Abstract
Purpose
The purpose of this article is to review the evidence for the hypothesis that the core mechanism of Dry Eye Disease (DED) is inflammation, including evidence from recent basic, clinical and translational research involving human patients, animal models, and cell cultures.
Method
Using the key words “dry eye + inflammation” , the authors conducted a comprehensive search of the PubMed and Web of Science databases for scientific articles published in English between January 1st 1900 and August 30th 2013 on the role of inflammation in DED in cell cultures, animal models and humans. The resulting articles were then categorized and reviewed.
Results
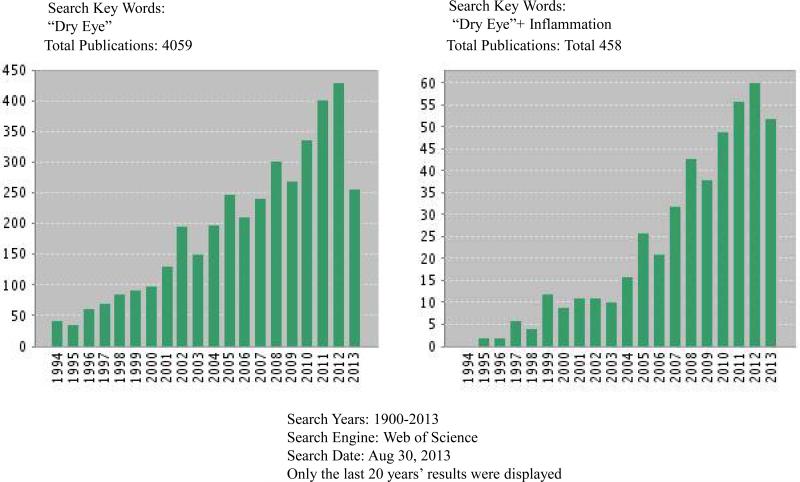
The literature search revealed a total of 458 publications, almost all published after 1992. The percentages of original studies and review articles are 77.29% (354) and 22.71% (104), respectively. Among the original studies, the number of reports on inflammation of human DED is 200 (43.7%); animal models, 115 (25.1 %); and cell cultures, 39 (8.5 %). The human DED studies reveal changes of numerous inflammatory cells and mediators in ocular surface and tear film and those changes can be attenuated or reversed by anti-inflammatory drug treatments. The animal DED studies demonstrate that DED is likely a dominant T-lymphocytes, especially T-helper 17-subset (Th17)-mediated autoimmune disease and that the autoimmune-driven inflammatory cycles are fundamentally linked with DED progression. These results are mirrored and confirmed by a number of corneal and conjunctival epithelial cell culture studies that clearly demonstrate an inflammatory immune response pattern when those cells are exposed to desiccation, inflammatory mediators or high osmolarity shock. A yearly distributing plot revealed that 76% were published from 2003 to 2011, 53% from 2008 to 2012, and 11% during the first 9 months of 2013. This distribution signifies a rapidly growing awareness of the importance of inflammation in DED pathogenesis.
Conclusion
The literature review study clearly demonstrate that inflammation is the core mechanism and plays a key role in the pathogenesis of DED as evidenced by research utilizing tissue culture, animal models and subjects with DED. The chronicity of the disease suggests that dysregulation of immune mechanisms leads to a cycle of continued inflammation, accompanied by alterations in both innate and adaptive immune responses. Therefore, developing biomarkers to monitor the ocular surface inflammatory status will not only improve our knowledge to fully understand the mechanisms leading to DED, to better classify the severity of DED, to measure the objective metrics for outcome of treatment, but also provide directions to develop effective and safe anti-inflammatory treatments that will be beneficial for patients with DED.
INTRODUCTION
Dry eye disease (DED) is a multifactorial disorder of the tear film and ocular surface.1 Tear film instability and ocular surface inflammation give rise to symptoms of discomfort, visual disturbance, eye dryness, irritation, foreign body sensation, light sensitivity and itching, all of which eventually reduce a person's quality of life. Effective treatment modalities that can reverse, or at least stop this progression, are scarce.
During the past 20 years, researchers have shown increasing interest in the hypothesis that the core mechanism of DED is inflammation. Using the key words “ dry eye” or “dry eye +inflammation,” the authors conducted a comprehensive search of the PubMed and Web of Science databases for scientific articles published in English between January 1st 1900 and August 30th 2013 on the role of inflammation in DED in cell cultures, animal models and humans. The search revealed a total of 4059 articles by key words “dry eye”, and 458 articles by key words “dry eye + inflammation”, almost 11.3% of DED articles are related to inflammation. These inflammation-related DED articles include 354 original studies (77.29%) and 104 reviews (22.71 %). Of the original studies, the percentage involving human subjects was 43.7% (200); animal or animal models, 25.1% (115); and cell or tissue cultures, 8.5% (39) (Table 1A). Lists of the 10 most published authors and affiliations on the topic are presented in Tables 1B and 1C. A yearly distributing plot (Figure 1) shows that, of the total, 76% were published from 2003 through 2012, 53% from 2008 through 2012, and 11% in the first 9 months of 2013.
Table 1A.
Subject distributions on “Dry Eye + Inflammation” since 1992
| Original Studies |
Reviews | |||
|---|---|---|---|---|
| Humans | Animals | Cell Cultures | ||
| Numbers (% of total) | 200 (43.7%) | 115 (25.1%) | 39 (8.5%) | 104 (22.7%) |
| Subtotal | 354 (77.2%) | 104 (22.7%) | ||
| Total | 458 (100%) | |||
Table 1B.
List of authors/Co-authers with 10 or more publications on “Dry Eye + Inflammation” since 1992
| Author(s) | Affiliations | Total Publications |
|---|---|---|
| Pflugfelder SC | Ocular Surface Center, Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, 6565 Fannin Street, NC505, Houston, TX 77030, USA | 40 |
| Sterm ME | Biological Sciences, Inflammation Research Program, Allergan, Inc., Irvine, California, USA | 34 |
| Baudouin C | Centre Hospitalier National d'Ophtalmologie des Quinze-Vingts and Vision Institute, Paris, France. | 21 |
| Dana R | Schepens Eye Research Institute, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts, USA | 20 |
| Li DQ | Ocular Surface Center, Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, Houston, Texas, USA | 18 |
| Tsubota K | Department of Ophthalmology, Keio University School of Medicine, Tokyo, Japan | 18 |
| De Paiva CS | Ocular Surface Center, Cullen Eye Institute, Department of Ophthalmology, Baylor College of Medicine, Houston, Texas, USA | 15 |
| Calonge M | Ocular Surface Group, IOBA, Universidad de Valladolid, Spain; CIBER-BBN (Biomedical Research Networking Center in Bioengineering, Biomaterials and Nanomedicine), Spain | 14 |
| Gao JP | Biological Sciences, Inflammation Research Program, Allergan, Inc., Irvine, California, USA | 12 |
| Chauhan SK | Schepens Eye Research Institute, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, Massachusetts, USA | 12 |
| Beuerman RW | Singapore Eye Research Institute, Singapore | 10 |
Table 1C.
List of organizations with 10 or more publications on “Dry Eye + Inflammation” since 1992
| Organizations | Numbers of publications | % of total |
|---|---|---|
| Harvard University | 41 | 8.9 |
| Baylor College of Medicine | 32 | 6.9 |
| Allergan Pharmaceut Inc. | 28 | 6.1 |
| Keio University | 17 | 3.7 |
| Universidad De Valladolid | 14 | 3.0 |
| University of California System | 13 | 3.0 |
| University of Paris Descartes Paris V | 12 | 2.6 |
| Louisiana State University | 11 | 2.4 |
| Institute National De La Sante Et De La Researche Medicale Inserm | 10 | 2.2 |
| Tokyo Dental College | 10 | 2.2 |
| Tufts University | 10 | 2.2 |
Figure 1.
Subject distributions on “Dry Eye + Inflammation” since 1992.
These reports strongly indicate that DED is an autoimmune disease of the ocular surface and that inflammation plays the key role in determining its progression and resolution.1-4 Based on this, DED has the same core mechanism as diverse other diseases, e.g., atherosclerosis and rheumatoid arthritis (RA). All these diseases display the same basic characteristics of inflammation.
INFLAMMATION
Inflammation is part of the complex biological response of vascular tissues to harmful stimuli, such as pathogens, damaged cells, or irritants.5 As such, it is a protective attempt by the organism to remove the injurious stimuli and to initiate the healing process. At cellular and molecular level, inflammation is mediated by increased immune cell filtration and liquid mediators, such as cytokines. The cellular components are leukocytes, granulocytes and epithelial cells. Leukocytes normally reside in blood and must move into the inflamed tissue via extravasation to aid in inflammation. Granulocytes release enzymatic granules and inflammatory mediators which develop and maintain the inflammatory response. Epithelial cells provide the first line innate immune defense to protect body against invasion of pathogens and harmful irritants. Acute inflammation is generally mediated by granulocytes, while chronic inflammation is mediated by mononuclear cells, dendritic cells and macrophages. The classical signs of acute inflammation are pain, heat, redness, swelling, and loss of function, but current research has shown that inflammatory responses can be critical to good heath, even when the typical signs of inflammation are not present.
Inflammation in Atherogenesis
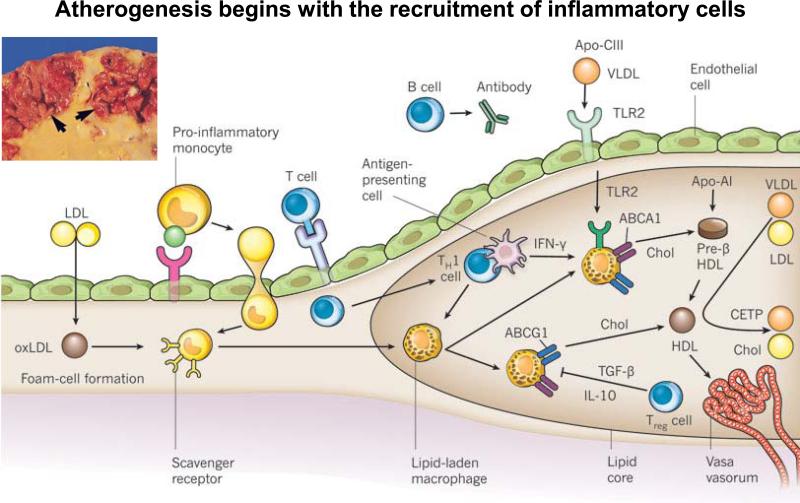
Recent research has demonstrated that atherogenesis begins with the recruitment of inflammatory cells to the intima (Figure 2).6 Activated vascular endothelial cells express leukocyte adhesion molecules that capture and recruit blood monocytes. The monocytes then express scavenger receptors and take up modified low-density lipoprotein (LDL) particles, such as oxidized LDL (oxLDL), leading to the formation of foam cells and the mature lipid-laden macrophages of the plaque's core. These cells can produce pro-inflammatory mediators, reactive oxygen species, and tissue factor pro-coagulants that amplify local inflammation and promote thrombotic complications.
Figure 2.
Atherogenesis and inflammation, adapted with permission from Libby P. et al. Nature 2011:473, 317–325. Atherogenesis begins with the recruitment of inflammatory cells to the intima. Activated endothelial cells express leukocyte adhesion molecules that capture and recruit blood monocytes to the intima. These activated monocytes express scavenger receptors that permit the uptake of modified LDL particles, such as oxidized LDL (oxLDL). Cholesterol loading leads to the formation of foam cells, and ultimately leads to the mature lipid-laden macrophages of the plaque's core. These cells can produce pro-inflammatory mediators, reactive oxygen species, and tissue factor pro-coagulants that amplify local inflammation and promote thrombotic complications. Although fewer in number than the mononuclear phagocytes, T cells also enter the intima and send decisive regulatory signals. After antigen-specific activation, T helper 1 (TH1) cells secrete the signature cytokine interferon-g (IFN-g), which can activate vascular wall cells and macrophages, and magnify and sustain the inflammatory response in the intima. Regulatory T (Treg) cells produce interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), two cytokines considered to exert anti-inflammatory actions.
T cells also enter the intima and send decisive regulatory signals. For example, T helper 1 (Th1) cells secrete the signature cytokine interferon-γ (IFN-γ), which can activate vascular wall cells and macrophages and magnify and sustain the inflammatory response in the intima. Regulatory T (Treg) cells produce interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), two cytokines considered to exert anti-inflammatory actions. At the same time, B cells accumulate and organize in the perivascular tissue surrounding atherosclerotic arteries, where they produce circulating antibodies that may limit inflammation and mute atherogenesis.
In addition, triglyceride-rich lipoproteins such as very-low-density lipoprotein (vLDL) — particularly those particles that bear apolipoprotein C-III (Apo-CIII) or apolipoprotein B (Apo-B) — can instigate vascular inflammation through Toll-like receptor 2 (TLR2) signalling. Macrophage foam cells can efflux cholesterol (chol) through ATP-binding cassette (ABC) transporters, which work in tandem.
Inflammation in Rheumatoid Arthritis (RA)
RA is an autoimmune disease in which the body mistakenly attacks the lining, or synovium, of joints.7 The symptoms include painful, warm, swollen joints, morning stiffness, fatigue, and nodules under the skin.
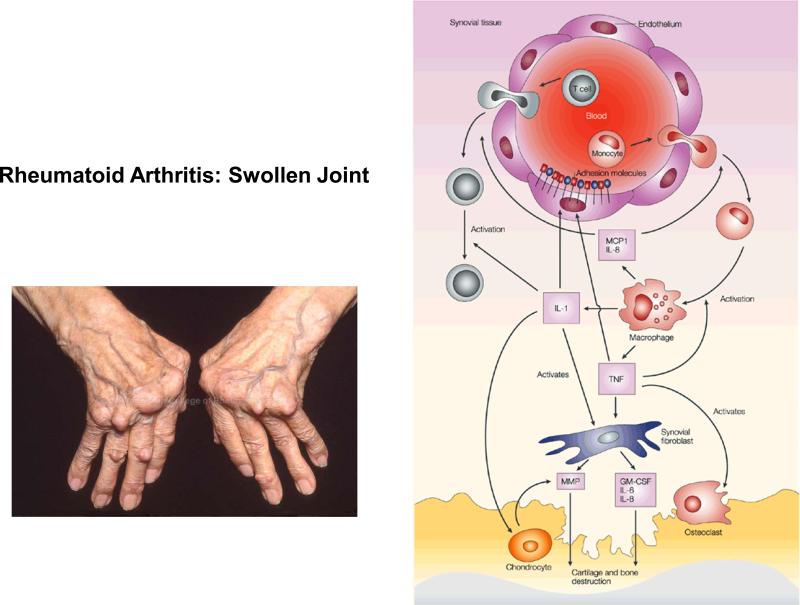
Symptoms appear when monocytes are attracted to the rheumatoid arthritis (RA) joint (Figure 3). Differentiating into activated macrophages, they secrete tumor-necrosis factor (TNF) and interleukin-1 (IL-1). TNF increases the expression of adhesion molecules on endothelial cells, which recruit more cells to the joint.
Figure 3.
Rheumatoid Arthritis and inflammation, adapted with permission from Pope R. Nature Reviews Immunology 2002:2, 527-535.
Monocytes are attracted to the rheumatoid arthritis (RA) joint, where they differentiate into macrophages and secrete inflammatory cytokines (TNF and IL-1) and chemokines (MCP-1 and IL-8). TNF increases the expression of adhesion molecules on endothelial cells, which recruit more inflammatory cells to the joint. Together with IL-1, TNF also induces synovial fibroblasts to express cytokines (such as IL-6), chemokines (such as IL-8), growth factors (such as GM-CSF) and matrix metalloproteinases (MMPs), which contribute to cartilage and bone destruction. Furthermore, TNF contributes to osteoclast activation and differentiation. In addition, IL-1 mediates cartilage degradation directly by inducing the expression of MMPs by chondrocytes.
Chemokines, such as monocyte chemotactic protein 1 (MCP1) and IL-8, are also secreted by macrophages and attract more cells into the joint. IL-1 and TNF induce synovial fibroblasts to express cytokines (such as IL-6), chemokines (such as IL-8), and growth factors (such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and matrix metalloproteinases (MMPs), which contribute to cartilage and bone destruction. TNF contributes to osteoclast activation and differentiation. In addition, IL-1 mediates cartilage degradation directly by inducing the expression of MMPs by chondrocytes.
Inflammation in Dry Eye Disease
The inflammatory components of DED include both cells and mediators. The cells are mainly antigen-presenting cells (APC) such as lymphocytes, dendritic cells, langerhans cells, macrophages, and T-cells (Th1, Th17 and Treg). They also include the epithelial cells of the cornea and conjunctiva. Human leukocyte antigens HLA-DR-positive cells have been proposed as a biomarker of DED in clinical trials.8
The inflammatory mediators associated with DED pathogenesis can be categorized as ubiquitous inflammatory cytokines,9, 10 Th1-related cytokines,11-13 Th17-related cytokines,13-16 chemokines and their receptors,13, 17-21 metalloproteinase20, 22 and secretory phospholipases.23-25 Like HLA-DR-positive cells, changes in tear cytokine expression in both humans 9, 12, 14, 15, 24 and animals, 26-30 can be used as minimally invasive objective biomarkers to classify disease severity, elucidate disease mechanisms, and assess treatments.31
EVIDENCE OF INFLAMMATION AS CORE MECHANISM IN PATHOPHYSIOLOGY OF DRY EYE DISEASE
The 354 original studies revealed by the authors’ search of relevant literature since January, 1900, provide substantial evidence that the core mechanism in the pathophysiology of DED is inflammation. Specific studies are discussed below by the tissue or subjects under study.
Cell or Tissue Culture Models
Numerous inflammatory mediators have been demonstrated with cell or tissue culture models to play pivotal roles in response to stresses like desiccation, pre-sensitazation or hyperosmolarity exposure, similar to those often seen in DED pathophysiology. Of the studies found by the authors, 39 concern DED and inflammation as evaluated by cell or tissue culture models.
In one study, a short-term desiccation (0-30 min) of human corneal epithelial cell line induced an increase in the expression of IL-6 and TNF-α as well as an increase cell death. Cell death was partially suppressed by the addition of anti-IL-6 antibody. A long-term desiccation (>8 hours) of the same cells induced an increase in both IL-6 and IL-8.32
On the other hand, human corneal epithelial cells treated with hyperosmolar shock alone (400-500 mOsm/kg for 12-48 hours) showed no effect on the constitutive expression of human beta-defensin-1 and 3(hBD-1 and -3) nor on induction of hBD-2. However, in the presence of IL-1β, induction of hBD-2 and IL-6 expression was reduced as compared with IL-1β alone, suggesting that hyperosmolar shock may affect ocular surface inflammation via altering functions of key inflammatory cytokines.33
The authors have shown that sPLA2-IIa together with TNF-α or IL-1β have a synergistic effect on the inflammatory cytokine/chemokine expression of human conjunctiva.34
Animal Models
Although animal DED models cannot fully mirror the DED of humans, recent progress on animal DED studies have strongly indicated that DED is dominantly a T-lymphocytes, especially T-helper 17 subset (Th17)-mediated autoimmune disease. The autoimmune-driven inflammatory cycles running around the ocular surface are fundamentally linked with the disease progression.
Dogs have been used in DED research. Normal dogs have a limited level of apoptosis in nictitans lacrimal gland (NLG) and conjunctival epithelial cells, while dogs with DED display an increased apoptosis in the same areas. Furthermore, such increase in apoptosis can be suppressed by a topical application of anti-inflammatory cyclosporine A, providing evidence of the mechanism of inflammation in DED. 35-37
The most frequently used animal models in DED studies involve mice. Based on the authors’ literature research, the most active teams using mouse models are Drs. Pflugfelder, Stern and Niderkorn and their colleagues at the Baylor College of Medicine and Allergan Inc.(with at least 11 publications on the topic) and Dr. Reza Dana's group at the Schepens Eye Research Institute of the Harvard University School of Medicine (with more than 10 publications) on this topic. The conclusions over time of the animal researches of the Pflugfelder and colleagues and Dana and colleagues are shown in Tables 2A and 2B.
Table 2A.
Conclusions of DED Studies by C. Plugfelder's Team at Baylor College of Medicine (2009-13)
| Year | Title | Conclusion |
|---|---|---|
| 2013 | Dry Eye as Mucosal Autoimmune Disease64 | Dry eye is a localized autoimmune disease originating from an imbalance in the protective immunoregulatory and proinflammatory pathways of the ocular surface. |
| 2013 | Toll-Like Receptor Expression and Activation in Mice with Experimental Dry Eye 68 | Toll-like receptors (TLRs) are involved in dysfunctional tear syndrome (DTS) inflammation |
| 2012 | Autoantibodies Contribute to the Immuno-pathogenesis of Experimental Dry Eye Disease 69 | Autoantibody deposition within the ocular surface tissues contributes to the predominantly T-cell-mediated immunopathogenesis of dry eye disease. |
| 2012 | Deletion of interferon-gamma Delays the Onset and Deverity of Dacryoadenitis in CD25KO Mice 70 | The deletion of IFN-γ in the CD25KO mice strain (γDKO ) delays glandular destruction and preserves glandular function. M3R autoantibodies increased with aging in both the γDKO and the CD25KO strains. The decrease in LG function in yDKO correlated with the degree of T-cell infiltration and the presence of M3R autoantibodies. |
| 2012 | Resolvin El (RX-10001) Reduces Corneal Epithelial Barrier Disruption and Protects Against Goblet Cell Loss in a Murine Model of Dry Eye 71 | Endogenous resolvins and resolvin analogues may have potential utility in the treatment of dry eye. |
| 2011 | Disruption of TGF-beta Signaling Improves Ocular Surface Epithelial Disease in Experimental Autoimmune Keratoconjunctivitis Sicca 72 | Disruption of TGF-β signaling in CD4(+) T cells causes paradoxical improvement of dry eye disease in mice subjected to desiccating stress. |
| 2011 | Homeostatic Control of Conjunctival Mucosal Goblet Cells by NKT-derived IL-13 73 | NKT cells are major sources of IL-13 in the conjunctival mucosa that regulates GC homeostasis. |
| 2010 | Induction of Th17 Differentiation by Corneal Epithelial-Derived Cytokines 74 | Th17 differentiation can be promoted by cytokines produced by corneal epithelium that are exposed to hyperosmotic, microbial, and inflammatory stimuli. |
| 2010 | Autoimmunity at the Ocular Surface: Pathogenesis and Regulation 75 | Environmental, microbial and endogenous stress, antigen localization, and genetic factors provide the triggers underlying the immunological events that shape the outcome of the diverse spectrum of autoimmune-based ocular surface disorders. |
| 2010 | An Immunoprotective Privilege of Corneal Epithelial Stem Cells Against Th17 Inflammatory Stress by Producing Glial Cell-Derived neurotrophic Factor 76 | Limbal progenitor cell-produced neurotrophic factor GDNF suppresses IL-17-mediated inflammation via NF-κB signaling pathway. This may represent a unique immunoprotective property of imbal stem cells against inflammatory challenges on the ocular surface. |
| 2009 | Spontaneous T Cell Mediated Keratoconjunctivitis in Aire-Deficient Mice 77 | Aire-deficiency leads to infiltration of CD4(+) and CD8(+) T cells on the ocular surface and meibomian glands, accompanied by goblet cell loss. Desiccating stress promotes this proinflammatory milieu. Immune-mediated mechanisms play a role in the severe blepharitis andkeratoconjunctivitis in the murine model of APECED. |
Table 2B.
Conclusions of DED Studies by R. Dana's Team at Harvard University Schepens Eye Research Institute (2009-13)
| Year | Title | Conclusion |
|---|---|---|
| 2013 | Chronic Dry Eye Disease is Principally Mediated by Effector Memory Th17 Cells 4 | Effector memory Th17 cells are primarily responsible for maintaining the chronic and relapsing course of DED |
| 2012 | Dry Eye Disease: An Immune-Mediated Ocular Surface Disorder 78 | Fundamental links between inflammation and dry eye disease. |
| 2012 | Efficacy of Topical Blockade of Interleukin-1 in Experimental Dry Eye Disease 79 | Topical treatment with IL-1Ra is effective in ameliorating the clinical signs of the dry eye disease as well as in reducing underlying inflammation. |
| 2012 | Expression of Toll-Like Receptor 4 Contributes to Corneal Inflammation in Experimental Dry Eye Disease 80 | DED increases the corneal expression of TLR4 and that TLR4 participates in the inflammatory response to ocular surface desiccating stress. |
| 2012 | Ocular Surface Immunity: Homeostatic Mechanisms and Their Disruption in Dry Eye Disease 81 | Disruption of afferent and efferent immune regulatory mechanisms are responsible for the chronicity of the disease, its symptoms, and its clinical signs. |
| 2011 | Interferon-Gamma-Secreting NK Cells Promote Induction of Dry Eye Disease 82 | IFN-γ-secreting NK cells can promote induction of DED via direct target tissue damage and indirect influence on the priming phase of an adaptive immune response in secondary lymphoid tissue. |
| 2011 | Therapeutic Efficacy of Topical Epigallocatechin Gallate in Murine Dry Eye 83 | Topical EGCG treatment is able to reduce the clinical signs and inflammatory changes in DED by suppressing the inflammatory cytokine expression and infiltration of CD11b+ cells in the cornea. |
| 2010 | Evidence of Corneal Lymphangiogenesis in Dry Eye Disease A Potential Link to Adaptive Immunity? 84 | Low-grade inflammation associated with DED is an inducer of lymphangiogenesis without accompanying hemangiogenesis. |
| 2009 | Autoimmunity in Dry Eye Is Due to Resistance of Th17 to Treg Suppression 85 | Pathogenic T cell subset (Th17) in DED is associated specifically with Treg dysfunction and disease pathogenesis . |
| 2009 | Amelioration of Murine Dry Eye Disease by Topical Antagonist to Chemokine Receptor 2 86 | Topical application of CCR2 antagonist is associated with significant improvement in dry eye disease and is reflected by a decrease in inflammation at the clinical, molecular, and cellular levels. |
Using a similar scopolamine-air ventilation desiccation-induction mouse model but with white BALB/C mice that have the same natural pla2g2a gene as in humans, the authors have conducted DED studies that confirmed the pivotal role of sPLA2-IIa (product of pla2g2a gene) in promoting inflammation on the ocular surface, especially when the ocular surface is under a desiccation stress or in the presence of additional TNF-α and IL-1β. 23-25, 38 sPLA2-IIa not only supports ridding the tear film of bacterial pathogens, but also serves as the first rate-limiting enzyme for the inflammatory cascade to produce PGE2 and many other inflammatory mediators that drive the cycle of inflammation on the ocular surface. Furthermore, the authors demonstrated that after DE induction, the mouse that received eye-drops containing solvent only developed a typical punctuated corneal keratopathy in corneal staining, whereas the mouse that received eye-drops containing sPLA2 inhibitors displayed almost no staining, indicating that sPLA2 played a significant role in DE pathogenesis. The inhibition of sPLA2 may provide a pathway for therapeutics of DED and other inflammatory diseases of the ocular surface. 38
Human Studies
Recent progress on human DED inflammation reveal that both innate and adaptive immunity are critical to DED pathogenesis and progression. These involve recruitments of many infiltrated inflammatory immune cells to the ocular surface and their released liquid mediators in situ. More supportive evidence also come from clinic trials on topically or systematically anti-inflammatory treatments of DED, which have emerged promising improvements on signs and symptoms of DED patients as well as in laboratory biomarker pattern changes.
A number of human studies have demonstrated increases in a variety of inflammatory cells in both non-Sjögren's and Sjögren's dry eye patients. Examples of such cells are the following:
CD3-positive and CD4-positive T cells in conjunctival epithelium of Sjögren and non-Sjögren syndrome-associated DED39
IL-7Rα-positive T cells 40
HLA-DR-positive antigen presenting cell (APC) in conjunctiva 39, 42
IFN-γ can also upregulate HLA-DR and intercellular adhesion molecule-1 (ICAM-1) in human conjunctival cells, which indicates that ocular surface cells can respond to and modulate inflammation. 43
Many human studies also point to the important inflammatory role played by cytokines, chemokines and their receptors; for example:
IL-2, IL-4, IL-5, IL-6, IL-8, IFN-γ, TNF-α, and IL-1β 44
inflammatory chemokines macrophage inflammatory protein 2 (MIP-2) and chemokine receptors (CCR5) (CCR5) 18, 39, 45
IL-1β, TNF-α, and matrix metalloproteinase 9 (MMP-9) and activates mitogen-activated protein kinase (MAPK) signaling pathways 45, 46
IL-6 protein and IL-1β, TNF-α, IL-8 mRNA47
IL-7 40
That inflammation plays a key role in DED pathogenesis is also supported by the authors’ own studies. We have shown the HLA-DR-positive cell population is correlated with severity of DED; 48 also that concentrations of inflammatory cytokine/chemokines, especially IL-1β, IL6, IL8, IL-10, TNF-α and IFN-γ, are increased in the tears of DED patients as compared with normal controls.44, 49
RESPONSES TO ANTI-INFLAMMATORY TREATMENTS CONSISTENT WITH THE PIVOTAL ROLE OF INFLAMMATION IN DED PROGRESSION
Several reports reveal improvement of DED symptoms and/or signs in patientstreated with topical or systematic anti-inflammatory drugs such as cyclosporin, and steroids:
CsA proven to improve symptoms and objective tests (Johnson LN 2007 http://clinicaltrials.gov/show/NCT01072526 ) 49, 50
Corticosteroids effective anti-inflammatory therapy in DED (Have to be used carefully and preferentially in the short-term due to steroid side effects) 51
Omega-3 essential fatty acids in dietary supplementation were found to be beneficial, though other reports were contradictory.52-55
Vitamin A & CsA 0,05% eye drops significantly improved blurred vision, TFBUT, Schirmer's Score and IC 49, 50
Anti-inflammatory therapy and/or immunomodulatory and anti-apoptotic strategies may play an important role in the management of DED.56, 57
Acupuncture and homeopathy, as alternative therapies, associated with topical cyclosporine, are currently being tested.58
Recently, the authors conducted a small randomized double-blind clinical trial in DED subjects of systemic Omega 3 supplements. Comparison of cytokine levels in tears of patients who received placebo with those who received Omega-3 supplementation for 3 months demonstrated an average 4-fold decrease in IL-1β, TNF-α and INF-γ but 4-fold increase in IL-6.31 These results suggest that Omega-3 may decrease inflammatory cytokines or delay progression of DED inflammation. However, a larger study populations and longer treatment would be needed to determine efficacy of Omega-3 in reducing tear cytokine concentrations and more clearly evaluate possible effects on signs and symptoms of DED.
OXIDATIVE STRESS AND THE PATHOGENESIS OF DRY EYE DISEASE
Oxidative stress is caused by an imbalance between the production of reactive oxygen species and the ability of a biological system's defense mechanisms to eliminate the stress. Such stress has been implicated in a variety of acute and chronic diseases, including atherosclerosis, myocardial infarction, fragile X syndrome, Parkinson's disease and Alzheimer's disease.59
Recently, Tsubota et al in Japan have investigated a possible pathogenic role of oxidative stress in DED with a conditional knockout mouse line of mev-1gene (Tet-Mev-1 mouse). The mev-1 gene encodes Cyt-1, the cytochrome b (560) large subunit of succinate-ubiquinone oxidoreductase in complex II of mitochondria (homologous to succinate dehydrogenase C subunit in humans). 60 As such, disruption of the mev-1 gene function causes excessive accumulation of superoxide anion and the oxidative stress in Caenorhabditis elegans. 61
In one study, the Tsubota team showed that a functional knockout of mev-1 gene in mice caused a decrease in tear production with morphological changes, including leukocytic infiltration and fibrosis, and induced lacrimal dysfunction and DED. They concluded that inflammation induced by oxidative stress may initiate a functional decline in tear production. 60
In another study, the same team showed that Se-lactoferrin eye drops in high doses weakly improved dry eye, probably by suppressing the up-regulation of heme oxygenase-1, cyclooxygenase-2, matrix metallopeptidase-9 and IL-6 while suppressing 8-Oxo-2’-deoxyguanosine (8-OHdG) production in the cornea.62
Previous studies have shown that lactoferrin, a dominant glycoprotein of tears, has anti-inflammatory effects and promotes cell growth. Oral lactoferrin administration preserves lacrimal gland function in aged mice by attenuating oxidative damage and suppressing subsequent gland inflammation. Lactoferrin administration may thus reduce inflammatory cell infiltration and the monocyte chemotactic protein 1 (MCP-1) and TNF-α expression levels in age-induced dry eye disease.63
Taken together, the above studies suggest that oxidative stress may play a significant role in the pathogenesis of DED, probably through influencing ocular surface inflammation.
ADAPTIVE OR INNAT E IMMUNITY, CHICKEN OR EGG: CAN'T TELL WHICH CAME FIRST, BUT THEY ARE INTEGRALLY CONNECTED
DED inflammation comprises both innate and adaptive immunity. 2, 64, 65 The initial, acute response is an innate immune episode of DED likely trigged by environmental and/or microbial stresses. These stresses change the dynamics of tear film and the associated lacrmial gland, neural network and ocular surface and initiate ocular surface non-specific immune responses by activating immature antigen-presenting cells (APCs, such as dendritic cells, monocytes, NK cells, γδT cells, langerhans cells, macrophages and ocular epithelial cells) through mitogen-activated protein kinase (MAPK) pathways and/or pattern recognition receptors (PRRs). 66 The activated APCs then produce and release a variety of inflammatory mediators, such as cytokines, chemokines, MMPs, ICAMs and phospholipases that further magnify the innate inflammatory responses.
Subsequently, mature APCs migrate into regional lymph nodes, where they initiate the adaptive immune episode by generating and maintaining dry eye- and ocular-specific autoreactive CD4+-T cells and autoantibody secreting B cells. Th17 cells then migrate through efferent blood vessels back to the ocular surface after breaking up the epithelial barriers and producing more mediators to promote lymphangiogenesis. They also facilitate pathogenic immunocytic infiltration that leads to further damage of the ocular surface and progression of a chronic cycle of inflammation.
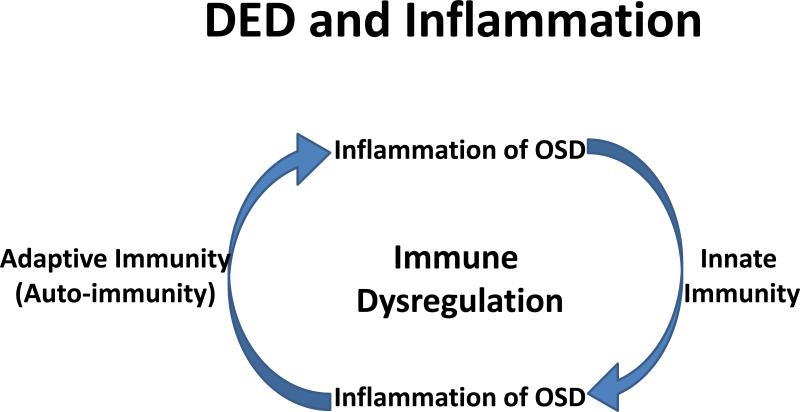
Thus, the authors’ working hypothesis is that the key mechanism in DED-related inflammation is not innate or adaptive immunity per se, but a chronic inflammatory cycle sustained by dysregulation of immunity (Figure 4).
Figure 4.
The current hypothesis of relationship between DED and inflammation. DED inflammation comprises both innate and adaptive immunity. It starts with an acute innate immune in response to environmental and/or microbial stresses, leading to activation and maturation of antigen-presenting cells (APCs). The matured APCs migrate into regional lymph nodes to generate and maintain dry eye- and ocular-specific autoreactive CD4+-T cells, autoantibody secreting B cells, leading to the adaptive immunity. Th17 cells back to the ocular surface through efferent blood vessels to promote production of inflammatory mediators, lymphangiogenesis and pathogenic immunocytic infiltration, leading to further damage of the ocular surface and progression of a chronic cycle of inflammation. Thus, the key mechanism in DED-related inflammation is not innate or adaptive immunity per se, but a abnormal inflammatory cycle sustained by dysregulation of immune response.
PERSPECTIVES AND NOVEL STRATEGIES TO EFFECTIVELY TREAT DED INFLAMMATION
Based on this new hypothesis of DED pathogenesis, a cure or delay in DED progression may be achieved by any means that breaks the chronic cycle of ocular surface inflammation. Given the complex network of regulation and feed-back, combined multiple targets are more plausible than single ones. Since sPLA2-IIa is the first enzyme in the cascade that produces inflammatory mediator PGE2 and a leading factor in the amplification of ocular surface inflammation under desiccation or compromised stress,67 strategies to inhibit the function of sPLA2-IIa and block the production of inflammatory cytokines may be one way to block the cascade of inflammation and thus interfer with the cycle of ocular surface inflammation.
The extensive and growing peer- reviewed published literature on DED from studies in tissue culture, animal models and humans strongly supports the role of inflammation as part of the core pathogenesis of DED. The chronicity of the disease suggests that dysregulation of immune mechanisms leads to a cycle of continued inflammation. Changes in both innate and adaptive responses are likely “at play,” and that understanding the dysregulation of the immune responses will lead to improved mechanisms for controlling inflammation of the ocular surface associated with DED.
Acknowledgments
Funding
Supported by National Eye Institute Grant to PAA (1U10EY022881-01).
Contributor Information
Yi Wei, Icahn School of Medicine at Mount Sinai, New York, NY.
Penny A. Asbell, Icahn School of Medicine at Mount Sinai, New York, NY.
REFERENCES
- 1.DEWS The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflugfelder SC, Stern ME. Immunoregulation on the ocular surface: 2nd Cullen Symposium. Ocul Surf. 2009;7:67–77. doi: 10.1016/s1542-0124(12)70297-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chauhan SK, Soo Lee H, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2013 doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 7.Pope RM. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nature reviews Immunology. 2002;2:527–535. doi: 10.1038/nri846. [DOI] [PubMed] [Google Scholar]
- 8.Epstein SP, Gadaria-Rathod N, Wei Y, Maguire MG, Asbell PA. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111:95–104. doi: 10.1016/j.exer.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol Vis Sci. 2011;52:7725–7730. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- 10.Narayanan S, Corrales RM, Farley W, McDermott AM, Pflugfelder SC. Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye. Cornea. 2008;27:811–817. doi: 10.1097/ICO.0b013e31816bf46c. [DOI] [PubMed] [Google Scholar]
- 11.Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 12.Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71:89–95. doi: 10.1590/s0004-27492008000700017. [DOI] [PubMed] [Google Scholar]
- 13.Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC. Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. 2009;147:198–205. e191. doi: 10.1016/j.ajo.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007;26:431–437. doi: 10.1097/ICO.0b013e31803dcda2. [DOI] [PubMed] [Google Scholar]
- 15.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- 16.De Paiva CS, Hwang CS, Pitcher JD, 3rd, et al. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford) 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon KC, Park CS, You IC, et al. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol-Chic. 2006;124:710–716. doi: 10.1001/archopht.124.5.710. [DOI] [PubMed] [Google Scholar]
- 19.El Annan J, Chauhan SK, Ecoiffier T, Zhang Q, Saban DR, Dana R. Characterization of effector T cells in dry eye disease. Invest Ophthalmol Vis Sci. 2009;50:3802–3807. doi: 10.1167/iovs.08-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 21.Acera A, Rocha G, Vecino E, Lema I, Duran JA. Inflammatory markers in the tears of patients with ocular surface disease. Ophthalmic Res. 2008;40:315–321. doi: 10.1159/000150445. [DOI] [PubMed] [Google Scholar]
- 22.VanDerMeid KR, Su SP, Ward KW, Zhang JZ. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Invest Ophthalmol Vis Sci. 2012;53:1512–1518. doi: 10.1167/iovs.11-7627. [DOI] [PubMed] [Google Scholar]
- 23.Wei Y, Pinhas A, Liu Y, Epstein S, Wang J, Asbell P. Isoforms of Secretory Group Two Phospholipase A (sPLA2) in Mouse Ocular Surface Epithelia and Lacrimal Glands. Invest Ophthalmol Vis Sci. 2012;53:2845–2855. doi: 10.1167/iovs.11-8684. [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Wei Y, Li X, Epstein S, Wolosin JM, Asbell P. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009;88:880–888. doi: 10.1016/j.exer.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Epstein SP, Fukuoka S, Birmingham NP, Li XM, Asbell PA. sPLA2-IIa amplifies ocular surface inflammation in the experimental dry eye (DE) BALB/c mouse model. Invest Ophthalmol Vis Sci. 2011;52:4780–4788. doi: 10.1167/iovs.10-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjogren's syndrome using adenovirus-mediated gene transfer. Lab Invest. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating Stress Promotes Th17 Differentiation by Ocular Surface Tissues through a Dendritic Cell-Mediated Pathway. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Paiva CS, Chotikavanich S, Pangelinan SB, et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan SK, Dana R. Role of Th17 cells in the immunopathogenesis of dry eye disease. Mucosal Immunol. 2009;2:375–376. doi: 10.1038/mi.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barabino S, Rolando M, Chen L, Dana MR. Exposure to a dry environment induces strain-specific responses in mice. Exp Eye Res. 2007;84:973–977. doi: 10.1016/j.exer.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y, Gadaria-Rathod N, Epstein S, Asbell P. Tear Cytokine Profile as a Non-invasive Biomarker of Inflammation for Ocular Surface Diseases: Standard Operating Procedures. Investigative Ophthalmology & Vision Sciences (accepted on Oct 24, 2013) 2013 doi: 10.1167/iovs.13-12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higuchi A, Kawakita T, Tsubota K. IL-6 induction in desiccated corneal epithelium in vitro and in vivo. Mol Vis. 2011;17:2400–2406. [PMC free article] [PubMed] [Google Scholar]
- 33.Narayanan S, Manning J, Proske R, McDermott AM. Effect of hyperosmolality on beta-defensin gene expression by human corneal epithelial cells. Cornea. 2006;25:1063–1068. doi: 10.1097/01.ico.0000228785.84581.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen DM, Wei Y, Li XH, Epstein S, Wolosin JM, Asbell P. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009;88:880–888. doi: 10.1016/j.exer.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calonge M, Enriquez-De-Salamanca A, Diebold Y, et al. Dry Eye Disease as an Inflammatory Disorder. Ocul Immunol Inflamm. 2010;18:244–253. doi: 10.3109/09273941003721926. [DOI] [PubMed] [Google Scholar]
- 36.Gao J, Schwalb TA, Addeo JV, Ghosn CR, Stern ME. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: the effect of topical Cyclosporin A therapy. Cornea. 1998;17:654–663. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Gao J, Gelber-Schwalb TA, Addeo JV, Stern ME. Apoptosis in the lacrimal gland and conjunctiva of dry eye dogs. Adv Exp Med Biol. 1998;438:453–460. doi: 10.1007/978-1-4615-5359-5_63. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Li P, Zou J, Epstein S, Gadaria N, Asbell P. Secretory group two phospholipase (sPLA2) inhibition decreases corneal superficial punctate keratitis (SPK) in dry eye mice.. ARVO 2013 Annual Meeting; Seatle, USA. 2013.pp. 4326–C0064. [Google Scholar]
- 39.Stern ME, Gao JP, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Investigative Ophthalmology & Visual Science. 2002;43:2609–2614. [PubMed] [Google Scholar]
- 40.Bikker A, Moret FM, Kruize AA, Bijlsma JW, Lafeber FP, van Roon JA. IL-7 drives Th1 and Th17 cytokine production in patients with primary SS despite an increase in CD4 T cells lacking the IL-7Ralpha. Rheumatology (Oxford) 2012;51:996–1005. doi: 10.1093/rheumatology/ker448. [DOI] [PubMed] [Google Scholar]
- 41.Rojas B, Cuhna R, Zafirakis P, et al. Cell populations and adhesion molecules expression in conjunctiva before and after bone marrow transplantation. Exp Eye Res. 2005;81:313–325. doi: 10.1016/j.exer.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Zoukhri D, Kublin CL. Impaired neurotransmission in lacrimal and salivary glands of a murine model of Sjogren's syndrome. Adv Exp Med Biol. 2002;506:1023–1028. doi: 10.1007/978-1-4615-0717-8_144. [DOI] [PubMed] [Google Scholar]
- 43.De Saint Jean M, Brignole F, Feldmann G, Goguel A, Baudouin C. Interferon-gamma induces apoptosis and expression of inflammation-related proteins in Chang conjunctival cells. Invest Ophthalmol Vis Sci. 1999;40:2199–2212. [PubMed] [Google Scholar]
- 44.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 45.Song XJ, Li DQ, Farley W, et al. Neurturin-deficient mice develop dry eye and keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2003;44:4223–4229. doi: 10.1167/iovs.02-1319. [DOI] [PubMed] [Google Scholar]
- 46.Luo LH, Li DQ, Doshi A, Farley W, Corrales RM, Pflugfelder SC. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Investigative Ophthalmology & Visual Science. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 47.Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- 48.Epstein SP, Gadaria-Rathod N, Wei Y, Maguire MG, Asbell PA. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res. 2013;111:95–104. doi: 10.1016/j.exer.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 50.Kim EC, Choi JS, Joo CK. A comparison of vitamin a and cyclosporine a 0.05% eye drops for treatment of dry eye syndrome. Am J Ophthalmol. 2009;147:206–213. e203. doi: 10.1016/j.ajo.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 51.Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren syndrome. Ophthalmology. 1999;106:811–816. doi: 10.1016/S0161-6420(99)90171-9. [DOI] [PubMed] [Google Scholar]
- 52.Rand AL, Asbell PA. Nutritional supplements for dry eye syndrome. Curr Opin Ophthalmol. 2011;22:279–282. doi: 10.1097/ICU.0b013e3283477d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocul Surf. 2010;8:18–28. doi: 10.1016/s1542-0124(12)70214-8. [DOI] [PubMed] [Google Scholar]
- 54.Roncone M, Bartlett H, Eperjesi F. Essential fatty acids for dry eye: A review. Cont Lens Anterior Eye. 2010;33:49–54. doi: 10.1016/j.clae.2009.11.002. quiz 100. [DOI] [PubMed] [Google Scholar]
- 55.Rashid S, Jin Y, Ecoiffier T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126:219–225. doi: 10.1001/archophthalmol.2007.61. [DOI] [PubMed] [Google Scholar]
- 56.Latkany R. Dry eyes: etiology and management. Curr Opin Ophthalmol. 2008;19:287–291. doi: 10.1097/ICU.0b013e3283023d4c. [DOI] [PubMed] [Google Scholar]
- 57.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 58.Kim TH, Kim JI, Shin MS, et al. Acupuncture for dry eye: a randomised controlled trial protocol. Trials. 2009;10:112. doi: 10.1186/1745-6215-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wakamatsu TH, Dogru M, Tsubota K. Tearful relations: oxidative stress, inflammation and eye diseases. Arq Bras Oftalmol. 2008;71:72–79. doi: 10.1590/s0004-27492008000700015. [DOI] [PubMed] [Google Scholar]
- 60.Uchino Y, Kawakita T, Miyazawa M, et al. Oxidative Stress Induced Inflammation Initiates Functional Decline of Tear Production. PLoS One. 2012:7. doi: 10.1371/journal.pone.0045805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senoo-Matsuda N, Yasuda K, Tsuda M, et al. A defect in the cytochrome b large subunit in complex II causes both superoxide anion overproduction and abnormal energy metabolism in Caenorhabditis elegans. J Biol Chem. 2001;276:41553–41558. doi: 10.1074/jbc.M104718200. [DOI] [PubMed] [Google Scholar]
- 62.Higuchi A, Inoue H, Kawakita T, Ogishima T, Tsubota K. Selenium Compound Protects Corneal Epithelium against Oxidative Stress. PLoS One. 2012:7. doi: 10.1371/journal.pone.0045612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawashima M, Kawakita T, Inaba T, et al. Dietary Lactoferrin Alleviates Age-Related Lacrimal Gland Dysfunction in Mice. PLoS One. 2012:7. doi: 10.1371/journal.pone.0033148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern ME, Schaumburg CS, Pflugfelder SC. Dry Eye as a Mucosal Autoimmune Disease. International reviews of immunology. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calonge M, Enriquez-de-Salamanca A, Diebold Y, et al. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18:190–199. doi: 10.3109/09273941003721926. [DOI] [PubMed] [Google Scholar]
- 66.Moschos MM, Eperon S, Guex-Crosier Y. Increased eotaxin in tears of patients wearing contact lenses. Cornea. 2004;23:771–775. doi: 10.1097/01.ico.0000133987.34274.c0. [DOI] [PubMed] [Google Scholar]
- 67.Wei Y, Du Z, Chen D, Afreen J, Chen V, Asbell P. The Role of the Secretory Group IIa Phospholipase A2 (sPLA2-IIa) in Ocular Surface Inflammation. JSM Ophthalmology (Open Acess Journal) 2013;1:1005. [Google Scholar]
- 68.Redfern RL, Patel N, Hanlon S, et al. Toll-Like Receptor Expression and Activation in Mice with Experimental Dry Eye. Investigative Ophthalmology & Visual Science. 2013;54:1554–1563. doi: 10.1167/iovs.12-10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stern ME, Schaumburg CS, Siemasko KF, et al. Autoantibodies Contribute to the Immunopathogenesis of Experimental Dry Eye Disease. Investigative Ophthalmology & Visual Science. 2012;53:2062–2075. doi: 10.1167/iovs.11-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pelegrino FSA, Volpe EA, Gandhi NB, Li DQ, Pflugfelder SC, de Paiva CS. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res Ther. 2012:14. doi: 10.1186/ar4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee CH. Resolvins as New Fascinating Drug Candidates for Inflammatory Diseases. Archives of pharmacal research. 2012;35:3–7. doi: 10.1007/s12272-012-0121-z. [DOI] [PubMed] [Google Scholar]
- 72.De Paiva CS, Volpe EA, Gandhi NB, et al. Disruption of TGF-beta Signaling Improves Ocular Surface Epithelial Disease in Experimental Autoimmune Keratoconjunctivitis Sicca. PLoS One. 2011:6. doi: 10.1371/journal.pone.0029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Paiva CS, Raince JK, McClellan AJ, et al. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng XF, Bian F, Ma P, et al. Induction of Th17 Differentiation by Corneal Epithelial-Derived Cytokines. J Cell Physiol. 2010;222:95–102. doi: 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stern ME, Schaumburg CS, Dana R, Calonge M, Niederkorn JY, Pflugfelder SC. Autoimmunity at the ocular surface: pathogenesis and regulation. Mucosal Immunol. 2010;3:425–442. doi: 10.1038/mi.2010.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bian F, Qi H, Ma P, et al. An immunoprotective privilege of corneal epithelial stem cells against Th17 inflammatory stress by producing glial cell-derived neurotrophic factor. Stem cells. 2010;28:2172–2181. doi: 10.1002/stem.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeh S, de Paiva CS, Hwang CS, et al. Spontaneous T cell mediated keratoconjunctivitis in Aire-deficient mice. Br J Ophthalmol. 2009;93:1260–1264. doi: 10.1136/bjo.2008.153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevenson W, Chauhan SK, Dana R. Dry Eye Disease An Immune-Mediated Ocular Surface Disorder. Arch Ophthalmol-Chic. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Okanobo A, Chauhan SK, Dastjerdi MH, Kodati S, Dana R. Efficacy of Topical Blockade of Interleukin-1 in Experimental Dry Eye Disease. Am J Ophthalmol. 2012;154:63–71. doi: 10.1016/j.ajo.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee HS, Hattori T, Park EY, Stevenson W, Chauhan SK, Dana R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5632–5640. doi: 10.1167/iovs.12-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barabino S, Chen Y, Chauhan S, Dana R. Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res. 2012;31:271–285. doi: 10.1016/j.preteyeres.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-gamma-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89:965–972. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee HS, Chauhan SK, Okanobo A, Nallasamy N, Dana R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea. 2011;30:1465–1472. doi: 10.1097/ICO.0b013e31821c9b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128:819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chauhan SK, El Annan J, Ecoiffier T, et al. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goyal S, Chauhan SK, Zhang Q, Dana R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch Ophthalmol. 2009;127:882–887. doi: 10.1001/archophthalmol.2009.125. [DOI] [PubMed] [Google Scholar]