A crystallization screen was set up for GαsAHD in complex with the specifically selected nanobody CA9177. A high-resolution (1.59 Å) data set was collected for one of the crystallization conditions.
Keywords: GPCR transmembrane signalling, nanobody-enabled X-ray crystallography, G protein
Abstract
GPCR–G-protein complexes are one of the most important components of cell-signalling cascades. Extracellular signals are sensed by membrane-associated G-protein-coupled receptors (GPCRs) and transduced via G proteins towards intracellular effector molecules. Structural studies of these transient complexes are crucial to understand the molecular details of these interactions. Although a nucleotide-free GPCR–G-protein complex is stable, it is not an ideal sample for crystallization owing to the intrinsic mobility of the Gαs α-helical domain (AHD). To stabilize GPCR–G-protein complexes in a nucleotide-free form, nanobodies were selected that target the flexible GαsAHD. One of these nanobodies, CA9177, was co-crystallized with the GαsAHD. Initial crystals were obtained using the sitting-drop method in a sparse-matrix screen and further optimized. The crystals diffracted to 1.59 Å resolution and belonged to the monoclinic space group P21, with unit-cell parameters a = 44.07, b = 52.55, c = 52.66 Å, α = 90.00, β = 107.89, γ = 90.00°. The structure of this specific nanobody reveals its binding epitope on GαsAHD and will help to determine whether this nanobody could be used as crystallization chaperone for GPCR–G-protein complexes.
1. Introduction
Heterotrimeric guanine nucleotide-binding proteins (G proteins) control many cellular processes by playing an important role in signal transduction (Simon et al., 1991 ▶; Hamm, 1998 ▶). G proteins are generally activated by G-protein-coupled receptors (GPCRs) upon binding of a ligand, and transduce the extracellular signal in the cell to start a cascade of intracellular responses (Oldham & Hamm, 2008 ▶). All G proteins consist of three subunits (Gα, Gβ and Gγ), where Gα is responsible for nucleotide binding and Gβγ forms an obligate functional dimer. G-protein activation occurs when GDP is exchanged for GTP in the Gα subunit. This cyclic process is catalysed by guanine nucleotide-exchange factors (GEFs), of which GPCRs are the most studied example. When a GPCR is activated by an extracellular signal, conformational changes trigger GDP release from the Gα subunit and a stable nucleotide-free GPCR–G-protein complex is formed (Rasmussen, DeVree et al., 2011 ▶). Upon binding of GTP in the nucleotide-binding pocket, Gα undergoes conformational changes which lead to its dissociation from the GPCR. This also causes the tightly associated G-protein heterotrimer to separate and free both Gα–GTP and Gβγ, which will regulate downstream effector molecules (Bohm et al., 1997 ▶). The intrinsic GTP hydrolase activity of Gα resets the G protein to its basal state, where GDP-bound Gα reassociates with the Gβγ heterodimer. The Gα subunit itself is composed of two domains: a Ras-like GTPase domain that hydrolyses GTP and a unique helical domain. Recently, structural studies revealed that the α-helical domain (AHD) acts as a lid that traps the nucleotide in a binding pocket. In the absence of a bound nucleotide, the AHD is a flexible switch that adopts a broad range of possible positions (Westfield et al., 2011 ▶).
To promote the stabilization of GPCR–G-protein complexes in the nucleotide-free form, nanobodies were generated that bind and preferably stabilize the GαsAHD. Nanobodies (Nbs) or VHHs are the small antigen-binding fragments (15 kDa) of heavy-chain-only antibodies (hcAbs) found in Camelidae (Muyldermans et al., 2001 ▶; Muyldermans, 2013 ▶). They specifically recognize native conformations and can reduce complexity or flexibility of proteins by trapping the protein in one particular conformation (Steyaert & Kobilka, 2011 ▶). In recent years, immune libraries have been exploited to select nanobodies that can be used as crystallization aids (Pardon et al., 2014 ▶). This approach has been especially successful in the determination of crystal structures of GPCRs in their active states (Rasmussen, Choi et al., 2011 ▶; Kruse et al., 2013 ▶) and of GPCR–G-protein complexes (Rasmussen, DeVree et al., 2011 ▶). As a continuation of this strategy, additional nanobodies were generated that target the AHD of Gαs. The aim was to reduce the observed mobility of the AHD (Westfield et al., 2011 ▶), which causes heterogeneity of a GPCR–G-protein sample and is one of the challenges to overcome when crystallizing such complexes. Additionally, these nanobodies could serve as imaging tools to unravel the conformational changes and signalling mechanisms of GPCR–G-protein complexes in living cells (Irannejad et al., 2013 ▶; Irannejad & von Zastrow, 2014 ▶). Here, we report the crystallization of a GαsAHD–Nb (CA9177) complex.
2. Materials and methods
2.1. Production and purification of GαsAHD in complex with a nanobody
2.1.1. Expression and purification of Gαs α-helical domain
The expression vector for the purification of the AHD of bovine Gαs (P04896) was a kind gift from Roger Sunahara’s laboratory (Table 1 ▶). The DNA sequence of GαsAHD was cloned into a pQE-60 expression vector, which introduces a C-terminal His6 tag and contains an ampicillin-resistance gene. The plasmid was transformed into chemically competent Escherichia coli BL21(DE3) cells. For expression, 1 l Terrific Broth (TB) containing 100 µg ml−1 ampicillin was inoculated with 10 ml of an overnight preculture. Cells were grown at 310 K with shaking (120 rev min−1) until they reached an OD600 of 0.7–1.1. Induction was performed by adding 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and lowering the temperature to 293 K for overnight expression of recombinant GαsAHD.
Table 1. Macromolecule-production information.
| GsAHD | CA9177 | |
|---|---|---|
| Source organism | Bos taurus | Lama glama |
| DNA source | Synthetic | Synthetic |
| Expression vector | pQE-60 | pMESy4 |
| Expression host | E. coli BL21 (DE3) | E. coli WK6 (su) |
Cells were harvested by centrifugation for 10 min at 6000g. The pellet was resuspended in 20 ml lysis buffer [50 mM Tris pH 8.0, 100 mM NaCl, 2 mM β-mercaptoethanol (BME), 200 µM phenylmethylsulfonyl fluoride (PMSF), 50 µg ml−1 DNAse, 20 mM MgCl2] per litre pellet. The solubilized cells were lysed at 138 MPa using a cell disruptor (Constant Systems) and centrifuged for 45 min at 40 000g. The clarified supernatant was loaded onto a 5 ml nickel–nitrilotriacetic acid (Ni–NTA) column (HisTrap HP, GE Healthcare) equilibrated with 50 mM Tris pH 8.0, 100 mM NaCl, 2 mM BME, 200 µM PMSF, 10 mM imidazole. GαsAHD was eluted with five column volumes (CV) of 50 mM Tris pH 8.0, 100 mM NaCl, 2 mM BME, 200 µM PMSF, 250 mM imidazole. The protein was further purified on a Superdex 75 HR 16/60 column equilibrated with 50 mM Tris pH 8.0, 150 mM NaCl, 2 mM DTT. The GαsAHD-containing fractions were concentrated (Amicon Ultra, MWCO 3000, Millipore) to a minimal concentration of 10 mg ml−1, flash-frozen and stored at 193 K.
2.1.2. Nanobody expression and purification
For production and purification, the DNA sequence encoding the nanobody was cloned into the E. coli expression vector pMESy4 (Pardon et al., 2014 ▶; Table 1 ▶). The nanobody is expressed with a C-terminal His6-EPEA tag (De Genst et al., 2010 ▶) and a pelB signal peptide directs the recombinant nanobody to the periplasm. The plasmid was transformed into the E. coli WK6 expression strain and a fresh colony was used to start a preculture in 50 ml Luria–Bertani broth (LB), 100 µg ml−1 ampicillin, 2% glucose. For large-scale production, 1 l TB was supplemented with 100 µg ml−1 ampicillin, 2% glucose, 1 mM MgCl2 and inoculated with 10 ml overnight-grown preculture. The cultures were grown at 310 K with shaking (120 rev min−1) until an OD600 of 0.6–0.8 was reached. The periplasmic expression of the nanobody was induced by adding 1 mM IPTG for 16 h at 301 K. Cells were harvested by centrifugation at 6000g for 10 min. The pellet was resuspended in 15 ml 200 mM Tris–HCl pH 8.0, 0.65 mM EDTA, 500 mM sucrose per litre pellet and incubated for 1 h on ice. Followed by the addition of 30 ml of 200 mM Tris–HCl pH 8.0, 0.65 mM EDTA, 125 mM sucrose, the cells experience an osmotic shock and release proteins from the periplasm. After an additional hour on ice, the cells were centrifuged for 45 min at 12 000g. The periplasmic extract was incubated with cobalt-loaded beads (Talon Metal Affinity Resin, Clontech) for 30 min and poured into an empty column (PD10, GE Healthcare). After washing with 10 CV 50 mM Na2HPO4/NaH2PO4 pH 7.4, 300 mM NaCl, 5 mM imidazole, the nanobody was eluted with 4 CV of 50 mM Na2HPO4/NaH2PO4 pH 7.4, 250 mM NaCl, 250 mM imidazole. To polish the nanobody and exchange the buffer, concentrated nanobody was injected onto a Superdex 75 HR 16/60 column equilibrated with 50 mM Tris pH 8.0, 150 mM NaCl, 2 mM DTT. The nanobody-containing fractions were concentrated (Amicon Ultra, MWCO 3000, Millipore) to a minimal concentration of 10 mg ml−1 and stored at 253 K.
2.1.3. Preparation of the GαsAHD in complex with a nanobody
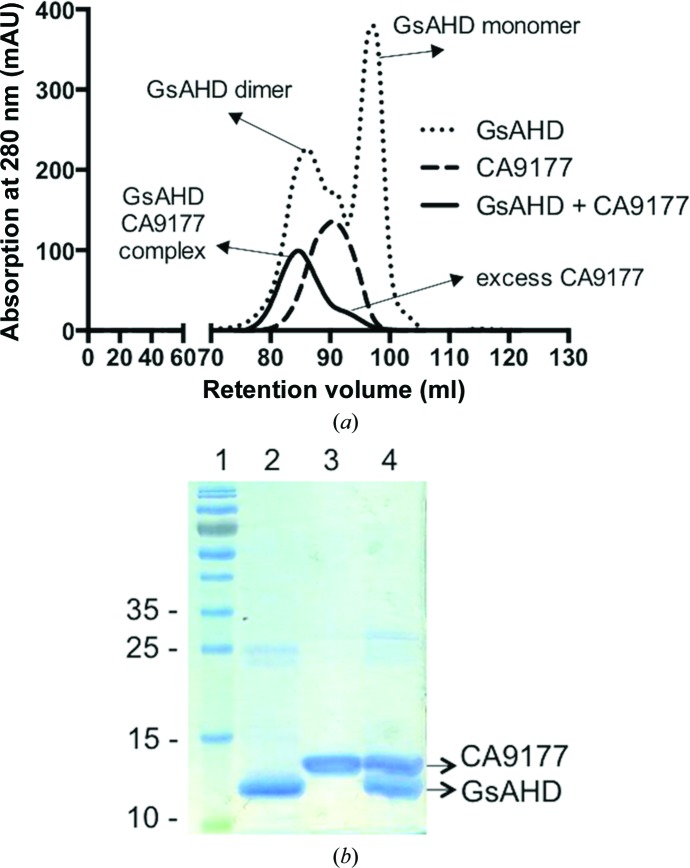
To prepare a 1:1 GαsAHD–CA9177 complex, purified GαsAHD was mixed with a 1.2 molar excess of CA9177 and incubated for 1 h. The complex was purified from free nanobody using a Superdex 75 HR 16/60 column equilibrated with 50 mM Tris pH 8.0, 150 mM NaCl, 2 mM DTT (Fig. 1 ▶ a). Fractions containing the GαsAHD–CA9177 complex were pooled and concentrated (Amicon Ultra, MWCO 3000, Millipore) to 40 mg ml−1 and verified by SDS–PAGE (Fig. 1 ▶ b). On the SDS–PAGE gel, GαsAHD (123 amino acids) and CA9177 (121 amino acids) run at molecular masses of 14.7 and 13.4 kDa, respectively.
Figure 1.
GαsAHD–CA9177 complex purification. (a) Gel-filtration elution profile of GαsAHD–CA9177 complex compared with free GαsAHD and CA9177. A Superdex 75 16/60 column was equilibrated with 50 mM Tris pH 8.0, 150 mM NaCl, 2 mM DTT. (b) Concentrated fractions were loaded onto a 15% SDS–PAGE gel and stained with Instant Blue. Lane 1, PageRuler Prestained Protein Ladder (Thermo Scientific); lane 2, GαsAHD (14.7 kDa); lane 3, CA9177 (13.4 kDa); lane 4, GαsAHD–CA9177 complex.
2.2. Crystallization
The initial crystallization trials consisted of one commercial screen: ProPlex HT-96 (Molecular Dimensions). The crystallization screen was set up using a Phoenix crystallization robot (Art Robbins Instruments): three different protein concentrations (35, 25 and 15 mg ml−1) were dispensed into three-well Intelli-Plate 96 plates (Hampton Research). For the sitting-drop vapour-diffusion method, 100 nl of screening solution was mixed with an equal volume of protein solution and the reservoir consisted of 70 µl of the screening solution. The plates were stored at 293 K and crystals appeared in 75% of the conditions after 1 day. Crystals for data collection were harvested from condition C7 [0.1 M sodium cacodylate pH 5.5, 25%(w/v) PEG 4000] and were flash-cooled in liquid nitrogen without additional cryoprotectant. Other crystals from condition D11 [0.1 M Tris pH 8.5, 15%(w/v) PEG 6000] could be reproduced by streak-seeding into 1 µl hanging drops in 24-well VDX plates (Hampton Research; Fig. 2 ▶). The PEG 6000 concentration was varied from 10 to 18% in 2% increments. These crystals were harvested and flash-cooled in liquid nitrogen with 0.1 M Tris pH 8.5, 15%(w/v) PEG 6000, 20% glycerol as a cryoprotectant. Crystallization conditions are summarized in Table 2 ▶.
Figure 2.

GαsAHD–CA9177 crystals. Crystals of GαsAHD–CA9177 obtained after streak-seeding in 0.1 M Tris pH 8.5, 15%(w/v) PEG 6000. Crystals were grown in 1 d using the hanging-drop method in 24-well plates.
Table 2. Crystallization.
| Method | Sitting drop | Hanging drop |
|---|---|---|
| Plate type | 96 3-well | 24-well |
| Temperature (K) | 293 | 293 |
| Protein concentration (mgml1) | 25 | 25 |
| Buffer composition of protein solution | 50 mM Tris pH 8.0, 150mM NaCl, 2mM DTT | 50 mM Tris pH 8.0, 150mM NaCl, 2mM DTT |
| Composition of reservoir solution | 0.1M sodium cacodylate pH 5.5, 25%(w/v) PEG 4000 | 0.1M Tris pH 8.5, 15%(w/v) PEG 6000 |
| Volume and ratio of drop | 100nl (1:1 ratio) | 1l (1:1 ratio) |
| Volume of reservoir (l) | 70 | 200 |
2.3. Data collection and preliminary X-ray analysis
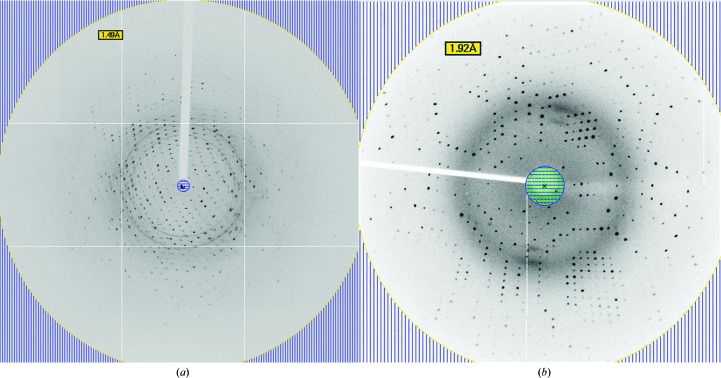
For data collection, a crystal from the screening plate (ProPlex HT-96 condition C7) was tested without the addition of cryoprotectant. Diffraction data were collected on the PROXIMA-1 beamline at the SOLEIL synchrotron, Paris, France. Data were processed with XDS (Kabsch, 2010 ▶). The crystal diffracted to a resolution of 1.59 Å (Fig. 3 ▶ a) and belonged to the monoclinic space group P21. The unit-cell parameters are consistent with one GαsAHD–CA9177 complex per asymmetric unit, with a Matthews coefficient of 2.07 Å3 Da−1 and an estimated solvent content of 40.69% (Matthews, 1968 ▶). Data-collection statistics are given in Table 3 ▶. The structure was solved by molecular replacement using Phaser (McCoy et al., 2007 ▶) using as input model the helical domain of Gαs (PDB entry 3c16; Mou et al., 2009 ▶) and the structure of a nanobody based on sequence identity (PDB entry 3p0g; Rasmussen, Choi et al., 2011 ▶). The sequence identity between GαsAHD and 3c16 is 100% and that between CA9177 and 3p0g is 70%. A solution was found for each search template with a rotation-function Z-score (RFZ) of 44.2 and a translation-function Z-score (TFZ) of 27.6 for 3c16 and an RFZ of 12.9 and a TFZ of 21.1 for 3p0g.
Figure 3.
Data collection. (a) X-ray diffraction pattern of a GαsAHD–CA9177 complex crystal obtained using a synchrotron-radiation source without cryoprotectant. (b) X-ray diffraction pattern using cryoprotectant [0.1 M Tris pH 8.5, 15%(w/v) PEG 6000, 20% glycerol] obtained on a Rigaku MicroMax-007 HF.
Table 3. Data collection and processing.
Values in parentheses are for the outer shell.
| Diffraction source | PROXIMA-1, SOLEIL |
| Wavelength () | 0.9793 |
| Detector | PILATUS 6M |
| Space group | P21 |
| a, b, c () | 44.07, 52.55, 52.66 |
| , , () | 90.00, 107.89, 90.00 |
| Resolution range () | 38.511.59 (1.681.59) |
| Total No. of reflections | 100990 |
| No. of unique reflections | 30170 |
| Completeness (%) | 98.3 (94.8) |
| Multiplicity | 3.3 (3.3) |
| I/(I) | 6.9 (2.1) |
| R merge † | 0.095 (0.641) |
| R meas ‡ | 0.113 (0.760) |
| R p.i.m. § | 0.061 (0.405) |
| Overall B factor from Wilson plot (2) | 11.9 |
R
merge = 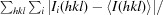
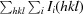 .
.
R
meas = 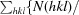
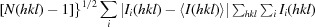 .
.
R
p.i.m. = 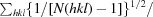
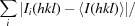
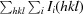 .
.
3. Results and discussion
Nanobody CA9177 was selected for binding the helical domain of Gαs. The GαsAHD–CA9177 complex was purified to homogeneity (Fig. 1 ▶). Interestingly, using one sparse-matrix screen (ProPlex HT-96), crystals could be grown in about 75% of the conditions tested in 24 h. One crystal from condition C7 of ProPlex HT-96 was cooled in liquid nitrogen without cryoprotectant and a high-resolution data set was collected at a synchrotron-radiation source. Ice rings did not hamper data processing, as shown in the data-collection statistics (Table 3 ▶). However, another crystallization condition (D11) was reproduced and optimized (Fig. 2 ▶), and the crystals diffracted to high resolution (1.9 Å) on a Rigaku MicroMax-007 HF (Fig. 3 ▶ b). The structure was solved from the synchrotron data set using molecular replacement with PDB entries 3c16 and 3p0g as search models. The CDR (complementary-determining regions) loops of CA9177 were manually rebuilt using Coot (Emsley & Cowtan, 2004 ▶), followed by several rounds of refinement using REFMAC5 (Murshudov et al., 2011 ▶), giving an R factor and R free of 0.18 and 0.19, respectively, in the final round of refinement.
The structure reveals in detail the epitope recognized by CA9177 on the GαsAHD and will prove useful for crystallizing GPCR–G-protein complexes.
Acknowledgments
This work was supported by the Fonds Wetenschappenlijk Onderzoek-Vlaanderen through research grant G.0068.14N and a personal doctoral fellowship to ST, by Innoviris Brussels through the Impulse Life Science Program BRGEOZ132, by the Belgian Federal Science Policy Office through IAP7-40 and by the SBO program IWT120026 from the Flemish Agency for Innovation by Science and Technology. We acknowledge the staff of the PROXIMA-1 beamline at the SOLEIL synchrotron (Saint-Aubin, France) and Dr Lionel Vercheval for their assistance in data collection. We are grateful to Dr Nicolas Villaneuva from Roger Sunahara’s laboratory for providing the GαsAHD clone.
References
- Bohm, A., Gaudet, R. & Sigler, P. B. (1997). Curr. Opin. Biotechnol. 8, 480–487. [DOI] [PubMed]
- De Genst, E. J., Guilliams, T., Wellens, J., O’Day, E. M., Waudby, C. A., Meehan, S., Dumoulin, M., Hsu, S.-T. D., Cremades, N., Verschueren, K. H. G., Pardon, E., Wyns, L., Steyaert, J., Christodoulou, J. & Dobson, C. M. (2010). J. Mol. Biol. 402, 326–343. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Hamm, H. E. (1998). J. Biol. Chem. 273, 669–672. [DOI] [PubMed]
- Irannejad, R., Tomshine, J. C., Tomshine, J. R., Chevalier, M., Mahoney, J. P., Steyaert, J., Rasmussen, S. G. F., Sunahara, R. K., El-Samad, H., Huang, B. & von Zastrow, M. (2013). Nature (London), 495, 534–538. [DOI] [PMC free article] [PubMed]
- Irannejad, R. & von Zastrow, M. (2014). Curr. Opin. Cell Biol. 27, 109–116. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 133–144. [DOI] [PMC free article] [PubMed]
- Kruse, A. C. et al. (2013). Nature (London), 504, 101–106.
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Mou, T.-C., Masada, N., Cooper, D. M. F. & Sprang, S. R. (2009). Biochemistry, 48, 3387–3397. [DOI] [PMC free article] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Muyldermans, S. (2013). Annu. Rev. Biochem. 82, 775–797. [DOI] [PubMed]
- Muyldermans, S., Cambillau, C. & Wyns, L. (2001). Trends Biochem. Sci. 26, 230–235. [DOI] [PubMed]
- Oldham, W. M. & Hamm, H. E. (2008). Nature Rev. Mol. Cell Biol. 9, 60–71. [DOI] [PubMed]
- Pardon, E., Laeremans, T., Triest, S., Rasmussen, S. G. F., Wohlkönig, A., Ruf, A., Muyldermans, S., Hol, W. G. J., Kobilka, B. K. & Steyaert, J. (2014). Nature Protoc. 9, 674–693. [DOI] [PMC free article] [PubMed]
- Rasmussen, S. G. F., Choi, H.-J. et al. (2011). Nature (London), 469, 175–180.
- Rasmussen, S. G. F., DeVree, B. T. et al. (2011). Nature (London), 477, 549–555.
- Simon, M. I., Strathmann, M. P. & Gautam, N. (1991). Science, 252, 802–808. [DOI] [PubMed]
- Steyaert, J. & Kobilka, B. K. (2011). Curr. Opin. Struct. Biol. 21, 567–572. [DOI] [PMC free article] [PubMed]
- Westfield, G. H. et al. (2011). Proc. Natl Acad. Sci. USA, 108, 16086–16091.