Abstract
Elite controllers (ECs) maintain undetectable HIV viral loads without antiretroviral therapy (ART), but are at increased risk of serious non-AIDS conditions (SNA). We assessed the impact of ART in ECs on gut immune dysfunction and biomarkers predicting SNA (blood CD4/CD8 ratio, plasma IL-6, D-dimer levels). At baseline, ECs had elevated IL-6 and D-dimer levels and reduced CD4/CD8 ratio compared to HIV-uninfected controls, but no difference in microbial translocation or gut CD4 subsets. ART increased CD4/CD8 ratio but did not normalize IL-6 and D-dimer levels. EC SNA pathogenesis may be independent of gut immune dysfunction, and resolution may require prolonged ART.
Keywords: HIV, elite controllers, antiretroviral therapy, gut immunology, microbial translocation, serious non-AIDS conditions, inflammation
INTRODUCTION
Serious non-AIDS conditions (SNA) are increased in HIV-infected individuals despite antiretroviral therapy (ART), and are important contributors to morbidity and mortality1. These SNA include cardiovascular, hepatic, psychiatric and renal diseases, and are associated with chronic inflammation and abnormal coagulation that persist despite virus suppression on ART2. A recent study combining data from three large cohorts of ART-experienced, HIV-infected individuals showed that plasma levels of the pro-inflammatory cytokine IL-6 and the coagulation biomarker D-dimer were the best predictors of subsequent SNA and death2. Furthermore, inversion of the normal blood CD4/CD8 ratio in ART experienced individuals is independently associated with SNA3.
Gut microbial translocation is a hallmark of progressive HIV infection and circulating microbial products are potent triggers of the innate immune system that may drive chronic inflammation4. During progressive HIV infection, mucosal CD4 T cell depletion, T cell functional dysregulation and impairment of the gut epithelial barrier all occur simultaneously to facilitate microbial translocation4,5. Gut CD4 T cells that produce the cytokines IL-22 (Th22 cells) and IL-17a (Th17 cells) normally maintain epithelial integrity and provide mucosal defense against invading pathogens, but are preferentially depleted soon after HIV infection5,6. Furthermore the functional capacity of gut Th17 cells, as defined by the ability to produce pro-inflammatory cytokines, determines their ability to protect mucosal tissues against microbial invasion and is drastically reduced from early HIV stages6.
Elite controllers (ECs) are small subset of HIV-infected individuals who maintain an undetectable plasma HIV RNA viral load (VL) in the absence of ART7. However, progressive CD4 decline can be seen in these individuals and they are also are at an increased risk of SNA7,8. One important contributor to chronic inflammation in EC individuals may be microbial translocation and gut immune damage. Increased plasma levels of microbial product lipopolysaccharide (LPS) and macrophage/monocyte activation marker soluble CD14 (sCD14), elevated plasma levels IL-6 and D-dimer, and reduced CD4/CD8 ratio have been implicated in SNA pathogenesis2-4,8,9, as has low-level HIV replication10,11. ECs have abnormal gut lymphoid structures with increased collagen deposits and fibrosis, both of which indicate chronic immune activation and end-organ damage12. Although gut CD4 T cell and Th17 cell numbers were found to be normal in ECs13, other mucosal CD4 subsets and mucosal Th17 function have not been studied. Since gut immune dysfunction and microbial translocation underpin inflammation in HIV-infected individuals, we assessed gut immunology, microbial translocation, and SNA biomarkers in EC individuals, and examined the impact of short-term ART.
METHODS
Participants
Four ECs were recruited from the Maple Leaf Medical Clinic (Toronto, Ontario), based on plasma VL below 50 copies/ml in the absence of ART at the majority of clinic visits over the preceding 6 years with (no more than 1 viral blip higher than 400 copies/ml; Supplemental Table 1)10. A standardized ART regimen of tenofovir, emtricitabine and raltegravir was administered for 9 months; blood and sigmoid biopsies were collected at baseline, 6 months after ART initiation, and 3 months after ART discontinuation. HIV-uninfected and HIV-infected, ART-naïve participants were recruited for comparison. All participants provided written informed consent, and the University of Toronto and St. Michael's Hospital Research Ethics Boards approved the study protocol.
Tissue and blood cell isolation
Blood was collected into Acid Citrate Dextran solution A (BD Biosciences), and peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density centrifugation. Sigmoid biopsies were collected approximately 25 cm from the anal verge, as previously described6, and immediately placed into RPMI solution (RPMI 1640 containing 100 U/ml penicillin, 100ug/ml streptomycin and 1X GlutaMAX-1; Invitrogen). Pre-weighed sigmoid biopsies were processed by two sequential Collagenase type I (Sigma-Aldrich) digestions for 30 minutes each on a shaking heated block at 37°C, passed through a 100 μm strainer, and cells counted.
Flow cytometry
Isolated cells from blood and sigmoid colon were stimulated with PMA (1 ng/ml) and ionomycin (1 μM/ml; Sigma) or with media alone for 6 h at 37°C, with brefeldin A (1 μm/ml) for the last 5 h in RPMI 1640 solution supplemented with 10% FBS. Cells were washed, permeabilized, and stained with fluorochrome-labeled antibodies, aqua LIVE/DEAD viability dye (Invitrogen), and fixed (CD3, CD4, CD8, HLA-DR, CD38, IL-17a, IL-22, IFNγ, IL-10 and TNFα; BD BioSciences, eBioscience and Beckman Coulter). Cells were acquired on a FACSCanto II (BD Systems) and analyzed using FlowJo v9.0.2 (Treestar). Dead cells and doublets were excluded from analysis and positive responses were background corrected where applicable. CD4/CD8 ratio was determined by gating on the frequency of CD4+ and CD8+ of CD3+ T cells. Th17 polyfunctionality analyses and data graphing were performed using SPICE software v5.22 (NIAID).
Plasma markers of microbial translocation, inflammation, and coagulation
The limulus amebocyte lysate assay kit (Cambrex) was used to measure plasma LPS levels, and commercially available ELISA kits were used to assay plasma levels of sCD14 (R&D Systems), C-reactive protein (CRP; R&D Systems) and D-dimer (Imuclone). Custom designed multiplex chemiluminescent ELISA plates (Meso Scale Discovery) were used to assay cytokine and chemokine levels.
Statistical analysis
Groups were compared by the Mann-Whitney U test and longitudinal data was assessed by Wilcoxon signed-ranked test using IBM SPSS Statistics 20.0 for Mac (SPSS). Assessment of Th17 polyfunctionality was performed using SPICE v5.22 using the Student's t test. P values of <0.05 were considered significant.
RESULTS
Participant characteristics
Four elite controllers (median age, 60) with a plasma VL <50 copies/ml for at least 6 years were recruited as previously published10 (Supplemental Table 1). The median duration of documented HIV infection in ECs was 18.5 years, and the median blood CD4 count at baseline was 660μl; 3/4 participants had HIV plasma RNA viral load below 20 copies/ml at enrolment, while one participant had a viral blip of 88 copies/ml. Subsequently, all participants maintained normal CD4 counts and an undetectable plasma viremia throughout the duration of the study (assessed monthly; data not shown).
HIV-uninfected controls (HIV-, N=11), ART-naïve chronically HIV-infected individuals (infected >1 year; HIV+ART-, N= 12), and long-term ART-treated individuals (HIV+ART+, N=16) were included as controls based on sample availability. Chronically HIV-infected individuals had a reduced CD4 count compared to HIV-uninfected controls (P=0.021; median, 500 cells/μl; range 310-900 cells/μl) and had detectable viremia (median, 6346 copies/ml, 1694 - 769 545 copies/ml). Long-term ART treated individuals were virally suppressed (VSL<50 copies/ml) on ART for greater than 6 years with a median CD4 count of 660 cells/μl. EC individuals were older (median age 60 years) than HIV- (43 years, P=0.005) and chronically HIV-infected participants (33 years, P=0.009), but were similar to long-term ART-treated individuals (51 years, P=0. 218).
Cellular and soluble markers of microbial translocation and SNA biomarkers in blood
T cell immune activation, defined as CD38+HLA-DR+ co-expression on CD8 T cells, CD4/CD8 ratio, and plasma markers of microbial translocation (LPS), monocyte activation (sCD14), inflammation (IL-6, IL-17, MIP-1b, IP-10, TNFα and IL-10) and coagulation (CRP and D-dimer) were measured in EC participants at baseline and compared with HIV-uninfected and chronic HIV-infected individuals (Supplemental Table 210). CD8 T cell activation and plasma levels of LPS, sCD14, TNFα, MIP-1b, IL-17, IP-10, IL-10, and CRP did not differ between EC and HIV-uninfected participants. However, plasma levels of the SNA biomarkers IL-6 and D-dimer were elevated (IL-6: EC 1.9 pg/ml vs. HIV- 1.1 pg/ml and D-dimer: 146.3 ng/ml vs. 98.8 ng/ml) and the CD4/CD8 ratio was reduced (1.0 vs. 1.8) in EC compared to HIV-uninfected participants. Within the HIV-uninfected group, age was not associated with differences in CD4/CD8 ratio or in plasma IL-6 or D-dimer levels (data not shown).
Baseline gut immunology
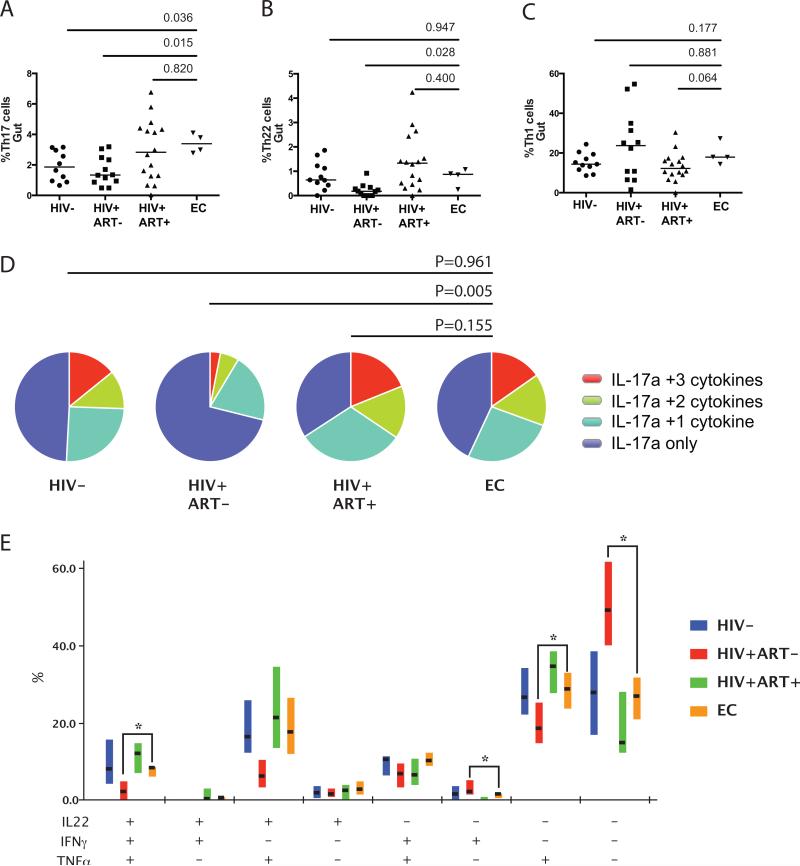
We next analyzed the number and proportion of CD4 T cell subsets within the gut mucosa. The proportion of bulk CD4 T cells, regulatory T cells (FoxP3+CD25+ CD4 T cells), Th1 cells (IFNγ+ CD4 T cells), and Th22 cells (IL-22+IFNγ-IL-17a- CD4 T cells) were similar in the mucosa of HIV-uninfected controls, long-term ART treated individuals, and ECs; the frequency of Th17 cells was increased in ECs compared to HIV-uninfected individuals (P=0.036; Figure 1A-1C).
Figure 1.
(A) Frequency of gut Th17 cells, (B) Th22 cells, and (C) Th1 cells in HIV-uninfected, chronic ART-naïve, long-term ART treated and ART-naïve EC men at baseline. (D) Polyfunctional capacity of gut Th17 cells in ECs was similar to the HIV-uninfected controls and higher than chronically HIV-infected participants. (E) Each combination of IL-22, IFNγ and TNFα production by Th17 cells is shown; horizontal lines indicate the median and box plots depict the interquartile range. * indicates P<0.05
The function of Th17 cells in the gut mucosa was then assessed based on their ability to co-produce the pro-inflammatory cytokines TNFα, IL-22 and IFNγ, and the regulatory cytokine IL-10. ECs maintained high levels of polyfunctional gut Th17 cells that were comparable to HIV-uninfected individuals (P=0.961), with similar levels of IL-22, TNFα and/or IFNγ production (Figure 1D and 1E). Immunoregulatory IL-10+ Th17 mucosal cells were also comparable between ECs and HIV-uninfected individuals (P=0.119; data not shown).
Impact of short-term ART on mucosal Th17 cells in ECs
We next evaluated the impact of ART on gut Th17 cells and their function. Th17 frequency and polyfunctionality were not altered after 6 months of ART (P=0.625 and P=0.255 respectively). However, their functional capacity was unexpectedly reduced after the discontinuation of ART compared to baseline (P=0.021).
Blood SNA biomarkers after ART
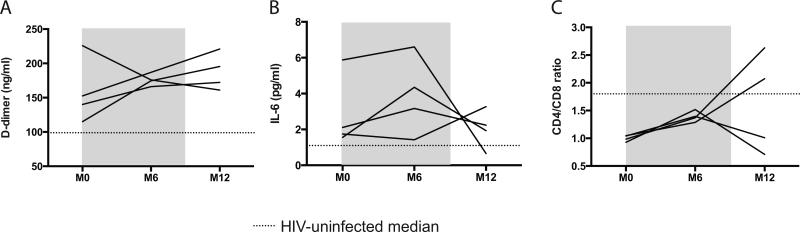
EC participants demonstrated elevated SNA biomarkers, with increased plasma levels of IL-6 and D-dimer and a lower CD4/CD8 ratio at baseline compared to HIV-uninfected controls (all P<0.05; Supplemental Table 1). Neither ART nor its subsequent discontinuation in ECs had any significant impact on plasma IL-6 or D-dimer levels (Figure 2A and 2B), while the blood CD4/CD8 ratio was increased in all EC to levels comparable to HIV-uninfected individuals (P=0.255) after 6 months of ART (Figure 2C).
Figure 2.
Impact of ART initiation and discontinuation on plasma SNA biomarkers and blood CD4/CD8 ratio. (A) Plasma D-dimer and (B) IL-6 levels were not reduced after ART initiation or discontinuation, but (C) the CD4/CD8 ratio was increased during short-term ART. Grey box indicates period of ART administration and dotted lines represent median values for HIV-uninfected group.
DISCUSSION
Serious non-AIDS conditions (SNAs) are an important cause of mortality and morbidity in HIV-infected individuals despite effective ART, and may be caused by chronic inflammation related to elevated gut microbial translocation1,4. ECs maintain an undetectable blood VL in the absence of ART, but have increased systemic inflammation and SNA8. This has led to the suggestion that ART may reduce SNA and mortality in EC individuals10,11,14. Since gut immune dysfunction and microbial translocation underpin inflammation in HIV-infected individuals, we assessed gut immunology, microbial translocation, and SNA biomarkers in EC individuals and the impact of short-term ART. Gut mucosal T cell immunology and plasma LPS levels in these individuals were not different to uninfected controls prior to ART, however blood SNA biomarkers IL-6 and D-dimer were elevated and CD4/CD8 ratio was reduced. Short-term ART did not reduce plasma IL-6 or D-dimer levels in ECs – indeed, these levels actually increased in 3/4 participants – but significantly improved the blood CD4/CD8 ratio.
These results suggest that the increased incidence of SNA conditions in EC individuals may be independent of HIV-associated gut immune dysfunction. Whether an increased duration of ART would impact D-dimer and IL-6 levels in these individuals is not clear, and will require future study. Furthermore, the benefits of ART that have been shown in ECs to date are limited to increased blood CD4 counts, reduced viral replication, and decreased immune activation10,11,14. Until larger studies are able to demonstrate improved clinical outcomes, the benefit of treating ECs will need to be carefully considered in light of the cost and potential toxicity of long-term ART.
The etiology of persistent immune activation in these EC individuals is not clear. While it is plausible that low-level viral replication in ECs would contribute to inflammation and SNA events, short-term ART in our EC cohort clearly diminished low-level viral replication10 and normalized CD4/CD8 ratio, but had no impact on elevated plasma IL-6 and D-dimer levels. The EC individuals that we studied had normal gut T cell immunology and no evidence of microbial translocation even prior to ART, in keeping with the findings from others13. While ECs had increased baseline proportions of polyfunctional gut Th17 cells, suggesting that enhanced host mucosal immune responses were preventing microbial translocation, we unexpectedly observed a reduction in mucosal Th17 polyfunction after ART discontinuation. The reason for this reduced Th17 function at the time of resumption of low-level virus replication is unknown, although blood HIV-specific CD8 T cell responses were also reduced during this period10.
Elite controllers constitute a rare subset of HIV-infected individuals, and a limitation of our study, as well as others investigating ART in ECs is the low sample number (N≤4)10,11,14. However, 6 months of ART normalized the CD4/CD8 ratio in all ECs and therefore a larger study with longer duration of ART should be considered to confirm these interesting findings.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the Ontario HIV Treatment Network (RK, ROGBG123); the Canadian Institutes of Health Research/ Canadian Digestive Health Foundation (CIHR/CDHF; CJK, salary award); a CIHR (Emerging HIV Team Grant #HET85518); Canadian Research Chair Program (RK, salary support) and the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases, National Institute of Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: The authors have declared that no competing interests exist.
Parts of the data were presented at the Canadian Association for HIV Research, St. John's, Newfoundland, May 1-4, 2014.
REFERENCES
- 1.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008 Nov 30;22(18):2409–2418. doi: 10.1097/QAD.0b013e3283174636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grund B, Baker J, Deeks S, et al. Combined Effect of Interleukin-6 and D-dimer on the Risk of Serious Non-AIDS Conditions: Data from 3 Prospective Cohorts.. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta, Georgia: [Google Scholar]
- 3.Serrano-Villar S, Perez-Elias MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One. 2014;9(1):e85798. doi: 10.1371/journal.pone.0085798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013 Jan;21(1):6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CJ, Nazli A, Rojas OL, et al. A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol. 2012 Nov;5(6):670–680. doi: 10.1038/mi.2012.72. [DOI] [PubMed] [Google Scholar]
- 6.Kim CJ, McKinnon LR, Kovacs C, et al. Mucosal Th17 Cell Function Is Altered during HIV Infection and Is an Independent Predictor of Systemic Immune Activation. J Immunol. 2013 Jul 26;191:2164–2173. doi: 10.4049/jimmunol.1300829. [DOI] [PubMed] [Google Scholar]
- 7.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007 Sep;27(3):406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012 Nov 28;26(18):2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson EMP, Krishnan S, Sheikh V, et al. Evidence of innate immune activation in HIV-1-infected elite controllers.. Paper presented at: 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2013; Kaula Lumpur, Malaysia: [Google Scholar]
- 10.Chun TW, Shawn Justement J, Murray D, et al. Effect of Antiretroviral Therapy on HIV Reservoirs in Elite Controllers. J Infect Dis. 2013 Aug 1; doi: 10.1093/infdis/jit306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hatano H, Yukl SA, Ferre AL, et al. Prospective antiretroviral treatment of asymptomatic, HIV-1 infected controllers. PLoS Pathog. 2013;9(10):e1003691. doi: 10.1371/journal.ppat.1003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez J, Hunt P, Jessurun J, Rothenberger M, Reilly C, Jasurda J. Persistent Abnormalities of Lymphoid Structures in HIV Viremic Controllers.. Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta: [Google Scholar]
- 13.Ciccone EJ, Greenwald JH, Lee PI, et al. CD4+ T cells, including Th17 and cycling subsets, are intact in the gut mucosa of HIV-1-infected long-term nonprogressors. J Virol. 2011 Jun;85(12):5880–5888. doi: 10.1128/JVI.02643-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okulicz JF, Grandits GA, Weintrob AC, et al. CD4 T cell count reconstitution in HIV controllers after highly active antiretroviral therapy. Clin Infect Dis. 2010 Apr 15;50(8):1187–1191. doi: 10.1086/651421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.