Abstract
Objective. Weight loss is the most effective approach to reducing diabetes risk. It is a research priority to identify factors that may enhance weight loss success, particularly among those at risk for diabetes. This analysis explored the relationships between self-efficacy, weight loss, and dietary fat intake among adults at risk for developing type 2 diabetes.
Methods. This pilot, site-randomized trial was designed to compare group-based Diabetes Prevention Program lifestyle intervention delivery by YMCA staff to brief counseling alone (control) in 92 adults at risk for diabetes (BMI ≥ 24 kg/m2, ≥ 2 diabetes risk factors, and a random capillary blood glucose of 110–199 mg/dl). Self-efficacy was measured using the Weight Efficacy Lifestyle questionnaire. Data were collected at baseline, 6 months, and 12 months. A paired t test was used to determine within-group changes in self-efficacy and weight at 6 and 12 months. Using a fitted model, we estimated how much of an increase in self-efficacy was related to a 5% weight reduction at 6 and 12 months.
Results. Self-efficacy was associated with a 5% reduction in baseline weight at 6 and 12 months but was not related to fat intake.
Conclusion. These findings suggest that it is important to assess the level of self-efficacy when counseling adults at high risk for diabetes about weight loss. Certain aspects of self-efficacy seem to play a greater role, depending on the stage of weight loss.
An estimated 79 million American adults have prediabetes, a condition placing them at a higher-than-normal risk of progression to type 2 diabetes and adverse cardiovascular events.1,2 It is also estimated that, by 2050, nearly 50 million Americans will have diabetes.3 However, this growing epidemic can be halted; the U.S. Diabetes Prevention Program (DPP)4 demonstrated that weight loss prevents or at least delays the onset of type 2 diabetes; for every kilogram of weight loss, there was a 16% risk reduction in the development of diabetes among DPP lifestyle intervention participants.
The impact of the DPP continues as new research builds on the study’s findings and seeks to translate its successful lifestyle intervention into more accessible community-based programs.5,6 However, to ensure the likelihood that translation of the DPP lifestyle intervention will be successful in real-world settings, researchers need a better understanding of the mechanisms of how such interventions work and how best to ensure weight loss success.7,8 Recently, examination of the ability of psychological and behavioral characteristics to explain weight loss among DPP lifestyle participants demonstrated that improvement in dietary restraint skills, also referred to as weight loss self-efficacy, was one of the most important modifiable, independent correlates of weight loss success.9 Authors concluded that exposure to the DPP core curriculum resulted in significant improvements in weight loss self-efficacy and that these improvements, in turn, were related to both short- and long-term weight loss. Understanding the role of these factors in weight loss will facilitate translation efforts and help to identify those most likely to succeed in this type of lifestyle intervention.9
Therefore, the purpose of this secondary analysis was to examine the relationship between weight loss self-efficacy and weight change among adults at high risk for diabetes who participated in the Diabetes Education & Prevention with a Lifestyle Intervention Offered at the YMCA (DEPLOY) study.10,11 This community-based adaptation of the DPP core curriculum was highly successful in promoting weight loss among adults at high risk for diabetes,10 but data on the relationship between self-efficacy and weight loss have only recently become available. Therefore, on the basis of recent DPP findings9 and previous research,12–16 we hypothesized that participants with stronger weight loss self-efficacy would experience greater weight loss at 6 and 12 months after baseline.
Study Methods
The DEPLOY design and methods have been described.9,10,17 Briefly, two YMCA facilities with similar racial and socioeconomic characteristics located in urban Indianapolis, Ind., served as the study sites; one YMCA was randomly assigned to receive training and support for delivering the adapted lifestyle intervention, and the other YMCA provided only information about existing YMCA wellness programs (control).
To identify participants, a letter describing risk factors for type 2 diabetes and the role of a healthy lifestyle in diabetes prevention was mailed to 7,500 randomly selected households within a 5-mile radius of the two YMCA facilities. Individuals with risk factors were invited to attend diabetes screening events offered at the two locations.
Study participants were allocated to the intervention or control condition depending on the location of the YMCA at which they were screened (i.e., group allocation). Diabetes risk was defined as a BMI ≥ 25 kg/m2, an American Diabetes Association risk score ≥ 10, and an abnormal whole-blood glucose concentration determined by a fingerstick (110–199 mg/dl or 100–199 mg/dl if fasting for < 9 hours).
Study participants
Of the 535 adults who attended the screening events, 131 were found to be at risk for developing diabetes. Ninety-two of these individuals met study eligibility criteria (i.e., no previous diagnosis of diabetes, not currently pregnant, and free of any comorbidity expected to limit life-span to < 3 years or contraindicate participation in light/moderate physical activity) and provided informed consent. The Indiana University Purdue University Indianapolis institutional review board approved the study.
Overview of protocol
All participants received personalized advice from a research team member about their diabetes risk and were advised that modest weight loss (5–7% of baseline body weight) via caloric restriction and the gradual adoption of moderate-intensity physical activity were generally safe and effective ways to lower diabetes risk. This brief advice typically lasted 2–5 minutes.
As previously reported,9,17 the adapted lifestyle intervention used in the DEPLOY study consisted of 16 classroom-style sessions that were scripted and directly followed the DPP lifestyle intervention core curriculum,18 with two important changes: 1) the intervention was delivered to groups of individuals (typically 8–12 adults) rather than on an individual basis, and 2) program content was delivered by lay educators (YMCA staff) rather than by health care professionals. To ensure that the adapted lifestyle intervention was delivered in a consistent manner and with fidelity to the DPP lifestyle intervention, YMCA instructors were required to complete a formal training program and certification process before program implementation.
Program goals included achieving a 5–7% reduction in baseline weight and performing 150 minutes of moderate exercise per week. Participation in the lifestyle intervention was highly encouraged but was not required for study eligibility. Consistent with the DPP,18 the intervention was based on social cognitive theory19 and was designed to enhance self-efficacy and problem-solving skills to increase the likelihood for successful lifestyle change.
Study measures
Participants were asked to complete self-administered questionnaires, and trained research assistants collected objective measures, including body weight, height, and blood glucose level at baseline and again at 6- and 12-month follow-ups. The Weight Efficacy Lifestyle (WEL) questionnaire20 measured weight loss self-efficacy (confidence in one’s ability to control weight by resisting overeating in certain tempting situations). Higher scores reflect stronger weight loss self-efficacy. The WEL questionnaire consists of five subscales: Negative Emotions (resist eating when feeling sad or anxious), Availability (resist eating when food is readily available), Social Pressure (resist eating when others are encouraging eating), Physical Discomfort (resist eating when fatigued or in pain), and Positive Activities (resist eating when watching television or reading). Item responses range from 0 (not confident) to 9 (very confident), with subscale and total scores calculated as the sum of individual item responses (subscale scores range from 0 to 36, and total score ranges from 0 to 180). Cronbach’s alpha was 0.95 for the current sample.
Body weight was measured using a calibrated, beam-balanced scale with participants wearing light clothing and no shoes. Height was measured using a wall-mounted stadiometer. A1C was assessed from a fingerstick capillary blood sample using a DCA 2000 analyzer (Siemens Healthcare, Malvern, Pa.).21,22 Change in percentage of fat consumption was determined using the 16-item National Health Interview Survey Multifactor Screener developed by the National Cancer Institute.23
Statistical analysis
Baseline characteristics and self-efficacy scores between intervention and control groups were compared using χ2 or Fisher’s exact tests for categorical outcomes and Student’s t test or two-sample Wilcoxon rank sum tests for continuous outcomes. A mixed-effects model for repeated measures was used to determine within- and between-group differences in mean changes at 6 and 12 months. The outcomes were change scores calculated using 6- or 12-month scores minus baseline scores of self-efficacy and weight loss. Analysis of covariance was used to examine the relationship between the change in self-efficacy scores at 6 and 12 months in separate models, as well as the outcome of weight loss, with intervention group and baseline value of fat intake as covariates.
To estimate how much of an increase in self-efficacy was related to a 5% weight reduction at 6 and 12 months, we used a fitted model that included self-efficacy and intervention group. Intervention group was the only other significant predictor for weight loss. Because there was a between-group difference in the percentage of participants who were male and African American, sensitivity analyses were performed in which sex and race (separate model) were added as covariates to the models. Adding these covariates did not significantly alter findings, so only the results of models adjusted for intervention group and baseline values of weight and fat intake are presented. Additionally, because an intent-to-treat methodology was followed, all analyses included all participants without missing data, regardless of the number of intervention sessions attended. We did not impute missing data because of similar dropout rates at 6 and 12 months between the two groups and similar baseline characteristics between those who did and did not drop out. Analyses were performed using SAS version 9.1 (SAS, Cary, N.C.).
Study Results
Key baseline characteristics of study participants (n = 92) are summarized in Table 1. About 50% of participants were employed, and 42% reported an annual income < $50,000. Control participants were more often female (61 vs. 50%) and African American (29 vs. 7%); no other significant differences were found between the groups. Thirty-five of the 46 participants in the intervention arm (76%) attended at least one intervention session, whereas the remaining 11 participants (24%) attended none. Of the 35 participants who attended at least one session, the mean number of sessions attended was 12 out of 16 possible sessions (75%).
Table 1.
Baseline Characteristics of DEPLOY Participants*
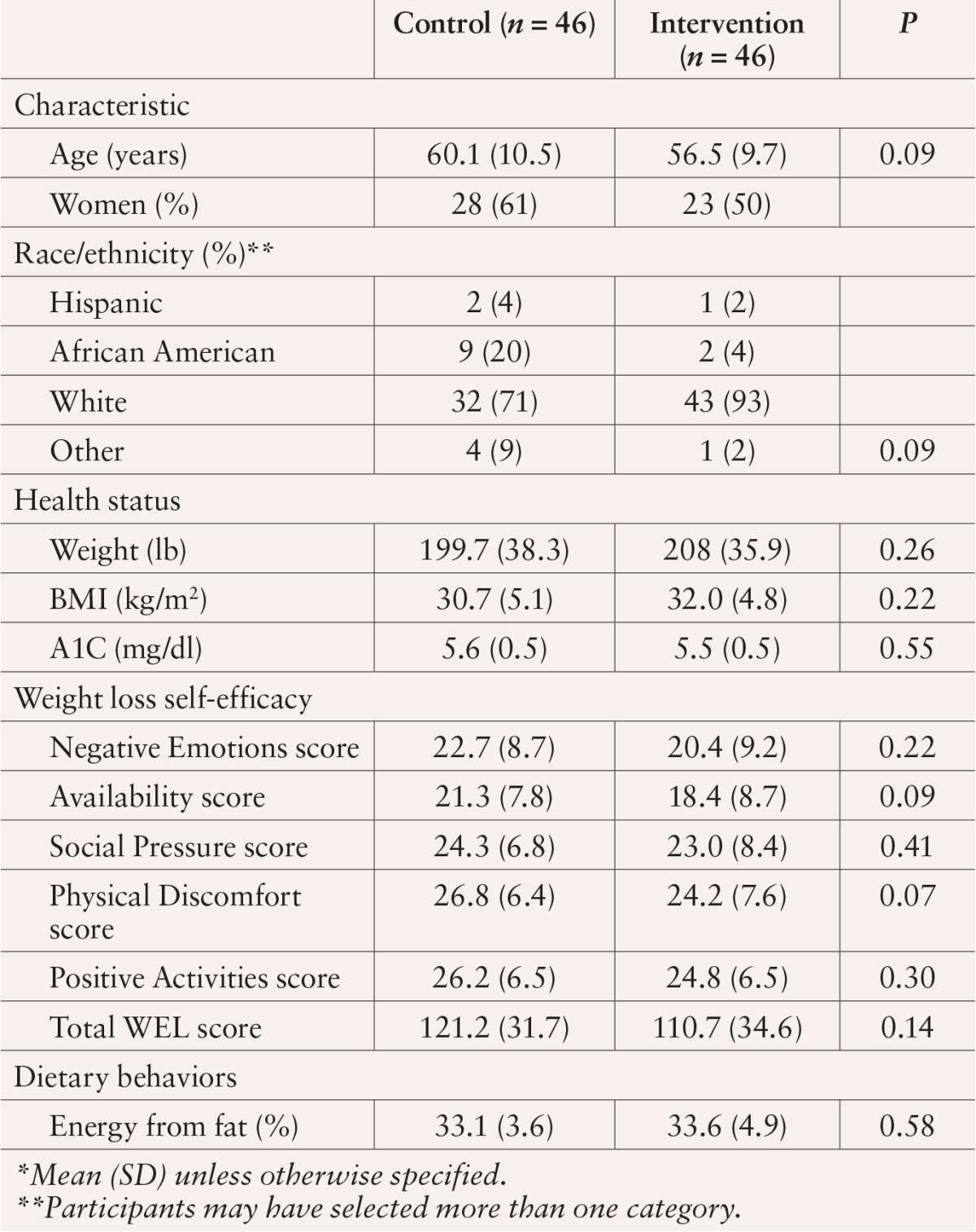
At baseline, participants reported moderately high levels of weight loss self-efficacy, indicating confidence in their ability to resist overeating in certain tempting situations. Means and standard deviations (SDs) for total WEL scores for intervention and control participants were 121.2 (SD 31.7) and 110.7 (SD 34.6), respectively, out of a possible 180 points. Average fat intake was ∼ 33% of total calories for both groups.
Weight loss and dietary fat intake results at 6 and 12 months
Eighty-five percent (n = 39) of the intervention group and 83% (n = 38) of control subjects provided 6-month follow-up data, and 65% (n = 30) of the intervention group and 72% (n = 33) of control subjects provided 12-month follow-up data. Weight loss outcomes for the study have been previously reported24 and were described above. Intervention and control participants reported a significant decrease in dietary fat intake (95% CIs 2.50–4.30 and 0.75–2.57, respectively, from baseline to 6 months). This reduction was greater among intervention participants (P < 0.01 for the difference between groups). At 12 months, however, there were no significant within- or between-group changes in fat intake.
Changes in WEL scores and their relationship to weight loss at 6 and 12 months
As shown in Table 2, from baseline to 6 months, intervention participants reported a significant within-group increase in total WEL scores (P = 0.028) and scores for the subscales Availability (P = 0.013) and Social Pressure (P = 0.005). There were, however, no significant between-group WEL score differences. At 12 months, there were no significant within- or between-group differences in WEL scores.
Table 2.
Change in Self-Efficacy Scores Among DEPLOY Participants From Baseline to 6 and 12 Months*
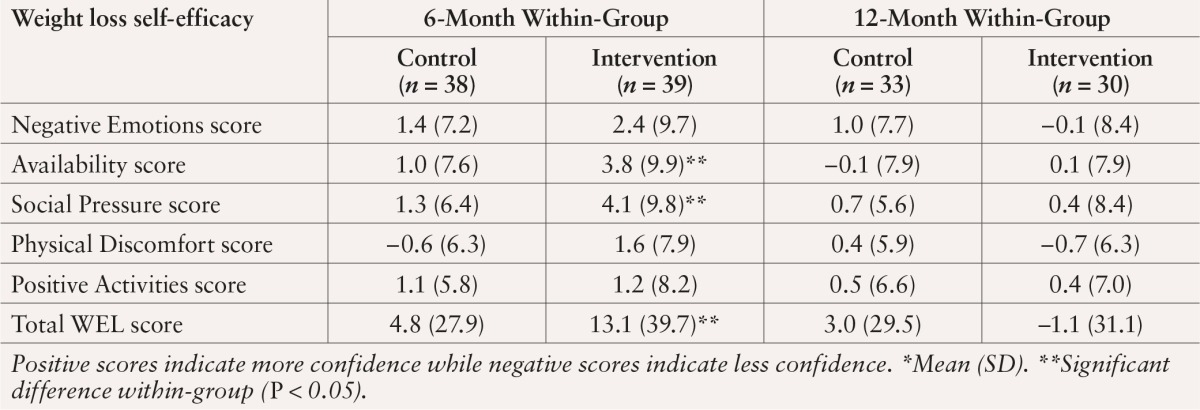
Six-month improvements in total WEL and Positive Activities subscale scores were associated with greater weight loss at 6 months (P = 0.024 and P = 0.008, respectively). At 12 months, improvements in Negative Emotions subscale scores were significantly related to weight loss (P = 0.02). To estimate how much of an increase in weight loss self-efficacy was related to a 5% weight reduction, we used a fitted model including self-efficacy and treatment group, the only additional significant predictor of weight loss. At 6 months, a 6-point increase in scores on the Positive Activities subscale and a 35-point increase in total WEL scores were associated with a 5% reduction in baseline weight. At 12 months, increases in Negative Emotions (5 points), Physical Discomfort (3.4 points), and total WEL scores (22 points) were associated with a 5% reduction in weight. Changes in weight loss self-efficacy at 6 and 12 months were not significantly related to reported dietary fat intake (data not shown).
Discussion
This study expands on previous research by examining relationships between modest increases in weight loss self-efficacy and both short- and long-term weight loss in the context of a community-based, real-world adaptation of the DPP lifestyle intervention. We demonstrated that weight loss self-efficacy is an important modifiable correlate of both short- (6 months) and long-term (12 months) weight loss. Additionally, certain aspects of self-efficacy seem to play a greater role, depending on the stage of weight loss. For example, being able to resist eating when watching television or reading was significantly related to short-term weight loss, whereas resisting the urge to eat when feeling sad, anxious, tired, or in pain was significantly related to long-term weight loss. These findings are not only theoretically important, but also clinically meaningful. A weight loss of 5–10% can reduce the risk of developing diabetes by as much as 58%.4 Importantly, previous research has shown that the degree of change in WEL scores achieved in the current study is realistic and attainable with exposure to cognitive-behavioral weight loss interventions (including the DPP).9,12–16 However, it is noteworthy that the lay educators (YMCA staff) that delivered this cost-effective, group-based adaptation of the DPP lifestyle intervention achieved similar, and in some cases even greater, improvements in confidence and weight loss when compared to more traditional, one-on-one interventions delivered by health care professionals.9,24,25 These findings have important implications for the development and implementation of effective, affordable prevention efforts that are essential to curbing the diabetes epidemic.
Consistent with previous research, we found a significant relationship between improvement in weight loss self-efficacy and weight loss 6 months after baseline.9,12–16 We also found a significant relationship between self-efficacy and 12-month weight loss, although previous attempts to link self-efficacy to long-term weight loss have resulted in conflicting reports.13,16 Variability in the type, strength, and duration of the tested lifestyle interventions may have adversely affected these relationships. The current study, however, tested these relationships within the context of a standardized lifestyle intervention with proven effectiveness.18 Consistent with the DPP core curriculum, our program focused on learning skills, including setting small, achievable goals, selecting options for reducing fat intake, self-monitoring and balancing fat gram intake, practicing stimulus control, managing stress/high-risk situations, and problem-solving.
As previously mentioned, DPP researchers examined the ability of psychosocial factors, including weight loss self-efficacy and behaviors, to predict both short- and long-term weight loss among lifestyle participants.9 Consistent with our findings, participants experienced a significant improvement in WEL scores after completing the 16-week core curriculum. Additionally, WEL scores were significantly related to weight loss at 6 months and at the end of the study, but were not independent predictors of weight loss success. Rather, changes in dietary restraint skills as measured by the Restraint subscale of the Dutch Eating Behavior Questionnaire (DEBQ)26 predicted long-term weight loss.9
Dietary restraint is defined as the intention to restrict food intake to control body weight and refers to the perception that the individual has to monitor and strain for limiting dietary intake to maintain body weight.27 It represents cognitive control of eating behavior, in contrast to physiological control such as hunger and satiety. The WEL measures individuals’ confidence in their ability to resist eating in response to five situational factors, whereas the Restraint subscale of the DEBQ is a behavioral measure that rates the frequency of using 10 different restraint behaviors such as “Do you intentionally eat food to help you lose weight?”
It seems apparent that focusing on dietary restraint skills is an important component of weight loss and may ultimately lead to a greater reduction of diabetes risk. However, additional research is needed to determine which measure more accurately captures the relationship between the concept of dietary restraint and weight loss.
We unexpectedly found a modest, although not statistically significant, increase in WEL scores among control participants. It is possible that the brief personalized advice regarding diabetes risk delivered at baseline and at the follow-up assessments was capable of increasing weight loss self-efficacy. Brief advice has been shown to encourage behavioral change such as unhealthy eating and physical inactivity.28 In a nationally representative study,28 71–82% of individuals with prediabetes who were advised to modify their lifestyles actually attempted to make changes. Additional research is needed to explore the ability of brief lifestyle counseling to strengthen self-efficacy and ideally potentiate weight loss.
Furthermore, this article is important because it demonstrates that a likely reason for the weight loss success seen in the DEPLOY study was the reduction in dietary fat intake. At 6 months, participants reported a significant decrease in dietary fat intake. Interestingly, we did not find a significant relationship between weight loss self-efficacy and fat intake. Assessing either a greater variety of dietary self-regulation behaviors or using a more specific measure of self-efficacy, such as confidence in one’s ability to follow a low-fat diet, may have resulted in stronger relationships.
Strengths and limitations
The results of this study provide information that can contribute to the weight loss literature, but certain limitations must be acknowledged. Pilot studies such as DEPLOY provide excellent opportunities to field-test interventions and guide intervention refinements before conducting more expensive, full-scale, randomized trials.29 Similar to most pilot studies, however, the validity of our results can be questioned because of the low response rate and the percentage of participants lost to follow-up. Additionally, we did not control for any additional determinants of weight loss, focusing solely on the effects of weight loss self-efficacy on weight loss. Thus, we did not examine the potential influence of other aspects of self-efficacy, such as stress management or exercise self-efficacy. Furthermore, we acknowledge that we were unable to present valid data on the contribution of physical activity to the weight loss that occurred. Finally, our small sample size prevented the performance of sophisticated statistical modeling, such as testing for interactions, subgroup analyses, or examination of a dose effect.
Future steps
Clinical efficacy trials such as the DPP have examined relationships between self-efficacy and weight loss under idealized, albeit unrealistic, circumstances.9 Exploring these relationships within DEPLOY, a real-world adaptation of the DPP core curriculum, revealed that self-efficacy changed in response to realistic levels of exposure to a lifestyle intervention and that these changes helped to explain weight loss success. Additional research is needed to determine whether certain aspects of weight loss self-efficacy should be tailored to individuals’ stage of weight loss. Furthermore, there is currently no clear formula for strengthening weight loss self-efficacy. Research is needed to identify the most effective types of experiences, as well as the format, intensity, and duration of weight loss interventions, needed to enhance weight loss self-efficacy.
Acknowledgments
This work was supported by grants awarded to Dr. Hays (Indiana University School of Nursing T32 Postdoctoral Training Grant and Indiana CTSI Young Investigator Award, PHS [NCCR], KL2RR025760) and Dr. Ackermann (National Institute of Diabetes and Digestive and Kidney Diseases [R34 DK70702-S1]). Additionally, the authors thank Phyllis Dexter of the Indiana University School of Nursing in Indianapolis for editorial support and Ashantí Harris and Alexandra Petrakos, both of Northwestern University School of Medicine in Chicago, Ill., for assistance in preparing this article.
References
- 1.Centers for Disease Control and Prevention: National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. Atlanta, Ga, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011 [Google Scholar]
- 2.Dall TM, Roary M, Yang W, Zhang S, Chen YJ, Arday DR, Sahai N, Sorn P, Jain A: Health care use and costs for participants in a diabetes disease management program, United States, 2007–2008. Prev Chronic Dis 8:A53, 2011 [PMC free article] [PubMed] [Google Scholar]
- 3.Narayan KM, Boyle JP, Geiss LS, Thompson TJ: Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care 29:2114–2116, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garfield SA, Malozowski S, Chin MH, Narayan KM, Glasgow RE, Green LW, Hiss RG, Krumholz HM: Considerations for diabetes translational research in real-world settings. Diabetes Care 26:2670–2674, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J: Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29:2102–2107, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baranowski T, Anderson C, Carmack C: Mediating variable framework in physical activity interventions. How are we doing? How might we do better? Am J Prev Med 15:266–297, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Jeffery RW: How can health behavior theory be made more useful for intervention research? Int J Behav Nutr Phys Act 1:10–14, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delahanty LM, Peyrot M, Shrader PJ, Williamson DA, Meigs JB, Nathan DM: Pretreatment psychological, and behavioral predictors of weight outcomes among lifestyle intervention participants in the Diabetes Prevention Program (DPP). Diabetes Care 36:34–40, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG: Translating the Diabetes Prevention Program into the community: the DEPLOY Pilot Study. Am J Prev Med 35:357–363, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ackermann RT, Finch EA, Caffrey HM, Lipscomb ER, Hays LM, Saha C: Long-term effects of a community-based lifestyle intervention to prevent type 2 diabetes: the DEPLOY extension pilot study. Chronic Illn 7:279–290, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Lindquist RA, Kurth CL, Johnson SL: Correlates of weight loss and its maintenance over two years of follow-up among middle-aged men. Prev Med 13:155–168, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Linde JA, Rothman AJ, Baldwin AS, Jeffery RW: The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol 25:282–291, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Rejeski WJ, Mihalko SL, Ambrosius WT, Bearon LB, McClelland JW: Weight loss and self-regulatory eating efficacy in older adults: the cooperative lifestyle intervention program. J Gerontol B Psychol Sci Soc Sci 66:279–286, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin H, Shin J, Liu PY, Dutton GR, Abood DA, Ilich JZ: Self-efficacy improves weight loss in overweight/obese postmenopausal women during a 6-month weight loss intervention. Nutr Res 31:822–828, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Warziski MT, Sereika SM, Styn MA, Music E, Burke LE: Changes in self-efficacy and dietary adherence: the impact on weight loss in the PREFER study. J Behav Med 31:81–92, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ackermann RT, Marrero DG: Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Educ 33:69, 74–75, 77–78, 2007 [DOI] [PubMed] [Google Scholar]
- 18.DPP Research Group: The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 25:2165–2171, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bandura A: Self-Efficacy: The Exercise of Control. New York, W.H. Freeman, 1997 [Google Scholar]
- 20.Clark MM, Abrams DB, Niaura RS, Eaton CA, Rossi JS: Self-efficacy in weight management. J Consult Clin Psychol 59:739–744, 1991 [DOI] [PubMed] [Google Scholar]
- 21.John WG, Edwards R, Price CP: Laboratory evaluation of the DCA 2000 clinic HbA1c immunoassay analyser. Ann Clin Biochem 31:367–370, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Marrero DG, Vandagriff JL, Gibson R, Fineberg SE, Fineberg NS, Hiar CE, Crowley LE: Immediate HbA1c results: performance of new HbA1c system in pediatric outpatient population. Diabetes Care 15:1045–1049, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Thompson FE, Midthune D, Subar AF, McNeel T, Berrigan D, Kipnis V: Dietary intake estimates in the National Health Interview Survey, 2000: methodology, results, and interpretation. J Am Diet Assoc 105:352–363, quiz 487, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Martin PD, Dutton GR, Brantley PJ: Self-efficacy as a predictor of weight change in African-American women. Obes Res 12:646–651, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Annesi JJ, Gorjala S: Relations of self-regulation and self-efficacy for exercise and eating and BMI change: a field investigation. Biopsychosoc Med 4:4–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman BS, Allison DB: Measures of restrained eating. In Handbook of Assessment for Eating Behaviors and Weight-Related Problems. Allison DB, Ed. Thousand Oaks, Calif, Sage Publishing, 1995, p. 149–184 [Google Scholar]
- 27.Herman CP, Mack D: Restrained and unrestrained eating. J Pers 43:647–660, 1975 [DOI] [PubMed] [Google Scholar]
- 28.Lobo IE, Loeb DF, Ghushchyan V, Schauer IE, Huebschmann AG: Missed opportunities for providing low-fat dietary advice to people with diabetes. Prev Chronic Dis 9:E161, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker PT: Publishing pilot intervention studies. Res Nurs Health 31:1–3, 2008 [DOI] [PubMed] [Google Scholar]


