Abstract
Context
There are anecdotal reports of adverse events (AEs) associated with exercise in people with spinal cord injury (SCI) and consequent concern by people with SCI and their providers about potential risks of exercise. Enumeration of specific events has never been performed and the extent of risk of exercise to people with SCI is not understood.
Objective
To systematically review published evidence to identify and enumerate reports of adverse events or AEs associated with training in persons with SCI.
Methods
Review was limited to peer-reviewed studies published in English from 1970 to 2011: (1) in adults with SCI, (2) evaluating training protocols consisting of repeated sessions over at least 4 weeks to maintain or improve cardiovascular health, (3) including volitional exercise modalities and functional electrical stimulation (FES)-enhanced exercise modalities, and (4) including a specific statement about AEs. Trained reviewers initially identified a total of 145 studies. After further screening, 38 studies were included in the review. Quality of evidence was evaluated using established procedures.
Results
There were no serious AEs reported. There were no common AEs reported across most types of interventions, except for musculoskeletal AEs related to FES walking. There were few AEs in volitional exercise studies.
Conclusion
There is no evidence to suggest that cardiovascular exercise done according to guidelines and established safety precautions is harmful. To improve the strength of these conclusions, future publications should include definition of AEs, information about pre-intervention screening, and statements of the nature and extent of AEs.
Keywords: Aerobic exercise, Functional electric stimulation, Locomotor training, Gait training, Treadmill training, Ergometry, Wheelchair exercise, Spinal cord injuries, Tetraplegia, Paraplegia
Introduction
In recent decades there has been a mounting interest in exercise for health, wellness, and fitness for people with spinal cord injury (SCI). This interest has led to a great deal of research on the potential benefits of exercise interventions.1 However, the potential risks of exercise have not been adequately summarized or described. In the non-disabled population, anecdotal reports of acute cardiac events or sudden cardiac death associated with exercise may cause anxiety and a reluctance to begin exercise in would-be exercisers. Fortunately for non-disabled persons, incidence data are available to show that the actual risk of such events is very low (0.01 to 0.03/10,000 participant hours)2 and that while acute cardiac events are associated with episodic physical activity, this association is the greatest in individuals who are the least physically active on a regular basis.2–4 Similarly, people with SCI and their healthcare providers need empirical data on potential risks of exercise in order to weigh the potential benefits of exercise against the potential harms. Such data would provide a more rational basis for clinical recommendations and future research.
Thus far, what is known about exercise-related adverse events (acute cardiac events occurring during exercise) and adverse effects (injurious or undesirable effects during or consequent to exercise) (AE) and the need for cardiovascular disease screening or exercise testing prior to beginning aerobic exercise in people with SCI is based mainly on anecdotal reports. In the absence of empirical data, reviews on the topic of exercise and physical activity for people with SCI5,6 have enumerated potential risks that are theoretically consonant with known impairments associated with SCI (e.g. autonomic dysreflexia, hypotension, fractures and joint dislocation, upper extremity and shoulder pain, hyperthermia). Reviews have also included risks that are common in exercise studies across populations such as muscle soreness.5,6 A systematic review of upper body exercise in SCI in 20077 reported “minimal adverse events”, but what these events were and the number of events per number of study participants were not documented. Similarly, a more recent systematic review by Hicks et al.8 concluded that there are insufficient data to draw an evidence-based conclusion regarding the risks associated with performing exercise for people with SCI. To our knowledge, the incidence of specific exercise-related AE has never been described in the SCI population. Therefore, the risks of acute cardiovascular (CV) events and AE associated with aerobic exercise in people with SCI are not understood.
We reasoned that an estimate of the potential for exercise-related AE could be gleaned from a systematic review of published exercise intervention studies among people with SCI. Similar to studies of drugs and other interventions, exercise studies should report on the safety of participants and list any AE. This systematic review focused on reviewing studies of cardiovascular-related exercise training programs in people with SCI. Our goals were to (1) determine the types and frequencies of associated AE, (2) identify exercise modalities or patient characteristics associated with AE of cardiovascular-related training, and (3) determine the type and extent to which pre-intervention exercise testing and screening is employed in studies. Information from this review might lead to advice for people with SCI who desire to begin cardiovascular exercise, and provide counsel as to whether they require extensive screening or pre-exercise medical evaluation or testing. Furthermore, this systematic review was planned to address the question as to how clinicians should counsel patients with SCI about potential risks related to exercise.
Methods
Search criteria
Literature searches were conducted in PubMed, CINAHL, PsycINFO, and EMBASE databases using the broad search terms “spinal cord injury/ies” AND “exercise” then limited to “aerobic exercise”. We have also carried out searches using more specific terms in order to be certain that we had included all potential studies of cardiovascular exercise modalities. These included searches using the terms: “locomotor or treadmill training”; “electric stimulation therapy”; “functional electrical stimulation” or “FES”; “walking or gait training”; “cardiovascular training”; and “ergometry”. The literature search was restricted to articles published in English from 1970 to 2011. The initial search included adults with SCI (traumatic or non-traumatic and any level of SCI) and all study types such as review articles and meta-analyses. This comprehensive electronic search identified 1174 potentially relevant peer-reviewed articles. An additional 47 articles were located from reviewing bibliographies of included articles for a total of 1221.
Criteria and methods for inclusion
Once the search for published articles was complete, more specific inclusion criteria were created to find the most relevant of the 1221 articles.
Studies
We identified experimental and observational research studies where maintaining or promoting cardiovascular health (e.g. exercise programs, strength and endurance training, whole body exercise, locomotor training, FES exercise and ambulation programs, activity-based interventions, and resistance training for improving cardiovascular endurance) was either a primary or secondary outcome of the intervention or activity.
Participants
Adults with SCI of any level or etiology were included. We included studies with participants who were aged 18 years or greater. Studies with children were excluded.
Interventions
Exercise interventions identified by the SCI Rehabilitation Evidence group9 as capable of providing cardiovascular benefits were included. These were both volitional exercise modalities (arm crank exercise, wheelchair exercise, circuit training, rowing, sports training, and treadmill training) and FES-enhanced exercise modalities (FES leg cycle ergometry, hybrid FES, treadmill training and walking with FES, and electrically assisted rowing). Only studies that evaluated a specified training protocol consisting of repeated sessions over a period of at least 4 weeks were included. Studies describing single-session interventions or brief protocols were excluded.
Outcome measures
Because this review focused specifically on AE reporting, we excluded studies that lacked either a description of AEs that occurred during the study or an explicit statement that no AEs occurred in study participants, since the absence of such statements leaves uncertainty whether they occurred. We considered an AE was reported if words implying AE, training safety or complications were included in the article.
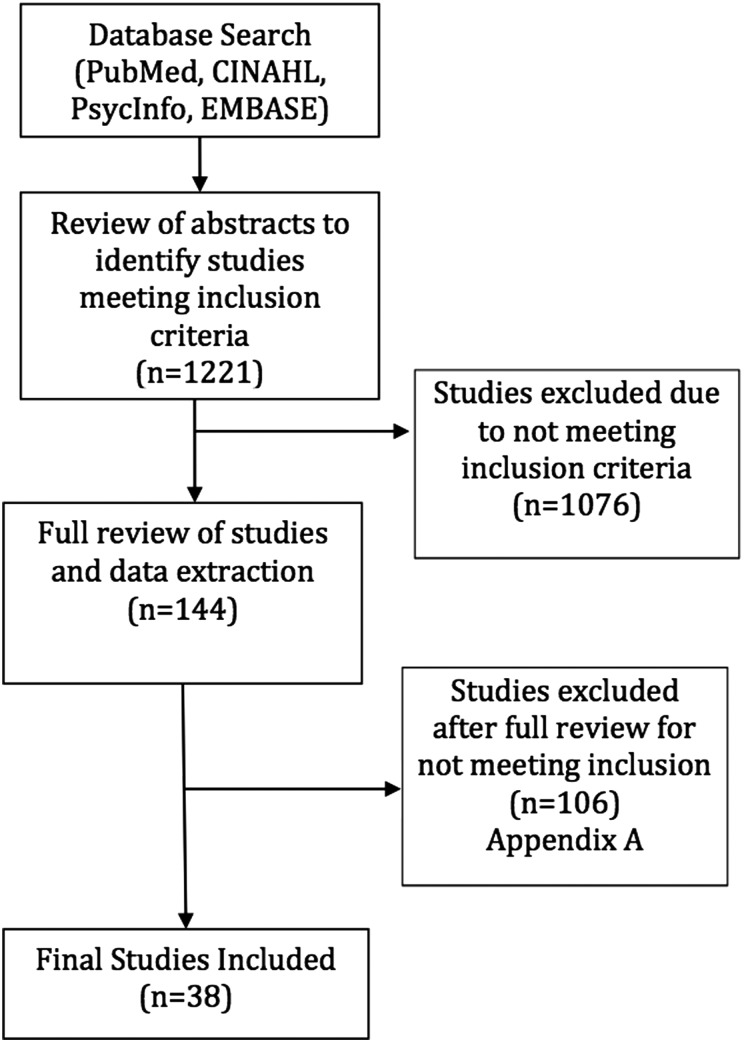
Using these criteria, abstracts from the 1221 articles were reviewed by three trained reviewers at the University of Washington Model Systems Knowledge Translation Center (MSKTC). Discrepancies were resolved by consensus of the reviewers. Author and journal names were not masked from the reviewers. If reviewers were unable to determine whether the article met the criteria from the abstract, the full article was reviewed. A total of 144 articles appeared to meet the inclusion criteria. The remaining articles were excluded from further review.
Data extraction and outcome results
Three MSKTC reviewers independently extracted data from each of the 144 articles including the research design, intervention setting, participant information, details of the interventions, outcome measures, and main outcomes, particularly the AE. Data were compiled in a custom web-based database specifically developed for systematic reviews. Differences between the three reviewers' data extraction were reconciled by consensus. During the data extraction process, articles were excluded if the detailed full review revealed that they did not meet the initial criteria and if they did not report or include a statement regarding AEs that occurred during the study. At the end of this full review 38 of the 144 articles met the final criteria for inclusion (see Fig. 1). A list of the 106 excluded articles can be obtained by request from the first author. An external expert reviewer was asked to review the methods and evidence tables. No further changes were recommended.
Figure 1 .
Study selection process.
Authors rated evidence using the American Academy of Neurology guidelines,10 and rated study methodological quality using criteria developed by Downs & Black (D&B)11 with modification as recommended by the SCIRE group.9 Maximum score on this scale is 28. Two raters rated articles independently. Any scoring discrepancies were resolved through a consensus derived through discussion.
To analyze AE reporting, we used the method suggested by Loke et al.12 Each included study was reviewed for how rigorous were the methods to detect AEs and how comprehensive was the reporting of those effects.
Results
In order to determine whether the findings of this review related to the reporting of AE were credible, the investigators rated the quality of research reports using D&B Scores.11 D&B scores for the studies included in this review range from 12 to 20, representing low-to-moderate quality. Most studies used a single group, pre–post design, and there were few randomized controlled trials. All studies identified based on the inclusion criteria for AE reporting met the minimum quality requirement using the D&B scale.
Table 1 provides details about the 38 studies included in the final review. Two studies were reported in two articles and are listed together in the table.14,15,47,48 Additionally, Ragnarsson et al.44 and Ragnarsson45 reported on two studies, the first is a report of one study and the other reporting that same study plus an additional study accounting for two reported studies. Studies were classified into one of two groups: volitional exercise (n = 19), and exercise employing functional electrical stimulation (FES) (n = 19). Volitional interventions included arm ergometry (n = 3), wheelchair ergometry (n = 3), kayak ergometry (n = 1), hand cycling (n = 2), arm-propelled 3-wheeled chair (n = 1), aerobic circuit training (n = 4), resistance circuit training (n = 1), and body weight supported treadmill training (BWSTT) (n = 4). FES interventions tested in the papers reviewed included FES lower extremity ergometry (LCE) (n = 11), FES arm ergometry and rowing (n = 2), FES with Parastep or reciprocating gait orthoses (n = 4), and FES with BWSTT (n = 2).
Table 1 .
Studies included
| Study | Study design | Intervention | Training | Location and | Participants | Lesion level | Screening or testing prior to |
|---|---|---|---|---|---|---|---|
| Downs & Black Scoring (D&B) | Protocol (session length, sessions per week, no. of weeks, intensity) | supervision | N and gender Mean age and age range (or SD) | AIS classification | training? Exclusion criteria | ||
| AAN classification | Adherence | Years post-injury | |||||
| Volitional exercise | |||||||
| Dyson-Hudson et al.13 | RCT (diet only vs. diet + exercise) D&B = 18 AAN class III | ACE | 20+ min. 3 × /week × 12 weeks, 60 rpm, 70% HRM Adherence not reported | Hospital gym, supervised | 21 males 4 females total, 10 males, 4 females in training group 42.9 ± 7.6 | C5-L2 AIS not reported 15.1 ± 8.9 years post-injury | No screening or testing. Exclusions: diabetes, CVD, cognitive impairment, “medical condition that precluded safe performance of upper limb exercise”. |
| El-Sayed and Younesian14; El-Sayed and Younesian et al.15 | Pre-post (two groups: SCI and control) | ACE | 30 min. 3 × /week × 12 weeks, 60 to 65% VO2 peak | Hospital gym, supervised | AB, n = 7; SCI, n = 5; Gender not reported | Below T-10 AIS not reported | Health history questionnaire and sub-maximal ACE monitored exercise testing pre and post |
| D&B = 14 | Adherence 100%. | SCI = 31 ± 2.9 years | Exclusions not stated | ||||
| AAN class IV | AB 32 ± 1.6 | Injury duration not reported | |||||
| McLean and Skinner16 | RCT D&B = 15 AAN class III | ACE | 20–35 min. 3 × /week × 10 weeks Adherence 100% | Laboratory, supervised | N = 15 Gender not stated Sit group 34.3± 12.1 Supine group 33.3 ± 7.0 | C5-T1 “Complete”, AIS not reported Sit group 9.3 ± 12.5 Supine group 14.1 ± 6.4 years post-injury | Approval from physician and Peak WCE exercise testing pre and post Exclusions: CVD, recurrent AD, hypotension, hypertension, use of alpha blockers, pressure sores, UTI, kidney stones, diabetes, incomplete SCI w/normal autonomic function |
| Le Foll-de Moro et al.17 | Pre–post D&B = 14 AAN class IV | WCE (interval training) | 30 min. 3 × /week × 6 weeks Adherence not reported | Hospital gym, supervised | 5 males 1 female 29 ± 14 (18–54) | T6-T12 AIS not reported 94 ± 23 days post-injury (range =73–137 days) | Maximal and submaximal WCE exercise testing pre and post Exclusions not stated |
| Bougenot et al.18 | Pre-post D&B = 14 AAN class IV | WCE (interval training) | 45 min. 3 × /week × 6 weeks Outstanding attendance (not defined) | Hospital gym, supervised | 7 males 35 ± 13 (21–55) | T6-L5 AIS A M = 12.3 (1–30) years post-injury | Maximal WCE exercise testing pre and post Exclusions not stated |
| Tordi et al.19 | Pre-post D&B = 14 AAN class IV | WCE (interval training) | 30 min. 3 × /week × 4 weeks Outstanding attendance (not defined) | Hospital gym, supervised | 5 males 27 ± 8.1 | T6-L4 AIS A “About 2 years” post-injury | Maximal WCE exercise testing pre and post, no ECG Exclusions not stated |
| Bjerkefors and Thorstensson20 | Pre-post | Kayak ergometry | 60 min. 3 × /week × 10 weeks | Clinical lab, supervised | 7 males | T3–T12 | No screening or testing |
| D&B = 18 | Adherence 100% | 3 females | AIS A, B, C | Exclusions not stated | |||
| AAN class IV | 38 ± 12 | M = 18.1 (3–26) years post-injury | |||||
| Valent et al.21 | Pre-post D&B = 18 AAN class IV | Hand cycle training | 35–45 min. 2–3 × /week × 8–12 weeks 24 sessions was goal. Adherence 19 ± 3 completed | Multiple locations (hospital gym, home setting, outdoors on track or trail) not supervised | 18 males 4 females 39 ± 12 | C5-C8 AIS A, B, C, D 10 ± 7 years post-injury | ACSM contraindications for exercise and hand cycle peak exercise test pre and post Exclusions CVD, overuse injuries of upper extremities, pressure sores, UTI, other medical conditions that did not allow performance of physical activity |
| Valent et al.22 | Controlled trial (not randomized) with matched control group D&B= 19 AAN class II | Hand cycle training | 35–45 min. 2 × /week × 9–39 weeks Adherence 87% | Hospital track, other outdoor locations. Initially supervised then no supervision | 26 males, 8 females total 13 males, 4 females in training group 46± 15 training group 45± 15 control group | 17 Paras 17 Tetras (levels not provided) AIS A/B = 22 AIS C/D = 12 5–47 weeks post-injury | Graded peak WCE test pre and post Exclusions: CVD, medical disease contraindicating exercise, serious musculoskeletal complaints |
| Mukherjee et al.23 | Pre–post D&B = 15 AAN class IV | Arm-propelled three wheeled chair | 15 min. 2 × /day × 12 weeks Adherence not reported | Outdoor setting, non-supervised | 12 males 30.5 ± 8.59 | Below T10 AIS not reported | No screening or testing Exclusions: CVD, musculoskeletal, neurological, or metabolic disorder |
| Tawashy et al.24 | Case report | Circuit training (aerobic) | 18–27 min. 3 × /week × 8 weeks | Hospital gym, supervised | 1 male | C5 | No screening or testing |
| D&B = 13 | 18/24 sessions completed | 22 years old | AIS A | Exclusions not stated | |||
| AAN class IV | 3 months post-injury | ||||||
| Duran et al.25 | Case series D&B = 20 AAN class IV | Circuit training (aerobic) | 120 min. 3 × /week × 16 weeks, THR 40–80% of max. HR Adherence 85% | Hospital gym, supervised | 12 males 1 female 26.3 ± 8.3 | T3–T12 AIS A–C M = 25 months (2 months–10 years) post-injury | ACE exercise test pre- and post-intervention Exclusions: Cardiac medications, major medical problems |
| Nash et al.26 | Pre–post D&B = 18 AAN class IV | Circuit training (resistance and aerobic) | 40–45 min. 3 ×/week × 16 weeks Adherence 94% | Hospital gym, supervised | 7 males Mn not provided (39–58) | T5-T12 AIS A, B 13.1± 6.6 years post-injury | Multi-stage graded exercise test with ECG monitoring pre and post Exclusions not stated |
| Jacobs et al.27 | Pre-post D&B = 16 AAN class IV | Circuit training (resistance) | 40–45 min 3 × /week; × 12 weeks Adherence not reported | University outpatient setting, supervised | 10 males M = 39.4± 6.0 (28–44) | T5-L1 AIS A M = 7.3± 6.0 years post-SCI (0.7–16.8) | Maximal WCE exercise test pre and post Exclusions: poor health, cardiac ischemia on ECG, shoulder joint dysfunction |
| Cooney and Walker28 | Pre–post D&B = 16 AAN class IV | Hydraulic resistance exercise (timed sets of resistance exercises w/ brief rest periods) | 30–40 min. 3 × /week × 9 weeks, 60–90% of HRM Adherence 100% | Hospital gym, supervised | 7 males, 3 females M = 28.8 (20–39) | C 5-L1 AIS not reported 2–9 years post-injury (M = 4.6 years) | ACE exercise test pre and post “Healthy”, exclusions not stated |
| Forrest et al.29 | Case report D&B = 17 AAN class IV | BWSTT | 15–25 min 3 × /week × 30 weeks 97 sessions completed | Therapy clinic; supervised, assisted | 1 male 25 | C6 AIS B 1 year post-injury | PE pre and post Exclusions: bone mineral density t-score <− 2.5 (osteoporosis) |
| Ditor et al.30 | Pre-post D&B = 15 AAN class IV | BWSTT | Up to 60 min 3 × /week × 6 months Adherence 83.6%± 9.1 | University based center, supervised, assisted | 6 males 2 females 27.6 ± 5.2 | C4-C5 AIS B = 1 AIS C = 7 9.6 ± 7.5 yrs post-injury | ECG pre and post Exclusions: CVD, musculoskeletal condition that would contraindicate exercise |
| Ditor et al.31 | Pre-post D&B = 18 AAN class IV | BWSTT | 15 min 3 × / week × 12 weeks Adherence 83.3 ± 7.6% | University based center, supervised, assisted | 4 males, 2 females (+4 dropouts, no gender information) 37.7 ± 15.4 | C4-T12 AIS A or B 7.6± 9.4 years post-injury | No screening or testing Exclusions: CVD, musculoskeletal condition that would preclude exercise |
| Protas et al.32 | Pre–post D&B = 12 AAN class IV | BWSTT | 60 min. 5 × /week × 12 weeks (Treadmill walking 20 minutes) Adherence not reported | Hospital supervised and assisted | 3 males M = 42.7 (34–48) | T8-T12 AIS C = 1 AIS D = 2 2–13 years post injury | No screening or testing Exclusions: lower extremity contracture, pressure ulcers |
| FES exercise | |||||||
| Needham-Shropshire et al. 33 | RCT with treatment control (3 groups) D&B = 15 AAN class IIII | FES-ACE | 32 min 3 × /week interval training Group 1= 8 weeks FES ACE Group 2 = 4 weeks FES ACE and 4 weeks non-FES ACE Group 3 = 8 weeks non-FES ACE Adherence not reported | Lab, supervised | N = 34 Group 1 = 11 males, 1 female Group 2 = 10 males, 1 female Group 3 = 10 males, 1 female M years group 1 = 24; group 2 = 22; group 3 = 24 | Cervical level injuries AIS not reported Group 1 = 6 years, group 2 = 9 years, group 3 = 4 years post-injury | No screening or testing Exclusions: biceps/triceps LMN dysfunction, shoulder or elbow contractures, shoulder joint subluxation, intolerance to surface FES |
| Wheeler et al.34 | Pre–post D&B = 13 AAN class IV | FES-rowing ergometry | 30 min 3 × /week × 12 weeks 70–75% of pretest peak O2 21–36 sessions completed | University based recreational activity facility; supervised | N = 6 (gender not reported) 42.5 ±17.9 (26–66) | C7-T12 ASIA A & C 13.8 ± 11.6 years post-injury | FES-row peak exercise test pre-participation Exclusions not stated |
| Duffell et al. 35 | Case series D&B = 14 AAN class IV | FES-LCE | Up to 1 hour 5 × s/week ×1 yr M completed sessions = 4.5/week | 3 research settings and 1 hospital supervised for initial sessions, then home w/o supervision | 9 males 2 females 41.8 ± 2.3 yrs | T3-T9 “Complete” 10.7± 2 yrs post injury | No screening or testing Exclusions: LMN injury Spasticity precluding pedaling Medical or psychiatric conditions Previous FES exercise |
| Frotzler et al. 36 | Prospective longitudinal cohort D&B = 18 AAN class IV | FES-LCE | 14 ± 7 weeks FES conditioning then FES cycling 10–60 mins, 3–4 × /week; then 60 mins, 5 × /week × 12 months at highest power output Adherence 76.6% | Home; not supervised; training diary only | 9 males 2 females 41.9 ± 7.5 yrs | T3-T12 AIS A 11.0 ± 7.1 years post injury | No screening or testing Exclusions: Severe spasticity Unhealed bone fxs Diseases known to affect metabolism LE contractures Previous FES exercise participation |
| Janssen and Pringle37 | Pre–post D&B = 16 AAN class IV | FES-LCE | Up to 25–30 min 2–3 × /week × 6 weeks for total =18 sessions Adherence not reported | Lab, supervised | 12 males 36 ± 16 | C4-T11 9 “motor complete”, 3 “motor incomplete” 11± 9 years post-injury | 2 Graded LCE exercise rests pre and post, screening for exercise contraindications Exclusions: Spasticity Heterotopic ossification Pressure sores Severe cardiopulmonary disease |
| Zbogar et al.38 | Pre–post D&B = 15 AAN class IV | FES-LCE | Habituation period (30 min 3 × week × 16 weeks prior to training) then 60 min 3 × /week × 12 weeks M sessions completed =29 | Rehab center, supervised | N = 4 females + N = 2 dropouts, gender not stated M = 32 (19–51) | C4-T7 AIS A-C 3–16 years post-injury | No screening or testing Exclusions: CVD Other neuro conditions Pressure ulcers Previous fragility fxs Abnormal bone formation Severe spasticity Lower extremity contractures |
| Hjeltnes et al.39 | Pre–post D&B = 13 AAN class IV | FES-LCE | 2 wk run in followed by 30 min sessions, 7 × / week × 8 weeks | Inpatients, hospital-based, supervised | N = 6 males 35 ± 3 | C5-C7 AIS A or B 10.2 ± 3.4 years post-injury | PE including x-rays, no testing pre-trial Exclusions: Osteoporosis Fxs |
| Mutton et al.40 | Pre–post D&B = 14 AAN class IV | FES-LCE | Progressive protocol, 30 min 2 × /week. Phase 1: up to 30 sessions Phase II – ∼35 sessions phase III ∼41 sessions (24–128 sessions completed) | Outpatient rehab setting, supervised | N = 11 males (phase I and II); N = 8/11 (phase III) 35.6 ±6.6 (25–46) | C5-L1 AIS A 9.7 ± 3.8 yrs post-injury | Peak and sub-maximal ACE exercise test, PE, blood chemistry, UA, chest and lower limb x-rays, 12-lead ECG pre & post Exclusions: CVD Metabolic disease Previous aerobic training |
| Mohr et al.41 | Pre–post D&B =17 AAN class IV | FES-LCE | 30 min 2–3× week × 1 year M = 2.3 sessions/week completed, adherence 75% | Research center, supervised | 8 males 2 females M = 35.3, (27–45) | C6 (6) T4 (4) AIS not specified M = 12.5 (3–23) years post injury | VO2 Max test after acclimation Exclusions: Diseases or disabilities other than SCI Previous training |
| Barstow et al.42 | Pre–post D&B = 15 AAN class IV | FES-LCE | 30 min 3 × .week × at least 24 sessions M = 2.1(.04) sessions/week completed | VA Hospital, supervised | 9 males 34.4 ±5.6 | C5-T12 AIS A 10.1± 4.1 years post-injury | PE, x-ray of spine and legs, CT legs, blood chemistry, UA, ACE ECG stress test pre–post Exclusions: Not stated |
| Hooker et al.43 | Pre–post D&B = 13 AAN class IV | FES-LCE | 30 min. 2 × /week, × 19 weeks M = 2.3 sessions/wk completed | VA Hospital Supervised | 8 males 36.0 ± 4.6 | C5–6 – T12-L1 Frankel A 9.8 ± 4.0 years post-injury | PE, blood chemistry, UA, chest, spine, and LE x-rays, 12 lead ECG, and ACE stress test with ECG monitor Exclusions not stated |
| Ragnarsson et al.44,45 | Pre–post D&B= 13 AAN class IV | FES-LCE | 12 sessions of quad strengthening (3 × /week × 4 weeks) + 36 sessions of LCE (3/week × 12 weeks). Adherence not reported | Hospital-based, supervised | 16 males, 3 females (study 1) 7 males, 4 females (study 2) M not stated (18–54) | C4-T10 11 paras 19 tetras Frankel A 0.6–17 years post-injury | LE x-rays pre Exclusions: Previous FES Abnormal LE x-ray |
| Brissot et al.46 | Pre–post D&B= 10 AAN class IV | FES-ambulation (parastep) | 20–40 min. one to two sessions/day × 4 to 12 weeks or longer M sessions = 20. | Hospital gym, supervised | 11 males, 4 females 28 ± 9 (16–47) | T3-T11, Frankel A–C M = 4.5 years post-injury (0.5–20 years) | Peak ACE exercise testing pre and post Exclusions: CVD Respiratory conditions morbid obesity Severe spasticity LE contracture Hx of Fx Severe scoliosis Skin problem at electrode site |
| Klose et al.47; Needham-Shropshire et al.48 | Pre–post D&B = 15 AAN class IV | FES-ambulation (parastep) | Incrementally increasing distances, 3 × /week × 32 sessions Adherence 100% | Therapy clinic, supervised | 13 males, 3 females 28.4± 6.6 | T4–T11 AIS A 4.0± 3.5 years post-injury | Peak ACE exercise testing pre and post. Resting ECG and PE before trial Exclusions: CVD Hx of Fxs Hx of DJD LMN injury LE contractures Severe spasticity Skin breakdown |
| Gallien et al.49 | Case series D&B = 11 AAN class IV | FES-ambulation (parastep) | 2 hours 3–5 × /week × up to 32 sessions (goal) 5–49 sessions achieved, M = 19 sessions | FES clinic, supervised | 11 males, 2 females 27± 7 (17–42) | T4-T10 AIS A 0.5–12 years post-injury | No screening or testing Exclusions: CVD Pulmonary disease LMN injury Epilepsy Skin breakdown near electrode sites |
| Field-Fote50 | Pre–post D&B = 16 AAN class IV | FES-ambulation (BWSTT) | 90 min 3 × /week × 12 weeks (36 sessions) Adherence not reported | Lab, supervised | 13 males, 6 females 31.7 ± 9.4 years | 13 tetra 6 paras AIS C 1–14.25 years post-injury | No screening or testing Exclusions not stated |
| Ferro et al.51 | Descriptive; longitudinal D&B = 10 AAN class IV | FES ambulation-(BWSTT) | 20 min 2 × /week × 6 months Adherence not reported | Lab, supervised | N = 9 Gender not specified M = 33.2 (25–46) | C4-C7 AIS A, B, D 1–10 years post-injury | No screening or testing Exclusions: Cardiac disease LMN injury Known knee injury |
| Thoumie et al.52 | Case series D&B = 12 AAN class IV | FES-ambulation (RGO) | Incrementally increasing distances, duration and frequency not specified, × 2–5 months for inpatients, 3–14 months for outpatients 21/23 completed entire program | Therapy clinic (inpatient or outpatient), supervised | 23 males, 3 females M = 31 (20–53) | Thoracic level except 1 with C8 AIS A M = 2.7, 1–12 years post-injury | No screening or testing Exclusions: LMN injury LE contracture |
AAN, American Academy of Neurology; ACE, arm cycle ergometry; AB, able-bodied; ACSM, American College of Sports Medicine; AD, autonomic dysreflexia; AIS, American Spinal Injuries Association impairment scale; BWSTT, Body weight supported treadmill training; C, cervical; CT, computed tomography; CVD, cardiovascular disease; D&B, Downs and Black scale score; DJD, degenerative joint disease, ECG, electrocardiogram; FES, functional electrical stimulation; fx: fracture; HRM, heart rate maximum; Hx, history; L, lumbar; LCE, leg cycle ergometry; LE, lower extremities; LMN, lower motor neuron; min, minutes; M, mean; MOS, months; PE, physical examination; RCT, randomized controlled trial; RGO, reciprocating gait orthoses; RPM, rotations per minute; SCI, spinal cord injury; SD, standard deviation; T, thoracic; UA, urinalysis; UTI, urinary tract infection; VO2 Max, maximal oxygen uptake; W/, with; W/O, without; WCE, wheelchair ergometry; Wk, week; Yrs, years
Information on pre-exercise cardiovascular screening or testing of potential study participants prior to the intervention is also presented in Table 1. Approximately 32% of the volitional studies and 42% of the FES studies did not report including any screening prior to enrollment (volitional n = 6, FES n = 8). Participant screening information when reported included exercise testing of some form, physical examination which sometimes included X-ray or electrocardiogram (ECG), or ECG alone. Slightly more than half of the volitional studies (58%) and the majority (78%) of the FES studies reported participant exclusion criteria. Exclusion criteria included musculoskeletal, cardiac, cardiorespiratory, skin, metabolic, and autonomic considerations.
The number of participants dropped from the study across both the volitional and FES studies was 15 of 176 (8%) and 12 of 252 (5%), respectively. The reasons for dropouts are found in Tables 2 and 3, in column 5. Two of 15 people who dropped out of volitional exercise studies did so due to study-related AE and 5 of 12 dropouts in FES studies were also due to study-related AE. The most common reason for dropping out across studies was health complications related to SCI.
Table 2 .
Adverse events reported in volitional exercise studies
| Study | Intervention | Adverse event definition provided | Method of adverse event data collection | Number excluded/total sample | Categories of adverse events reported | Specific adverse events (N of individuals) | All important serious adverse events reported and defined | Numerical data reported by group? |
|---|---|---|---|---|---|---|---|---|
| Reason | ||||||||
| Dyson-Hudson et al. 13 | ACE | Yes. Overuse injuries related to training that cause shoulder pain | Systematic survey using WUSPI | 0/14 | MSK | None | Unknown | Yes |
| El-Sayed and Younesian14; El-Sayed et al.15 | ACE | No | Prospective monitoring | 0/5 | All | None | Yes | Yes |
| McLean and Skinner16 | ACE | No | Not reported | 1/15 | All | AD (1) | Unknown | Yes |
| Fracture of metacarpal, unknown if related to training | metacarpal fracture (1) | |||||||
| Note: same person | ||||||||
| Le Foll-de Moro et al.17 | WCE (interval training) | No | Not reported | 0/6 | All | None | Unknown | Not applicable |
| Bougenot et al.18 | WCE (interval training) | No | Not reported | 0/7 | All | None | Unknown | Not applicable |
| Tordi et al.19 | WCE (interval training) | No | Not reported | 0/5 | All | None | Unknown | Not applicable |
| Bjerkefors and Thorstensson20 | Kayak ergometry | No | Not reported | 0/10 | MSK | None | Reported “no other problems” not defined | Not applicable |
| Valent et al.21 | Hand cycle training | Yes. Pain in the arms or shoulders | Questionnaire | 7/22 | MSK | Upper extremity pain (3) | Yes | Not applicable |
| Various non-training related health complications (6), transportation (1) | ||||||||
| Valent et al.22 | Hand cycle training | No | Diary | 0/17 | MSK | Transient muscle soreness (number not provided) | Yes | Yes |
| Mukherjee et al.23 | Arm-propelled three wheeled chair | Yes. Notable MSK, CV or respiratory complications, or any other adverse experience | Implies prospective monitoring, but not explicitly reported | 0/12 | All | None | Reported “none”, not defined | Not applicable |
| Tawashy et al.24 | Circuit training (aerobic) | No | Prospective monitoring | 0/1 | All | None | Yes | Not applicable |
| Duran et al.25 | Circuit training (aerobic) | No | Prospective monitoring | 0/13 | All | MSK pain (2) | Reported “none”, not defined | Not applicable |
| Transient sinus bradycardia during ACE exercise test, reverted spontaneously (1) | ||||||||
| Nash et al.26 | Circuit training (resistance and aerobic) | No | Not reported | 0/7 | Injuries | None | Unknown | Not applicable |
| Jacobs et al.27 | Circuit training (resistance) | No | Not reported | 0/10 | “Mishaps” | None | Yes | Not applicable |
| “Medical” complications | ||||||||
| Cooney et al.28 | Hydraulic resistance Training (timed sets of resistance exercises w/ brief rest periods) | No | Prospective monitoring | 0/10 | All | None | Unknown | Not applicable |
| Forrest et al.29 | BWSTT | No | Prospective monitoring | 0/1 | All | 7 episodes of AD (1) | Yes | Not applicable |
| Ditor et al.30 | BWSTT | No | Prospective monitoring assumed due to 3 trainers in constant attendance, but not explicitly reported | 0/8 | All | None | Yes | Not applicable |
| Ditor et al.31 | BWSTT | No | Prospective monitoring assumed due to 3 trainers in constant attendance, but not explicitly reported | 4/10 | All | Syncope (1) | Yes | Not applicable |
| Personal reasons (3) | Stage 1 Pressure ulcer (1) | |||||||
| Health issues unrelated to training (1) | ||||||||
| Protas et al.32 | BWSTT | Yes. Safety of the training measured by monitoring BP & HR, examination for skin irritation or joint swelling and asking about pain. | Prospective/routine monitoring | 0/3 | All | Knee pain (1) | Yes | Not applicable |
ACE, arm cycle ergometry; AD, autonomic dysreflexia; BP, blood pressure; BWSTT, body weight supported treadmill training; CV, cardiovascular; HR, heart rate; M, mean; MSK, musculoskeletal; WCE, wheelchair ergometry; WUSPI, wheelchair users shoulder pain index.
Table 3 .
Adverse events reporting in FES-enhanced exercise studies
| Study | Intervention | Adverse event definition provided | Method of adverse event data collection | Number of excluded participants and reason | Categories of adverse events reported | Adverse events (N) | All important or serious adverse events reported | Numerical data reported by intervention group? |
|---|---|---|---|---|---|---|---|---|
| Needham-Shropshire et al.33 | FES-ACE | No | Not reported | 0/34 | All | None | Yes | Yes |
| Wheeler et al.34 | FES-rowing ergometry | Yes. Safety concerns and injuries related to training | Prospective monitoring | 0/6 | Derm | None | Yes | Not applicable |
| MSK | ||||||||
| Duffell et al.35 | FES-LCE | No | Prospective monitoring | 0/11 | Derm | Skin reaction under electrodes (4) | Yes | Not applicable |
| Frotzler et al.36 | FES-LCE | No | Not reported | 1/11 Foot fracture unrelated to training | All | None | Unknown | Not applicable |
| Janssen and Pringle37 | FES-LCE | No | Prospective monitoring | 0/12 | CV | Lightheadedness in “some” subjects | Unknown | Not applicable |
| Zbogar et al.38 | FES-LCE | No | Prospective monitoring | 2/6 | CV | Mild AD symptoms (3) | Unknown | Not applicable |
| Transportation (1) | ||||||||
| “Illness” (1) | ||||||||
| Hjeltnes et al.39 | FES-LCE | No | Prospective monitoring | 1/6 Urinary tract complications | CV | None | Yes | Not applicable |
| Mutton et al.40 | FES-LCE | No | Spontaneous reporting | 0/11 | All | None | Yes | Not applicable |
| Mohr et al.41 | FES-LCE | Yes | Prospective monitoring | 0/10 | All | Small hematoma in quadriceps (1) | Yes | Not applicable |
| Post-exercise hypotension, (3) | ||||||||
| Barstow et al.42 | FES-LCE | No | Not reported | 0/9 | All | None | Unknown | Not applicable |
| Hooker et al.43 | FES-LCE | No | Prospective/routine monitoring | 0/8 | CV | None | Unknown | Not applicable |
| Ragnarsson et al.44,45 | FES-LCE | Yes. Risks of training with FES-LCE in people w/ SCI and subjective response to program | Prospective/ routine monitoring | 0/30 | All | None | Yes | Not applicable |
| Brissot et al.46 | FES-ambulation (parastep) | No | Not reported | 2/15 | Derm | Transient ankle edema (4) | Yes | Not applicable |
| Pain due to electric stimulation (1) pressure ulcer (1) | MSK | Lumbar pain (4) (3 with prior hx). | ||||||
| Pain | Four falls in 3 participants, 1 caused sacral FX (3) | |||||||
| Falls | ||||||||
| Klose et al.47 and Needham-Shropshire et al.48 | FES-Ambulation (Parastep) | No | Not reported | 0/16 | All | None | Unknown | Not applicable |
| Gallien et al.49 | FES-ambulation (Parastep) | Yes. Complications of FES walking | Prospective/routine monitoring | 1/13 Calcaneum FX | MSK | Calcaneus FX (1) | Unknown | Not applicable |
| Falls | Sacral FX (1) | |||||||
| Pain | Back pain (2) benign ankle sprain (2) | |||||||
| Field-Fote50 | FES-ambulation (BWSTT) | No | Not reported | 0/19 | All | None | Yes | Not applicable |
| Ferro et al.51 | FES – ambulation (BWSTT) | Yes. Knee injury caused by FES treadmill training | Prospective/routine monitoring | 0/9 | MSK (knee only) | Meniscal tears (2) medial condyle contusion (1) Medial collateral ligament tear (1) | No | Not applicable |
| Thoumie et al.52 | FES-ambulation (RGO) | Yes. Complications related to the training program | Prospective routine monitoring | 5/26 | All | Spontaneous tibia FX (1) | Yes | Not applicable |
| Syringomyelia (1), spontaneous FX of both legs (1) | Skin breakdown (4) (causing 2 to drop out) | |||||||
| pressure ulcers (2), | Mild orthostatic hypotension (21) | |||||||
| tibial FX during training (1) |
ACE, arm cycle ergometry; AD, autonomic dysreflexia; BP, blood pressure; BWSTT, body weight supported treadmill training; CV, cardiovascular; Derm, dermatological; FES, functional electrical stimulation; FX, fracture; HR, heart rate; Hx, history; LCE, leg cycle ergometry; MSK, musculoskeletal; RGO, reciprocating gait orthosis; WCE, wheelchair ergometry; WUSPI, wheelchair users shoulder pain index.
Details of the definition of AE, the method of collecting information related to the AE, and the types of AE that occurred in the volitional studies are presented in Table 2. Four of the 19 articles related to volitional exercise provided a definition of AE, whereas the remainder did not. Two studies included musculoskeletal issues, such as pain, joint swelling, or skin issues in their definition of an AE, and two included cardiovascular abnormalities (heart rate and blood pressure changes), or respiratory complications. One also included “or any other adverse experience”. Twelve studies reported how AE were recorded. Methods of collecting AE data included survey or questionnaire or prospective monitoring by the investigators.
There were few AEs noted in the volitional studies. Only seven studies reported any AEs. Those were fracture unrelated to training (n = 1), upper extremity pain (n = 3+), cardiovascular-related AE (n = 2), autonomic dysreflexia (n = 2; seven episodes reported in one participant), and a pressure ulcer (n = 1). One study reported “transient muscle soreness”, but did not include either a clear description or the number of participants affected by the soreness. Whether all AE were reported was clearly delineated in 12 of the 19 articles. In the remaining seven, it is not known if all important AEs were reported.
Details related to AEs in the FES studies are presented in Table 3. Similar to the volitional studies, only 4 of the 19 studies provided a definition of an AE for their study. These definitions included safety concerns and injuries related to training, complications of FES walking programs, and injury. The majority of the studies (n = 12) stated that they monitored AE with prospective routine monitoring or spontaneous reporting. Eight of the studies reported the occurrence of AE. These AE reported for the FES studies were related to skin reactions to electrodes or skin breakdown (n = 8), lightheadedness or orthostatic hypotension (n = 24), autonomic dysreflexia (n = 3), edema (n = 4), joint injury or fracture (n = 8), muscle injury (n = 1), back pain (n = 6), or falls (n = 4 in 3 participants). That all AEs were reported was clear in 10 of 19 studies, and unclear in the remainder.
AEs by training modality are shown in Table 4. The training modality for which the largest number of AEs, i.e. the greatest number of study participants for which AEs were reported, was FES walking (n = 48). The next greatest was FES LCE (n = at least 11; note: one study did not provide number of participants who reported lightheadedness). In volitional studies BWSTT was the training modality associated with the highest reporting of AE. There were no AE reported for wheelchair or kayak ergometry, FES arm or rowing ergometry, or resistance circuit training.
Table 4 .
Adverse events by training modality
| Training modality | No. of studies | No. of participants | No. of AEs | Type of AEs (n) |
|---|---|---|---|---|
| Volitional exercise | ||||
| ACE (arm cycle ergometry) | 3 | 34 | 2 | Autonomic dysreflexia (1) |
| Metacarpal fracture (1) | ||||
| WCE (wheelchair ergometry) | 3 | 18 | 0 | |
| Kayak ergometry | 1 | 10 | 0 | |
| Hand cycle training | 2 | 39 | 3 + | Upper extremity pain (3) |
| Transient muscle soreness (# not provided) | ||||
| Arm-propelled thee-wheeled chair | 1 | 12 | 0 | |
| Circuit training | 4 | 31 | 3 | Musculoskeletal pain (2) |
| Sinus bradycardia (1) | ||||
| Hydraulic resistance training | 1 | 10 | 0 | |
| BWSTT (Body weight supported treadmill training) | 4 | 22 | 4 | Autonomic dysreflexia (1) |
| Syncope (1) | ||||
| Stage 1 pressure ulcer (1) | ||||
| Knee pain (1) | ||||
| Totals | 19 | 176 | 12 | |
| Functional electrical stimulation (FES-enhanced) exercise | ||||
| FES-ACE | 1 | 34 | 0 | None |
| FES-rowing ergometry | 1 | 6 | 0 | None |
| FES-LCE (leg cycle ergometry) | 11 | 114 | 11 | Skin reaction under electrodes (4) |
| Mild AD symptoms (3) | ||||
| Small hematoma in quadriceps (1) | ||||
| Post-exercise hypotension, (3) | ||||
| FES-ambulation | 6 | 98 | 48 | Transient ankle edema (4) |
| Lumbar pain (6) | ||||
| Falls without injury (3) | ||||
| Fall with fracture (1) | ||||
| Calcaneus fracture (1) | ||||
| Sacral fracture (1) | ||||
| Tibia fracture (1) | ||||
| Benign ankle sprain (2) | ||||
| Meniscal tears (2) | ||||
| Medial condyle contusion (1) | ||||
| Medial collateral ligament tear (1) | ||||
| Skin breakdown (4) | ||||
| Mild orthostatic hypotension (21) | ||||
| Totals | 19 | 252 | 59 | |
Across all studies, there were no serious AE reported. Furthermore, there were no common AE reported across most types of interventions, except for FES walking, which did report a variety of musculoskeletal-related AE.
Discussion
Principal findings
The primary goal of this systematic review was to identify, enumerate, and describe the potential negative or AEs that may occur in people with SCI undergoing cardiovascular-related training for research purposes. Similar to Martin Ginis et al.6, we are unable to come to a clear, well-substantiated, evidence-based conclusion regarding the risks associated with cardiovascular training for people with SCI. We also agree with their statement that “when proper precautions are taken, the risks are relatively low and likely comparable with the variant risks observed in the general population.”6 Across all studies in this review, there were no serious AE reported. Furthermore, there were no common AE reported across most types of interventions, except for FES walking, which did report a variety of musculoskeletal-related AE.
We were not able to identify specific participant characteristics associated with AE. The participants in these studies represent a rather diverse group of individuals with SCI and AE monitoring was comprehensive in some studies and focused in others. Most study participants were males and most were 40 years of age or younger. People with varying levels of injury and impairment classification were included. Some AE could be expected based on the participant pool and specific exercise modality for a given study. For instance, in Valent et al.21 the intervention was hand cycle ergometry, and the AE reported was upper extremity pain; in Valent et al.22 and Duran et al.25 the interventions were hand cycle ergometry and aerobic circuit training, and the AE was muscle soreness. In McLean and Skinner,16 the metacarpal fracture during arm cycle ergometry was reported as unrelated, but given that this was an upper extremity task, might not be unexpected. Skin irritation after FES cycling35 which uses surface electrodes adhered to the skin, also would not be unexpected. Several studies29,31,37,38,41 that included primarily participants with higher levels of injury (C4–T4) reported AE related to autonomic dysfunction, cardiac irregularity, or hypotension. These types of AE may be more expected and should be assessed in people with higher levels of injury. Further investigation is warranted to explore the nature and extent of these AEs in people with different levels and classification of SCI so that tailored exercise guidelines can be developed.
Of greatest concern, however, are the musculoskeletal-related AEs that occurred related to walking interventions, whether volitional or FES-related. Several studies reported fractures, back pain, knee pain or injury, ankle swelling or sprain, and falls.32,46,49,51,52 People with SCI and their providers should be made aware of these potential AE, especially given the wide use of walking interventions for people with SCI. These events may be in part preventable with appropriate precautions. Caution should be taken to protect weak and unstable joints in people with SCI, whether they have tetraplegia or paraplegia. Increased demand on the weak and insensate lower extremity puts it at risk for injury.53
Methodological issues
This review demonstrated inconsistent reporting of AE in studies assessing outcomes of cardiovascular-related exercise in people with SCI. AEs were rarely defined, and often were narrowly focused on a few specific categories and not inclusive of all possible events. Most studies did not report AEs at all (more than half of studies initially considered for inclusion were ruled out for this review due to lack of an AE statement). Similar to studies of medications and other healthcare interventions, the monitoring and reporting of harmful effects may not match the quality of the study as a whole.12,54,55 This suggests the need for standards related to AE reporting in scientific publications related to exercise. Future publications should include the definition of AE, reports of screening, and statements related to the nature and extent of AE, not only to inform clinicians, but also researchers and SCI consumers.
Limitations of the review
These studies represent the “ideal” world rather than the real world in that most were supervised programs and participants were selected to minimize the possibility of risks. Exclusion criteria for exercise studies may have eliminated individuals who would have been likely to experience AE, which may limit generalizability to the SCI population as a whole. In many studies, especially those on volitional exercise modalities, exclusion criteria were not stated. Of those that did state exclusion criteria, most excluded participants with cardiovascular disease, risk factors for specific AE being monitored (osteoporosis, musculoskeletal pain), and those with existing complications of SCI (pressure ulcers, urinary tract infections (UTIs), kidney stones).
The level of evidence presented in most of the studies included is limited to mostly class IV evidence, uncontrolled studies, pre-post studies, case series, and case reports. This is due to both the nature of exercise research studies where outcome assessments often cannot be masked and also due to the limitations of research on exercise in the SCI where randomized controlled trials pose methodological, ethical, and practical challenges to researchers.55 People with SCI are a very heterogeneous population in terms of injury level, completeness of lesion, and functional ability making conclusions about any specific sub-group difficult due to intragroup variability. Also, given the numerous secondary health problems that are associated with SCI that may also make participation in training studies inconsistent or impossible, the level of adherence to the training programs in these studies was better than would be predicted.
In summary, the strength of evidence on AE presented in this review is low due to limitations of exercise research studies in people with SCI. However, the number of studies with few or no reported AEs is large enough to provide useful possibly predictive indication that cardiovascular training for people with SCI is no more dangerous from a cardiovascular perspective than it is in the general population. Given this low-risk profile similar to people without SCI, it seems appropriate to use screening measures that are widely used with the general population such as the Physical Activity Readiness Questionnaire.2 The specific AE associated with CV training are mostly those expected due to lesion level and completeness or due to the specific training modality and factors associated with safety for that modality.
Conclusions and recommendations for future research
In the studies reviewed, there were no serious AE reported. Furthermore, there were no common AE reported across most types of interventions, except for FES walking, which did report a variety of musculoskeletal-related AEs. The musculoskeletal-related AE that occurred related to walking interventions, whether volitional or FES-related are of greatest concern and may be preventable with appropriate protection for the weak and insensate lower extremity. There is no evidence to suggest that cardiovascular exercise done according to guidelines and established safety precautions is harmful. To improve the strength of these conclusions, future publications reporting on exercise intervention studies should include the definition of AE, reports of screening, and statements related to the nature and extent of AE, not only to inform clinicians, but also researchers and SCI consumers.
Disclaimer statements
Contributors All authors listed made a substantial contribution to the concept and design, acquisition of data or analysis and interpretation of data. Each person listed was personally responsible for reviewing and evaluating included studies. Each person listed was involved in writing various sections of the paper and in editing the final document. As chief author, CW was responsible for planning and drafting the initial paper and incorporating sections written by the other authors. DB wrote the discussion section and contributed substantially to the results section. SR provided careful editing of tables and references. KS managed the data and worked closely with two reviewers from the MSKTC to perform literature searches, do initial abstract reviews and maintain data integrity. CB rewrote the introductory section and reviewed manuscripts. SB edited the entire manuscript and provided substantial expertise for planning and guiding the study.
Conflicts of interest None.
Ethics approval None.
Funding This work was funded in part by grants from the National Institute for Disability and Rehabilitation Research, Office of Special Education and Rehabilitative Services, US Department of Education, Washington, DC to the University of Washington Model Systems Knowledge Translation Center (H133A060070) and the University of Washington Northwest Regional Spinal Cord Injury System (H133N060033).
References
- 1.Nash MS. Exercise as a health-promoting activity following spinal cord injury. J Neurol Phys Ther 2005;29(2):87–103,106. [DOI] [PubMed] [Google Scholar]
- 2.Goodman JM, Thomas SG, Burr J. Evidence-based risk assessment and recommendations for exercise testing and physical activity clearance in apparently healthy individuals. Appl Physiol Nutr Metab 2011;36(Suppl1):S14–S32. [DOI] [PubMed] [Google Scholar]
- 3.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA 2011;305(12):1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA III, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation 2007;115(17):2358–68. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med 2004;34(11):727–51. [DOI] [PubMed] [Google Scholar]
- 6.Martin Ginis KA, Hicks AL, Latimer AE, Warburton DE, Bourne C, Ditor DS, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011;49(11):1088–96. [DOI] [PubMed] [Google Scholar]
- 7.Valent L, Dallmeijer A, Houdijk H, Talsma E, van der Woude L. The effects of upper body exercise on the physical capacity of people with a spinal cord injury: a systematic review. Clin Rehabil 2007;21(4):315–30. [DOI] [PubMed] [Google Scholar]
- 8.Hicks AL, Martin Ginis KA, Pelletier CA, Ditor DS, Foulon B, Wolfe DL. The effects of exercise training on physical capacity, strength, body composition and functional performance among adults with spinal cord injury: a systematic review. Spinal Cord 2011;49(11):1103–27. [DOI] [PubMed] [Google Scholar]
- 9.Warburton DER, Sproule S, Krassioukov A, Eng JJ. Cardiovascular health and exercise following spinal cord injury. Eng JJ, Teasell RW, Miller WC, Wolfe DL, Townson AF, Hsieh JTC, et al., (eds.)Spinal cord injury rehabilitation evidence. Version 2.0. Vancouver, Canada; 2008. p. 7.1–7.34. [Google Scholar]
- 10.American Academy of Neurology (AAN). Clinical practice guideline process manual. St. Paul, MN: American Academy of Neurology; 2011. 57p. [Google Scholar]
- 11.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loke YK, Price D, Herxheimer A. Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 2007;7(32): Open access online, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyson-Hudson TA, Sisto SA, Bond Q, Emmons R, Kirshblum SC. Arm crank ergometry and shoulder pain in persons with spinal cord injury. Arch Phys Med Rehabil 2007;88(12):1727–9. [DOI] [PubMed] [Google Scholar]
- 14.El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 2005;43(5):299–305. [DOI] [PubMed] [Google Scholar]
- 15.El-Sayed MS, Younesian A, Rahman K, Ismail FM, El-Sayed Ali Z. The effects of arm cranking exercise and training on platelet aggregation in male spinal cord individuals. Thromb Res 2004;113(2):129–36. [DOI] [PubMed] [Google Scholar]
- 16.McLean KP, Skinner JS. Effect of body training position on outcomes of an aerobic training study on individuals with quadriplegia. Arch Phys Med Rehabil 1995;76(2):139–50. [DOI] [PubMed] [Google Scholar]
- 17.Le Foll-de Moro D, Tordi N, Lonsdorfer E, Lonsdorfer J. Ventilation efficiency and pulmonary function after a wheelchair interval training program in subjects with recent spinal cord injury. Arch Phys Med Rehabil 2005;86(8):1582–6. [DOI] [PubMed] [Google Scholar]
- 18.Bougenot MP, Tordi N, Betik AC, Martin X, LeFoll D, Parratte B, et al. Effects of a wheelchair ergometer training programme on spinal cord-injured persons. Spinal Cord 2003;41(8):451–6. [DOI] [PubMed] [Google Scholar]
- 19.Tordi N, Dugue B, Klupzinski D, Rasseneur L, Rouillon JD, Lonsdorfer J. Interval training program on a wheelchair ergometer for paraplegic subjects. Spinal Cord 2001;39(10):532–7. [DOI] [PubMed] [Google Scholar]
- 20.Bjerkefors A, Thorstensson A. Effects of kayak ergometer training on motor performance in paraplegics. Int J Sports Med 2006;27(10):824–9. [DOI] [PubMed] [Google Scholar]
- 21.Valent LJ, Dallmeijer AJ, Houdijk H, Slootman HJ, Janssen TW, Post MW, et al. Effects of hand cycle training on physical capacity in individuals with tetraplegia: a clinical trial. Phys Ther 2009;89(10):1051–60. [DOI] [PubMed] [Google Scholar]
- 22.Valent L, Dallmeijer AJ, Houdijk H, Slootman HJ, Janssen TW, Van Der Woude LH. Effects of hand cycle training on wheelchair capacity during clinical rehabilitation in persons with spinal cord injury. Disabil Rehabil 2010;32(26):2191–200. [DOI] [PubMed] [Google Scholar]
- 23.Mukherjee G, Bhowmik P, Samanta A. Physical fitness training for wheelchair ambulation by the arm crank propulsion technique. Clin Rehabil 2001;15(2):125–32. [DOI] [PubMed] [Google Scholar]
- 24.Tawashy AE, Eng JJ, Krassioukov AV, Miller WC, Sproule S. Aerobic exercise during early rehabilitation for cervical spinal cord injury. Phys Ther 2010;90(3):427–37. [DOI] [PubMed] [Google Scholar]
- 25.Duran FS, Lugo L, Ramırez L, Eusse E. Effects of an exercise program on the rehabilitation of patients with spinal cord injury. Arch Phys Med Rehabil 2001;82(10):1349–54. [DOI] [PubMed] [Google Scholar]
- 26.Nash MS, van de Ven I, van Elk N, Johnson BM. Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch Phys Med Rehabil 2007;88(1):70–5. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs PL, Nash MS, Rusinowski JW. Circuit training provides cardiorespiratory and strength benefits in persons with paraplegia. Med Sci Sports Exerc 2001;33(5):711–7. [DOI] [PubMed] [Google Scholar]
- 28.Cooney MM, Walker JB. Hydraulic resistance exercise benefits cardiovascular fitness of spinal cord injured. Med Sci Sports Exerc 1986;18(5):522–5. [PubMed] [Google Scholar]
- 29.Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med 2008;31(5):509–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ditor DS, Kamath MV, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J Appl Physiol 2005a;98(4):1519–25. [DOI] [PubMed] [Google Scholar]
- 31.Ditor DS, MacDonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005b;43(11):664–73. [DOI] [PubMed] [Google Scholar]
- 32.Protas EJ, Holmes SA, Qureshy H, Johnson A, Lee D, Sherwood AM. Supported treadmill ambulation training after spinal cord injury: a pilot study. Arch Phys Med Rehabil 2001;82(6):825–31. [DOI] [PubMed] [Google Scholar]
- 33.Needham-Shropshire BM, Broton JG, Cameron TL, Klose KJ. Improved motor function in tetraplegics following neuromuscular stimulation-assisted arm ergometry. J Spinal Cord Med 1997;20(1):49–55. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler GD, Andrews B, Lederer R, Davoodi R, Natho K, Weiss C, et al. Functional electric stimulation–assisted rowing: increasing cardiovascular fitness through functional electric stimulation rowing training in persons with spinal cord injury. Arch Phys Med Rehabil 2002;83(8):1093–9. [DOI] [PubMed] [Google Scholar]
- 35.Duffell LD, Donaldson Nde N, Perkins TA, Rushton DN, Hunt KJ, Kakebeeke TH, et al. Long-term intensive electrically stimulated cycling by spinal cord-injured people: effect on muscle properties and their relation to power output. Muscle Nerve 2008;38(4):1304–11. [DOI] [PubMed] [Google Scholar]
- 36.Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson Nde N, et al. High volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 2008;43(1):169–76. [DOI] [PubMed] [Google Scholar]
- 37.Janssen TW, Pringle DD. Effects of modified electrical stimulation-induced leg cycle ergometer training for individuals with spinal cord injury. J Rehabil Res Dev 2008;45(6):819–30. [DOI] [PubMed] [Google Scholar]
- 38.Zbogar D, Eng JJ, Krasioukov AV, Scott JM, Esch BT, Warburton DE. The effects of functional electrical stimulation leg cycle ergometry training on arterial compliance in individuals with spinal cord injury. Spinal Cord 2008;46(11):722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hjeltnes N, Aksnes AK, Birkeland KI, Johansen J, Lannem A, Wallberg-Henriksson H. Improved body compostion after 8 wk of electrically stimulated leg cycling in tetraplegic patients. Am J Physiol 1997;273(3 pt 2):R1072–9. [DOI] [PubMed] [Google Scholar]
- 40.Mutton DL, Scremin AM, Barstow TJ, Scott MD, Kunkel CF, Cagle TG. Physiologic responses during functional electrical stimulation leg cycling and hybrid exercise in spinal cord injured subjects. Arch Phys Med Rehabil 1997;78(7):712–8. [DOI] [PubMed] [Google Scholar]
- 41.Mohr T, Andersen JL, Biering-Sorensen F, Galbo H, Bangsbo J, Wagner A, et al. Long term adaptation to electrically induced cycle training in severe spinal cord injured individuals. Spinal Cord 1997;35(1):1–16. [DOI] [PubMed] [Google Scholar]
- 42.Barstow TJ, Scremin AM, Mutton DL, Kunkel CF, Cagle TG, Whipp BJ. Changes in gas exchange kinetics with training in patients with spinal cord injury. Med Sci Sports Exerc 1996;28(10):1221–8. [DOI] [PubMed] [Google Scholar]
- 43.Hooker SP, Scremin AM, Mutton DL, Kunkel CF, Cagle G. Peak and submaximal physiologic responses following electrical stimulation leg cycle ergometer training. J Rehabil Res Dev 1995;32(4):361–6. [PubMed] [Google Scholar]
- 44.Ragnarsson KT, Pollack S, O'Daniel W Jr, Edgar R, Petrofsky J, Nash MS. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multicenter pilot study. Arch Phys Med Rehabil 1988;69(9):672–7. [PubMed] [Google Scholar]
- 45.Ragnarsson KT. Physiologic effects of functional electrical stimulation-induced exercises in spinal cord-injured individuals. Clin Orthop Relat Res 1988;233:53–63. [PubMed] [Google Scholar]
- 46.Brissot R, Gallien P, Le Bot MP, Beaubras A, Laisne D, Beillot J, et al. Clinical experience with functional electrical stimulation-assisted gait with Parastep in spinal cord-injured patients. Spine 2000;25(4):501–8. [DOI] [PubMed] [Google Scholar]
- 47.Klose KJ, Jacobs PL, Broton JG, Guest RS, Needham-Shropshire BM, Lebwohl N, et al. Evaluation of a training program for persons with SCI paraplegia using the Parastep1 ambulation system: part 1. Ambulation performance and anthropometric measures. Arch Phys Med Rehabil 1997;78(8):789–93. [DOI] [PubMed] [Google Scholar]
- 48.Needham-Shropshire BM, Broton JG, Klose KJ, Lebwohl N, Guest RS, Jacobs PL. Evaluation of a training program for persons with SCI paraplegia using the Parastep1 ambulation system: part 3. Lack of effect on bone mineral density. Arch Phys Med Rehabil 1997;78(8):799–803. [DOI] [PubMed] [Google Scholar]
- 49.Gallien P, Brissot R, Eyssette M, Tell L, Barat M, Wiart L, et al. Restoration of gait by functional electrical stimulation for spinal cord injured patients. Paraplegia 1995;33(11):660–4. [DOI] [PubMed] [Google Scholar]
- 50.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82(6):818–24. [DOI] [PubMed] [Google Scholar]
- 51.Ferro FP, Gonzalez HJ, Ferreira DM, Cliquet A Jr. Electrical stimulation and treadmill gait in tetraplegic patients: assessment of its effects on the knee with magnetic resonance imaging. Spinal Cord 2008;46(2):124–8. [DOI] [PubMed] [Google Scholar]
- 52.Thoumie P, Perrouin-Verbe B, Le Claire G, Bedoiseau M, Busnel M, Cormerais A, et al. Restoration of functional gait in paraplegic patients with the RGO-II hybrid orthosis. A multicenter controlled study. I. Clinical evaluation. Paraplegia 1995;33(11):647–53. [DOI] [PubMed] [Google Scholar]
- 53.Hastings JD, Field-Fote EC. Maximizing mobility. In Field-Fote EC (ed): Spinal cord injury rehabilitation. Philadelphia, PA: FA Davis Company; 2009, p. 143. [Google Scholar]
- 54.McIntosh HM, Woolacott NF, Bagnall AM. Assessing harmful effects in systematic reviews. BMC Med Res Methodol 2004;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Golder S, Loke Y, McIntosh HM. Poor reporting and inadequate searches were apparent in systematic reviews of adverse effects. J Clin Epidemiol. 2008;61(5):440–8. [DOI] [PubMed] [Google Scholar]
- 56.Ginis KA, Hicks AL. Exercise research issues in the spinal cord injured population. Exerc Sport Sci Rev 2005;33(1):49–53. [PubMed] [Google Scholar]