Abstract
Alternative donor stem cell transplantation from cord blood or haploidentical peripheral blood donors is increasingly being used for patients who lack a matched related or unrelated donor. A higher nonrelapse mortality (NRM) rate has been noted with these 2 types of transplants, primarily because of infectious complications. Here, we hypothesized that the time to lymphocyte recovery (absolute lymphocyte count [ALC] of ≥ 1000/μL for the first 3 consecutive days) after transplant correlates with outcomes. We retrospectively analyzed 65 consecutive patients treated at our institution with cord blood (n = 37) and haploidentical (n = 28) transplantation with myeloablative fludarabine, melphalan, and thiotepa conditioning. Patients with lymphocyte recovery at day 60 posttransplant were more likely to survive long term than those without lymphocyte recovery. In multivariate analysis, ALC recovery was the only independent prognostic factor associated with mortality; patients without ALC recovery were 10.5 times (95% confidence interval [CI]: 4.3–25.4) more likely to die than those with ALC recovery (P < .0001). This difference appeared to be related to NRM (hazard ratio [HR] =0.1, 95% CI: 0.02–0.6, P = .008), whereas ALC recovery did not influence the rate of disease relapse. These results suggest that ALC recovery is an important prognostic indicator for patients treated with cord blood and T cell–depleted peripheral haploidentical transplants.
Keywords: Lymphocyte recovery, Nonrelapse mortality, Cord blood transplantation, T cell–depleted haploidentical stem cell transplantation
INTRODUCTION
Transplantation using alternative sources of stem cells, from mismatched relatives or umbilical cord blood, is an established treatment modality for patients who lack a matched sibling or unrelated donor [1,2]. The morbidity and mortality rates with these types of transplant have been higher than those for matched transplants because of a higher incidence of infectious complications [3–8]. Delayed immunologic reconstitution also likely contributes to the unfavorable outcomes seen with these types of transplants. Impaired functional recovery of lymphocytes, in addition to prolonged lymphopenia, was previously observed in both T cell–depleted haploidentical and cord blood transplant recipients [9,10]. We and others have also found a higher nonrelapse mortality (NRM) rate primarily related to infectious complications, which was the predominant cause of unfavorable outcomes in these patients [9,11].
Lymphocyte recovery has been previously correlated with lower rates of disease relapse after autologous or allogeneic stem cell transplantation in patients with hematologic malignancies, and faster absolute lymphocyte count (ALC) recovery has been found to be associated with lower rates of relapse and improved survival [12–23]. Limited data exist on the relationship between lymphocyte recovery and outcomes in alternative donor transplants [24], whereas no data exist for these 2 types of transplants. Moreover, it is unclear whether delayed ALC recovery contributes to higher treatment-related mortality (TRM) and relapse rates in patients treated using alternative sources of stem cells.
Here, we hypothesized that ALC recovery may be a useful predictor of outcomes in these patients, and analyzed the relationship between ALC recovery and relapse, NRM, and survival in cord blood and T cell–depleted haploidentical transplant recipients treated at a single institution.
PATIENTS AND METHODS
Patients
We searched the database of the Department of Stem cell Transplantation and Cellular Therapy and identified 65 consecutive patients who had received a transplant, T cell–depleted haploidentical (n = 28) or cord blood (n = 37) at The University of Texas M.D. Anderson Cancer Center between 10/2001 and 5/2008. Patients were treated simultaneously on separate protocols. Patients treated with a cord blood transplant could have up to 10% bone marrow blasts at the time of transplant, whereas no cutoff existed for haploidentical transplant recipients. All patients were treated on clinical trials approved by the institutional review board (IRB) of M.D. Anderson, and provided written informed consent. An IRB-approved protocol and a waiver of informed consent were obtained for this retrospective study.
Conditioning Regimen
All patients were treated with a preparative regimen consisting of melphalan at 140 mg/m2 8 days before transplantation (day −8), thiotepa at 10 mg/kg on day −7, fludarabine at 160 mg/m2 in divided doses given on days −6, −5, −4, and −3, and ± rabbit antithymocyte globulin (ATG) at 1.5 mg/kg/day on days −6, −5, −4, and −35. Cord blood transplant patients received tacrolimus-based graft-versus-host disease (GVHD) prophylaxis with either methotrexate (MTX) or mycophenolate mofetil (MMF), whereas haploidentical stem cell transplant (HSCT) patients had only T cell depletion as GVHD prophylaxis. Patients developing GVHD greater than grade 2 were routinely treated with steroids (methylprednisolone 2 mg/kg in divided doses). All treated patients received standard antimicrobial prophylaxis with voriconazole, valacyclovir, trimethoprim-sulfamethoxazole, or pentamidine. Patients received foscarnet prophylaxis for cytomegalovirus (CMV) infection and gancyclovir only for treatment of CMV antigenemia or disease.
Lymphocyte Recovery
Lymphocyte recovery was defined as the first day of 3 consecutive days (or ALC values) in which the ALC was ≥1000/μL, regardless of how long after transplantation this occurred. A cutoff of 1000/μL was chosen because a higher ALC variability was observed with lower thresholds for ALC, which made the interpretation of the results more difficult. Patients who eventually experienced lymphocyte recovery had a general upward trend until they reached this value. In addition to ALC recovery, other parameters such as neutrophil and platelet recovery, defined in the usual fashion (first day of 3 consecutive days of absolute neutrophil count above 500/μL, and platelet count 20,000 for 7 consecutive days without platelet transfusions), number of CD34+ cells infused, and disease status at transplant (complete remission versus not) were evaluated in relation to the following outcomes: NRM, disease relapse, and survival. To avoid a potential intrinsic selection bias, patients who did not have the chance to recover the lymphocytes, such as those who developed primary graft failure (n = 10) or who died before day 60 posttransplant (n = 3), were excluded from the analysis.
Infectious Complications
We identified episodes of infection, documented both clinically (signs and symptoms of infection, radio-logic evidence, and no microorganism identified) and microbiologically (signs and symptoms and microbiologic or pathologic identification of the etiologic agent), and then determined the number of patient-days from transplant to last follow-up or death. For the group with ALC recovery, we also determined the number of patient-days from transplant to ALC recovery and from ALC recovery to last follow-up or death and defined the infection rate as the number of infection episodes per thousand patient-days.
Statistical Analysis
Competing risk analysis was used to assess the competing risks of relapse and NRM [25]. Cox proportional hazards regression analysis was used to evaluate the effects of transplant type and CD34+ cell count on the recoveries of different blood cells and to assess the effects of certain prognostic factors on overall mortality [26]. The prognostic factors tested included age, disease status at transplantation, type of transplant, CD34+ cell count, ALC recovery, platelet recovery, and neutrophil recovery. Of these, the recoveries of the different blood cells were time-dependent variables in the Cox model analysis. In addition, if ALC recovery was found to be a significant factor in multivariate survival analyses, a certain cutoff value was chosen and patients were dichotomized based on it, and then the relevant survival curves were estimatedby the Kaplan-Meier method and compared using the log-rank test [27]. The incidence rates of infectious complications were calculated and compared by a normal-theory test using normal approximation to the binomial distribution. Poisson distribution and test-based methods were used to construct the confidence intervals for incidence rate ratios. All tests were 2-sided, and the statistical significance was set at P ≤ .05. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and R (http://www.r-project.org) version 2.9.2 software.
RESULTS
Patient and Treatment Characteristics
The median age of the patients studied was 34 years (range: 4–57 years), 37.3 years for the haploidentical transplant group, and 29.3 years for the cord blood group (P = .059, Wilcoxon rank sum test). Patients’ disease and treatment characteristics are listed in Table 1. Forty-nine patients (75%) had acute leukemia, and 13 (20%) had lymphoma. Overall, 43% of patients were in remission at transplantation. All HSCT recipients received peripheral blood as the stem cell source. Cord blood transplant patients received an average of 2 logs fewer CD34+ cells/kg than HSCT recipients (Table 1).
Table 1.
Patient Characteristics and Treatment Outcomes
| Characteristics | No. of Patients (N = 65) |
|---|---|
| Type of transplant | |
| Haploidentical/cord blood | 28/37 |
| Age, years (range) | 34 (4–57) |
| Sex | |
| Male/female | 40/25 |
| Diagnosis | |
| AML/MDS | 29 |
| ALL | 20 |
| NHL/HD | 13 |
| CML/MPD | 2 |
| CLL | 1 |
| Disease status at transplantation | |
| Complete remission (%) | 28 (43%) |
| Conditioning chemotherapy | |
| FMT ± ATG | 100% |
| GVHD prophylaxis | |
| T cell depletion with no posttransplant immunosuppression | 28 |
| Tacrolimus-based* | 37 |
| Number of CD34+ cells infused, median (× 106/kg) | |
| Haploidentical SCT patients | 10.5 |
| Cord blood transplant patients | 0.11 |
| Neutrophil engraftment | |
| n (%) | 55 (85%) |
| median time,† days (range) | 16 (5–57) |
| Platelet engraftment | |
| n (%) | 42 (76%) |
| median time,‡ days (range) | 29 (7%–134) |
| ALC 1000 | |
| n (%) | 28 (51%) |
| median time, days (range) | 57 (26%–434) |
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndromes; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; HD, Hodgkin disease; CML, chronic myeloid leukemia; MPD, myeloproliferative disease; CLL, chronic lymphocytic leukemia; FMT, fludarabine, melphalan, and thiotepa conditioning; ATG, antithymocyte globulin; GVHD, graft-versus-host disease; SCT, stem cell transplant; ALC, absolute lymphocyte count; MTX, methotrexate; MMF, mycophenolate mofetil.
Tacrolimus + MTX 22 patients, tacrolimus + MMF 14 patients, tacrolimus alone 1 patient.
Median 13 days for haploidentical and 21 days for cord blood transplant recipients.
Median 12 days for haploidentical and 35 days for cord blood transplant.
ALC Recovery
Overall, ALC recovery to 1000/μL or greater occurred in 51% of patients. Only 5 (28%) of 18 evaluable patients in T cell–depleted haploidentical transplant group reached ALC recovery, whereas 23 (68%) of 34 evaluable patients treated with cord blood transplantation did (P = .002). The median time to ALC recovery was 57 days for all patients (range: 26–434 days), with a median of 62 days for haploidentical transplant and 56 days for cord blood transplant recipients. In 55 patients (85%), the neutrophils engrafted after a median of 16 days (range: 8–57 days) and 42 patients (76%) recovered platelets to 20,000/μL after a median time of 29 days (range: 7–134 days). The survival analysis showed that the type of transplant (cord blood versus haploidentical) had a significant effect on the recovery of the different blood cells. Although haploidentical transplant patients had faster neutrophil (P < .0001) and platelet (P <.0001) recovery, cord blood transplant patients recovered ALC sooner than those treated with haploidentical transplantation (P = .02). No correlation between recovery of ALC, engraftment of white blood cells or platelets, and number of CD34+ cells infused was identified for either cord blood or haploidentical stem cell transplant recipients. In addition, there was no evidence that the number of prior chemotherapies affected lymphocyte recovery; the mean number of chemotherapies for patients with lymphocyte recovery at day 100 was 7.8, whereas the mean for patients without lymphocyte recovery at day 100 was 6.6 (P = .41, logistic regression).
A higher proportion of cord blood patients developed GVHD (22/33, 67%) than haploidentical patients (7/18, 39%) (P = .09, Fisher’s exact test). GVHD did not seem to impact lymphocyte recovery in these patients, overall 14/29 (48%) patients with GVHD had lymphocyte recovery compared with 7/23 (30%) who did not (P = .26, Fisher’s exact test). When this analysis was repeated only for the subset of 34 cord blood patients, again there was no evidence of a difference regarding lymphocyte recovery between patients who developed GVHD (12/22, 54.5%) and those who did not (6/12, 50%).
Analysis of Outcomes Based on ALC Recovery
Recovery of ALC was evaluated in relationship to outcomes (survival, disease relapse, and NRM) for all patients except the 13 who were excluded from analysis because of primary graft failure or early death. After a median follow-up of 361 days (range: 62–2220 days) for the remaining 52 patients, 19 patients (36%) had disease relapse, 17 (33%) died of nonrelapse-related causes, and 16 (31%) were alive and relapse-free.
Overall survival
Thirty-five patients (67%) had died by the end of follow-up in this study. The median time from transplant to death was 235 days (range: 62–908 days). Univariate analysis indicated that ALC recovery (P < .0001) and type of transplant (P = .02) had a significant effect on overall survival (OS), whereas multivariate analysis indicated that, from the factors evaluated, ALC recovery was the only independent prognostic factor for OS. Patients without ALC recovery were 10.5 times (95% confidence interval [CI]: 4.4–25.4) more likely to die than those with ALC recovery (P < .0001) (Figure 1).
Figure 1.
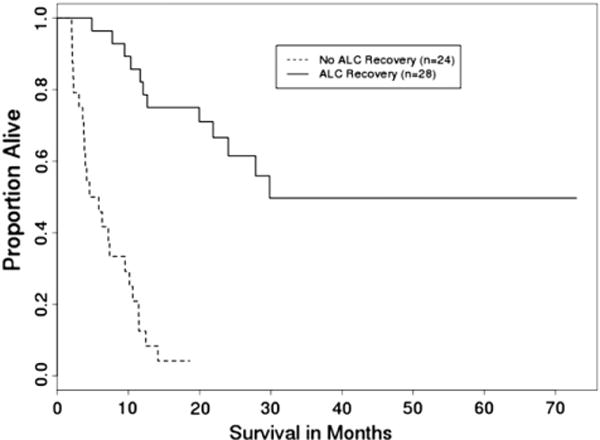
OS by ALC recovery (n = 52) (P <.001).
Relapse
The median time from transplant to relapse was 194 days (range: 55–607 days). In a competing risks analysis, with NRM considered as a competing event, univariate analysis of different factors (ALC recovery, platelet recovery, white blood cell recovery, age, type of transplant, disease status at transplant, CD34+ cell count/kg) indicated that CD34+ cell count/kg (P < .01), type of transplant (P = .02), and disease status at transplant (P = .02) had a significant effect on relapse. Multivariate analysis using regression modeling in this competing risks model indicated that the disease status at the time of transplantation was the only independent prognostic factor for relapse; patients not in remission were 2.6 times (95% CI: 1.02–6.60) more likely to have a relapse than those in remission (P = .046). CD34+ cell count had a strong trend toward being associated with relapse (P = .08), whereas ALC recovery had no impact on disease relapse.
NRM
The NRM rate was 33% after a median time from transplant to death of 178 days (range: 64–731 days). A similar competing risk analysis was performed for these data, with relapse considered as a competing event. Univariate analysis revealed that ALC recovery (P < .001) and platelet recovery (P = .005) had a significant effect on NRM. Multivariate analysis indicated that, from the factors evaluated, ALC recovery and platelet recovery were both independent prognostic factors for NRM. After adjustment for the type of transplant, patients without ALC recovery were 12.8 times (95% CI: 4.4–37.1) more likelytohave NRM than those with ALC recovery (P = .005) (Figure 2); patients without platelet recovery were 4.0 times (95% CI: 1.7–9.2) more likely to have NRM than those with platelet recovery (P = .001). The type of transplant was not an independent prognostic factor for NRM (P = .23). Lack of ALC recovery to 1000/μL by day 60 was associated with higher NRM rates in cord blood and haploidentical stem cell transplantation (Figures3and 4) (P < .001 for both types of transplant).
Figure 2.
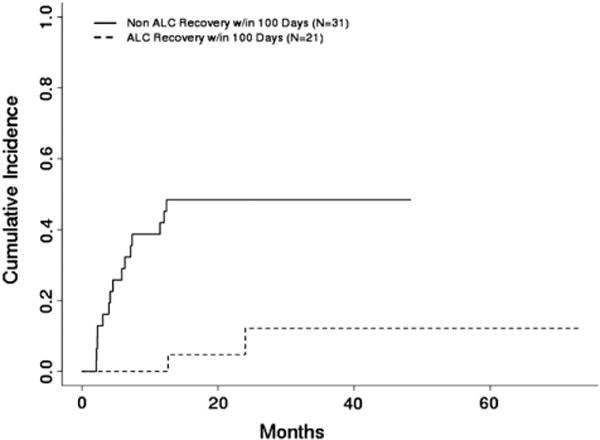
Cumulative incidence of NRM by ALC recovery within 100 days posttransplant (n = 52) (P = .005).
Infectious Complications and Lymphocyte Recovery
The incidence of infectious complications was analyzed for all patients before and after ALC recovery, if this occurred, and expressed as an incidence rate ratio (number of infections per number of days analyzed). Results were similar whether the analysis was done for the first year posttransplant or overall.
Within the first 365 days, for patients with ALC recovery, the infection rate before ALC recovery was significantly higher than that after ALC recovery (35.5/1,000 patient-days versus 8.6/1,000 patient-days, P < .0001). The incidence rate ratio was 4.1 (95% CI: 2.9–6.0). No significant difference in infection rate was observed between ALC-recovery patients before their ALC recovery and those who did not have ALC recovery (35.5/1000 patient-days versus 38.4/1,000 patient-days, P = .6).
Differences in infectious complication rates remained significant for the entire posttransplant period. For patients with ALC recovery, the infection incidence rate before ALC recovery was significantly higher than that after ALC recovery (35.5/1000 patient-days versus 6.5/1,000 patient-days, P = .0001). The incidence rate ratio was 5.4 (95% CI: 3.9–7.6). No significant difference was identified in the infection incidence rate between ALC-recovery patients before their ALC recovery and those who did not have ALC recovery (35.5/1,000 patient-days versus 37.8/1,000 patient-days, P = .68) (Table 2).
Table 2.
Comparison of Infection Rate before and after Lymphocyte Recovery
| Incidence Rate Overall
| |||||
|---|---|---|---|---|---|
| Group
|
ALC Recovery Group
|
||||
| Subgroup | Before ALC Recovery | After ALC Recovery | IRR | 95% CI | P Value |
| Infection episodes | 74 | 76 | |||
| Patient-days | 2083 | 11,657 | |||
| Rate | 35.5 | 6.5 | 5.4 | (3.9, 7.6) | <.0001 |
|
| |||||
| Group
|
ALC Recovery Group
|
||||
| Subgroup | Before ALC Recovery | Non-ALC Recovery | |||
|
| |||||
| Infection episodes | 74 | 109 | |||
| Patient-days | 2083 | 2882 | |||
| Rate | 35.5 | 37.8 | 0.9 | (0.7, 1.3) | .68 |
IRR indicates incidence rate ratio; ALC, absolute lymphocyte count; CI, confidence interval.
The great majority of patients without ALC recovery who did not experience graft failure or relapse (8 haploidentical and 10 cord blood transplant recipients) died of infectious complications. Except 2 patients with noninfectious cause (both in the cord blood transplant group), all patients died of documented infection, 7 in the haploidentical and 7 in the cord blood transplant group, whereas in 2 patients, the organism was not identified.
DISCUSSION
With the current study, we have found that outcomes of patients treated with cord blood and T cell–depleted haploidentical transplantation with lymphocyte recovery by day 60 posttransplant are better than those without lymphocyte recovery, and showed that improved survival is because of less NRM related to infectious complications.
Previous studies demonstrated that early lymphocyte recovery in patients with hematologic malignancies treated with myeloablative conditioning was associated with improved outcomes, primarily related to decreased rate of relapse, in both autologous and allogeneic stem cell transplant settings [12–23]. Cutoffs of 200, 500, or 1000 cells/μL were used in different analyses for different types of transplant to evaluate lymphocyte recovery [12–23]. Because of the variability in ALC values soon after transplantation, we used ALC recovery to 1000 cells/μL as a more reliable cutoff for lymphocyte recovery. In patients with lymphocyte recovery, the ALC reached 1000/μL at a median of approximately 2 months posttransplant. This was also the earliest time point when differences in outcomes started to become apparent, and these were increasingly more evident at day 100 posttransplant and overall, strongly suggesting that the risk of NRM increases as lymphocyte recovery becomes increasingly delayed (Figure 5).
Figure 5.
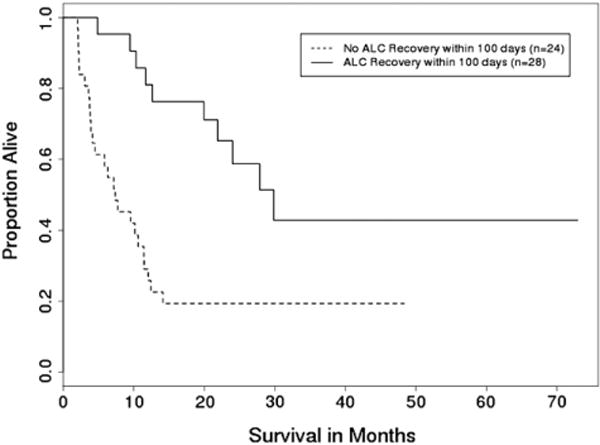
OS by ALC recovery within 100 days posttransplant (N = 52) (P <. 001).
Interestingly, studies performed in other types of transplants appreciated the recovery of lymphocytes in relation to disease relapse, whereas no association was demonstrated between ALC recovery and NRM. Our study identified lack of or delayed ALC recovery as an important factor closely associated with mortality from nonrelapse causes. Only 3 (11%) of 28 patients who recovered ALC after transplant died of nonrelapse causes compared with 17 (63%) of 27 patients who did not recover ALC (P < .0001). Patients who recovered ALC had a substantial decrease in the infection rate from 35 to 6 events per 1000 patient-days after ALC recovery, whereas the group that never recovered ALC had a rate of 37 events per 1000 patient-days, which was comparable to the infection rate before ALC recovery. Taken together, the marked decrease in the infection rate after ALC recovery and lower mortality in this group suggest that increased protection form infection may be the reason behind decreased NRM in the ALC recovery group.
Although similar correlation has been identified between lymphocyte recovery and outcomes for both groups, a different pattern of lymphocyte recovery was observed between the 2 transplant types. The great majority of patients with T cell–depleted HSCT did not have ALC recovery, whereas two-thirds of cord blood transplant recipients did have ALC recovery; however, delays in lymphocyte recovery of up to more than a year were observed in this group. This finding could explain, at least in part, the prolonged risk of infectious complications after more than 1 year posttransplant for cord blood transplant recipients [28]. Similar to other reports, we have found that a higher number of CD34+ cells infused did not appear to favor faster lymphocyte recovery in these patients, suggesting that infusion of higher stem cell doses may not translate into a faster lymphocyte recovery in these patients [12,14].
Although limited by a relatively small number of patients and its retrospective nature, this study identified a marked difference in outcomes between patients who had lymphocyte recovery and those who did not. Lack of ALC recovery to 1000/μL by day 60 appeared to be associated with worse outcomes in both cord blood and T cell–depleted haploidentical stem cell transplantation. Further studies are needed to identify subsets of lymphocytes likely to play a greater role in lymphocyte recovery in these patients. Patients identified in this fashion could benefit from approaches aimed at enhancing immunologic reconstitution posttransplant. Strategies directed at augmenting lymphocyte recovery after transplant might improve outcomes for patients treated with alternative donor transplantation.
Figure 3.
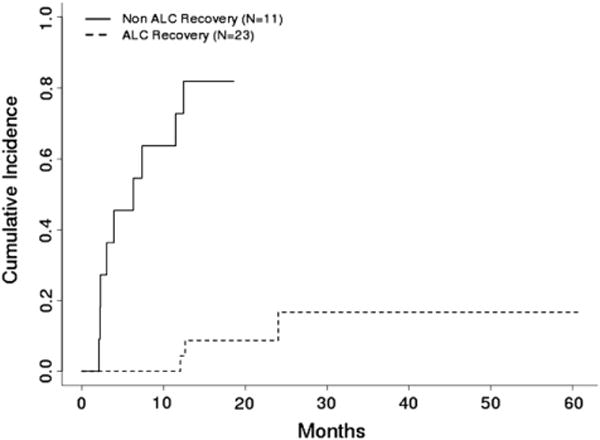
Cumulative incidence of NRM by ALC recovery in cord blood transplant patients (n = 34) (P <.001).
Figure 4.
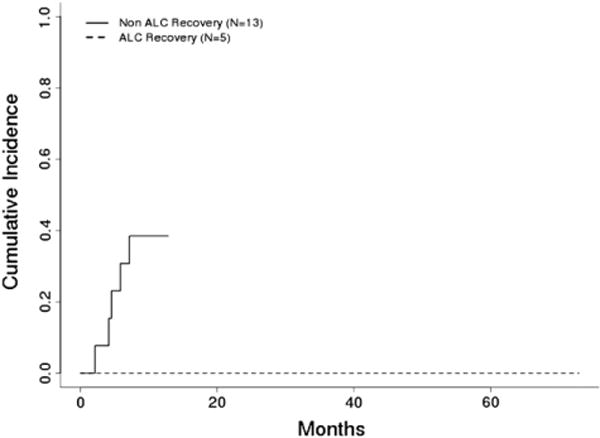
Cumulative incidence of NRM by ALC recovery in T cell–depleted haploidentical transplant patients (n = 18) (P <.001).
Acknowledgments
We thank Dawn Chalaire for excellent manuscript proofreading.
Financial disclosure: This work was supported in part by an Institutional Research Grant to S.O.C.
Footnotes
AUTHORSHIP CONTRIBUTION
S.O.C. designed the study, collected the data, analyzed the results, and wrote the paper. V.M. contributed to the study design, data collection, and interpretation of results, and reviewed and approved the manuscript. Y.J. and R.B. performed the statistical analyses, and reviewed and approved the manuscript. G.R. and J.M. contributed with data collection, and reviewed and approved the manuscript. M.L. contributed with data collection, interpretation of results, and reviewed and approved the manuscript. E.J.S. and R.E.C. contributed to study design, critically revised the manuscript, and approved the final form of the article.
References
- 1.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 2.Laughlin MJ, Barker J, Barnbach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 3.Roux E, Helg C, Dumont-Girard F, Chapuis B, Jeannet M, Roosnek E. Analysis of T-cell repopulation after allogeneic bone marrow transplantation: significant differences between recipients of T-cell depleted and unmanipulated grafts. Blood. 1996;87:3984–3992. [PubMed] [Google Scholar]
- 4.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- 5.Ciurea SO, Saliba R, Rondon G, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45:429–436. doi: 10.1038/bmt.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safdar A, Rodriguez GH, de Lima MJ, et al. Infections in 100 cord blood transplantations: spectrum of early and late post-transplant infections in adult and pediatric patients 1996–2005. Medicine (Baltimore) 2007;86:324–333. doi: 10.1097/MD.0b013e31815c52b0. [DOI] [PubMed] [Google Scholar]
- 7.Cahu X, Rialland F, Touzeau C, et al. Infectious complications after unrelated umbilical cord blood transplantation in adult patients with hematologic malignancies. Biol Blood Marrow Transplant. 2009;15:1531–1537. doi: 10.1016/j.bbmt.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Yazaki M, Atsuta Y, Kato K, et al. Incidence and risk factors of early bacterial infections after unrelated cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:439–446. doi: 10.1016/j.bbmt.2008.12.508. [DOI] [PubMed] [Google Scholar]
- 9.Roux E, Dumont-Girard F, Starobinski M, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96:2299–2303. [PubMed] [Google Scholar]
- 10.Eyrich M, Lang P, Lal S, et al. Immunological aspects of haploidentical stem cell transplantation in children. Br J Haematol. 2001;114:422–432. doi: 10.1046/j.1365-2141.2001.02934.x. [DOI] [PubMed] [Google Scholar]
- 11.Komanduri K, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powles R, Singhal S, Treleaven J, Kulkarni S, Horton C, Mehta J. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery after transplantation. Blood. 1998;91:3481–3486. [PubMed] [Google Scholar]
- 13.Pavletic ZS, Joshi SS, Pirrucello SJ, et al. Lymphocyte reconstitution after allogeneic blood stem cell transplantation for hematologic malignancies. Bone Marrow Transplant. 1998;21:33–41. doi: 10.1038/sj.bmt.1701037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 15.Porrata LF, Litzow MR, Tefferi A, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 16.Porrata LF, Inwards DJ, Micallef IN, Ansell SM, Geyer SM, Markovic SN. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin’s disease. Br J Haematol. 2002;117:629–633. doi: 10.1046/j.1365-2141.2002.03478.x. [DOI] [PubMed] [Google Scholar]
- 17.Nieto Y, Shpall EJ, McNiece IK, et al. Prognostic analysis of early lymphocyte recovery in patients with advanced breast cancer receiving high-dose chemotherapy with autologous hematopoietic progenitor cell transplant. Clin Cancer Res. 2004;10:5076–5086. doi: 10.1158/1078-0432.CCR-04-0117. [DOI] [PubMed] [Google Scholar]
- 18.Kim H, Sohn HJ, Kim SE, et al. Lymphocyte recovery as a positive predictor of prolonged survival after autologous peripheral blood stem cell transplantation in T-cell non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;34:43–49. doi: 10.1038/sj.bmt.1704530. [DOI] [PubMed] [Google Scholar]
- 19.Porrata LF, Gertz MA, Litzow MR, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis. Clin Cancer Res. 2005;11:1210–1218. [PubMed] [Google Scholar]
- 20.Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB. Rapid helperT-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;37:1119–1128. doi: 10.1038/sj.bmt.1705381. [DOI] [PubMed] [Google Scholar]
- 21.Joao C, Porrata LF, Inwards DJ, et al. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 22.Boulassel MR, Herr AL, deB Edwardes MD, et al. Early lymphocyte recovery following autologous peripheral stem cell transplantation is associated with better survival in younger patients with lymphoproliferative disorders. Hematology. 2006;11:165–170. doi: 10.1080/10245330600667559. [DOI] [PubMed] [Google Scholar]
- 23.Porrata LF, Inwards DJ, Ansell SM, et al. Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant. 2008;14:807–816. doi: 10.1016/j.bbmt.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang XJ, Zhao XY, Huo MR, et al. Influence of lymphocyte recovery on outcome of haploidentical stem cell transplantation for hematologic malignancies. Medicine (Baltimore) 2009 Nov 9; doi: 10.1097/MD.0b013e3181c167e2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Prentice RL, Kalbfleisch JD, Peterson AV, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life tables [with discussion] J R Stat Soc B. 1972;34:187–202. [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Ciurea SO. Presentation at the 7th Annual International Cord Blood Symposium; Los Angeles, CA. 2009. [Google Scholar]


