |
| 0 = ≤ 44 breaths/min (BPM); 1 = 45 to 64 (BPM); 2 = 65 to 80 (BPM); 3 = ≥ 81 (BPM) |
| Dyspnea |
| 0 = normal, 1 = mild — short and rapid; 2 = moderate — labored, abdominal, 3 = severe — very labored, grunting |
| Cough (cough scores were assigned to calves with spontaneous coughing during the clinical exam observation period; approximately 1 h/d) |
| 0 = < 3 episodes, 1 = 3+ episodes |
Sample collection
Deep nasal swab specimens were taken from both nares prior to challenge and on days 2 through 8 after challenge for virus isolation (15,16). Serum samples were obtained from blood collected by jugular venipuncture. Arterial blood samples were collected from the caudal thoracic aorta (18) and PaO2 (mmHg) measurements, corrected for rectal temperature, were performed as previously described (16).
Quantitative virus isolation
Virus shedding was quantitatively determined by use of a microisolation plaque assay with bovine embryonic lung fibroblasts (19).
Postmortem analysis
On necropsy, the respiratory tract of each calf was harvested and analyzed for percentage of pneumonic tissue as previously described (16). Selected sections of lung were immunohistochemically stained for BRSV antigens as previously described (20).
Cytokine assays
Bovine cytokines, interferon gamma (IFNγ) and interleukin-4 (IL-4), produced by T helper 1 and T helper 2 lymphocytes, respectively (21), were measured in plasma from 24-h BRSV-stimulated whole blood cultures (16) using antigen capture ELISAs that were obtained from the manufacturer (Pierce Biotechnology, Rockford, Illinois, USA).
Antibody assays
The BRSV neutralization tests, BRSV-specific IgG ELISAs for serum and colostral antibodies and a BRSV-specific IgA ELISA for nasal secretions were performed and analyzed as previously described (16,22).
Experimental design
Three- to 9-day-old seropositive calves were randomized (by coin flip) into 2 groups: 17 calves (vaccinates) were given the 5-way commercial vaccine subcutaneously in the right cervical area, and 17 (controls) were given the 3 way commercial vaccine. The groups were housed in alternate individual pens in the same air space in a calf barn (14) and challenged as previously described (16) at approximately 11 wk after vaccination. Briefly, the calves were challenged by aerosol delivery of BRSV into an enclosed, approximately 7.3 × 2.4 × 2.4 m, transport (stock) trailer (42 m3 of air space). For aerosol delivery, 30 mL of in vivo-passaged BRSV inoculum (103.4 pfu/mL) were placed in each of 3 ultrasonic nebulizers (Ultra-Neb 99, Devilbiss, Somerset, PA) that were placed equidistant approximately 1.8 m off the floor of the trailer. After approximately 30 min in the sealed trailer, the calves were removed from the trailer and maintained as a single group in 1 large covered pen (16).
Data analysis
Clinical and laboratory outcome variables were stratified by experimental group (i.e., controls and vaccinates) and descriptive statistics were performed. Measurements with repeated observations were summarized to reflect clinically important outcomes and these calculated parameters were then utilized for examining statistical differences between vaccinate and control groups (16). Outcomes that were determined for each calf included, maximum post challenge rectal temperature, proportion of days alive that the post challenge rectal temperature was > 39.6°C, and maximum post-challenge change from baseline rectal temperature. Maximum post-challenge change in calf rectal temperature was calculated by subtracting the calf’s baseline temperature (mean calf temperature for the 2 days immediately preceding challenge) from the maximum rectal temperature achieved post challenge. The maximum concentration of BRSV particles shed per individual prior to euthanasia, and the proportion of days alive that virus particles were shed were also calculated for each calf. A maximum clinical score and the proportion of days alive that a calf had a maximum clinical score of ≥ 1 were also determined. Prior to examining any potential differences between vaccinate and controls, descriptive statistics were performed and data were assessed for normality. Since data were not normally distributed appropriate non-parametric tests were used to compare the differences between treatment groups for the summarized outcomes of interest (16). Summary variables described above were compared between the vaccinate and control groups using the MannWhitney U-test and differences were considered statistically significant where P < 0.05. Differences in mortality rates between the groups were assessed using a Pearson Chi-square test. Associations between pneumonic lesions and PaO2 and antibody responses were assessed using Spearman’s rank correlations (ρ). All statistical analyses were performed using a commercial software program (SPPS 17 for Windows; Analytical Software, Chicago, Illinois, USA).
Results
Clinical signs and mortality
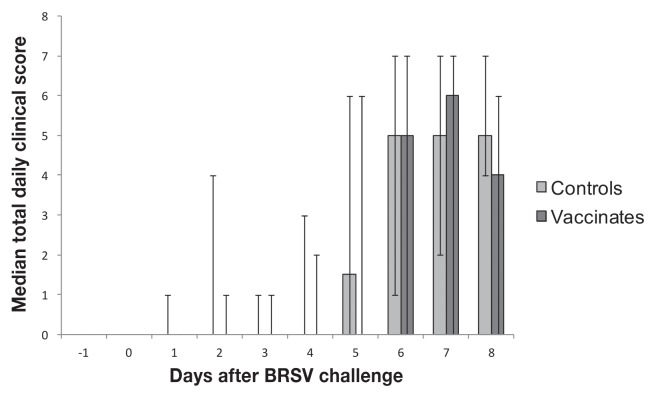
One control calf required euthanasia on day 51 after vaccination due to severe hemorrhagic enterocolitis (coccidiosis), but was free of respiratory disease. Calves in both groups developed variable, often pronounced, signs of respiratory tract disease characteristic of BRSV infection, including pyrexia, cough, dyspnea, and increased respiratory rates after challenge. There were no significant differences between the groups in any of the clinical parameters measured, including the total clinical scores (Figure 1).
Figure 1.
Median of total daily clinical scores in calves before and after BRSV challenge approximately 11 weeks after parenteral vaccination at 3 to 9 d of age with a commercial combination modified-live virus vaccine containing BRSV, or a control-vaccine. The total daily clinical score for each calf was computed from the addition of each parameter evaluated using the clinical scoring guidelines. From these values a median was calculated for each study day for both the control group and the vaccinated group. Error bars represent minimum and maximum total clinical scores recorded for each day.
There was no significant difference (P = 0.60) in mortality (as defined by death or requiring euthanasia prior to termination of the study) between the groups prior to day 8, as 8/17 (47%) vaccinates required euthanasia, 1 on day 6 and 7 on day 7, whereas 9/16 (56%) controls required euthanasia, 5 on day 6 and 4 on day 7.
Nasal shedding of BRSV
No shedding of BRSV was detected in any calf prior to challenge in either of the groups. No differences were found between the groups in maximum shedding of virus (P = 0.93) or between the 2 groups in the proportion of days alive that virus particles were shed (P = 0.74).
PaO2 and pneumonic lesions
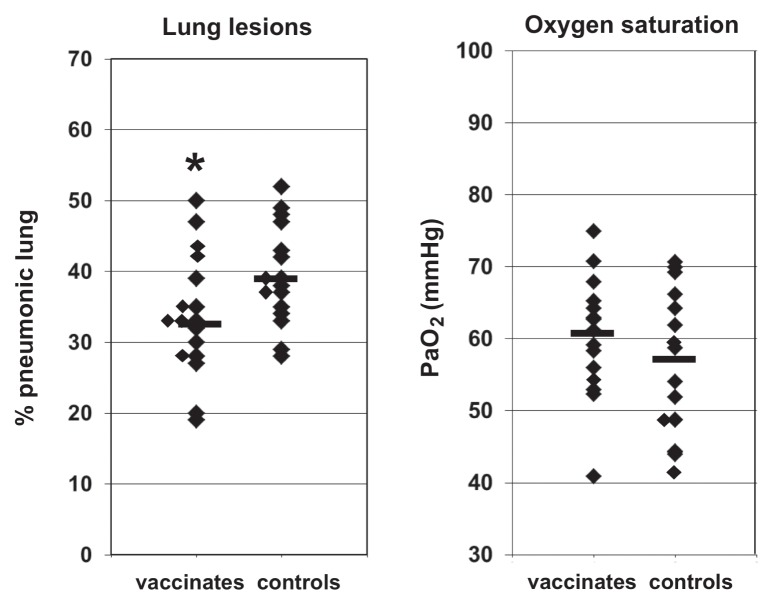
There were no significant differences in arterial blood oxygen concentrations on day 6 after challenge; vaccinated calves had a median PaO2 concentration of 61 mmHg (range: 41 to 75 mmHg), whereas control calves had a median of 57 mmHg (range: 42 to 71 mmHg).
Calves in both groups had extensive pneumonic lesions typical of acute BRSV infection. The median percent of lung affected in the vaccinate group was 33%, (range: 19% to 50% pneumonic lung) and in the control group was 39% (range: 28% to 52% pneumonic lung). Although the vaccinate group had a significantly (P = 0.05) lower percent of lung affected by pneumonic lesions than did the control group, given the extent of the lesions, these differences were not considered biologically significant (Figure 2). Representative sections of affected lung from calves that required euthanasia on day 6 (1 vaccinate, 5 controls) had bronchiolytic lesions typical of BRSV that stained immunohistochemically for BRSV antigens (data not shown).
Figure 2.
Scatter plots of the percentage of lungs affected with pneumonic lesions and arterial oxygen concentrations after BRSV challenge of BRSV-seropositive calves that were either vaccinated parenterally with a commercial combination modified-live virus vaccine containing BRSV, or control-vaccinated, and that were challenged with BRSV approximately 11 weeks after vaccination. Lines indicate median values in each group. *indicates significant difference between groups.
Cytokine responses
No IFNγ or IL-4 was detected in plasma from BRSV-stimulated leukocyte in any calf after vaccination prior to challenge, or after exposure to BRSV.
Antibody responses and correlation analyses
Antibody responses are summarized in Table 2. There were no significant differences between the groups in BRSV-specific IgG or IgA, or virus neutralizing antibodies at any time point, and no anamnestic responses after challenge. There were no significant associations between post challenge serum IgG (P = 0.90) or nasal swab IgA (P = 0.19) antibody responses and percent pneumonic lung lesions, and no significant associations between blood oxygen concentrations and post-challenge serum IgG (P = 0.82) or IgA (P = 0.51) antibody responses.
Table 2.
Bovine respiratory syncytial virus (BRSV)-specific antibody responses at the times of vaccination, challenge and termination after BRSV infection in vaccinate and control group
| Treatment group (Gp) | At vaccination | At challenge | At termination | Conversion rated |
|---|---|---|---|---|
| Vaccinates | 11 wk post VX | |||
| IgGa | 79 (54–86) | 32 (13–43) | 26 (5–47) | 0 |
| VNb | 5.7 (1:24–1:96) | 3.4 (1:8–1:24) | 3.1 (0–1:16) | 0 |
| IgAc | Not done | 0 (0–0.09) | 0 (0–0.06) | 0 |
| Controls | 11 wk post VX | |||
| IgG | 79 (73–87) | 34 (25–49) | 29 (13–46) | 0 |
| VN | 5.8 (1:24–1:128) | 3.4 (1:8–1:16) | 3.0 (1:6–1:16) | 0 |
| IgA | Not done | 0 (0–0.11) | 0 (0–0.06) | 0 |
BRSV-specific IgG ELISA units median and range in serum.
Geometric mean titer (log2 value) and range.
BRSV-specific IgA ELISA median and range in nasal secretions.
≥ 3-fold increase in ELISA units; 4-fold increase in VN.
Discussion
This study has shown that maternal antibodies can substantially inhibit or completely block priming of protective acquired immune responses to BRSV following parenteral administration of a typical commercial modified-live BRSV vaccine. Not only was there inhibition of disease-sparing responses, but, in addition, there was no evidence that the humoral or cell-mediated compartments of the immune system had been primed at all, since, in contrast to seronegative calves successfully immunized with this (7) or similar MLV vaccines (14), or an inactivated BRSV vaccine (23), there were no anamnestic antibody (B lymphocyte) or cytokine (T-helper lymphocyte) responses after exposure to BRSV in the passively immune neonatal calves that had been parenterally vaccinated.
Previous studies support the argument that the observed “blocking” was a maternal antibody, and not a vaccine effect. These studies document the induction of significant disease-sparing responses to BRSV, associated with anamnestic antibody and cell-mediated responses, when BRSV-seronegative calves were vaccinated parenterally with a minimal immunizing dose (MID; about 1/100 of release dose) of the vaccinal BRSV antigen tested herein (7), or other similar vaccines at MID (J. Ellis, unpublished data, 2000–2012), or full commercial doses of similar modified-live combination BRSV vaccines (14), and subsequently similarly challenged with the virus. As well, the aforementioned studies were conducted in young calves (vaccinated as young as 3 days of age), indicating that, while not immunologically mature (1), young cattle can respond with significant disease-sparing immune responses to vaccination early in postnatal life. The mechanism by which MatAb inhibits induction of primary immune responses is unresolved (1,24). It may result from differental antigen signaling in B-cells, involving the B-cell receptor (1,24), during primary versus subsequent exposures to antigen, as has been demonstrated in another model of paramyxoviral infection (24). It is probably not an “all or none” phenomenon, and is likely related to the vaccine antigen:MatAb ratio at the time of vaccination (25) such as could occur in a group of similarly aged calves with variable passive transfer, or in a group of variously aged calves in differing stages of MatAb decay (5). We could not explore those possibilities in this study because all calves were, by design, close in age, and had consistently moderate to high concentrations of BRSV-specific MatAb at the time of neonatal vaccination. It has been suggested, using ELISA as measurement of antibody concentration on a continuous scale, that maternally derived VN antibody must decline to between 1:8 and 1:16 to allow at least 90% of calves to respond to a dose of modified-live BRSV by 28 days after vaccination (26); however, that study did not examine T-cell responses or responses to challenge in vaccinated calves.
It is possible that vaccine formulations for parenteral administration other than the most commonly used combination modified-live immunogens, such as conventional inactivated vaccines, can “override” maternal antibodies and prime young animals; however, at this point convincing data are lacking in the only 2 challenge studies of inactivated vaccines in seropostitive calves published in the last decade (12,13). In one study (12), the efficacy of a single dose of an inactivated combination vaccine that is available in Europe, but not North America, was compared to that of single component modified-live BRSV vaccine or no vaccine in groups of 4- to 5-week-old calves that had moderate concentrations of maternal antibodies post suckling. Six of 7 calves that received the inactivated vaccine compared to 1 calf in each of the other 2 groups seroconverted to BRSV (> 4-fold increase). Unfortunately, the challenge model used produced little or no disease; there were no differences in clinical responses or viral shedding among the groups, and pulmonary pathology was not evaluated. In a second study (13) of the same vaccine using a different challenge model, 5 BRSV MatAb-positive 2-week-old calves were given a single dose of the inactivated vaccine and were compared to 5 unvaccinated controls. Again, there was essentially no clinical disease (only sporadic coughing in individual animals), and the only significant differences between groups in any of the parameters measured was some reduction in the frequency and amount of viral shedding in the vaccinated calves. Presentation of inactivated BRSV antigens in micelle-like immunostimulating complexes (ISCOMs) has recently been shown to stimulate antibody and cell-mediated immune responses that confer a significant reduction in clinical disease and pulmonary pathology in young (2 to 7-week-old) calves with MatAb (27). This technology is an attractive alternative to conventional inactivated vaccines, but has yet to be widely used in commercial vaccine production.
In summary, our results are consistent with the dogma that responses to parenterally administered modified-live viral vaccine antigens can be substantially inhibited by MatAb in young animals (1); however, significant differences in the “lifesyles” of viruses in combination vaccines militate against extrapolations from one pathogen to another, or one species to another, in the absence of data. To our knowledge, this is the first time that inhibition of neonatal responses to parenteral administration of BRSV vaccines by MatAb has been demonstrated using a commercial vaccine in a disease-producing challenge model with sufficient numbers of uniformly passively immune calves to draw statistically sound conclusions. Finally, in conclusion, the simple and practical implication of this study is that in calf herds in which there is good colostrum management and resultant good passive transfer, parenteral immunization of young calves with combination modified-live viral vaccines is unlikely to engender protective primary immune responses to BRSV, and alternative routes of administration (6,7) or formulations other than conventional modified-live vaccines should be used for this application.
Acknowledgments
The study was supported, in part, by Merck Animal Health. The first author (JE) has conducted and conducts similar studies for Pfizer Animal Health, Merial, Merck Animal Health, Fort Dodge Animal Health, Boehringer Ingelheim Vetmedica. The authors thank Trudy and Ed Hupaelo for care of animals in this study and Stacey Lacoste and Carrie Rhodes for technical assistance. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Tizard I. Veterinary Immunology: An Introduction. 9th ed. Philadelphia, Pennsylvania: WB Saunders; 2013. Immunity in the fetus and newborn; pp. 225–239. [Google Scholar]
- 2.Baker JC, Ames TR, Markham RJ. Seroepizootiologic study of bovine respiratory syncytial virus in a dairy herd. Am J Vet Res. 1986;47:240–245. [PubMed] [Google Scholar]
- 3.Van der Poel WH, Kramps JA, Middel WG, Van Oirschot JT, Brand A. Dynamics of bovine respiratory syncytial virus infections: A longitudinal epidemiological study in dairy herds. Arch Virol. 1993;133:309–321. doi: 10.1007/BF01313771. [DOI] [PubMed] [Google Scholar]
- 4.Belknap EB, Baker JC, Patterson JS, Walker RD, Haines DM, Clark EG. The role of passive immunity in bovine respiratory syncytial virus-infected calves. J Infect Dis. 1991;163:470–476. doi: 10.1093/infdis/163.3.470. [DOI] [PubMed] [Google Scholar]
- 5.Beam AL, Lombard JE, Kopral CA, et al. Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations. J Dairy Sci. 2007;92:3973–3980. doi: 10.3168/jds.2009-2225. [DOI] [PubMed] [Google Scholar]
- 6.Kimman TG, Westenbrink F, Staver PJ. Priming for local and systemic antibody memory responses to bovine respiratory syncytial virus: Effect of amount of virus, virus replication, route of administration and maternal antibodies. Vet Immunol Immunopathol. 1989;22:145–160. doi: 10.1016/0165-2427(89)90057-3. [DOI] [PubMed] [Google Scholar]
- 7.Ellis JA, Gow SP, Mahan S, Leyh R. Duration of immunity to experimental infection with bovine respiratory syncytial virus following intranasal vaccination of young passively immune calves. J Am Vet Med Assoc. 2013;243:1602–1608. doi: 10.2460/javma.243.11.1602. [DOI] [PubMed] [Google Scholar]
- 8.Stokka GL. Prevention of respiratory disease in cow/calf operations. Vet Clin N Am Food Anim Pract. 2010;26:229–241. doi: 10.1016/j.cvfa.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorden PJ, Plummer P. Control, management, and prevention of bovine respiratory disease in dairy calves and cows. Vet Clin N Am Food Anim Pract. 2010;26:243–259. doi: 10.1016/j.cvfa.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fulton RW, Briggs RE, Payton ME, et al. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1b, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine. 2004;22:643–649. doi: 10.1016/j.vaccine.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 11.Ellis JA, Hassard L, Cortese V, Morley PS. Effects of perinatal vaccination on humoral and cellular immune responses in cows and young calves. J Am Vet Med Assoc. 1996;208:393–400. [PubMed] [Google Scholar]
- 12.Mawhinney IC, Burrows MR. Protection against bovine respiratory syncytial virus challenge following a single dose of vaccine in young calves with maternal antibody. Vet Rec. 2005;156:139–143. doi: 10.1136/vr.156.5.139. [DOI] [PubMed] [Google Scholar]
- 13.van der Sluijs MT, Kuhn EM, Makoschey B. A single vaccination with an inactivated bovine respiratory syncytial virus vaccine primes the cellular immune response in calves with maternal antibody. BMC Vet Res. 2010;6:2–8. doi: 10.1186/1746-6148-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West K, Petrie L, Konoby C, Haines DM, Cortese V, Ellis JA. The efficacy of modified-live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine. 2000;18:907–919. doi: 10.1016/s0264-410x(99)00324-2. [DOI] [PubMed] [Google Scholar]
- 15.Njaa BL, Clark EG, Janzen E, Ellis JA, Haines DM. Diagnosis of persistent bovine viral diarrhea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens. J Vet Diag Invest. 2000;12:393–399. doi: 10.1177/104063870001200501. [DOI] [PubMed] [Google Scholar]
- 16.Ellis J, Gow S, Goji N. Response of experimentally induced infection with bovine respiratory syncytial virus following intranasal vaccination of seropositive and seronegative calves. J Am Vet Med Assoc. 2010;236:991–999. doi: 10.2460/javma.236.9.991. [DOI] [PubMed] [Google Scholar]
- 17.West K, Petrie L, Haines DM, et al. The effect of formalin-inactivated vaccine on respiratory disease associated with bovine respiratory syncytial virus infection in calves. Vaccine. 1999;17:809–820. doi: 10.1016/s0264-410x(98)00265-5. [DOI] [PubMed] [Google Scholar]
- 18.Will J, Bisgard G. Cardiac catheterization of unanesthetized large domestic animals. J Appl Physiol. 1972;33:400–401. doi: 10.1152/jappl.1972.33.3.400. [DOI] [PubMed] [Google Scholar]
- 19.West K, Bogdan J, Hamel A, et al. A comparison of diagnostic methods for the detection of bovine respiratory syncytial virus in experimental clinical specimens. Can J Vet Res. 1998;62:245–250. [PMC free article] [PubMed] [Google Scholar]
- 20.Haines DM, Clark EG, Chelack BJ. The detection of bovine respiratory syncytial virus in formalin fixed bovine lung with commercially available monoclonal antibodies and avidin biotin complex immunohistochemistry. Can J Vet Res. 1989;53:366–368. [PMC free article] [PubMed] [Google Scholar]
- 21.Tizard I. Veterinary Immunology: An Introduction. 9th ed. Philadelphia, Pennsylvania: WB Saunders; 2013. Helper T cells and their response to antigen; pp. 137–149. [Google Scholar]
- 22.West K, Ellis J. Functional analysis of antibody responses of feedlot cattle to bovine respiratory syncytial virus following vaccination with mixed vaccines. Can J Vet Res. 1997;61:28–33. [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis J, West K, Konoby C, et al. Efficacy of an inactivated respiratory syncytial virus vaccine in calves. J Am Vet Med Assoc. 2001;218:1973–1980. doi: 10.2460/javma.2001.218.1973. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Huey D, Oglesbee M, Niewiesk S. Insights into the regulatory mechanism controlling the inhibition of vaccine-induced seroconversion by maternal antibodies. Blood. 2011;117:6143–6151. doi: 10.1182/blood-2010-11-320317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegrist C-A. Mechanisms by which maternal antibodies influence infant vaccine responses: Review of hypotheses and definition of main determinants. Vaccine. 2003;21:3406–3012. doi: 10.1016/s0264-410x(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 26.O’Neill RG, Fitzpatrick JL, Glass EJ, Williams JL, Woolliams JA. Optimisation of the response to respiratory virus vaccines in cattle. Vet Rec. 2007;161:269–270. doi: 10.1136/vr.161.8.269. [DOI] [PubMed] [Google Scholar]
- 27.Hagglund S, Hu K, Vargmar K, et al. Bovine respiratory syncytial virus ISCOMs-Immunity, protection and safety in young conventional calves. Vaccine. 2011;29:8719–8730. doi: 10.1016/j.vaccine.2011.07.146. [DOI] [PMC free article] [PubMed] [Google Scholar]