Abstract
Angiotensin Converting Enzyme type 2 (ACE2) is a pivotal component of the renin-angiotensin system, promoting the conversion of Angiotensin (Ang)-II to Ang-(1-7). We previously reported that decreased ACE2 expression and activity contribute to the development of Ang-II-mediated hypertension in mice. The present study aimed to investigate the mechanisms involved in ACE2 down-regulation during neurogenic hypertension. In ACE2-transfected Neuro-2A cells, Ang-II treatment resulted in a significant attenuation of ACE2 enzymatic activity. Examination of the subcellular localization of ACE2 revealed that Ang-II treatment leads to ACE2 internalization and degradation into lysosomes. These effects were prevented by both the Ang-II type 1 receptor (AT1R) blocker losartan and the lysosomal inhibitor leupeptin. In contrast, in HEK293T cells, which lack endogenous AT1R, Ang-II failed to promote ACE2 internalization. Moreover, this effect could be induced after AT1R transfection. Further, co-immunoprecipitation experiments demonstrated that AT1R and ACE2 form complexes and these interactions were decreased by Ang-II treatment, which also enhanced ACE2 ubiquitination. In contrast, ACE2 activity was not changed by transfection of AT2 or Mas receptors. In vivo, Ang-II-mediated hypertension was blunted by chronic infusion of leupeptin in wildtype C57Bl/6, but not in ACE2 knockout mice. Overall, this is the first demonstration that elevated Ang-II levels reduce ACE2 expression and activity by stimulation of lysosomal degradation through an AT1R-dependent mechanism.
Keywords: Post-transcriptional regulation, proteasome, autonomic nervous system, renin-angiotensin system, hypertension
Introduction
Overactive renin-angiotensin system (RAS) and chronically elevated Angiotensin (Ang)-II levels are widely recognized as major factors in the development and maintenance of hypertension. The RAS comprises renin which metabolizes Angiotensinogen to the decapeptide Ang-I, which is further cleaved by Angiotensin Converting Enzyme (ACE) to the octapeptide Ang-II. ACE2, an ACE homologue insensitive to ACE inhibitors was identified in 2000.1 ACE2 acts primarily on Ang-II to generate the vasodilatory heptapeptide Ang-(1-7). The effects of Ang-II and Ang-(1-7) are mediated by interactions with specific plasma membrane receptors, i. e . AT1, AT2 and Mas, all of which are G-protein-coupled receptors (GPCR). Ang-II type 1 receptor (AT1R) activation by Ang-II leads to elevated blood pressure (BP), hypertrophy and fibrosis, whereas Mas receptor (MasR) activation by Ang-(1-7) results in effects opposite to AT1R activation, such as vasodilation, growth inhibition and anti-fibrotic actions.2 Thus, the ACE2/Ang-(1-7)/MasR axis provides emerging therapeutic targets to prevent the pathological actions of Ang-II.
We previously demonstrated that overexpression of ACE2 in the mouse central nervous system (CNS) significantly reduces the pressor effects of Ang-II and attenuates AT1R expression.3-6 In addition, we have shown that ACE2 gene deletion in the CNS promotes age-dependent oxidative stress, autonomic dysfunction and hypertension.2, 7 However, while our group and others previously showed that Ang-II reduces ACE2 expression in the CNS,3, 5, 8 themechanisms by which Ang-II levels modulate the expression, subcellular localization and activity of ACE2 remain unknown.
A common physiological mechanism to prevent exaggerated cellular responses during chronic stimulation is internalization of the respective receptor protein followed by degradation and down-regulation, as it has been extensively documented for the GPCR family. However, AT1R expression levels are regulated in a cell- and tissue-dependent manner and no down-regulation after exposure to chronic Ang-II was observed in kidney proximal tubule.9 While the mechanisms involved in AT1R internalization are partly characterized,10 almost nothing is known about how chronic elevated Ang-II levels modulate the expression, subcellular localization and activity of other RAS members with plasma membrane localization, like ACE and ACE2.
ACE mRNA was found to be markedly up-regulated whereas ACE2 mRNA down-regulated in hypertensive patients.11 This transcriptional regulation was also observed in vitro in kidney tubular epithelial cells after Ang-II treatment and could be prevented through inhibition of MAP kinases. ACE and ACE2 are type I membrane proteins with a single-pass N-terminus domain, a short transmembrane domain and a short C-terminus. Other members of the metallopeptidase family, like β-site APP-cleaving enzyme (BACE1) were extensively shown in pathological conditions to internalize into early endosomes and undergo ubiquitination followed by lysosomal degradation, contributing to the progression of Alzheimer’s disease.12, 13
ACE2 has been also identified as the cellular receptor for the severe acute respiratory syndrome coronavirus (SARS-CoV).14, 15 Interestingly, ACE2 expression is reduced after SARS-CoV infection, suggesting a regulated internalization mechanism. The route of internalization of the ACE2/SARS-CoV complex remains under debate; with reports of clathrin- and caveolae-independent pathways in HEK293T cells, as well as clathrin-dependent and C-terminus independent internalization in COS7 and HepG2 cell lines.16, 17 In addition, ACE2 polymorphisms have been associated with hypertension in humans. Despite an increase in ACE2 mRNA and protein expression in the early phase of several cardiovascular diseases, seen as a compensatory mechanism, the expression of the carboxypeptidase is usually reduced in later phases of disease progression. Interestingly, this latter decrease can be prevented or reversed by ACE inhibitors or AT1R blockers treatment. Although these data suggest that the ACE/Ang-II/AT1R axis is responsible for down-regulation of ACE2, the mechanisms involved remain unknown. Therefore, the goal of the present study was to investigate the effects of Ang-II on ACE2 cellular levels, localization and activity and to define the cellular mechanisms associated with these effects.
Methods
An expanded Materials and Methods section is available in the online data supplement (please see http://hyper.ahajournals.org).
Cell culture and transfection
Neuro-2A and HEK293T cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 10 units/mL penicillin and 100 μg/mL streptomycin. The cells were transiently transfected using Lipofectamine 2000 reagent (Invitrogen), in Dulbecco’s modified Eagle’s medium with no antibiotics and fetal bovine serum at ~80% confluence according to manufacturer’s instructions. After 6 h, the cells were trypsinized and plated at the desired density in full medium for the next 30 h. The cells were serum-starved 24 h before each experiment.
Blood pressure recordings
Experiments were performed in adult (14-16 weeks old, 25-30 g) male C57Bl/6 (Jackson Laboratories) and ACE2 knockout (ACE2−/y) mice (kind gift of Drs. Thomas M. Coffman and Susan B. Gurley, Duke University). Animals were housed in a temperature- and humidity-controlled facility under a 12 h dark/light cycle, fed standard mouse chow and water ad libitum. All procedures were approved by the LSU Health Sciences Center-NO Animal Care and Use Committee and are in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Baseline BP was measured in ACE2−/y and non-transgenic (NT) mice during 3 days using radio-telemetry, as described.5 Mice were infused subcutaneously with Ang-II (600 ng/kg/min), or vehicle (0.9 % saline) for 14 days using osmotic pumps (Alzet). Following anesthesia with isoflurane, as above, mice were additionally implanted with an icv cannula connected to a subcutaneous osmotic pump containing leupeptin (460 ng/kg/min) or artificial cerebrospinal fluid (aCSF). BP was continually recorded for 17 days.
Statistics
Data are presented as mean ±SEM. Data were analyzed, by repeated measures ANOVA, or two-way ANOVA, followed by Bonferroni post-tests, as appropriate. Statistical comparisons were performed using Prism 5 (GraphPad Software). Differences were considered statistically significant at P<0.05.
Results
Ang-II decreases ACE2 protein expression and activity in neurons
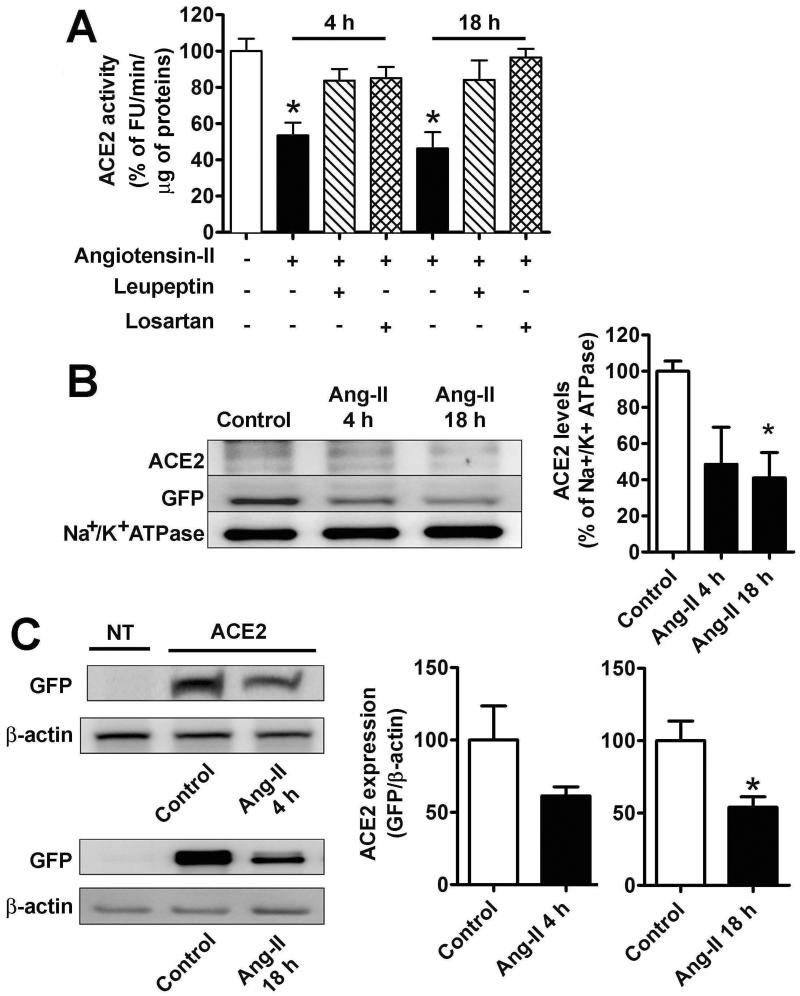
Neuro-2A cells, a murine neuroblastoma cell line, have been previously used by our group and others to study oxidative stress and ACE2 expression in response to endogenous AT1R stimulation.7 As these cells express low levels of ACE2, activity is beyond detectable levels in non-transfected Neuro-2A cells. In order to study ACE2 subcellular localization, Neuro-2A cells were transfected with an ACE2-GFP fusion plasmid allowing for fluorescent detection and quantification of the enzyme in various subcellular compartments. Ang-II treatment markedly decreased ACE2 activity as early as 4 h and this effect was prevented by pre-treatment with losartan (Figure 1A), suggesting that this inhibition is mediated by AT1R. Surprisingly, this decrease was also eliminated by treatment with the protease inhibitor, leupeptin (Figure 1A). The effects of leupeptin suggest that Ang-II-mediated ACE2 internalization may lead to lysosomal degradation. Since we recently reported that ACE2 undergoes shedding from the plasma membrane in neurogenic hypertension,5 activity of the enzyme was assessed in the cell medium. In these conditions, ACE2 activity was minimal in the medium (9 ±2 % of the total activity) and this was not changed by Ang-II treatment, indicating that shedding was not responsible for the decreased ACE2 activity.
Figure 1.
A. ACE2 activity in transfected Neuro-2A cells in control (white bar), or after treatment with Ang-II alone (100 nM, black bars), or Ang-II and losartan (1 μM, horizontal hatched bars), or Ang-II and leupeptin (100 μM, vertical hatched bars) after 4 h (middle columns) or 18 h (right columns). ACE2 activity was determined as described in Experimental Procedures and the results were expressed as % from the activity measured in control cells (100% corresponds to 449 ±51 FU/min/μg of proteins). n=12 from 5 independent transfections, *−indicates P<0.05. B. Plasma membrane levels of ACE2 in control conditions and after treatment with 100 nM Ang-II for 4 and 18 h. Neuro-2A cells were transfected with 2 μg/well ACE2-GFP in 6-well plates and 48 h later the plasma membrane proteins were isolated by biotinylation, as described in Methods section. Subsequently, ACE2 levels were determined by western blot using ACE2 (top) or GFP (middle) antibodies. Na+/K+ ATP-ase (bottom) was used as a loading control. The summary data of three independent experiments are shown in the bottom panel. *−indicates P<0.05. C. Total cellular levels of ACE2-GFP in Neuro-2A cells in non-treated controls and after Ang-II (100 nM) treatment for 18 h. Similar results were obtained in two other independent experiments. FU: Fluorescence units.
To test whether this reduction was due to changes in subcellular localization, we next investigated the effects of Ang-II on the plasma membrane levels of ACE2. To this end, we used biotinylation, a procedure that labels exclusively plasma membrane proteins. In agreement with the above observations, by 4 h, Ang-II treatment had clearly decreased (~50 %) the amount of ACE2 present at the plasma membrane with no further reduction at 18 h post-treatment (Figure 1B). Interestingly, the total cellular ACE2 levels determined by western blot were significantly decreased after 18 h (Figure 1C) but not after 4 h of Ang-II treatment, supporting the hypothesis that Ang-II promotes ACE2 internalization followed by degradation.
Ang-II alters ACE2 subcellular localization by an AT1R-mediated mechanism
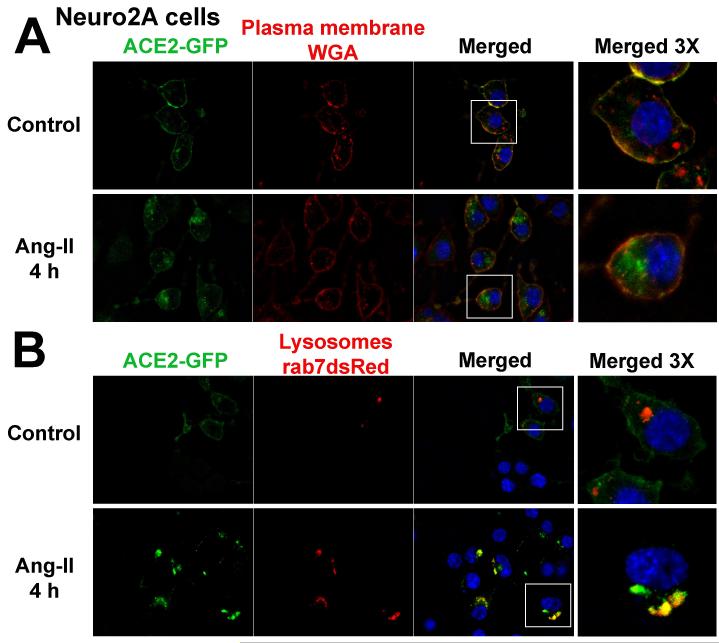
Confocal microscopy was next used to evaluate the subcellular localization of the enzyme before and after Ang-II treatment. In untreated Neuro-2A cells, ACE2 displays a predominant cell surface localization as indicated by co-localization with WGA, a specific plasma membrane marker (Figure 2A). However, after 4 h Ang-II treatment, ACE2 was barely detectable at the plasma membrane and had clearly accumulated within the cytoplasm, confirming internalization of the carboxypeptidase. Further investigation revealed that ACE2 accumulates into lysosomes following Ang-II treatment, as shown by co-localization with Rab7-positive Neuro-2A cells (Figure 2B).
Figure 2.
Subcellular localization of ACE2-GFP in Neuro-2A cells treated with WGA (wheat germ agglutinin) for plasma membrane localization (A) and co-transfected with Rab7-dsRed for labeling of lysosomal compartments (B). Cells were processed as described in the Methods section. These images are representative of 12 different coverslips from 5 independent transfections. The far right panels represent 3 times magnification of the white boxes in the merged column.
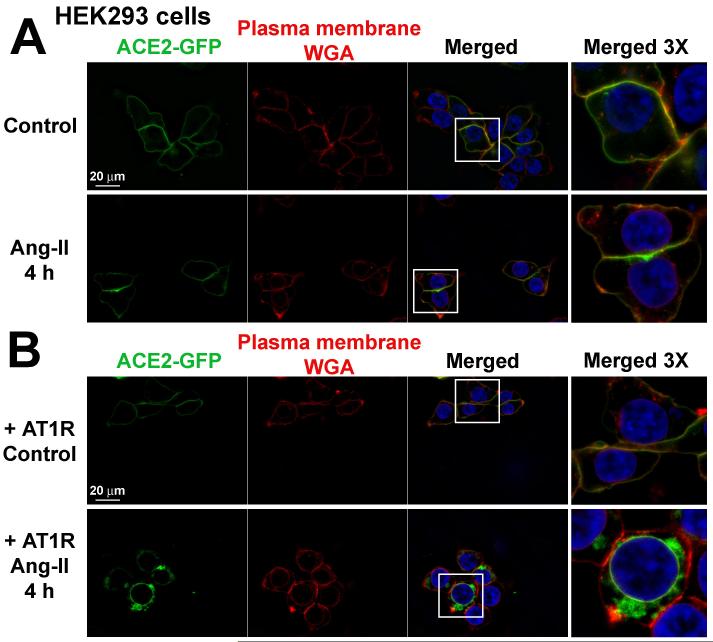
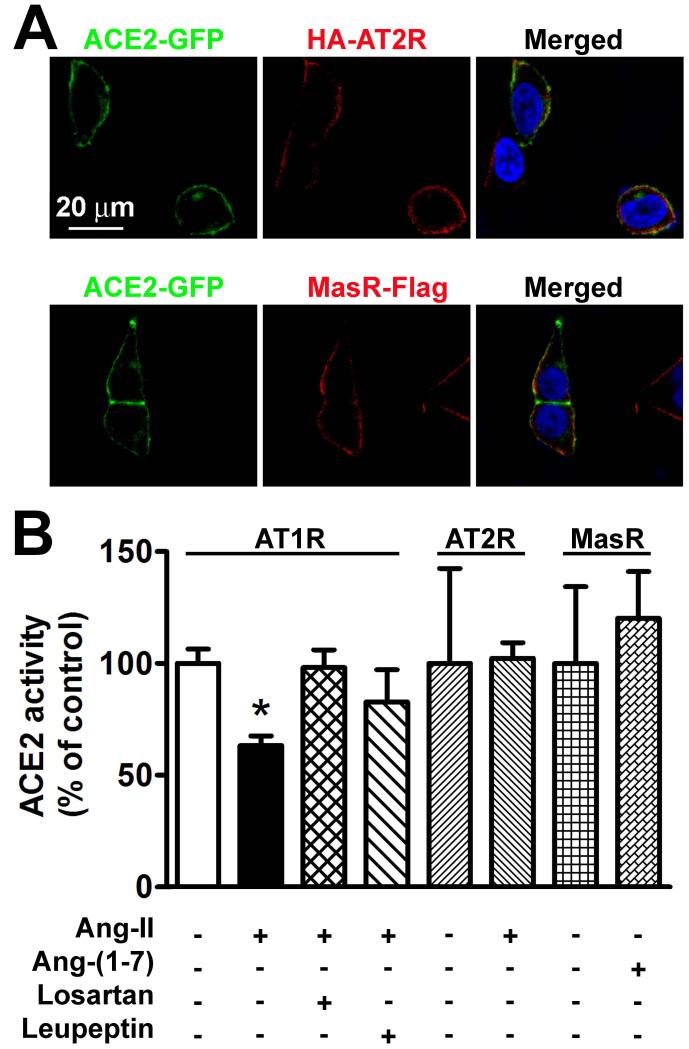
To further investigate the contribution of AT1R in ACE2 internalization, we repeated these experiments in HEK293T cells, which lack endogenous expression of this receptor.18 In contrast to Neuro-2A cells, ACE2 plasma membrane localization was not changed by treatment with Ang-II in HEK293T cells (Figure 3A), suggesting that AT1R is required for ACE2 internalization. Indeed, transfection of AT1R to these cells prompted Ang-II-mediated ACE2 internalization (Figure 3B). In addition, this observation was specific for AT1R, since ACE2 was in proximity but not clearly co-localized with Ang-II type 2 receptor (AT2R) or MasR in HEK293T cells (Figure 4A). Furthermore, ACE2 activity was not changed in cells co-transfected with AT2R or MasR, but was clearly inhibited in HEK293T cells transfected with AT1R after Ang-II treatment (Figure 4B). As in Neuro-2A cells, these effects were eliminated by pre-treatment with losartan or leupeptin (Figure 4B).
Figure 3.
Subcellular localization of ACE2-GFP in HEK293T cells co-transfected with pcDNA3.1 (A) or AT1R (B). The cells were treated with wheat germ agglutinin (WGA) for plasma membrane staining as described in the Methods section. These images are representative of 12 different coverslips from 4 independent transfections. The far right panels represent 3 times magnification of the white boxes in the merged column.
Figure 4.
A. AT2R (top panel) and MasR (bottom panel) are localized at the plasma membrane in transfected HEK293T cells, but are not co-localized with ACE2. These images are representative of 6 different coverslips from 3 independent transfections. B. ACE2 activity in HEK293T cells co-transfected with AT1R (left columns), AT2R (middle columns) and MasR (right columns) in presence of different treatments indicated under each bar. n=6 from 2 independent transfections. *−indicates P<0.05.
ACE2 internalization involves ubiquitination and AT1R interaction
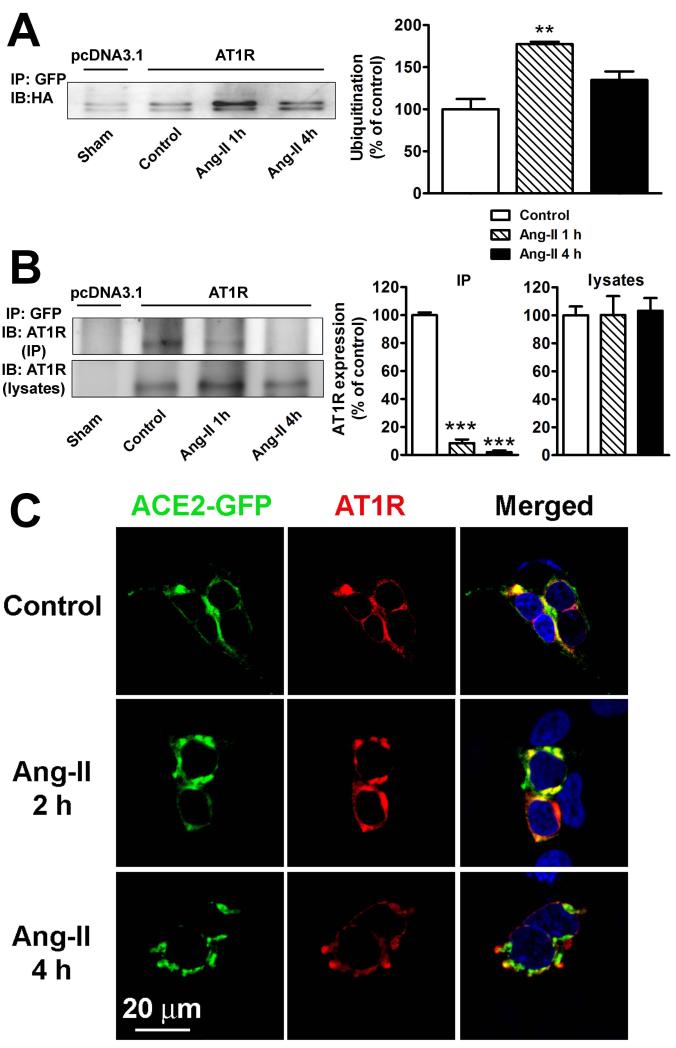
Since ubiquitination is the major post-translational modification responsible for lysosomal targeting of plasma membrane proteins19 and Ang-II treatment targets ACE2 to lysosomes (Figure 2B), we next investigated ACE2 ubiquitination levels in transfected HEK293T cells. In basal conditions, ACE2 displayed small levels of ubiquitination irrespective of AT1R presence, and Ang-II clearly enhanced ACE2 ubiquitination after 1 h post treatment (Figure 5A).
Figure 5.
A. Ang-II stimulates ACE2 ubiquitination. HEK293T cells were transfected in 10 cm2 plates with 10 μg ACE2-GFP, 10 μg HA-ubiquitin, and either 10 μg pcDNA3.1 (1st lane) or 10 μg AT1R (lanes 2-4). Cells were serum starved for 24 h and treated with Ang-II (100 nM) for the indicated time periods. Cells were then lysed and ACE2 interacting proteins were pulled down by treatment with a GFP antibody (2 μg/mg). Cell lysates were separated by 10% SDS-PAGE and ubiquitin levels were revealed by western blotting using anti-HA antibody. The experiment shown is representative from 3 independent transfections. **−indicates P<0.01 B. ACE2 interaction with AT1R in HEK293T cells determined by co-immunoprecipitation. Cells were transfected in 10 cm2 plates with 10 μg ACE2-GFP and 10 μg pcDNA3.1 (lane 1) or 10 μg AT1R (lanes 2-4). Cells were serum starved for 24 hours and treated with Ang-II (100 nM) for the indicated time periods. Following lysis, ACE2 interacting proteins were pulled down by treatment with a GFP antibody (2 μg/mg). AT1R was detected in immunoprecipitates (top panel) and lysates (bottom panel) using an anti-AT1R antibody (SC-579). Similar results were obtained in 2 other experiments. ***−indicates P<0.001 C. ACE2 and AT1R localization in HEK293T cells in control conditions (top panel), or after 2 h (middle panel) and 4 h (bottom panel) treatment with Ang-II (100 nM).
Because AT1R appears to be essential for Ang-II-mediated ACE2 internalization, we also tested whether ACE2 and AT1R physically interact. Co-immunoprecipitation experiments confirmed this dimerization in control conditions (Figure 5B, 2nd lane). However, Ang-II treatment decreased this interaction in a time-dependent manner (Figure 5B, 3rd and 4th lanes). This conclusion is supported by confocal microscopy data, showing that ACE2 and AT1R are co-localized at the plasma membrane in control conditions (Figure 5C, top) and that following Ang-II treatment both proteins are still co-localized after 2 h (Figure 5C, middle), but no longer co-localized after 4 h (Figure 5C, bottom).
Leupeptin restores ACE2 activity and prevents the development of Ang-II-dependent hypertension
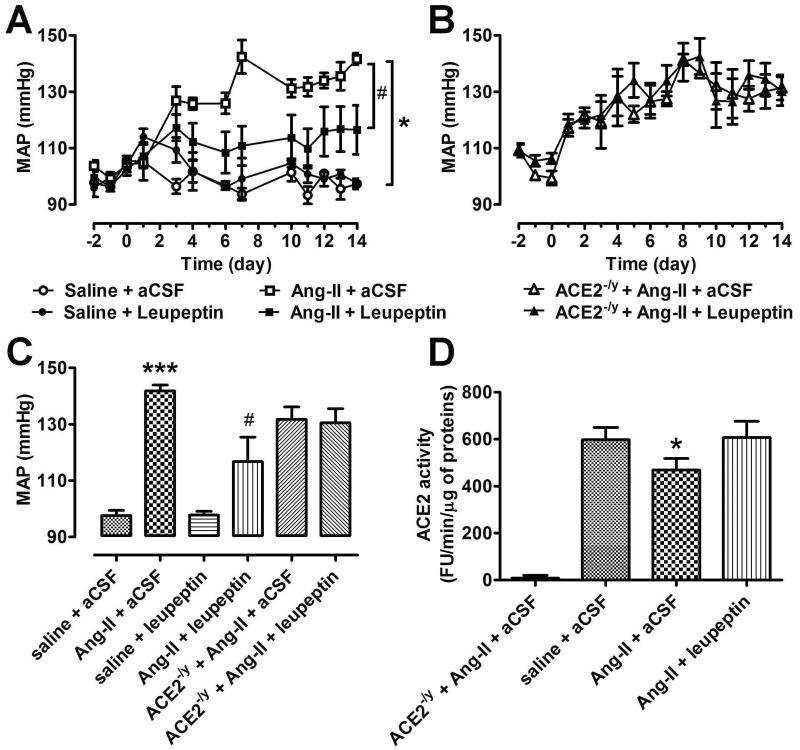
Based on our findings at the cellular level, we next assessed whether ACE2 internalization may contribute to the development of Ang-II-induced hypertension in vivo. Using the slow-pressor dose model,6 chronic infusion of Ang-II led to a progressive increase in BP in C57Bl/6 mice that peaked to ~140 mmHg after 2 weeks (Figure 6A,C). Although chronic central infusion of leupeptin alone (460 ng/h for 2 weeks) did not have a significant effect on BP, co-administration of Ang-II sc and leupeptin icv resulted in a significant blunting of the BP increase (Figure 6A,C). To address the contribution of ACE2 to this observation, ACE2 knockout mice (ACE2−/y) were similarly infused with Ang-II and leupeptin. Interestingly, while the Ang-II pressor response was similar in NT and ACE2−/y, leupeptin was not able to inhibit the Ang-II-induced hypertension in ACE2−/y mice (Figure 6B,C). These data indicate that leupeptin beneficial effects are, at least in part, mediated by preservation of ACE2 activity in the presence of elevated Ang-II levels. This conclusion is supported by measurements of ACE2 activity in the hypothalami of these mice. Indeed, Ang-II infusion resulted in a clear reduction of hypothalamic ACE2 activity that could be restored following co-administration of leupeptin (Figure 6D).
Figure 6.
A. The effects of Ang-II and leupeptin on blood pressure in wild type mice (n=6-8 animals per group). B. The effects of Ang-II and leupeptin on blood pressure in ACE2 KO mice (n=6-8 animals per group). C. Average MAP following 2 weeks of treatment in all 6 groups. ***−indicates P<0.001 between saline + aCSF vs. Ang-II + aCSF; #-indicates P<0.05 between Ang-II + leupeptin vs. Ang-II + aCSF. D. ACE2 activity in the hypothalamus of animals used in A and B. n=4-5 per group, *−indicates P<0.05 between Ang-II + aCSF vs. saline + aCSF.
Discussion
The primary goal of the present study was to clarify the mechanisms involved in the reduction of ACE2 compensatory properties during the development of Ang-II-mediated hypertension. We observed that ACE2 is internalized upon Ang-II treatment, leading to a reduction of its enzymatic activity on the cell surface. Within the cytoplasm, ACE2 co-localizes with lysosomes where it undergoes degradation, a process involving ubiquitination, further reducing ACE2 activity within the cell. Internalization and degradation of ACE2 were inhibited by the AT1R blocker, losartan, and leupeptin, a protease inhibitor. In addition, leupeptin infusion in the brain blunted the development of Ang-II-mediated hypertension in C57Bl/6 mice, confirming that ACE2 degradation contributes to neurogenic hypertension. To our knowledge, our data provide the first demonstration that elevated Ang-II levels decrease ACE2 activity through an AT1R-dependent internalization mechanism followed by lysosomal targeting for degradation.
Human ACE2 has 805 amino acids (AA), organized in a short cytoplasmic domain (44 AA), a transmembrane helix (21 AA) and a very long extracellular domain containing a single catalytic metallopeptidase unit responsible for the cleavage of Ang-II into Ang-(1-7). Limitation of Ang-II levels by ACE2 has been shown to have multiple positive effects in various cardiovascular pathological models,4, 8, 20-28 including Ang-II-induced neurogenic hypertension.3, 5-7, 29 However, as the disease progresses, ACE2 compensatory mechanisms are failing, as evidenced by reduction in protein expression and activity.3, 8, 28
Several mechanisms have been proposed for ACE2 down-regulation. A reduction of ACE2 mRNA in astrocytes following Ang-II treatment was previously reported30 and similar observations were made in kidneys isolated from Goldblatt hypertensive rats31 and brains from chronic heart failure rabbits,32 two models with elevated systemic Ang-II levels, suggesting that Ang-II can modulate the transcriptional regulation of ACE2. In addition, a recent study demonstrated that Ang-II treatment leads to a reduction in ACE2 mRNA and protein levels in catecholaminergic neurons, an effect that was prevented by p38 and MAP kinase inhibitors,33 confirming that these signaling mechanisms are involved in ACE2 gene expression. Post-transcriptional mechanisms have also been suggested, in particular during exercise training where expression of ACE2 was correlated with a reduction of miR 143 in the heart.34 While some steps have been taken to understand ACE2 transcriptional regulation in vivo, there is very limited knowledge regarding the enzyme’s post-translational regulation. ACE2 shedding was shown to contribute to the enzyme cell surface activity, through an ADAM17-dependent mechanism.35, 36 Consequently, ADAM17 has been speculated to contribute to the cleavage and release of the extracellular domain of ACE2, in plasma37 and urine.38, 39 Very recently, our group demonstrated that ACE2 shedding is taking place in neurogenic hypertension and can be reversed by inhibition of ADAM17 in the CNS.5
In contrast, and despite our early observations that ACE2 expression can be localized to the cytoplasm of neurons,40 the effects of Ang-II on ACE2 plasma membrane localization and internalization have not been investigated. This lack of information is surprising considering that ACE2 is also a cellular receptor for the SARS coronavirus and that ACE2 expression and plasma membrane levels are reduced after SARS-CoV infection, indicating a regulated internalization mechanism.41, 42 Similarly, other members of the metallopeptidase family, like β-site APP-cleaving enzyme (BACE1), were extensively shown, in pathological conditions, to internalize into early endosomes and undergo ubiquitination followed by lysosomal degradation.15, 43 In the present study, we observed that in Neuro-2A cells, ACE2 activity is markedly reduced by Ang-II and this effect is completely blocked by leupeptin, an inhibitor of the proteases found in lysosomes, indicating that elevated Ang-II levels target ACE2 for degradation. In agreement with this conclusion, ACE2 was shown to be co-localized with Rab7, a well-characterized lysosomal marker. In addition, Ang-II treatment enhanced ACE2 ubiquitination, a post-translational modification essential for lysosomal targeting.
Several lines of evidence indicate that Ang-II-induced ACE2 internalization and lysosomal degradation are mediated by AT1R. First, this effect is blocked by the AT1R blocker, losartan. Second, ACE2 internalization by Ang-II is absent in HEK293T cells which do not express AT1R, but this effect is restored in cells transfected with this receptor. Third, co- immunoprecipitation experiments demonstrated that ACE2 and AT1R are interacting and these interactions are reduced in the presence of Ang-II. These results suggest that along the endocytic pathway ACE2 and AT1R dissociate, with the former being transported to the lysosomes, whereas the receptor is slowly recycled back to the plasma membrane from early endosomes. Consistent with this idea and in contrast to other GPCR, AT1R expression levels are not always down-regulated after chronic exposure to Ang-II in vivo.9 The role of AT1R in the regulation of ACE2 subcellular localization in presence of Ang-II, is unique, as its effects cannot be mimicked by AT2R or MasR. Recently, similar interactions between GPCR and the enzyme involved in the degradation of the respective endogenous ligand were reported. Specifically, adenosine deaminase was shown to allosterically bind to adenosine A1 and A2 receptors and modulate receptor signaling.44 In contrast, in the case of AT1R/ACE2 interactions, AT1R controls ACE2 expression levels, subcellular localization and enzymatic activity. Furthermore, internalization of ACE2 also supports the concept that Ang-II has intracellular actions independent of activation of plasma membrane receptors. Indeed, along with a functional intracellular RAS, detectable intracellular Ang-II levels ranging from 5 to 20 fmol/g have been reported in several tissues.45 Whether or not the internalized ACE2 can also act in the endosomes to generate Ang-(1-7) remains to be determined. This unexpected feature of AT1R appears very important in the regulation of BP at least at the CNS level. Our group3, 6, 29, 46 and others8, 47 have shown that brain ACE2 overexpression blunts the central effects of Ang-II. Moreover, the effects of central Ang-II on cardiac sympathetic tone, brain NADPH oxidase and SOD activities are significantly increased in ACE2 knockout mice,7 although differences in baseline BP levels were only observed in aging mice. In the present study, we found that leupeptin significantly attenuated the hypertensive effects of Ang-II. However, the effects of this protease inhibitor were eliminated in ACE2 knockout mice, indicating that its effects are at least mediated by prevention of ACE2 lysosomal degradation, as also indicated by the finding that leupeptin enhanced ACE2 activity in presence of chronic Ang-II treatment. While the lack of change in baseline BP for ACE2 knockout mice is difficult to reconcile with a major regulatory role of the enzyme, at least in basal conditions, our data clearly show that ACE2 internalization contributes to Ang-II-mediated hypertension.
Perspective
This study is the first demonstration that central hypertensive effects of Ang-II are at least in part mediated by decreasing ACE2 activity and stimulation of the carboxypeptidase lysosomal degradation through an AT1R-dependent mechanism. This feed-forward mechanism, supporting the reduction of ACE2, limits the formation and therefore availability of Ang-(1-7) and enhances the pathologic actions of Ang-II. Soluble recombinant ACE2 has been proposed as a new therapeutic approach in cardiovascular diseases and could be used to diminish the circulating levels of Ang-II. Our study indicates that this may have great value during chronic stimulation of AT1R since soluble recombinant ACE2 is not associated with the plasma membrane and therefore unlikely to be down-regulated. Alternatively, an ACE2 derivative resistant to internalization and degradation may potentially have a therapeutic value.
Supplementary Material
Novelty and Significance.
What is new?
This study demonstrates that ACE2 undergoes an internalization and degradation process upon exposure to Ang-II levels involving ubiquitination of the enzyme and physical interaction with the AT1R.
Reduction of Ang-II-mediated hypertension could be achieved by prevention of ACE2 degradation in lysosomes.
What is relevant?
Elevation of Ang-II levels in hypertension results in reduction of ACE2 compensatory activity. Identification of the mechanisms responsible for ACE2 down-regulation provides new opportunities for hypertension treatment.
Summary
Elevation of Ang-II levels leads to ACE2 down-regulation via internalization and degradation in lysosomes. Blockade of ACE2 degradation by leupeptin restores ACE2 compensatory activity on the plasma membrane and attenuates the development of hypertension
Acknowledgements
We are grateful to Mrs. Evangeline M. Bailey for expert technical assistance and to Dr. Kim B. Pedersen for helpful discussions.
Sources of Funding
The present study was supported by NIH grants P20-GM103514 (EL and CMF), R01-HL093178 (EL), American Heart Association Established Investigator Award (EL), LSU Research Enhancement Fund (EL) and startup funds from Howard University (CMF).
Footnotes
Disclosures
None.
References
- 1.Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des. 2007;13:1231–1245. doi: 10.2174/138161207780618911. [DOI] [PubMed] [Google Scholar]
- 2.Xu P, Sriramula S, Lazartigues E. ACE2/Ang-(1-7)/Mas pathway in the brain: The Axis of Good. Am J Physiology - Regul Integr Comp Physiol. 2011;300:R804–817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II Type 1 Receptor-Mediated Reduction of Angiotensin-Converting Enzyme 2 Activity in the Brain Impairs Baroreflex Function in Hypertensive Mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng Y, Hans C, McIlwain E, Varner KJ, Lazartigues E. Angiotensin-Converting Enzyme 2 Over-Expression in the Central Nervous System Reduces Angiotensin-II-Mediated Cardiac Hypertrophy. PLos ONE. 2012;7:e48910. doi: 10.1371/journal.pone.0048910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia H, Sriramula S, Chhabra K, Lazartigues E. Brain ACE2 Shedding Contributes to the Development of Neurogenic Hypertension. Circ Res. 2013;113:1087–1096. doi: 10.1161/CIRCRESAHA.113.301811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E. Brain-Selective Overexpression of Human Angiotensin-Converting Enzyme Type 2 Attenuates Neurogenic Hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. ACE2-Mediated Reduction of Oxidative Stress in the Central Nervous System Is Associated with Improvement of Autonomic Function. PLoS One. 2011;6:e22682. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of Angiotensin-Converting Enzyme 2 in the Rostral Ventrolateral Medulla Causes Long-Term Decrease in Blood Pressure in the Spontaneously Hypertensive Rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 9.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shenoy SK, Lefkowitz RJ. b-arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koka V, Huang XR, Chung ACK, Wang W, Truong LD, Lan HY. Angiotensin II Up-Regulates Angiotensin I-Converting Enzyme (ACE), but Down-Regulates ACE2 via the AT1-ERK/p38 MAP Kinase Pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danilczyk U, Eriksson U, Crackower MA, Penninger J. A story of two ACEs. J Mol Med. 2003;81:227–234. doi: 10.1007/s00109-003-0419-x. [DOI] [PubMed] [Google Scholar]
- 13.Tan J, Evin G. β-Site APP-cleaving enzyme 1 trafficking and Alzheimer’s disease pathogenesis. J Neurochem. 2012;120:869–880. doi: 10.1111/j.1471-4159.2011.07623.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai Y, Kuba K, Penninger JM. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue Y, Tanaka N, Tanaka Y, Inoue S, Morita K, Zhuang M, Hattori T, Sugamura K. Clathrin-Dependent Entry of Severe Acute Respiratory Syndrome Coronavirus into Target Cells Expressing ACE2 with the Cytoplasmic Tail Deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atwood B, Lopez J, Wager-Miller J, Mackie K, Straiker A. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. doi: 10.1186/1471-2164-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol. 2012;21:647–655. doi: 10.1016/j.tcb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-Selective Overexpression of Angiotensin-Converting Enzyme 2 Attenuates Sympathetic Nerve Activity and Enhances Baroreflex Function in Chronic Heart Failure. Hypertension. 2011;58:1057–1065. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazato Y, Ferreira AJ, Hong K-H, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, Katovich MJ, Raizada MK. Prevention of Pulmonary Hypertension by Angiotensin-Converting Enzyme 2 Gene Transfer. Hypertension. 2009;54:365–371. doi: 10.1161/HYPERTENSIONAHA.108.125468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarajapu YPR, Bhatwadekar AD, Caballero S, Hazra S, Shenoy V, Medina R, Kent D, Stitt AW, Thut C, Finney EM, Raizada MK, Grant MB. Activation of the ACE2/Angiotensin-(1-7)/Mas Receptor Axis Enhances the Reparative Function of Dysfunctional Diabetic Endothelial Progenitors. Diabetes. 2012;62:1258–1269. doi: 10.2337/db12-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 24.Grobe JL, Der Sarkissian S, Stewart JM, Meszaros JG, Raizada MK, Katovich MJ. ACE2 overexpression inhibits hypoxia-induced collagen production by cardiac fibroblasts. Clin Sci. 2007;113:357–364. doi: 10.1042/CS20070160. [DOI] [PubMed] [Google Scholar]
- 25.Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 26.Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardiac Overexpression of Angiotensin Converting Enzyme 2 Protects the Heart From Ischemia-Induced Pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 27.Chhabra KH, Xia H, Pedersen KB, Speth RC, Lazartigues E. Pancreatic angiotensin-converting enzyme 2 improves glycemia in angiotensin II-infused mice. Am J Physiol Endocrinol Metab. 2013;304:E874–E884. doi: 10.1152/ajpendo.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E. Angiotensin-I Converting Enzyme Type 2 (ACE2) Gene Therapy Improves Glycemic Control in Diabetic Mice. Diabetes. 2010;59:2540–2548. doi: 10.2337/db09-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E. Angiotensin-Converting Enzyme 2 Overexpression in the Subfornical Organ Prevents the Angiotensin II-Mediated Pressor and Drinking Responses and Is Associated With Angiotensin II Type 1 Receptor Downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher PE, Chappell MC, Ferrario CM, Tallant EA. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol. 2006;290:C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 31.Prieto MC, Gonzalez-Villalobos RA, Botros FT, Martin VL, Pagan J, Satou R, Lara LS, Feng Y, Fernandes FB, Kobori H, Casarini DE, Navar LG. Reciprocal changes in renal ACE/ANG II and ACE2/ANG 1-7 are associated with enhanced collecting duct renin in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2011;300:F749–F755. doi: 10.1152/ajprenal.00383.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kar S, Gao L, Zucker IH. Exercise training normalizes ACE and ACE2 in the brain of rabbits with pacing-induced heart failure. J Appl Physiol. 2010;108:923–932. doi: 10.1152/japplphysiol.00840.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao L, Haack KKV, Zucker IH. Angiotensin II regulates ACE and ACE2 in neurons through p38 mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 signaling. Am J Physiol Cell Physiol. 2013;304:C1073–C1079. doi: 10.1152/ajpcell.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandes T, Hashimoto NY, Magalhaes FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI, Oliveira EM. Aerobic Exercise Training-Induced Left Ventricular Hypertrophy Involves Regulatory MicroRNAs, Decreased Angiotensin-Converting Enzyme-Angiotensin II, and Synergistic Regulation of Angiotensin-Converting Enzyme 2-Angiotensin (1-7) Hypertension. 2011;58:182–189. doi: 10.1161/HYPERTENSIONAHA.110.168252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2) J Biol Chem. 2005;280:30113–30119. doi: 10.1074/jbc.M505111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lambert DW, Clarke NE, Hooper NM, Turner AJ. Calmodulin interacts with angiotensin-converting enzyme-2 (ACE2) and inhibits shedding of its ectodomain. FEBS Lett. 2008;582:385–390. doi: 10.1016/j.febslet.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: where does angiotensin-converting enzyme 2 fit in? Clin Exp Pharmacol Physiol. 2013;40:551–559. doi: 10.1111/1440-1681.12069. [DOI] [PubMed] [Google Scholar]
- 38.Xiao F, Hiremath S, Knoll G, Zimpelmann J, Srivaratharajah K, Jadhav D, Fergusson D, Kennedy CRJ, Burns KD. Increased Urinary Angiotensin-Converting Enzyme 2 in Renal Transplant Patients with Diabetes. PLos ONE. 2012;7:e37649. doi: 10.1371/journal.pone.0037649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chodavarapu H, Grobe N, Somineni HK, Salem ESB, Madhu M, Elased KM. Rosiglitazone Treatment of Type 2 Diabetic db/db Mice Attenuates Urinary Albumin and Angiotensin Converting Enzyme 2 Excretion. PLOS ONE. 2013;8:e62833. doi: 10.1371/journal.pone.0062833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol - Regul Integr Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol. 2008;75:781–786. doi: 10.1016/j.bcp.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamming I, Cooper ME, Haagmans BL, Hooper NL, Korstanje R, Osterhaus A, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang EL, Biscaro B, Piazza F, Tesco G. BACE1 Protein Endocytosis and Trafficking Are Differentially Regulated by Ubiquitination at Lysine 501 and the Di-leucine Motif in the Carboxyl Terminus. J Biol Chem. 2012;287:42867–42880. doi: 10.1074/jbc.M112.407072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gracia E, Farré D, Cortés A, Ferrer-Costa C, Orozco M, Mallol J, Lluís C, Canela EI, McCormick PJ, Franco R, Fanelli F, Casadó V. The catalytic site structural gate of adenosine deaminase allosterically modulates ligand binding to adenosine receptors. FASEB J. 2013;27:1048–1061. doi: 10.1096/fj.12-212621. [DOI] [PubMed] [Google Scholar]
- 45.Filipeanu CM, Henning RH, de Zeeuw D, Nelemans A. Intracellular Angiotensin II and cell growth of vascular smooth muscle cells. Br J Pharmacol. 2001;132:1590–1596. doi: 10.1038/sj.bjp.0703984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sriramula S, Cardinale JP, Lazartigues E, Francis J. ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res. 2011;92:401–408. doi: 10.1093/cvr/cvr242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng H, Liu X, Patel KP. Angiotensin-Converting Enzyme 2 Overexpression Improves Central Nitric Oxide Mediated Sympathetic Outflow in Chronic Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2011 doi: 10.1152/ajpheart.00330.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.