Ischemic stroke (IS) is a complex genetic disorder caused by a combination of multiple genetic and environmental factors. Despite the enormous burden associated with stroke, we know little about its underlying pathogenesis. Unlike many other common age-related diseases (eg, diabetes mellitus and heart disease), there are no good preclinical biomarkers that can provide a foothold for studying the molecular and physiological processes leading up to a stroke event. Because a genetic predisposition to stroke is widely acknowledged, 1 current strategy to study stroke pathogenesis is to start by identifying genes associated with stroke and then to investigate the function of these genes. Toward this goal, numerous candidate gene and genome-wide association studies (GWAS) of stroke have been performed.
A strategy successfully used to identify genetic determinants of other common complex diseases has been to focus on subtypes, such as early-onset disease which may be enriched for high-penetrance variants. Variants that are highly penetrant or highly predictive of diseases may be easier to detect and also produce useful insights about disease pathways, even if the same variants play a lesser role in late-onset disease.
The goal of this review is to assess the evidence for a genetic basis to early-onset IS. We mainly focus on early-onset stroke occurring between the ages of 15 and 49 years but specify age ranges considered by other studies as they are discussed. We begin with an epidemiological characterization of early-onset stroke, comparing and contrasting risk factors and the heterogeneity of stroke subtypes between early- and late-onset IS. We then review the evidence for a genetic basis of early-onset stroke, contrasting where possible the magnitude of the genetic contribution in younger versus older IS through familial aggregation studies. Finally, we review current status and prospects for identifying genes contributing to susceptibility to early-onset stroke. Throughout the review, we highlight some of the key gaps in knowledge.
Methods
Articles eligible for inclusion in this review were identified by searching PubMed published online before July 2013, as well as references cited from these studies. For familial aggregation of IS, we used as search terms (ischemic stroke or brain infarction or cerebral infarction) and (family history or twin study or heritability). This search generated a total of 244 studies. After review of abstracts to determine relevance, we identified 11 studies that addressed the genetic contribution to IS in relation to age of stroke onset. References for genes associated with early-onset IS were identified by searching PubMed using the search terms (ischemic stroke or ischemia or cerebral infarction or brain infarction) and (gene or polymorphism or mutation or variant) and (young adults or early onset or young onset), yielding 126 studies. We further restricted eligibility to include only studies based on meta-analysis or individual studies with ≥500 cases, a threshold chosen because it is estimated to provide 80% power to detect a common variant with a minor allele frequency of 30% and an odds ratio (OR) of 1.3 under an additive genetic model. After review of abstracts to determine relevance, 6 studies met these criteria, 4 of which were restricted to IS cases ≤50 years of age.
Epidemiological Features of Young-Onset IS
Incidence rates for IS increase exponentially with increasing age.1 Incidence rates for IS in the 15- to 45-year age range are ≈10 per 100 000 person-years in individuals of predominantly European ancestry, with similar rates for men and women.2,3 Compared with persons of European ancestry, blacks have incidence rates 2-fold higher for IS in this 15- to 45-year age range.2
Risk factors for early-onset stroke generally parallel those for late-onset stroke, although the frequencies of these risk factors and their effects on stroke may differ between young and old. Compared with older adults, patients 18 to 44 years of age with IS have a lower prevalence of dyslipidemia, hypertension, diabetes mellitus, and atrial fibrillation, although not necessarily current cigarette smoking.1,4,5 Younger patients with stroke are also less likely to have a history of coronary or peripheral artery disease.4 Despite their lower prevalence, some stroke risk factors seem to have a stronger effect in the young population. For example, the relative risk (RR) of hypertension associated with stroke is highest among those aged <50 years (RR=4.0) and decreases with increasing age.1
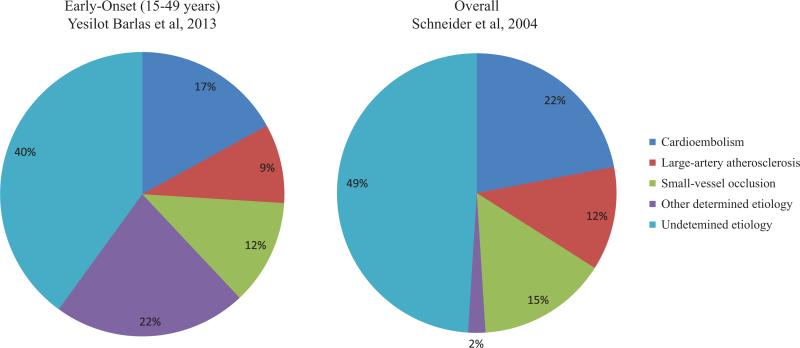
One key difference between early- versus late-onset IS is the distribution of stroke subtypes. In contrast to late-onset IS, there is a higher proportion of stroke because of other determined causes in young adults. In a large collection of first-ever IS cases, aged from 15 to 49 years, from hospital-based registries across Europe, the frequency of stroke because of other determined causes, particularly dissection, was the predominate subtype, whereas cardioembolic, large artery atherosclerotic, and small-vessel strokes were less represented compared with what is typically seen in general population (Figure).6,7 The evidence on the proportions of stroke of undetermined pathogenesis between these 2 age groups is somewhat conflicting. Two US population–based studies, one enrolling strokes of all ages6 and the other enrolling only young adults aged 15 to 44 years,8 found a similar proportion of stroke of undetermined pathogenesis (≈50%). In contrast to these results, the European study of hospital-based cases mentioned above reported a slightly lower proportion (40%) of stroke of undetermined pathogenesis.7 Such conflicting results may relate to differing geographically based work-up practices for early-onset stroke. Some of the observed phenotype heterogeneity between young- versus older-onset stroke likely reflects differing in the frequencies of stroke risk factors. Thus, the relatively low proportion of large artery atherosclerosis strokes occurring in the young could be because of the lower prevalence of and shorter exposure to athero-sclerotic risk factors at younger ages.
Figure.
Subtype distribution for early-onset vs overall stroke based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification system. Data sources: subtype distribution for early-onset stroke is based on data from 3331 first-ever IS patients, aged 15 to 49 years, from hospital-based registries across Europe (Yesilot Barlas et al7) and for overall stroke based on data from 1594 white first-ever IS patients from the Greater Cincinnati/Northern Kentucky Stroke Study (Schneider et al6).
In summary, early-onset stroke not only represents the lower part of the age of onset spectrum but also differs in several key ways from late-onset stroke, as reflected by the difference in subtype distribution and the effects of traditional stroke risk factors.
Familial Aggregation of IS in the Young
Evidence That IS Aggregates in Families
The available studies indicate that a moderate proportion of stroke susceptibility can be attributable to familial aggregation. Bak et al9 reported a heritability of 32% for stroke death and 17% for stroke hospitalization or stroke death in twins. Flossmann et al10 summarized the evidence for a genetic contribution to IS across 9 cohort studies, 27 case–control studies, and 3 twin studies and reported that having a positive family history of stroke was associated with an ≈30% to 76% increase in stroke risk (OR=1.3–1.76). In the Framingham Study, a parental history of IS by 65 years of age was associated with a 2.22-fold increase in IS risk in offspring (P<0.05) after adjusting for stroke risk factors.11 The interpretation of these family-based studies is subject to several caveats. First, the estimates of genetic contribution can vary widely depending on study design. Second, presence or absence of stroke in family members is often based solely on recall by the index case and without verification from medical records. Not only does this provide opportunities for misclassification error, but even for true strokes, it may be impossible to distinguish between ischemic versus hemorrhagic stroke. The Framingham Study was among the few studies able to minimize this bias because strokes were adjudicated in both the probands and parents.11 Perhaps, the biggest limitation lies in the interpretation of the familial aggregation itself because it is not only an indicator for shared genes between family relatives but also shared environments. More recent approaches using population samples and genetic polymorphisms across the entire genome can circumvent this limitation.12,13 This genotype-based heritability method estimates the similarity among individuals based on their actual genotypes at markers throughout the genome (ie, genetic relatedness). Heritability is then defined as the proportion of phenotype variation (eg, in stroke liability) explained by the genotypes across the chromosome. Two recent studies using this approach estimated the heritability of IS to be 37% to 38% (estimate statistically different than zero at P<0.001), indicating a significant genetic contribution to IS.14,15 The genotype-based method for estimating heritability requires large sample sizes, which have become available with large GWAS studies.
Is Familial Aggregation More Pronounced in Early-Onset IS Compared With Late-Onset Stroke?
A central question addressed by this review is the extent to which genes contribute to risk of early-onset IS. At least 2 case–control studies have been performed in patients with stroke aged <50 years, each reporting stroke to be associated with a positive family history of stroke, with ORs ranging from 1.2 to 3.2.16,17 Few studies have explicitly contrasted the association of stroke with family history of stroke between early-and late-onset stroke subjects. One of the earliest efforts to address this question was undertaken by Flossmann et al10 and reported a slightly stronger association of stroke with family history of stroke in studies restricted to cases with onset age <70 years (OR=1.9; 95% confidence interval [CI]=1.7–2.2) than in studies without this age restriction (OR=1.7; 95% CI=1.6–1.8). Despite the high age cutoff (70-year old) used in this analysis, Flossmann et al10 indicated a potential age modification on the familial aggregation. A significant limitation of this analysis was that estimates of the young versus old differences were not made within each study but rather were made by combining estimates obtained from early-onset stroke studies and comparing them to estimates obtained from other stroke studies. As noted by these and other authors, estimates of familial aggregation can vary widely across studies for many reasons, including study design, ascertainment criteria, exposure assessment, and analytic approaches.
Through our search criteria, we identified 11 studies that specifically addressed whether familial aggregation of stroke differed across the age spectrum. These included 7 case-only, 2 case–control, and 2 cohort studies. The case-only studies evaluated whether the frequency of a positive family history varied according to the age of stroke onset in the index case, whereas the case–control and cohort studies evaluated whether the association between family history and stroke was modified by age. It should be noted that these studies applied various age cutoff criteria contrasting the younger versus older strokes, but all examined the potential for age modification on aggregation of strokes within families. A summary of these studies, along with the study-specific age reference for contrasting younger versus older strokes, is provided in Table 1.
Table 1.
Characteristics and Results of Family Studies Examining Familial Aggregation of IS in Relation to Age
| Study | Subjects | Mean Age* | Study Location | Design | Key Result | Result Details |
|---|---|---|---|---|---|---|
| Case-only | ||||||
| Meschia et al18 | 310 IS probands | 72 | United States | Correlate age of stroke in proband with presence of stroke in sibling or parent | Older stroke probands more likely than younger probands to report affected siblings | 1.OR of having a concordant sibling per 10-y increase in proband age -All probands: 1.65 (1.20-2.28) -First-ever IS probands: 1. 50 (1.05-2.15) 2.OR of having a concordant parent per 10-y increase in proband age -All probands: 1.13 (0.92-1.38) -First-ever IS probands: 1.13 (0.90-1.42) |
| Schulz et al19 | 596 first-ever IS patients | ... | United Kingdom | Compare frequency of a positive family history of stroke or MI between young- vs old-onset stroke cases | Positive family history of stroke more common in younger- vs older-onset stroke cases for overall stroke and all stroke subtypes | Comparing the odds of having an FHstroke in age group ≤60 y vs age group >60 y: -OR=1.73 (1.02-2.91; all) -OR=2.17 (0.38-12.6; CE) -OR=2.57 (0.84-7.88; LA) -OR=1.43 (0.50-L09; SV) -OR=2.51 (1.00-6.26; undetermined) |
| Lisabeth et al20 | 353 patients with IS | 73.2 | United States | Compare the median age between patients with vs without a family history | Positive family history of stroke more common in younger- vs older-onset stroke but not statistically significant | Median age at stroke onset was 71.4 y among those with a family history and 73.9 y among those with no family history. |
| Lee et al21 | 533 first-ever IS patients | 64.7 (<80 y group) vs 84.3 (>80 y group) | Taiwan | Compare frequency of a positive family history of IS between young- vs old-onset stroke cases | Positive family history of stroke more common in younger- vs older-onset stroke | Prevalence of FHstroke is significantly higher among patients with stroke <80 y vs patients >80 y (39.9% vs 13.9%; P<0.01) |
| Knottnerus et al22 | 195 first-ever SV strokes | 63.1 | The Netherlands | Correlate age of stroke in probands with presence of stroke in sibling or parent | Positive family history of stroke in parents (but not siblings) more common in younger- vs older-onset stroke | 1.Decrease in likelihood of having ≥1 concordant parent with stroke with increasing age of proband: 0.97 (0.95-1.00) per year 2.Nonsignificant increase in likelihood of having ≥1 concordant siblings with stroke with increasing age of proband: 1.02 (0.98-1.05) per year. |
| Yao et al23 | 1027 first-ever IS | 67.5 (median) | China | Compare frequency of a positive family history of stroke between young- vs old-onset stroke cases | Positive family history of stroke more common in younger- vs older-onset stroke | Prevalence of FHstroke 33.7% among youngest (onset age <50 y) vs 25.6% in older (onset age: 50-80 y) vs 13.3% in oldest (onset age >80 y) stroke cases (P<0.05) |
| Putaala et al24 | 3944 first-ever IS | 43 (median) | Europe | Compare frequency of a positive family history of stroke by age | Positive family history of stroke more common in older- vs younger-onset stroke | Frequency of FHstroke positively correlated with age of stroke onset (P for trend=0.002) |
| Case-control | ||||||
| Jerrard-Dunne et al25 | 1000 IS/TIA cases; 800 controls | 65.1 in cases vs 64.4 in controls | United Kingdom | Compare association between stroke and family history of stroke across age strata | Association of stroke with family history of stroke stronger in younger- vs older-onset stroke | Associations of FHstroke <65 y and risk of stroke, by proband's age of onset: -OR for SV=3.99, 2.7, 2.69, 1.91, 1.55, 1.55, and 1.45 for proband's age of stroke onset ≤55, 60, 65, 70, 75, and 80 y, respectively. -OR for LA=4.46, 2.55, 2.34, 1.86, 1.88, 1.82, and 1.67 for proband's age of stroke onset ≤55, 60, 65, 70, 75, and 80 y, respectively |
| MacClellan et al17 | 487 IS female cases and 615 female controls | 39.1 in cases vs 36.8 in controls | United States | Compare association between stroke and family history of stroke across age strata | Association of stroke with family history of stroke stronger in younger- vs older-onset stroke | Crude OR of having a positive FHstroke, stratified by proband's age of onset=2.53, 1.63, and 1.47 for proband's age group of 15-24, 25-34, and 35-49 y, respectively (P for trend<0.0001) |
| Cohort | ||||||
| Jousilahti et al26 | 14371 middle-aged men and women | 25-64 (range) | Finland | Compare incidence of stroke according to parental history of stroke across age strata | Effect of parental history of stroke on stroke in the offspring is stronger in young- (25-49 y) vs old-onset stroke (50-64 y) | RR of incident stroke associated with parental history of stroke: -Men, aged 25-49 y: 2.82 (1.27-6.23) -Men, aged 50-64 y: 1.65 (0.99-2.76) -Women, aged 25-49 y: 2.76 (1.16-6.60) -Women, aged 50-64 y: 1.54 (0.94-2.51) |
| Seshadri et al11 | 3433 offspring | 48 | United States | Compare incidence of stroke according to parental history of stroke across age strata | Effect of parental history of stroke on stroke in the offspring is stronger in young-onset stroke (<65 y) | 1.Effect of FHparental stroke <65 y on risk of all stroke in the offspring: HR=2.21 (1.32-3.70) for offspring at any age vs HR=3.79 (1.90-7.58) for offspring <65 y 2.Effect of FHparental IS <65 y on risk of IS in the offspring: HR=2.22 (1.19-4.15) for offspring at any age vs HR=3.16 (1.27-7.90) for offspring <65 y |
CE indicates cardioembolic stroke; FH, family history; HR, hazard ratio; IS, ischemic stroke; LA, large artery atherosclerotic stroke; MI, myocardial infarction; OR, odds ratio; RR, relative risk; SV, small-vessel stroke (lacunar stroke); and TIA, transient ischemic attack.
Mean age is provided unless otherwise specified.
Five of the case-only studies reported that younger stroke cases were more likely than older cases to have a positive family history of stroke or that age at stroke was inversely related to a family history of stroke,19–23 whereas 1 case-only study reported that older stroke cases were more likely than younger cases to have a positive family history of stroke.24 An additional case-only study by Meschia et al18 reported that concordance rates for siblings increased with increasing age of the proband, although this observation is difficult to interpret given that risk of stroke increases with age. The 2 case–control studies evaluating the association between stroke and family history of stroke both observed stronger associations in younger individuals than in older individuals.17,25 Particularly in the Genetics of Early-Onset Stroke Study, which included only adults aged <50 years, the OR for the association between family history and overall IS was strongest among the youngest age group (15–24 years: OR=2.5, CI=0.4–15.0) and weakest in the oldest age group (35–49 years: OR=1.5, CI=1.1–1.9; P<0.0001 for trend).17
The 2 cohort studies that evaluated the association between family history and stroke also observed stronger associations in younger individuals. Jousilahti et al26 studied 14 371 Finnish individuals, aged 25 to 64 years, and observed that the RR of incident stroke associated with family history was stronger in men aged 25 to 49 years than in men aged 50 to 64 years (RR=2.82 versus 1.65) with similar associations observed for women, although they did not distinguish between ischemic and hemorrhagic types of stroke in their study. In the Framingham Heart Study, 3433 offspring were followed for stroke onset, and their parental history of stroke was obtained using medical records. A stronger effect of parental history of IS was observed for risk of IS in the offspring at younger age (<65 years) compared with stroke risk in offspring at any age (RR=3.16 versus 2.22).11 Moreover, this study also showed that the observed association between parental history of stroke and stroke risk in offspring seemed to be independent of conventional stroke risk factors.
Is the Stronger Familial Aggregation Observed in Early- Compared With Late-Onset Stroke Because of Differences in the Distribution of Stroke Subtypes?
Most studies of familial aggregation have not considered whether the association of family history of stroke differed by stroke subtypes. Among the few examining such associations, the results are inconclusive.17,25,27,28 A key question that has not been definitively answered is whether the same IS subtypes tends to aggregate within families. This was first addressed in a retrospective study of ≈300 sib pairs affected with stroke and demonstrated poor agreement among IS subtypes in the 2 siblings.29 This was an important finding in that it suggested that genetic risk factors for IS may not be subtype specific. Nevertheless, clear evidence is emerging that most IS subtypes are heritable. In a more recent study of 3540 IS cases and 6221 controls, Bevan et al14 used the genotype-based heritability approach and estimated heritabilities of 40% for large-vessel stroke, 33% for cardioembolic stroke, and 16% for small vessel disease (all estimates statistically >0; P<0.001). Also estimating heritability based on genotypes, Holliday et al15 reported a higher heritability for large artery atherosclerotic type (66%; P<0.001) and cardioembolic stroke (60%; P=0.003) and a small, nonsignificant heritability for small-vessel diseases (10%; P=0.26) in a set of 1162 cases and 1244 controls from Australia. One caveat of these estimates is that they have large confidence intervals, particularly for small vessel disease, which accounts for a relatively smaller proportion of all IS cases. As with more conventional pedigree-based heritability estimates, genotype-based heritability estimates are also subject to error because of imprecision of stroke subtype definitions. Nevertheless, on balance, these studies provide support for a genetic contribution to individual stroke subtypes, although the magnitude of the genetic contribution to each subtype remains unclear.
Given that the genetic contributions to IS may differ across stroke subtypes and the stroke subtype distribution varies by age, it is possible that the higher familial aggregation observed in the young IS population could be because of an overrepresentation of stroke subtypes that have stronger genetic contributions. The best data addressing this issue comes from the study of Jerrard-Dunne et al,25 who assessed the genetic contribution to stroke subtype according to age of stroke onset. They reported a positive association of family history of stroke with large artery atherosclerotic and small vessel sub-types and, most importantly, observed the association to be strongest among the youngest age of onset group (≤55 years) and that the association diminished with increasing age of onset within each subtype (Table 1). However, they did not examine the age-stratified associations for cryptogenic stroke, which represents a significant portion of young-onset form of stroke cases (Figure). Additional insights could be provided by estimating subtype-specific heritability of young versus old stroke using the genotype-based approach; unfortunately, such estimates are not yet available. Such an approach might address more definitively the issue of whether the genetic contribution to stroke is increased at younger ages and whether the genetic contribution to early-onset stroke is independent of differences in the distribution of stroke subtype.
In summary, substantial evidence from family and population studies supports a significant genetic contribution to IS, which appears to be stronger for early-onset IS. There is emerging evidence from genotype-based heritability analyses that heritability of IS may vary by stroke subtype. What is not clear at this time is the degree of genetic overlap between different stroke subtypes. This gap may be addressed through family studies that examine segregation of specific stroke subtypes within families (eg, do stroke-enriched families present with predominately the same stroke subtype or do subtypes crossover?). Unfortunately, available data to address this important question are sparse.
Monogenic Conditions Related to Early-Onset Stroke May Provide Insight Into Understanding Complex Forms of Stroke in Young Adults
Insights into the pathogenesis of some common diseases have been gained by studying genes previously identified as being responsible for monogenic forms of the early-onset disease. For example, the discovery of the gene encoding the low-density lipoprotein receptor (LDLR) in 1986 led to discoveries of LDLR mutations as a cause of familial hypercholesterolemia and one of the first characterized genetic causes of early coronary artery disease (CAD).30 Monogenic forms of type 2 diabetes mellitus, principally maturity-onset diabetes mellitus of the young (MODY), have also been identified, nearly all involving mutations in transcription factors that regulate various aspects of pancreatic β-cell function.31 Discovery of these monogenic forms of disease has motivated efforts to identify milder variants in these same genes that might have more subtle effects on gene function, perhaps acting through gene regulation. In recent years, common variants in LDLR and in multiple of the MODY genes have been identified that are reliably associated with common forms of both coronary heart diseases32 and diabetes mellitus,33 albeit with small effect sizes.
Genes associated with numerous monogenic disorders that include early-onset stroke as a phenotype have been identified. For some disorders, stroke can be the predominant clinical feature, whereas in others, stroke can occur infrequently. Excellent reviews of stroke-associated monogenic disorders have been previously published.34–36 Among the more well-known disorders are Fabry disease, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes, sickle-cell disease, homocystinuria, and Marfan syndrome.34,37,38 Even collectively, these disorders are rare and account for only a small percentage of young stroke cases. Although the biological basis for stroke is apparent for most of these disorders, the extent to which disruption of these pathways is important to common forms of stroke is debatable. There is some support that genes in homocysteine metabolism pathway (eg, CBS and MTHFR)39,40 and in the mitochondria genome41,42 may play a role in stroke susceptibility, but evidence for their role in complex forms of early-onset stroke is less conclusive. It is possible that there may be other high-penetrance mutations segregating in some families that also contribute to the burden of early-onset stroke, although it seems unlikely that these will account for a significant proportion of early-onset stroke because most early-onset stroke cases do not show Mendelian forms of inheritance. Exome sequencing studies that include early-onset stroke cases may address this issue in the future.
Current Status for Identifying Genes Associated With Young-Onset IS
The heterogeneity within IS imposes a significant challenge for identifying genes associated with this disorder because different genes are likely to predispose to different stroke subtypes. During the past several years, large GWAS have reported 6 to 7 loci associated with IS (Table 2). Four of these loci have P values reaching genome-wide significance in the large METASTROKE Consortium, the largest GWAS of IS to date, and all 4 genome-wide significant associations were subtype specific: 4q25 (PITX2) and 16q22 (ZFHX3) with cardioembolic stroke,43,44 7p21 (HDAC9) and 6p21 with large artery atherosclerotic stroke.15,45,46 Two additional loci, that is, the 9p21 locus (CDKN2B-AS1) with large artery atherosclerotic stroke45,47,48 and 9q34 locus (ABO) with total IS, large artery atherosclerotic stroke, and cardioembolic stroke49 did not reach genome-wide significance but were robustly replicated in other studies, albeit with small effect sizes. Notably, 1 locus, NINJ2 (12p13), which was initially identified in population-based cohort studies to be associated with all IS, has not been replicated in well-powered hospital-based case–control studies.45,46,50,51 The absence of replication suggests that this locus may be a false-positive, but it is also possible that this locus was associated with stroke risk and severity and thus the association was not seen in case–control studies that were largely based on stroke survivors. Among the associated loci, 4 have biological appeal considering their associated stroke subtype. For example, PITX2 and ZFHX3 have previously been associated with atrial fibrillation, an important risk factor for cardioembolic stroke.43,44 CDKN2B-AS1, located in the CDKN2A-CDKN2B gene cluster at 9p21, encodes CDKN2B antisense RNA 1 and is a known locus showing strong associations with CAD and myocardial infarction, consistent with a role in large artery disease.32,35 Many of the disease-associated variants in CDKN2B-AS1 may affect the expression of this gene, implicating their role in disease mechanisms via regulating the gene expression.52,53 Last, the ABO locus, which was initially identified by GWAS associated with the clotting trait (ie, von Willebrand factor), was associated with cardioembolic and large artery atherosclerotic strokes.49 However, little is known about the biological relevance of HDAC9 and the 6p21 locus to the large artery atherosclerotic stroke. HDAC9 encodes histone deacetylase 9 and may affect transcriptional regulation by altering chromosome structure. The 6p21 locus is located in an intergenic region (between SUPT3H and CDC5L) enriched for enhancer- and promoter-associated marks of histone modification.15 It is possible that both loci may play a role in modulating gene expressions critical to stroke, but further research is warranted to support or refute such hypothesis.
Table 2.
Loci Associated With IS Identified Through GWAS Studies
| CHR | Gene | SNP | Position | Trait | Discovery Cohort |
Risk Allele (Frequency)* |
Discovery OR (P Value) |
METASTROKE OR (P Value)† |
Reference |
|---|---|---|---|---|---|---|---|---|---|
| 12p13 | NINJ2 | rs11833579 | 645460 | IS | CHARGE Consortium | A (0.23) | 1.41 (2.3×10–10) | 1.06 (6.1×01–4) | 45, 50 |
| 4q25 | PITX2 | rs2200733 | 111929618 | CE | Iceland | T (0.12) | 1.50 (0.00011) | 1.36 (2.8×10–16)‡ | 43, 45 |
| 16q22 | ZFHX3 | rs7193343 | 71586661 | CE | Iceland | T (0.23) | 1.22 (0.00021) | 1.25 (2.28×10–8)‡ | 44, 45 |
| 9p21 | CDKN2B-AS1 | rs2383207 | 22105959 | LA | ISGC Consortium | G (0.57) | 1.17 (0.0025) | 1.15 (3.32×10–5) | 45, 47 |
| 7p21 | HDAC9 | rs11984041 | 18998460 | LA | WTCCC United Kingdom/Munich | A (0.09) | 1.50 (1.1×10–5) | 1.39 (2.03×10–16)‡ | 45, 46 |
| 6p21 | Intergenic | rs556621 | 44702127 | LA | Australia ASGC | A (0.33) | 1.62 (3.9×10–8) | 1.21 (4.7×10–8) | 15 |
| 9q34.2 | ABO | rs505922 | 135139050 | IS, CE, and LA | MORGAM and WTCCC2 | C (0.32) | IS: 1.06 (0.023) | IS: 1.07 (0.00061) CE: 1.13 (0.0002) LA: 1.23 (0.001) |
49 |
ASGC indicates Australian Stroke Genetics Collaborative; CE, cardioembolic stroke; CHARGE, Cohorts for Heart and Aging Research in Genomic Epidemiology; CHR, chromosome; GWAS, genome-wide association studies; IS, ischemic stroke; ISGC, International Stroke Genetics Consortium; LA, large artery atherosclerotic stroke; MORGAM, MOnica Risk, Genetics, Archiving and Monograph Cohort; OR, odds ratio; and WTCCC, Wellcome Trust Case Control Consortium.
Risk allele (and frequency) is based on data reported in discovery study.
Except ABO loci, ORs and P values reported in METASTROKE include discovery studies.
Proxy SNPs were used in METASTROKE results (rs6843082 for PITX2, rs879324 for ZFHX3, and rs2107595 for HDAC9).
The stroke-associated loci summarized above have all been identified in large studies comprising predominantly older stroke cases. Given the relatively low prevalence of early-onset stroke, it is perhaps not surprising that efforts to identify pre-disposing genes for early-onset IS have been much more limited. Below we summarize candidate gene and GWAS studies that have been performed specifically for early-onset IS.
Candidate Gene Approach
Numerous candidate genes and pathways for IS have been studied and extensively reviewed elsewhere.34,40,54–57 They generally fall into one of the following categories: (1) genes involved in coagulation and fibrinolytic system (eg, F5, F2, FGA, and FGB) or genes encoding platelet glycoproteins (eg, ITGB3 and ITGA2),34,40,54 (2) genes in the homocysteine metabolism (eg, MTHFR),54–56 (3) genes involved with lipid metabolism (eg, APOE),40,54 and (4) and inflammation genes.57 As with many candidate gene studies, the associations of these genes with stroke have generally been inconclusive because of insufficient sample size, heterogeneity in the study design, diversity of study populations, inconsistent phenotype classifications, and diverse inclusion/exclusion criteria for each study. Furthermore, relatively few of these studies have focused on younger populations, a group in which rare high-penetrance variants could theoretically account for disproportionately more of the disease burden.
Here, we focus on candidate gene studies of early-onset stroke that are based on either meta-analysis or large well-powered individual studies. Only 4 candidate gene studies met our PubMed search criteria examining the genetic associations with IS risk among cases ≤50-year old (Table 3).58–61 The MTHFR C677T variant (rs1801133), a missense variant encoding A222V amino acid change, was reported to be significantly associated with early-onset IS in the meta-analysis of 8 studies with a total of 1093 cases (OR=1.44; P=0.002; Xin et al58). The same study also reported significant associations with APOE (rs7412 and rs429358; OR=2.53; P<0.001) and ITGA2 C807T (rs1126643; OR=1.50; P=0.01), although they also detected evidence for potential publication bias for this latter result, calling the ITGA2 association into question.58 The FVL mutation (R506Q, rs6025) was investigated in 3 studies.58–60 Although no significant association was found in a study by Xin et al,58 a more recent meta-analysis found a significant association between FVL early-onset IS among studies where cases were selected on the basis of having cryptogenic stroke or recruited from a subset of patients referred for a thrombophilic work-up (OR=2.73; 95% CI=1.98–3.75) but a much smaller association among unselected cases (OR=1.40; 95% CI=0.998–1.95).59 Consistent with this meta-analysis, the largest single study of unselected early-onset IS cases found no association between FVL and IS (OR=1.0).60 Harriott et al61 studied the relationship between 134 polymorphisms of ATP1A2, a migraine-associated gene, with early-onset IS and only 1 variant reached nominal significance (rs2070704), suggesting no strong association between the ATP1A2 and early-onset IS.
Table 3.
Genetic Variants Associated With Early-Onset IS (≤50-Year Old) in Candidate Gene Studies
| Study | Age Range | Ethnicity | Genetic Variant | Risk Variant (Frequency)* |
No. of IS Cases/ Controls |
Effect of the Risk Allele (Genetic Model) |
Minimal Detectable OR Based on Available Sample Size† |
|---|---|---|---|---|---|---|---|
| Xin et al58 (meta-analysis) | 18–50y | Mixed | F5 G1691A | A (0–0.008) | 1543/5267 | OR=1.17, P=0.28 (dominant) | 1.75 |
| F2 G20210A | A (0.016–0.022) | 1279/5036 | OR=1.40, P=0.06 (dominant) | 1.48–1.56 | |||
| MTHFR C677T | T (0.11–0.51) | 1093/1757 | OR=1.44, P=0.002 (recessive) | 1.27–2.25 | |||
| ITGB3 PLA1/A2 | A2 (0.01–0.16) | 488/837 | OR=1.27, P=0.49 (dominant) | 1.41–2.50 | |||
| ITGA2 C807T | T (0.27–0.49) | 308/614 | OR=1.50, P=0.01 (dominant) | 1.48–1.62 | |||
| APOE e2/e3/e4 | e4 (0.07–0.08) | 326/338 | OR=2.53, P<0.001 (dominant) | 1.75–1.80 | |||
| NOS3 4ab | 4b (0.09–0.26) | 365/419 | OR=1.37, P=0.61 (recessive) | 2.00–4.70 | |||
| Hamedani et al59 (meta-analysis) | <50 y | Majority European ancestry | F5 G1691A | A (0.008) | 2045/5307 | All studies: OR=2.00, P<0.001 selected studies: OR=2.73, P<0.001 unselected studies: OR=1.40, P=0.05 (dominant) | 1.66 |
| Hamedani et al60 | 15–49y | Mixed (European and African ancestry) | F5 G1691A | A (0.007–0.027) | 830/907 | OR=1.0, P=1.0 (dominant) | 1.73–2.57 |
| Harriott et al61 | 15–49y | Mixed (European and African ancestry) | ATP1A2 rs2070704 A/G | G (0.21–0.29) | 830/907 | OR=0.75, P<0.001 (additive) | 0.79–0.80 |
HR indicates hazard ratio; IS, ischemic stroke; MI, myocardial infarction; OR, odds ratio; and TIA, transient ischemic attack.
Risk allele frequency is based on data reported in each study, except for the 2 meta-analyses (ie, Xin et al58 and Hamedani et al59). For the 2 meta-analyses, risk allele frequency was obtained from HapMap data, except rs1799963 (based on National Center for Biotechnology Information dbSNP (Single Nucleotide Polymorphism Database)), APOE e4 (based on Pezzini et al62 and Gu et al63), and NOS3 4ab (Shi et al,64 Yeh et al,65 and Howard et al66). For studies with mixed ethnic group, a range of risk allele frequencies is provided based on the allele frequencies provided across different ethnic groups.
The minimal detectable OR was estimated using Quanto v1.2.4 program (http://hydra.usc.edu/gxe/) based on the risk allele frequency, available sample size, and the genetic model used in each study under the assumption of an unmatched case-control design with 80% statistical power at α=0.05 (2-sided). For studies with mixed ethnicity, a range of detectable ORs was calculated using ethnic group-specific allele frequencies.
In addition to these studies, a large meta-analysis was performed to test whether 11 genetic variants known to be associated with myocardial infarction or CAD were also associated with IS under the assumption that myocardial infarction/ CAD and IS may share common susceptibility genes. In this analysis, none of the myocardial infarction/CAD-associated single-nucleotide polymorphisms were associated with IS in young populations (<50-year old); associations with IS sub-types (eg, large artery stroke) were not investigated in this young population.67
Genome-Wide Association Approach
Compared with stroke in older adults, GWAS data on early-onset stroke is limited. A suggestive association has been reported from the Genetics of Early Onset Stroke Study between early-onset IS and an intronic variant in FMNL2 (OR=0.69; P=1.2×10−7), although power to detect associations was modest and the association could not be replicated in case–control studies of older-onset stoke.68 Because of the relatively small sample sizes of the available early-onset stroke studies, there is insufficient power to determine whether the effect sizes of the established stroke susceptibility loci identified in older populations are similar for early-onset stroke. Given the success of large GWAS studies in identifying IS-associated genes in older adults and stronger familial aggregation of stroke in the young population, larger-scale GWAS studies conducted in early-onset stroke to identify additional stroke loci are warranted.
In summary, there have been few large-scale candidate gene studies or GWAS performed exclusively in early-onset IS. The few that have been performed provide some support that variants in the methionine metabolism (eg, MTHFR), lipid metabolism (eg, APOE), and the coagulation (eg, FVL) pathways may play a role in early-onset stroke. It remains to be determined whether these play a more prominent role in early-onset versus late-onset stroke. Notably, none of the published gene studies of early-onset IS have been sufficiently powered to assess associations with specific subtypes of IS.
Re-emerging Role of Family Studies in the Post-GWAS Era
Early linkage studies performed in families with predominantly older-onset stroke have mapped stroke susceptibility genes to the 5q12 region (PDE4D),69–72 although subsequent association studies attempting to identify the causative variant(s) for these linkages have been inconclusive.73–76 His torically, linkage studies have been most successful in localizing high-penetrance variants, which tend to be rare and are often associated with monogenic forms of disease. However, when such variants are identified, they can elucidate mechanistic pathways relevant to more common polygenic forms of disease, such as the identification of mutations in the LDLR and familial hypercholesterolemia.30 Another example, specifically relevant to stroke, was the discovery through linkage analysis of mutations in the gene encoding the vascular smooth muscle cell–specific isoform of α-actin (ACTA2) as a cause of familial aortic aneurysms and dissection.77 Additional analyses of these families have shown that mutations in this gene, which is a modulator of smooth muscle cell contraction, are also associated with premature CAD and premature IS, including moyamoya disease.78 Although ACTA2 variants have not been associated with more common or complex forms of stroke, these findings do suggest a new biological pathway to target in future genetic and translational research.
The recent advances in sequencing technology, coupled with the track record of GWAS in identifying mostly small-effect variants, have prompted enthusiasm by many in the complex disease field to explore the role of rare variants with large effect size and high-penetrance in disease pathogenesis. In this context, it will be fruitful to identify families enriched with multiple affected members who are more likely to be segregating disease-causing variants. This type of design may be particularly attractive for early-onset stroke, in which the associated variants may be more highly penetrant and thus more easily detectable through sequencing.
Future Directions
Important insights into mechanisms underlying several complex diseases have been gleaned after the identification of genetic variants predisposing to early-onset forms of the disease (eg, breast cancer and BRCA mutations, diabetes mellitus and MODY variants, heart disease and LDLR mutations). Whether similar success can arise from the study of early-onset stroke remains to be seen. One important issue that has not been established is whether early-onset stroke represents the lower part of the age of onset spectrum of all IS or whether there is an enrichment in early-onset cases of low frequency variants with larger effect sizes and higher penetrance. Identifying such variants could potentially shed light on new pathways relevant to the prevention and treatment of the more common forms of stroke.
Large-scale GWAS studies may also provide new insights into stroke genetics, including those that differentiate between early- and late-onset stroke. Such studies have been limited to date by small sample sizes in the early-onset category precluding the subtype-specific analyses that have been most productive in studying genes associated with late-onset stroke. Therefore, international collaborations combining early-onset samples across studies through meta-analysis are essential.
Acknowledgments
Sources of Funding
This work was supported, in part, by funding from the Department of Veterans Affairs (Career Development Award 1IK2BX001823 to Dr Cheng, Medical Research Service funding and Stroke Research Enhancement Award to Dr Cole, and a Merit Review Award to Dr Kittner) and from National Institute Health grants U01 HG004436, U01 NS069208, and P30 DK072488.
Footnotes
Disclosures
None.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kittner SJ, McCarter RJ, Sherwin RW, Sloan MA, Stern BJ, Johnson CJ, et al. Black-white differences in stroke risk among young adults. Stroke. 1993;24(12 Suppl):I13–I15. discussion I20. [PubMed] [Google Scholar]
- 3.Harmsen P, Wilhelmsen L, Jacobsson A. Stroke incidence and mortality rates 1987 to 2006 related to secular trends of cardiovascular risk factors in Gothenburg, Sweden. Stroke. 2009;40:2691–2697. doi: 10.1161/STROKEAHA.109.550814. [DOI] [PubMed] [Google Scholar]
- 4.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40:1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 5.Fromm A, Waje-Andreassen U, Thomassen L, Naess H. Comparison between ischemic stroke patients <50 years and ≥50 years admitted to a single centre: The Bergen Stroke Study. StrokeRes Treat. 2011;2011:183256. doi: 10.4061/2011/183256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider AT, Kissela B, Woo D, Kleindorfer D, Alwell K, Miller R, et al. Ischemic stroke subtypes: a population-based study of incidence rates among blacks and whites. Stroke. 2004;35:1552–1556. doi: 10.1161/01.STR.0000129335.28301.f5. [DOI] [PubMed] [Google Scholar]
- 7.Yesilot Barlas N, Putaala J, Waje-Andreassen U, Vassilopoulou S, Nardi K, Odier C, et al. Etiology of first-ever ischaemic stroke in European young adults: the 15 cities young stroke study. Eur J Neurol. 2013;20:1431–1439. doi: 10.1111/ene.12228. [DOI] [PubMed] [Google Scholar]
- 8.Kittner SJ, Stern BJ, Wozniak M, Buchholz DW, Earley CJ, Feeser BR, et al. Cerebral infarction in young adults: the Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;50:890–894. doi: 10.1212/wnl.50.4.890. [DOI] [PubMed] [Google Scholar]
- 9.Bak S, Gaist D, Sindrup SH, Skytthe A, Christensen K. Genetic liability in stroke: a long-term follow-up study of Danish twins. Stroke. 2002;33:769–774. doi: 10.1161/hs0302.103619. [DOI] [PubMed] [Google Scholar]
- 10.Flossmann E, Schulz UG, Rothwell PM. Systematic review of methods and results of studies of the genetic epidemiology of ischemic stroke. Stroke. 2004;35:212–227. doi: 10.1161/01.STR.0000107187.84390.AA. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri S, Beiser A, Pikula A, Himali JJ, Kelly-Hayes M, Debette S, et al. Parental occurrence of stroke and risk of stroke in their children: the Framingham study. Circulation. 2010;121:1304–1312. doi: 10.1161/CIRCULATIONAHA.109.854240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bevan S, Traylor M, Adib-Samii P, Malik R, Paul NL, Jackson C, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genome-wide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 15.Holliday EG, Maguire JM, Evans TJ, Koblar SA, Jannes J, Sturm JW, et al. Australian Stroke Genetics Collaborative; International Stroke Genetics Consortium; Wellcome Trust Case Control Consortium 2. Common variants at 6p21.1 are associated with large artery atherosclerotic stroke. Nat Genet. 2012;44:1147–1151. doi: 10.1038/ng.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Friedlander Y, Longstreth WT, Jr, Edwards KL, Schwartz SM, Siscovick DS. Family history as a risk factor for stroke in young women. Am J Prev Med. 2004;27:391–396. doi: 10.1016/j.amepre.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 17.MacClellan LR, Mitchell BD, Cole JW, Wozniak MA, Stern BJ, Giles WH, et al. Familial aggregation of ischemic stroke in young women: the Stroke Prevention in Young Women Study. Genet Epidemiol. 2006;30:602–608. doi: 10.1002/gepi.20171. [DOI] [PubMed] [Google Scholar]
- 18.Meschia JF, Atkinson EJ, O'Brien PC, Brott TG, Brown RD, Jr, Hardy J. Familial clustering of stroke according to proband age at onset of presenting ischemic stroke. Stroke. 2003;34:e89–e91. doi: 10.1161/01.STR.0000078312.07274.A4. [DOI] [PubMed] [Google Scholar]
- 19.Schulz UG, Flossmann E, Rothwell PM. Heritability of ischemic stroke in relation to age, vascular risk factors, and subtypes of incident stroke in population-based studies. Stroke. 2004;35:819–824. doi: 10.1161/01.STR.0000121646.23955.0f. [DOI] [PubMed] [Google Scholar]
- 20.Lisabeth LD, Kardia SL, Smith MA, Fornage M, Morgenstern LB. Family history of stroke among Mexican-American and non-Hispanic white patients with stroke and TIA: implications for the feasibility and design of stroke genetics research. Neuroepidemiology. 2005;24:96–102. doi: 10.1159/000081056. [DOI] [PubMed] [Google Scholar]
- 21.Lee YS, Chen DY, Chen YM, Chuang YW, Liao SC, Lin CS, et al. First-ever ischemic stroke in Taiwanese elderly patients: predicting functional independence after a 6-month follow-up. Arch Gerontol Geriatr. 2009;49(Suppl 2):S26–S31. doi: 10.1016/S0167-4943(09)70009-3. [DOI] [PubMed] [Google Scholar]
- 22.Knottnerus IL, Gielen M, Lodder J, Rouhl RP, Staals J, Vlietinck R, et al. Estimating the magnitude of genetic factors by calculating the genetic relative risk of stroke in first-ever lacunar stroke patients. PLoS One. 2011;6:e21439. doi: 10.1371/journal.pone.0021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao XY, Lin Y, Geng JL, Sun YM, Chen Y, Shi GW, et al. Age- and gender-specific prevalence of risk factors in patients with first-ever ischemic stroke in china. Stroke Res Treat. 2012;2012:136398. doi: 10.1155/2012/136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putaala J, Yesilot N, Waje-Andreassen U, Pitkäniemi J, Vassilopoulou S, Nardi K, et al. Demographic and geographic vascular risk factor differences in european young adults with ischemic stroke. Stroke. 2012;43:2624–2630. doi: 10.1161/STROKEAHA.112.662866. [DOI] [PubMed] [Google Scholar]
- 25.Jerrard-Dunne P, Cloud G, Hassan A, Markus HS. Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke. 2003;34:1364–1369. doi: 10.1161/01.STR.0000069723.17984.FD. [DOI] [PubMed] [Google Scholar]
- 26.Jousilahti P, Rastenyte D, Tuomilehto J, Sarti C, Vartiainen E. Parental history of cardiovascular disease and risk of stroke. A prospective follow-up of 14371 middle-aged men and women in Finland. Stroke. 1997;28:1361–1366. doi: 10.1161/01.str.28.7.1361. [DOI] [PubMed] [Google Scholar]
- 27.Jood K, Ladenvall C, Rosengren A, Blomstrand C, Jern C. Family history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2005;36:1383–1387. doi: 10.1161/01.STR.0000169944.46025.09. [DOI] [PubMed] [Google Scholar]
- 28.Polychronopoulos P, Gioldasis G, Ellul J, Metallinos IC, Lekka NP, Paschalis C, et al. Family history of stroke in stroke types and subtypes. J Neurol Sci. 2002;195:117–122. doi: 10.1016/s0022-510x(01)00691-8. [DOI] [PubMed] [Google Scholar]
- 29.Wiklund PG, Brown WM, Brott TG, Stegmayr B, Brown RD, Jr, Nilsson-Ardnor S, et al. Lack of aggregation of ischemic stroke subtypes within affected sibling pairs. Neurology. 2007;68:427–431. doi: 10.1212/01.wnl.0000252955.17126.6a. [DOI] [PubMed] [Google Scholar]
- 30.Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 31.Winter WE, Nakamura M, House DV. Monogenic diabetes mellitus in youth. The MODY syndromes. Endocrinol Metab Clin North Am. 1999;28:765–785. doi: 10.1016/s0889-8529(05)70101-8. [DOI] [PubMed] [Google Scholar]
- 32.Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 2013;45:25–33. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnycastle LL, Willer CJ, Conneely KN, Jackson AU, Burrill CP, Watanabe RM, et al. Common variants in maturity-onset diabetes of the young genes contribute to risk of type 2 diabetes in Finns. Diabetes. 2006;55:2534–2540. doi: 10.2337/db06-0178. [DOI] [PubMed] [Google Scholar]
- 34.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–161. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 35.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123(Pt 9):1784–1812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 36.Tonk M, Haan J. A review of genetic causes of ischemic and hemorrhagic stroke. J Neurol Sci. 2007;257:273–279. doi: 10.1016/j.jns.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 37.Wozniak MA, Kittner SJ, Tuhrim S, Cole JW, Stern B, Dobbins M, et al. Frequency of unrecognized Fabry disease among young European-American and African-American men with first ischemic stroke. Stroke. 2010;41:78–81. doi: 10.1161/STROKEAHA.109.558320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ballabio E, Bersano A, Bresolin N, Candelise L. Monogenic vessel diseases related to ischemic stroke: a clinical approach. J Cereb Blood Flow Metab. 2007;27:1649–1662. doi: 10.1038/sj.jcbfm.9600520. [DOI] [PubMed] [Google Scholar]
- 39.Giusti B, Saracini C, Bolli P, Magi A, Martinelli I, Peyvandi F, et al. Early-onset ischaemic stroke: analysis of 58 polymorphisms in 17 genes involved in methionine metabolism. Thromb Haemost. 2010;104:231–242. doi: 10.1160/TH09-11-0748. [DOI] [PubMed] [Google Scholar]
- 40.Bersano A, Ballabio E, Bresolin N, Candelise L. Genetic polymorphisms for the study of multifactorial stroke. Hum Mutat. 2008;29:776–795. doi: 10.1002/humu.20666. [DOI] [PubMed] [Google Scholar]
- 41.Anderson CD, Biffi A, Rahman R, Ross OA, Jagiella JM, Kissela B, et al. International Stroke Genetics Consortium. Common mitochondrial sequence variants in ischemic stroke. Ann Neurol. 2011;69:471–480. doi: 10.1002/ana.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson CD, Biffi A, Nalls MA, Devan WJ, Schwab K, Ayres AM, et al. International Stroke Genetics Consortium. Common variants within oxidative phosphorylation genes influence risk of ischemic stroke and intracerebral hemorrhage. Stroke. 2013;44:612–619. doi: 10.1161/STROKEAHA.112.672089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gretarsdottir S, Thorleifsson G, Manolescu A, Styrkarsdottir U, Helgadottir A, Gschwendtner A, et al. Risk variants for atrial fibrillation on chromo-some 4q25 associate with ischemic stroke. Ann Neurol. 2008;64:402–409. doi: 10.1002/ana.21480. [DOI] [PubMed] [Google Scholar]
- 44.Gudbjartsson DF, Holm H, Gretarsdottir S, Thorleifsson G, Walters GB, Thorgeirsson G, et al. A sequence variant in ZFHX3 on 16q22 associates with atrial fibrillation and ischemic stroke. Nat Genet. 2009;41:876–878. doi: 10.1038/ng.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Traylor M, Farrall M, Holliday EG, Sudlow C, Hopewell JC, Cheng YC, et al. Australian Stroke Genetics Collaborative, Wellcome Trust Case Control Consortium 2 (WTCCC2); International Stroke Genetics Consortium. Genetic risk factors for ischaemic stroke and its subtypes (the META-STROKE collaboration): a meta-analysis of genome-wide association studies. Lancet Neurol. 2012;11:951–962. doi: 10.1016/S1474-4422(12)70234-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellenguez C, Bevan S, Gschwendtner A, Spencer CCA, Burgess AI, Pirinen M, et al. Genome-wide association study identifies a variant in HDAC9 associated with large vessel ischemic stroke. Nat Genet. 2012;44:328–333. doi: 10.1038/ng.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gschwendtner A, Bevan S, Cole JW, Plourde A, Matarin M, Ross-Adams H, et al. International Stroke Genetics Consortium. Sequence variants on chromosome 9p21.3 confer risk for atherosclerotic stroke. Ann Neurol. 2009;65:531–539. doi: 10.1002/ana.21590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson CD, Biffi A, Rost NS, Cortellini L, Furie KL, Rosand J. Chromosome 9p21 in ischemic stroke: population structure and meta-analysis. Stroke. 2010;41:1123–1131. doi: 10.1161/STROKEAHA.110.580589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams FM, Carter AM, Hysi PG, Surdulescu G, Hodgkiss D, Soranzo N, et al. EuroCLOT Investigators; Wellcome Trust Case Control Consortium 2; MOnica Risk, Genetics, Archiving and Monograph; MetaStroke; International Stroke Genetics Consortium. Ischemic stroke is associated with the ABO locus: the EuroCLOT study. Ann Neurol. 2013;73:16–31. doi: 10.1002/ana.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.International Stroke Genetic Consortium Wellcome Trust Case-Control Consortium 2. Failure to validate association between 12p13 variants and ischemic stroke. N Engl J Med. 2010;362:1547–1550. doi: 10.1056/NEJMc0910050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cunnington MS, Santibanez Koref M, Mayosi BM, Burn J, Keavney B. Chromosome 9p21 SNPs Associated with Multiple Disease Phenotypes Correlate with ANRIL Expression. PLoS Genet. 2010;6:e1000899. doi: 10.1371/journal.pgen.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, et al. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet. 2013;9:e1003588. doi: 10.1371/journal.pgen.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stankovic S, Majkic-Singh N. Genetic aspects of ischemic stroke: coagulation, homocysteine, and lipoprotein metabolism as potential risk factors. Crit Rev Clin Lab Sci. 2010;47:72–123. doi: 10.3109/10408361003791520. [DOI] [PubMed] [Google Scholar]
- 55.Casas JP, Hingorani AD, Bautista LE, Sharma P. Meta-analysis of genetic studies in ischemic stroke: thirty-two genes involving approximately 18,000 cases and 58,000 controls. Arch Neurol. 2004;61:1652–1661. doi: 10.1001/archneur.61.11.1652. [DOI] [PubMed] [Google Scholar]
- 56.Casas J. Homocysteine and stroke: evidence on a causal link from Mendelian randomisation. Lancet. 2005;365:224–232. doi: 10.1016/S0140-6736(05)17742-3. [DOI] [PubMed] [Google Scholar]
- 57.Matarin M, Singleton A, Hardy J, Meschia J. The genetics of ischaemic stroke. J Intern Med. 2010;267:139–155. doi: 10.1111/j.1365-2796.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xin XY, Song YY, Ma JF, Fan CN, Ding JQ, Yang GY, et al. Gene polymorphisms and risk of adult early-onset ischemic stroke: a meta-analysis. Thromb Res. 2009;124:619–624. doi: 10.1016/j.thromres.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Hamedani AG, Cole JW, Mitchell BD, Kittner SJ. Meta-analysis of factor V Leiden and ischemic stroke in young adults: the importance of case ascertainment. Stroke. 2010;41:1599–1603. doi: 10.1161/STROKEAHA.110.581256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamedani AG, Cole JW, Cheng Y, Sparks MJ, O'Connell JR, Stine OC, et al. Factor V leiden and ischemic stroke risk: the Genetics of Early Onset Stroke (GEOS) study. J Stroke Cerebrovasc Dis. 2013;22:419–423. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harriott AM, Dueker N, Cheng YC, Ryan KA, O'Connell JR, Stine OC, et al. Polymorphisms in migraine-associated gene, atp1a2, and ischemic stroke risk in a biracial population: the genetics of early onset stroke study. Springerplus. 2013;2:46. doi: 10.1186/2193-1801-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pezzini A, Grassi M, Del Zotto E, Archetti S, Spezi R, Vergani V, et al. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young adults. Stroke. 2005;36:533–539. doi: 10.1161/01.STR.0000155741.31499.c2. [DOI] [PubMed] [Google Scholar]
- 63.Gu L, Su L, Chen Q, Liang B, Qin Y, Xie J, et al. Association between the apolipoprotein E gene polymorphism and ischemic stroke in Chinese populations: new data and meta-analysis. Exp Ther Med. 2013;5:853–859. doi: 10.3892/etm.2012.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi C, Kang X, Wang Y, Zhou Y. The coagulation factor V Leiden, MTHFRC677T variant and eNOS 4ab polymorphism in young Chinese population with ischemic stroke. Clin Chim Acta. 2008;396:7–9. doi: 10.1016/j.cca.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Yeh PS, Lin HJ, Li YH, Lin KC, Cheng TJ, Chang CY, et al. Prognosis of young ischemic stroke in Taiwan: impact of prothrombotic genetic polymorphisms. Thromb Haemost. 2004;92:583–589. doi: 10.1160/TH04-03-0099. [DOI] [PubMed] [Google Scholar]
- 66.Howard TD, Giles WH, Xu J, Wozniak MA, Malarcher AM, Lange LA, et al. Promoter polymorphisms in the nitric oxide synthase 3 gene are associated with ischemic stroke susceptibility in young black women. Stroke. 2005;36:1848–1851. doi: 10.1161/01.STR.0000177978.97428.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng YC, Anderson CD, Bione S, Keene K, Maguire JM, Nalls M, et al. GARNET Collaborative Research Group; GENEVA Consortium; International Stroke Genetics Consortium. Are myocardial infarction–associated single-nucleotide polymorphisms associated with ischemic stroke? Stroke. 2012;43:980–986. doi: 10.1161/STROKEAHA.111.632075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng YC, O'Connell JR, Cole JW, Stine OC, Dueker N, McArdle PF, et al. Genome-wide association analysis of ischemic stroke in young adults. G3 (Bethesda) 2011;1:505–514. doi: 10.1534/g3.111.001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nilsson-Ardnor S, Wiklund PG, Lindgren P, Nilsson AK, Janunger T, Escher SA, et al. Linkage of ischemic stroke to the PDE4D region on 5q in a Swedish population. Stroke. 2005;36:1666–1671. doi: 10.1161/01.STR.0000174188.04716.8d. [DOI] [PubMed] [Google Scholar]
- 70.Gretarsdottir S, Sveinbjörnsdottir S, Jonsson HH, Jakobsson F, Einarsdottir E, Agnarsson U, et al. Localization of a susceptibility gene for common forms of stroke to 5q12. Am J Hum Genet. 2002;70:593–603. doi: 10.1086/339252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nilsson-Ardnor S, Janunger T, Wiklund PG, Lackovic K, Nilsson AK, Lindgren P, et al. Genome-wide linkage scan of common stroke in families from northern Sweden. Stroke. 2007;38:34–40. doi: 10.1161/01.STR.0000251643.37454.16. [DOI] [PubMed] [Google Scholar]
- 72.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 73.Bevan S, Dichgans M, Gschwendtner A, Kuhlenbäumer G, Ringelstein EB, Markus HS. Variation in the PDE4D gene and ischemic stroke risk: a systematic review and meta-analysis on 5200 cases and 6600 controls. Stroke. 2008;39:1966–1971. doi: 10.1161/STROKEAHA.107.509992. [DOI] [PubMed] [Google Scholar]
- 74.Lövkvist H, Olsson S, Höglund P, Melander O, Jern C, Sjögren M, et al. A large-sample assessment of possible association between ischaemic stroke and rs12188950 in the PDE4D gene. Eur J Hum Genet. 2012;20:783–789. doi: 10.1038/ejhg.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lövkvist H, Smith JG, Luthman H, Höglund P, Norrving B, Kristoffersson U, et al. Ischaemic stroke in hypertensive patients is associated with variations in the PDE4D genome region. Eur J Hum Genet. 2008;16:1117–1125. doi: 10.1038/ejhg.2008.62. [DOI] [PubMed] [Google Scholar]
- 76.Song Q, Cole JW, O'Connell JR, Stine OC, Gallagher M, Giles WH, et al. Phosphodiesterase 4D polymorphisms and the risk of cerebral infarction in a biracial population: the Stroke Prevention in Young Women Study. Hum Mol Genet. 2006;15:2468–2478. doi: 10.1093/hmg/ddl169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 78.Guo DC, Papke CL, Tran-Fadulu V, Regalado ES, Avidan N, Johnson RJ, et al. Mutations in smooth muscle alpha-actin (ACTA2) cause coronary artery disease, stroke, and Moyamoya disease, along with thoracic aortic disease. Am J Hum Genet. 2009;84:617–627. doi: 10.1016/j.ajhg.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]