Abstract
Context
Electrical stimulation (ES) can confer benefit to pressure ulcer (PU) prevention and treatment in spinal cord injuries (SCIs). However, clinical guidelines regarding the use of ES for PU management in SCI remain limited.
Objectives
To critically appraise and synthesize the research evidence on ES for PU prevention and treatment in SCI.
Method
Review was limited to peer-reviewed studies published in English from 1970 to July 2013. Studies included randomized controlled trials (RCTs), non-RCTs, prospective cohort studies, case series, case control, and case report studies. Target population included adults with SCI. Interventions of any type of ES were accepted. Any outcome measuring effectiveness of PU prevention and treatment was included. Methodological quality was evaluated using established instruments.
Results
Twenty-seven studies were included, 9 of 27 studies were RCTs. Six RCTs were therapeutic trials. ES enhanced PU healing in all 11 therapeutic studies. Two types of ES modalities were identified in therapeutic studies (surface electrodes, anal probe), four types of modalities in preventive studies (surface electrodes, ES shorts, sacral anterior nerve root implant, neuromuscular ES implant).
Conclusion
The methodological quality of the studies was poor, in particular for prevention studies. A significant effect of ES on enhancement of PU healing is shown in limited Grade I evidence. The great variability in ES parameters, stimulating locations, and outcome measure leads to an inability to advocate any one standard approach for PU therapy or prevention. Future research is suggested to improve the design of ES devices, standardize ES parameters, and conduct more rigorous trials.
Keywords: Electrical stimulation, Pressure ulcer, Spinal cord injury, Systematic review
Introduction
Approximately 1200 people are paralyzed from a spinal cord injury (SCI) every year in the UK, with a total of ∼40 000 people living with paralysis,1 while in the USA, it is reported that there are ∼12 000 new cases each year, excluding those who die at the scene of an accident.2 Following SCI, the loss of motor, sensory, and autonomic control may lead to pressure ulcers (PUs). PUs are the most common secondary medical complication associated with SCI.3,4 It is reported that up to 85% of adults with SCI will develop a PU at some point during their lifetime.5–7
A PU is otherwise and perhaps more commonly known as a pressure sore. It is described as an area of localized damage to the skin as a result of prolonged pressure alone, or pressure in combination with shearing forces.8 PUs can occur in patients with SCI very early, often within a few days following the injury. According to the Model SCI System Statistical Center, the annual incidence rate of PUs is seen at 14.7% in the first post-injury year and noted to be steadily increasing thereafter.3 Once a PU has developed, it can be extremely difficult to achieve full repair. Those who suffer a PU may be subjected to longer hospital stays, delayed rehabilitation, and a significant loss of independence, which add other burdens to the psychological trauma of injury and reduced quality of life. If a PU is severe, it can lead to further disabilities, need for surgical interventions, and even fatal infections.9
Apart from the significant personal consequences, PUs also present a significant cost burden for health and social care systems. Treating a PU varies from £1214 (Category I) to £14 108 (Category IV) in the UK,10 with a total annual cost for the treatment being £1.4–£2.1 billion; this accounts for 4% of annual National Health Service (NHS) budget.10–12 It is estimated that PUs account for ∼25% of overall treatment costs for people with SCI.13,14 Therefore, given the significant personal consequences and serious health care burden, effective preventive and therapeutic interventions are vitally important for individuals who live with a SCI.
Thus far, the tremendous efforts to prevent PUs tend to focus on methods to reduce external pressure. These range from using pressure-relieving devices, to patients performing “pressure relief” maneuvers themselves, such as frequent repositioning or “push-ups” or “leaning forward”.15–17 However, these efforts are only partially effective at best in SCI. Although it is well documented that simple pressure relief measures confer benefits on reducing local pressures at bony prominences, they do not prevent the muscle atrophy that has emerged as a specific risk factor for PU development in SCI.12,18 In fact, the incidence of PUs remains unacceptably high.2,5–7
To date, once a PU is diagnosed, conventional standard nursing care will be provided, which includes offloading, improving nutrition, revascularization, compression, and/or debridement. Generally, it is predicted that the ulcer should completely heal if a 50% reduction in ulcer size is achieved by 4 weeks of treatment, in the absence of infection. If this reduction in size cannot be achieved, the wound is likely to have stagnated into a chronic phase, for which advanced therapies will usually be advocated to speed up the healing process.19 A number of advanced treatments are documented in the literature, such as bioengineered skin substitutes, negative pressure wound devices, oxygen, ultrasound, and electrical stimulation (ES). Determining which of the advanced therapies to use often depends on availability of modalities and the cost and time invested.19 In the era of evidence-based practice, understanding the updated evidence for advanced therapies of PU in SCI is prudent.
Regan et al.20 conducted a systematic review of preventive and therapeutic interventions for PUs after SCI, and identified ES as an intervention for both PU prevention and treatment in the SCI population. Indeed, as early as 50 years ago, ES was documented to enhance healing of various chronic wounds including PUs in individuals with SCI,13,21,22 while the preventive effects of ES for PU in SCI has been reported since 1980s.16,17,23,24 A recent systematic review suggested that ES is cost-effective for treating PUs in SCI,25 yet clinical practice guidelines regarding the use of ES for PU prevention and treatment in SCI remain limited.19,26 This systematic review was therefore conducted to identify the updated evidence, and make recommendations for future studies implementing ES for PU prevention and treatment in SCI.
Objectives
The overall aim of this review is to critically appraise and synthesize the research evidence available on ES for prevention and treatment of PUs in people living with SCI. This review therefore sought to address three specific questions:
(1) Which devices used to deliver ES for PU prevention and treatment in SCI population are documented in the literature?
(2) What are the parameters of ES used?
(3) How effective is ES for PU prevention and treatment in SCI population?
Methods
A systematic review protocol was devised for the identification, retrieval, and appraisal of the evidence. The systematic review was registered in the PROSPERO database in July 2013 (http://www.crd.york.ac.uk/PROSPERO/) and the registration number is CRD42013005088. The search strategies in each database are available on request.
Search methods for identification of studies
Electronic searches
All relevant literature published from 1970 to 2013 was searched up to 18 July 2013 in five databases without any language restrictions. Free-text and keyword/MESH terms for each of the following databases were used: Medline, Embase, CINAL, PsycINFO, and the Cochrane Central Register of Controlled Trials. Subject sub-headings and word truncations were entered according to database requirements in order to map all possible keywords. Search terms for SCI included quadriplegi*, tetraplegi*, paraplegi*, spinal cord trauma*, and spinal cord injury*. Search terms for ES included electric* stimulation, nerve/neuro-muscular/ neuromuscular/muscular/muscle, and electric* and stimulation*. Those for PUs covered: pressure sore*, PU*, decubitus ulcer*, ischemic ulcer*, bed sore*, and skin sore*.
Other resources
The National PU Advisory Panel (NPUAP), European PU Advisory Panel (EPUAP), National Institute for Health and Clinical Excellence (NICE), and Scottish Intercollegiate Guidelines Network (SIGN) were searched for relevant published guidelines. In addition, the reference list of included studies and other relevant papers (e.g. available reviews) were screened for eligible studies and authors and experts in the field were contacted to identify any additional studies.
Inclusion criteria
Types of studies
To capture all relevant evidence, eligible studies included randomized controlled trials (RCTs), non-RCTs, prospective cohort studies, case series, case control studies, and case report studies. Further study inclusion criteria were applied as follows:
-
•
primary studies;
-
•
target population including persons with SCI irrespective of their age, sex, and degree of severity of traumatic or non-traumatic SCI.
Interventions
Any type of intervention using ESs was accepted and intervention terminology included functional electric stimulation (surface/implant), neuromuscular electric stimulation (NMES), and nerve root stimulation.
Outcome measurement
Any outcome measuring the effectiveness of PU prevention and treatment was taken into account. Outcomes of prevention criteria were PU incidence (direct), seating pressure, muscle bulk, skin blood flow, and PTCO2 (indirect). Outcomes of treatment were healing time, healing rate, ulcer size, and the stage of the ulcer.
Data extraction and management
The following data were extracted from eligible articles by one reviewer (L.Q.L.) and double-checked by the second reviewer (J.M.): year of publication, country of author affiliated, and type of study design. All other data including sample size, participants' age, sex, type and level of SCI, the type of electric stimulation, period of the stimulation, pattern of stimulation, duration of study, adverse events, outcome measures, and findings along with methodological quality were assessed independently by two reviewers (L.Q.L. and J.M.). Any disparity in assessed findings between the two independent reviewers was resolved by discussion or through consultation with a third reviewer.
A quality assessment was conducted for each article (except case reports). For RCTs, a Jadad score was employed together with the item allocation concealment and whether the analysis was based on the randomized groups,27,28 and a modified Downs and Black tool for non-RCTs.20,29 Both scales are well-established tools for assessing and reporting on the quality of clinical and health-related studies in the literature.
The Jadad score addresses the items relating to randomization, blinding, and description of withdrawals and dropouts, with scores ranging from 0 to 5 with trials scoring 3 or greater, considered to be of reasonably good quality. Allocation concealment was considered adequate if patients and investigators who enrolled patients could not foresee treatment assignment. “Intention to treat” (ITT) is defined as an analysis which demonstrates inclusivity of all randomized participants based on the following criteria: the groups to which they were originally randomly assigned regardless of whether they satisfied the entry criteria, and the treatment actually received and subsequent withdrawal or deviation from the protocol.28
The Downs and Black tool29 consists of 27 questions, which evaluates the level of four domains: (1) reporting; (2) external validity; (3) internal validity (both bias and confounding); (4) power. This was modified slightly because of what was felt to be an ambiguity in the final question; thus, the highest score that any reviewed article could receive was 28. It should be noted that scores increased in line with the methodological quality of the study, higher scores indicating higher methodological quality.20
Data analysis
All studies were categorized by the type of study design and further grouped according to the objectives of the intervention and intervention model used. All studies were classified using the guidelines published by the Oxford Centre for Clinical Evidence in cooperation with the grade of evidence published by Harding et al.30 and Clucas et al.31 as follows:
-
•
Grade I (strong evidence): corresponded to RCTs:
IA: RCTs with Jadad score >3 combined with adequate allocation concealment and using ITT for data analysis.
IB: RCTs with Jadad score >3 without AC or ITT.
-
•
Grade II (fairly strong evidence): RCT with Jadad score <3 with/without AC and ITT, prospective non-randomized controlled studies, and cohort study.
-
•
Grade III (weaker evidence): retrospective case-controlled, pre–post studies and case series.
-
•
Grade IV (weak evidence): cross-sectional studies and case reports.
All descriptive statistics were carried out using Excel 2007 (Microsoft Corp., Redmond, WA, USA). A formal meta-analysis with statistical pooling of results across studies was not possible because of the absence of both a uniform mode of intervention as well as standardization of outcome measures.
Results
Included studies
The literature search identified a total of 384 unique references that were all exported to Endnote (Endnote version X7 for Windows, Thomson Reuters, Philadelphia, PA, USA), and three additional articles were identified from other sources. Of these 387 articles, 119 were identified as duplicates, thus resulting in 268 abstracts and titles that were available for sifting for eligibility.
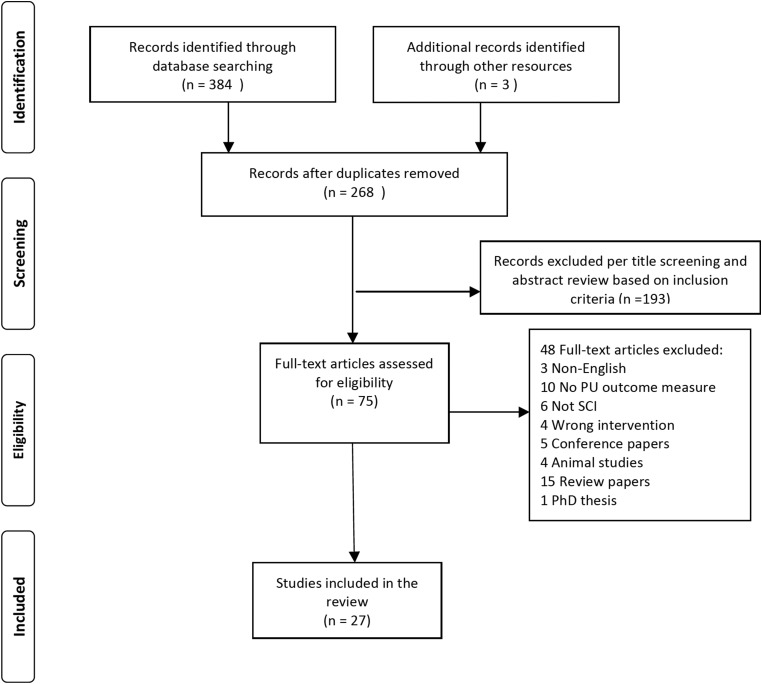
All 268 abstracts were further screened and this subsequently generated 75 abstracts that were potentially relevant. The full texts of these 75 abstracts were retrieved and considered for eligibility for inclusion in the final systematic review. The outcome following this procedure was that a total of 27 studies met the inclusion criteria and were subjected to full-data extraction. Fig. 1 provides a flow chart of the process and results for screening eligibility and study selection.
Figure 1 .
A flow chart of the process and results for screening eligibility and study selection.
Sample characteristics
All 27 articles22,26,32–56 described the study target population as SCIs, with eight studies (30%) reporting the level of injury. Of the 27 studies, 11 (41%) of the studies were conducted in the USA, four studies (15%) in the UK, and (15%) Slovenia, respectively; three studies (11%) in the Netherlands, two studies in Canada (7%), with the remaining three studies were from Germany, Australia, and Nigeria. In terms of study objectives, 16 studies were designed for PU prevention, while 11 studies were designed for PU treatment. Among the 11 therapeutic studies, 6 were RCTs, 2 were case reports and prospective non-RCTs, respectively, and the remaining study was a case series. For prevention studies, 9 out of 16 studies (56%) were case series, 3 studies were RCTs (19%) and case reports (19%), respectively. There was one cohort study (6%). As a whole, the number of patients per study ranged from 1 to 150. Details of sample characteristics are shown in Table 1.
Table 1 .
Characteristics of studies included in the systematic review (n = 27)
| Study | Sample characteristics | Study design | Methodological quality |
|---|---|---|---|
| Therapeutic studies | |||
| Houghton et al.35 | 34 (20 males, 14 females) SCI with mean age of 51 years old | RCT | Jadad 3, AC yes, ITT yes |
| Griffin et al.33 | 20 SCI with pelvis PUs | RCT | Jadad 4, AC yes, ITT no |
| Adegoke and Badmos32 | 7 SCI, 21–60 years with Grade IV pelvic ulcer | RCT | Jadad 2, AC yes, ITT no |
| Baker et al.22 | 80 (66 male, 14 female) SCI, 17–76 years old with one or more PUs | RCT | Jadad 2, AC no, ITT no |
| Karba et al.37 | 50 SCI with PU | Prospective control trial | Jadad 2, AC no, ITT no |
| Jercinovic et al.36 | 73 SCI with 109 PUs aged 18–68 years old | RCT | Jadad 1, AC no, ITT yes |
| Stefanovska et al.39 | 150 SCI with one or more PUs | Prospective control trial | D&B score 13 |
| Trontelj et al.50 | 106 SCI with PUs | Prospective control trial | D&B score 8 |
| Recio et al.47 | 3 male SCI, 29 -51 years old with recalcitrant PUs | Case series | D&B score 4 |
| Lippert-Grüner53 | 1 male SCI at T9 level, 34 years old who had bilateral large decubitus ulcers in gluteus region for 6 months | Case report | Not applicable |
| Pollack et al.55 | 1 male SCI at C4 level, 27 years old who had a left ischial PU poorly responded to conventional treatment | Case report | Not applicable |
| Preventive studies | |||
| Kim et al.38 | 6 male SCI aged 36–75 years old without open ulcers | RCT | Jadad 4, AC yes, ITT no |
| Gyawali et al.34 | 17 (10 male, 7 female) SCI mean age of 37 years | RCT | Jadad 1, AC no, ITT no |
| Londen et al.26 | 13 SCI, 20–74 years old | Crossover RCT | Jadad 1, AC no, ITT no |
| Petrofsky40 | 124 SCI, 12–57 years old | Cohort study | D&B score 8 |
| Smit et al.48 | 10 SCI, 34 ± 9 years old, no current ischial PUs | Case series | D&B score 14 |
| Smit et al.49 | 12 male SCI, 26–52 years old, no current ischial PUs | Case series | D&B score 14 |
| Liu et al44 | 11 (10 males, 1 female) suprasacral SCI, 23–62 years old, no current ischial PUs | Case series | D&B score 13 |
| Liu et al.45 | 5 suprasacral SCI (4 males, 1 female), 34–62 years old, no current ischial PUs | Case series | D&B score 13 |
| Bogie and Triolo41 | 8 (7 males, 1 female) SCI, 27–47 years old, had gluteal muscle electrodes implanted bilaterally | Case series | D&B score 12 |
| Mawson et al46 | 32 SCI, 18–57 years old, with or without current PUs | Case series | D&B score 10 |
| Levine et al.43 | 6 acute SCI at or above T7 level who had no history of PUs under ischial tuberosities | Case series | D&B score 9 |
| Wu et al.51 | 7 (5 males, 2 females) SCI, 26–58 years old, had implanted lower extremity NMES | Case series | D&B score 9 |
| Ferguson et al.42 | 9 SCI, 21–56 years old, had completed injury and had no current PU | Case series | D&B score 8 |
| Bogie et al.52 | 1 male SCI at C4 level, 42 years old, with regular Grade II and occasional IV ischial PU | Case report | Not applicable |
| Rischbieth et al.54 | 1 male SCI at C6 level with history of PUs | Case report | Not applicable |
| Vanoncini et al.56 | 1 male SCI at T5 level with sensory and motor complete injury | Case report | Not applicable |
AC, allocation concealment; D&B, Modified Down & Black score range from 0 to 28; ITT, intention to treat; Jadad score range from 0 to 5; PU, pressure ulcer; RCT, randomized controlled trial; SCI, individual with spinal cord injury.
Review of therapeutic studies
Methodological quality
All case reports were not assessed for methodological quality, as a single case report has been considered to be of poor quality in comparison with any other type of study design reported in this review.
Randomized controlled trials
In a total of 11 therapeutic studies, 6 trials were RCTs, with 1 trial describing an appropriate method to generate the randomization sequence. Two of the six studies were double-blinded and described the method of double-blinding, three trials adequately described allocation concealment, and two trials used ITT to analyze the data. Two RCTs were considered to be of reasonably good methodological quality according to the Jadad score.
Non-RCTs
Three non-RCTs (one case series and two prospective control trials) were assessed for their reporting quality using the Down and Black tool. The scores of these trials were 13, 8 and 4 out of a total achievable score of 28. The remaining two case reports were not assessed for methodological quality.
Grade of evidence
Two out of 11 therapeutic studies were graded as strong evidence, one of which scored >3 according to the Jadad scale, in combination with adequate allocation concealment and using ITT data analysis, hence it was classified as Grade IA. The other trial was classified as Grade IB, in which the trial was scored >3 on the Jadad scale, yet did not meet with the other two criteria. Six studies were graded as fairly strong evidence (Grade II), which include those inadequately designed RCTs (Jadad score <3) and prospective non-RCTs. One case series was graded as weaker evidence (Grade III), and the remaining two case reports were considered to demonstrate weak evidence only.
Intervention features
The ES parameters and stimulation sites are shown in Table 2. The ES parameters were often modified in each study, despite the same type of ES being utilized; therefore, the parameters were often set differently across studies. The use of different stimulation frequencies, intensities, pulse width, waveform and duration together with the varied stimulating site, and outcome measurement, limits the comparability of results from the interventions across different samples. This inevitably prevented meta-analysis of the data.
Type of ES device
In total, 10 of the 11 therapeutic studies delivered ES using surface electrodes. One case study reported the use of an anal probe to heal large decubitus ulcers in gluteal region, which were resistant to conventional treatment.
Stimulation sites
Nine out of 11 therapeutic trials placed the electrodes on the ulcer tissue or intact skin around the wound. Within the nine trials, seven studies laid the electrodes directly over the wound, two studies stimulated intact skin nearby ulcer tissue. One case report used an anal probe to activate gluteal muscles, and another case report placed the surface electrodes on the bilateral gluteus, hamstring, and quadriceps muscles to treat an ischial PU, which had previously demonstrated poor response to conventional treatments.
Parameters of ES
In a total of 11 therapeutic studies, 1 case report used an anal probe to activate the gluteal muscles to treat the ischial PUs; however, there are no parameters reported; 3 of 11 studies applied high-voltage pulsed current with different pulse width of 10–50 µs, stimulation frequency of 10–100 Hz, intensity of 50–200 V; three studies applied direct current. Within the three studies, one trial applied interrupted direct current with intensity adjusted by user to keep minimum muscle contraction, another study utilized constant direct current with intensity of 0.6 mA, the remaining one study delivered direct current with either a low density of an amplitude of 600 µA or with a pulse duration of 0.25 ms low frequency of 40 Hz, amplitude 15–25 mA. There was one case report, in which the authors used ES to stimulate gluteus, hamstring, and quadriceps as a treatment of ischial PU. In terms of ES parameters, the authors used a frequency of 60 Hz with 400 µs pulse width was used according to the instructions provided by the manufacturer. There are three therapeutic studies that adjusted the ES intensity to achieve minimal muscle contraction in individual participants.22,32,36
Intervention effectiveness
All 11 therapeutic studies aimed to heal the PUs by measuring the size of the wound or the healing rate, with 10 of the 11 studies reporting the follow-up period as varying from 20 days to 1 year. One study did not report the study period at all.37 Three non-controlled studies (two case series and one case report), reported that the PUs were all completely healed with stimulation by the end of the study. Eight of 11 trials have a control group, among the 8, 6 were designed as RCTs and the other 2 studies were assigned the interventions without any randomization. The control group was given either sham simulation or no stimulation. Seven of the eight trials reported a significantly better healing process than the control groups. One study reported no statistical differences noted in PU healing rates between the stimulation and control groups when electrodes were placed on the intact skin nearby ulcer tissue.22 However, the subgroup analysis in this study showed that participants in the control group who had ulcers healed by functional ES after the control period, subsequently achieved a greater healing rate (43.3 ± 12.5% change/week) than during the control period (9.7 ± 3.4% change/week).
Adverse events
Two out of 11 studies reported on adverse events. One study indicated that some patients experienced minor adverse reactions related to ES, which included red, raised, itchy skin beneath the large dispersive electrode. The other study showed that participants tolerated ES well, with no complications reported.
Review of preventive studies
Methodological quality
Randomized controlled trials
In a total of 16 preventive studies, 3 were RCTs. One of the three RCTs described an appropriate method for generating the randomization sequence. The trial was double-blinded, described the method of double-blinding, and adequately described the allocation concealment. It was the only one RCT was considered to be of reasonably good methodological quality according to Jadad score. None of the three trials used ITT to analyze the data.
Non-RCTs
Ten non-RCTs (nine case series, one cohort study) were assessed for their reporting quality using the Down and Black tool. Two of the 10 trials were scored 14 out of a total achievable score of 28, 2 were scored 13 out of 28, 1 scored 12 out of 28, 1 scored 10, 2 attracted a score of 9, 2 were scored 8 out of 28. Again, three case reports were not assessed for methodological quality.
Grade of evidence
One out of 16 preventive studies were graded as strong evidence, in which the RCT scored >3 according to the Jadad scale, in combination with adequate allocation concealment, but not using ITT data analysis, hence it was classified as Grade IB. Three studies were graded as fairly strong evidence (Grade II), which included two inadequately designed RCTs and one cohort study. Nine case series were classified as weaker evidence (Grade III), and the remaining three case reports were considered to demonstrate weak evidence only.
Intervention features
Type of ES device
Four types of ES were identified in 16 PU preventive articles retained within this review. The ES delivered through conventional surface electrodes was the most commonly used stimulation intervention and was utilized in 10 of 16 studies. Other types of ES identified for PU prevention through this review included a custom-made garment with built-in electrodes,48 the electrical current delivered through a sacral anterior nerve root stimulator (SARS) implant,44,45 or alternatively, via implanted intramuscular electrodes.41,51,52
Stimulation sites
Eight out of 16 studies stimulated gluteal muscles alone, and five trials activated gluteal muscles together with other muscle groups, e.g. quadriceps, hamstrings, and lumber spinal muscles. The remaining three studies stimulated spine, erector spine, or quadriceps alone, respectively.
Parameters of ES
The ES parameters and sites varied greatly across individual preventive studies. The use of different stimulation frequencies, intensities, pulse width, waveform, and duration alongside diverse stimulating sites was seen in this review (Table 2). Overall, 14 studies used the frequency >20 Hz, e.g. 25, 30, 40, 50 Hz, which can cause titanic muscle contraction; two other studies utilized low frequency of 10 Hz; the amplitude was in a range of 20–150 mA, while pulse width ranged from 64 to 600 µs.
Table 2 .
Summary data of included studies (n = 27)
| Study | Intervention description I, Intervention; C, control | FES settings | Study period | Outcome measures | Level of evidence | Results |
|---|---|---|---|---|---|---|
| Therapeutic studies | ||||||
| Houghton et al.35 | I: HVPC applied to the wound bed plus SWC program; C: SWC only | HVPC with 50 µs pulse duration, 50–150 V intensity was applied for 20 minutes with 100 Hz, 10 Hz and off each hour for 8hours/day. | 3 months | WSA | Grade IA | 1. The percentage decrease in WSA was greater in the EST +SWC group (mean ±SD, 70 ±25%) than in the SWC group (36 ± 61%; P = 0.048). 2. The proportion of stages III, IV, or X PUs improving by at least 50% WSA was significantly greater in the EST +SWC group than in the SWC group |
| Griffin et al.33 | I: HVPC of wound; C: Sham HVPC given 1 hour a day for 20 consecutive days, both group received standard nursing care | Stimulation frequency and intensity was 100 pps, 200 V, respectively | 20 days | WSA | Grade IB | Percentage reduction in WSA achieved by the HVPC group was greater than the sham treatment group at day 5 (32 vs. 14%, P = 0.03), day 15 (66 vs. 44%, P = 0.05), and day 20 (80 vs. 52%, P = 0.05) |
| Adegoke and Badmos32 | I: IDC of wound; C: Sham IDC for 45-minutes 3 days/week, both group received SWC | ES intensity gradually increased until a minimal muscle contraction, then kept just below contraction | 4 weeks | WSA | Grade II | 1. WSA decreased by 22.2% in the IDC group vs. 2.6% in the sham treatment group. 2. Most of the decrease in WSA occurred during the first 2 weeks of the study (15.8 vs. 1.9% change in the DC group vs. the sham DC group, respectively) |
| Baker et al.22 | I: asymmetric biphasic ES; II: symmetric biphasic ES; III: microcurrent ES; C: no stimulation. Each treatment last 1.5 hours, 5 days/week | ES intensity increased until a minimal muscle contraction, then decreased until no muscle contraction | 4 weeks | Healing rate and WSA | Grade II | 1. No statistical differences in healing rates and wound areas among the four groups. 2. Subgroup analysis showed the healing rate by ES in the control group was greater after the control period (43.3 ± 12.5 vs. 9.7 ± 3.4% change/week) |
| Karba et al.37 | I: ES were delivered using the 1 positive stimulation electrode and 4 negative electrodes (DC+). II: same ES program with one positive and one negative pad. C: sham group, no ES delivered | Constant direct electric current of 0.6 mA | Not reported | Relative healing rate (%/day) | Grade II | The relative healing rates of PU treated by direct current with electrode overlaid wound was higher than those with electrodes placed on intact skin, or treated by sham ES |
| Jercinovic et al.36 | I: SWC plus ES edge of PU for 2 hours; C: SWC and standard rehabilitation. Crossover group after 4 weeks | ES was applied with 40 Hz, 250 µs, amplitude adjusted up to 45 mA individually to achieve minimal contraction | 1 year | Wound healing rate | Grade II | 1. Mean healing rate for ES group in first 4 weeks was greater comparing to the control group. 2. ES group have 1.5–2 times shorter healing period |
| Stefanovska et al.39 | I: conventional treatment plus direct currents with low density (DC); II: conventional treatment plus direct currents with low frequency (AC) were applied across wound for 2 hours daily; III: conventional treatment only | Direct currents with low density an amplitude of 600 µA; AC currents with a pulse duration of 0.25 ms, low frequency of 40 Hz, amplitude 15–25 mA | 4 weeks or till wound closure | Healing rates | Grade II | Healing rate in the AC group was significantly better than the DC and control group (P = 0.003) after excluding those with very deep, superficial, or long-term wounds |
| Trontelj et al.50 | I: ES delivered with two electrodes placed on health skin at the edge of each wound for 2 hours daily; C: conventional treatment only | ES with pulse duration of 1.25 ms, frequency of 40 Hz was delivered 4 on 4seconds off. (15–25 mA) adjusted individually to achieve minimal muscle contraction | 8 weeks | Wound healing rate | Grade II | ES-treated wounds healed at almost twice the rate of those in the control group. Mean relative healing rate of the ES group was higher than the control group (4.89 ± 3.80 vs. 2.6 ± 2.59) |
| Recio et al.47 | HVES to the wound bed for 60-minute sessions 3–5 times per week | ES was delivered by twin peaked, monophasic, 10 µs pulse width | 12 months | PU status | Grade III | WSA decreased (11.5 cm2 at baseline vs. 0.4 cm2 at end of treatment). 2. The long-standing PUs were completely healed after 7–22 weeks |
| Lippert-Grüner53 | ES of gluteal muscles was delivered using anal probe for 15–20 minutes tid. | No details given | 6 weeks | Size of PUs | Grade IV | After 2 weeks of stimulation, the size of ulcers were reduced on both side, within 6 weeks, all ulcers were completed healed |
| Pollack et al.55 | ES of bilateral gluteus hamstring and quadriceps muscles twice weekly | ES with a frequency of 60 Hz and a pulse duration of 400 µs | 6.5 months | PU status | Grade IV | After 6.5 months of ES, the PU completed closed |
| Prevention studies | ||||||
| Kim et al.38 | I: bilateral sub-threshold ES of the gluteus muscles was applied using surface electrodes. C: sham ES | Biphasic, charge-balanced stimulation was applied at 10 Hz frequency with a pulse duration of 200 µs | 12 weeks after recruitment | TcPO2, muscle thickness, and interface pressure | Grade IB | 1. A 78% increase in TcPO2 immediately following ES in the intervention group, but this was not maintained at follow-up. 2. No significant changes in regional TcPO2, gluteal muscle thickness, or pressure distribution pre- and post-treatment using sub-threshold ES |
| Gyawali et al.34 | I: continuous stimulation; II: bursting stimulation, 3 bursts of stimuli were delivered bilaterally to the gluteus maximus muscles | ES with pulse duration of 200 µs and 40 Hz frequency | Dynamic | Interface pressure over the IT | Grade II | 1. Both continuous and bursting ES paradigms decreased pressure around IT. 2. Within the continuous paradigm, the 7 seconds of stimulation produced greater pressure reduction than 13 seconds stimulation. 3. ES increased signal intensity by MRI in the atrophied and loaded muscles |
| Londen et al.26 | I: the alternating stimulation of 0.5 seconds ES of one gluteal muscle and a 15 seconds rest, followed by 0.5 seconds stimulation of the other side and a 15 seconds rest. II: the simultaneous stimulation of a 0.5 seconds stimulation of both gluteal muscles followed by a 15-second rest | Rectangular monophasic pulses were applied with 50 Hz stimulation frequency and 80 mA current amplitude | Dynamic | Interface pressure | Grade II | 1. Both alternating and simultaneous stimulation caused a significant (P < 0.01) decrease in interface pressure (−17 ± 12 mmHg, −19 ± 14 mmHg) and pressure gradient (−12 ± 11 mmHg, −14 ± 12 mmHg) during stimulation periods compared with rest periods. 2. There was no significant difference in effects between the alternating and simultaneous stimulation |
| Petrofsky40 | ES of quadriceps for 10–15 minutes per day; after 4 weeks, sequence stimulation of the quadriceps, gluteus maximus, and hamstring muscles for 30 minutes, 3 days/week | ES with pulse width of 350 µs, at frequency of 40 Hz, and amplitude varies from 0 to 150 mA | 1 year | Incidence of PU | Grade II | The incidence of PU was 5.2% in SCI who had ES, 32% in control population |
| Smit et al.48 | ES to gluteal and hamstring muscles using a custom-made electrode garment with build-in electrodes | 1 hour stimulation to gluteal muscles (g) or gluteal +hamstring muscle (g + h).Gluteal muscles were stimulated first and then g + h muscles | On time | Interface pressure over the IT and pressure gradient | Grade III | 1. Pressure reduced by 34.5% after g + h muscles activation compared with rest pressure, 2. Pressure reduced by 10.2% after activation of g muscles only. 3. Pressure gradient reduced significantly only after stimulation of g + h muscles (49.3%) |
| Smit et al.49 | ES to gluteal and hamstring muscles was delivered through surface electrodes | ES with a duty cycle of 1 second stimulation and 4 seconds rest for 3 minutes was delivered at standard 150 V, with 50 Hz, amplitude ranging from 55 to 125 mA to induce a titanic contraction | On time 4 hours | Interface pressure over the tuberosities, blood flow, and oxygenation | Grade III | 1. Pressure was significantly lower during ES as compared with rest. 2. There were no significant changes of oxygenation during ES as compared with rest. 3. There was a significant difference in peak blood flow during ES as compared with rest (P = 0.007), but no significant change on mean blood flow for ES |
| Liu et al.44 | ES to sacral nerve root was delivered using an sacral nerve root implant or a magnetic stimulator | Sacral ES frequency was 20 pps with pulse with of ranging from 128 to 600 µs | On time | Interface pressure under ischial tuberosities and skin blood flow | Grade III | 1. Peak pressure and gradient at peak pressure significantly decreased during FMS as compared with baseline. 2. Peak pressure and gradient at peak pressure significantly decreased during sacral nerve root via SARS implant as compared with baseline. 3. Ischial skin blood perfusion significantly increased during the FMS and SARS |
| Liu et al.45 | ES to the second sacral nerve root (S2) was delivered using an sacral anterior nerve root implant | Sacral ES frequency was 20 pps with pulse with of ranging from 64–600 µs | On time | Interface pressure under ischial tuberosities | Grade III | 1. Peak pressure and gradient at peak pressure significantly decreased during sacral nerve root via a SARS implant as compared with baseline |
| Bogie and Triolo41 | ES of gluteal muscles, leg, and back muscles was delivered by NMES implant | The exercise regime included 3 different stimulation patterns with frequency 16 or 30 Hz. Ramp up “2 seconds”, on time “5 seconds” or “10 seconds”, ramp down “2 seconds” or “4 seconds” off time “‘10 seconds” | 8 weeks | Interface pressure and TcPO2 | Grade III | 1. There was no significant difference in overall mean interface pressure between baseline and post-exercise. 2. Mean region interface pressure statistically decreased post-conditioning as compared with baseline. 3. Baseline mean unloaded TcPO2, increased by 1–36% at post-exercise assessment for five participants, but showed a decrease in other three participants. 3. Differences between baseline and post-exercise TcPO2, levels were not statistically significant |
| Mawson et al.46 | HVPGS was applied using electrodes taped on the spine when participants were supine or prone | HVPGS of 50 V and 10 Hz, then at 75 V and 10 Hz was applied to the back T6 during prone. HVPGS of 75 V and 10 Hz was delivered during prone | On time | Sacral transcutaneous oxygen tension TcPO2 | Grade III | Sacral TcPO2 was increased during HVPGS and the results were reproducible |
| Levine et al.43 | ES of gluteus maximus began with a 20 minutes rest, followed by 12 minutes stimulation | 50 Hz with a duty cycle of 2 on 4 seconds off | On time | Ischia region muscle blood flow | Grade III | All participants showed an increase in muscle blood flow during ES |
| Wu et al.51 | ES to bilateral lumber spinal muscle and gluteal muscle was delivered by NMES implant | 20 Hz, 20 mA pulse amplitude | On time | Interface pressure under tuberosities and region TcPO2 | Grade III | 1. Maximum interface pressure gradient showed a variable response overall. 2. Subgroup analysis for sacral sitters, sacral interface pressure, and maximum interface pressure gradient tend to decrease on ES application; mean TcPO2 increased during ES and remained elevated after the intervention |
| Ferguson et al.42 | ES of quadriceps was applied bilaterally and simultaneously 30 minutes per day for at least 5 days/week | Pulse width 300 µs, frequency 20 Hz and amplitude 100 mA. The stimulation was applied for 10 seconds intervals with 20 seconds rest period, which was repeated after a one minute rest | On time | Pressure at ischia | Grade III | 1. Mean pressure across all participants at both ischia reduced during the stimulation as compared with resting (55 vs. 99 mmHg on the right, 49 vs. 76 mmHg on the left, respectively). 2. Two participants had an increase in left pressure during quadriceps stimulation. 3. In general, the greatest reductions occurred in participants with large knee movement |
| Bogie et al.52 | ES of gluteal muscles was delivered using an NMES implant | Alternating left and right gluteal stimulation at 20 Hz, 15 seconds on and 15 seconds off to each muscle for a 3-minute period on and 17-minute interval for up to 10 hours/day. | 5 years | Seated interface pressure, tissue oxygen, gluteal muscle thickness, and sitting tolerance | Grade IV | 1. Seating interface pressure was reduced significantly at 6 weeks, 6 months, and 40 months follow-up. 2. Tissue oxygen level improved over the study time. 3. Gluteal muscle thickness was increased at 1 year and 5 years. 4. Sitting tolerance had increased from 6 hours a day to more than 12 hours a day |
| Rischbieth et al.54 | ES of gluteal muscles for 15 minutes tid between 0 and 4 months, 30 minutes bid between 7 and 24 months | Frequency was 30 pps, duty cycle was 10:15 seconds between 0 and 1 months, 10:8 between 4 and 24 months; intensity was 54% at start, 80% at 1 month and 100% between 4 and 24 months | 24 months | Dimension of buttocks and sitting tolerance | Grade IV | The circumferential dimensions across the buttocks were increased 21% |
| Vanoncini et al.56 | ES of erector spine through surface electrodes | A train of square pulses with a frequency of 50 Hz and a fixed pulse width of 450 µs and manually altered pulse amplitude | On time | Seated interface pressure | Grade IV | 1. The pressure decreased on the side opposite to the stimulation. 2. Sitting tolerance increased from 30 minutes to more than 2 hours |
Intervention effectiveness
Overall, there were 11 studies that investigated dynamic effect of ES, 5 studies evaluated long-term effects. Within 11 studies that investigated dynamic effect, 8 demonstrated a significant reduction of pressure under the ischial tuberosities; five studies measured local tissue oxygenation or blood flow with three of the five studies reporting a significant increase in regional tissue oxygenation or blood flow during the stimulation. There were two studies that reported an increase of tissue oxygenation in some participants, though not all.
In relation to the long-term effect, while the majority of the five studies demonstrated positive changes including reduced seating pressure or incidence of PUs, increased muscle thickness, ischial tissue oxygenation, and sitting tolerance, one study reported no change on gluteal thickness or pressure distribution after a 12-week follow-up. In addition, another case series assessed the effects of an 8-week period of exercise using ES in eight subjects. The reported mean of unloaded tissue oxygen levels increased post-exercise for five participants, but showed a decrease in the other three participants. The authors also indicated that there were no statistically significant differences between baseline and post-exercise tissue oxygen levels.
Adverse events
In total, 4 out of 16 preventive studies reported on adverse events. Among these four studies, two studies delivered ES using surface electrodes and two studies used a SARS implant. All four of these studies reported no adverse events experienced by the participants.
Discussion
In this systematic review of 27 studies, 11 studies applied ES for the treatment of PUs. It was found that the outcome measure of therapeutic effectiveness varied, which include size of the wounds, healing times, healing status, and healing rates defined as percentage changes weekly or daily. The heterogeneity of study design together with diverse stimulation parameters and outcome measures across the studies prevented us from performing a formal meta-analysis. Nevertheless, as a whole of the 11 therapeutic studies, ES significantly enhanced PU healing in the SCI. Two therapeutic RCTs were classified as Grade I evidence, in which the authors used high-voltage pulse currents to stimulate the wound bed and reported a significant improvement in PU healing. The percentage of reduction of PU in the ES group was as twice large as that in the control group. In an inadequately reported therapeutic RCT that was classified as Grade II evidence, the authors compared the effect of placement of electrodes and concluded that healing of PUs was significantly enhanced by ES with the positive stimulation electrode overlaying the wound surface and the negative electrode placed on the intact skin around the wound. By contrast, both stimulation electrodes being positioned on the healthy skin at the ulcer edge across the wound had only a non-significant effect on PU healing.37 Although this is a double-blinded trial, the interventions were not assigned randomly, there was no adequate description of allocation concealment, nor a sample size calculation. Future well-designed RCTs are urgently needed to confirm the beneficial effect of ES on PU healing in SCI.
In terms of the type of modalities to deliver ES in therapeutic studies, one case study used an anal probe to stimulate the gluteal maximus to treat large gluteal decubitus ulcers on both sides, in a patient with T9-level SCI.53 The authors indicated that after 2 weeks stimulation, size of the ulcers reduced and that within 6 weeks, both ulcers had healed. This was a single case report and was published in 2003. There have been no follow-up papers published, the reliability of the results are uncertain. The other 10 of the 11 therapeutic studies applied surface electrodes. The surface ES system utilizes electrodes that are placed on the skin and connected with flexible leads to a stimulator. Nine of 10 studies placed the stimulating electrodes over the wound directly or the intact skin nearby wound. The remaining study stimulated bilateral gluteus, hamstring, and quadriceps muscles to treat an ischial PU.
Indeed, ES has been proposed as a therapeutic modality for wound healing over a century ago and has been well documented since the 1960s especially for wounds not responding to standard forms of treatment.13,21,22 A number of theories as to why ES may stimulate wound healing have been suggested. One theory is that when a wound occurs, there is a weak but measurable current between the skin and inner tissues, called the current of injury. The current is thought to continue until the skin defect is repaired.57 Application of an external electrical current to wounds can facilitate some aspects of the repair process and pre-clinical studies have given some indication of the mechanism of ulcer healing being enhanced by functional ES. For instance, ES has been demonstrated to enhance cellular activities such as collagen and DNA synthesis, ATP concentration, and generation of chemotaxic factors. ES has also been shown to increase tissue perfusion, decrease edema, and promote angiogenesis and galvanotaxis, directing cell migration in the wound tissue to promote wound healing.19,57,58
Within the 27 studies included in this review, 16 studies applied ES for PU prevention. Eleven of 16 studies investigated dynamic effect of stimulation and demonstrated beneficial effects on decreased interface pressure under ischial tuberosities. The underlying mechanism of reducing pressure under ischial tuberosities during the dynamic stimulation has been suggested to be pressure redistribution, which was caused by either pelvic and/or leg tilt or changes of gluteal muscle force. Five of the 11 studies measured tissue oxygenation or blood flow, and 3 of these showed a significant increase during the ES, with the remaining 2 studies reporting inconsistent findings. The exact mechanism of improving local tissue oxygenation and blood flow during the dynamic ES remains unclear, but a dynamic “pressure relief” caused by gluteus muscle contractions and/or pelvic tilt, which dilates the micro-vessels underlying the ischial skin, may be partly attributable. Alternatively, increased blood perfusion may result from muscle contraction allowing higher oxygen delivery rates and metabolite removal, or neuronal excitation and cardiovascular response. However, of the three studies which investigated the interface pressure and tissue oxygenation or blood flow simultaneously, none of them approved the hypothesis that ES-induced muscle activation would directly increase blood flow and oxygenation.
There were five studies which explored the long-term effect of functional ES in PU prevention in SCI and three of them reported beneficial changes, e.g. increased muscle thickness, reduced seating pressure, increased ischial tissue oxygenation, and sitting tolerance or a reduction in the incidence of PUs after a period of ES. Conversely, the other two studies reported no statistically significant difference noted in muscle thickness, pressure distribution under ischial tuberosities or tissue oxygenation after ES. It is worth noting, the follow-up period of the two studies with non-significant findings seems to be much shorter than that in the other three studies with positive findings (8 weeks and 12 weeks with non-significant findings vs. 1, 2, and 5 years in those with positive findings). However, among the three studies with positive findings, two were single case reports that provide the weakest evidence. The long-term beneficial effect of ES for PU prevention is therefore inconclusive, based on the low level of evidence with diverse findings in the five long-term studies. Well-designed and large sample sized studies to investigate the long-term of effect of ES for PU prevention are unquestionably needed.
With respect to the type of modalities to deliver ES in 16 preventive studies, 4 different types of neuromuscular ES have been identified in this review, with the traditional surface ES system being the most commonly used intervention, with the electrodes being placed on the skin over the nerves or over the “motor points” of muscles to be activated. The advantage of the surface ES system is that it is non-invasive and relatively technologically simple. However, the repeated placement of the electrodes in the appropriate locations to get the desired response requires skill and patience. Also, it can be difficult to achieve isolated contractions or to activate the deep muscles. In addition, local skin reaction caused by electrodes together with managing the electrodes, wires, stimulators, and applying the electrodes to the skin each session is inconvenient for long-term use.
Interestingly, one PU preventive article reported ES as capable of stimulating gluteal muscles and hamstrings using a custom-made electrode garment with built-in electrodes (ES shorts).48 The ES shorts (Axiobionics, Ann Arbor, MI, USA) were custom-developed lycra shorts in which wires and surface electrodes were integrated. These were operated by placing two built-in surface electrodes over gluteal muscles and hamstring muscles on both sides. While this innovative device is easy to put on, and also avoids the need for skin preparation (thus overcoming some of the common disadvantages of traditional surface ES), the authors indicated the key limitation of such a device is that the electrodes were fixed to one place in a one size of shorts. They suggested that the electrodes should be individually positioned in flexible shorts to make it more practical and efficient. Future research on an improved design of the ES shorts in order to improve the flexibility and efficiency, as well as more clinical studies, is needed.
Two types of implants were identified in this review, one of them named SARS implant,44,45 which was implanted first in patients with SCI in 1976 to aid bladder management. A typical SARS implant (Finetech-Brindley SARS implant) usually utilizes electrodes that are implanted into the S2, S3, and S4 roots. Because the S2 carries only a subordinate role for the urinary bladder, patients had S3 and S4 nerve roots implanted, as many studies confirmed the highest detrusor response was registered at these two nerve roots. It is known that the S2 nerve roots always innervate the gluteus maximus, triceps surae, and also innervate other glutei, the biceps femoris and the pelvic floor. In this review, two studies conducted by Liu et al.44,45 explored benefit of stimulation of the S2 nerve root for activating gluteal maximus and consequently decreasing the interface pressure under the ischial tuberosities and increasing localized blood flow to the skin. The authors concluded that in addition to bladder and bowel management, using a SARS implant may confer long-term benefit for tissue health in SCI. Another type of implant is the NMES system.41,51,52 The stimulators of NMES system are either placed externally (percutaneous) or fully implanted within the body. The former utilizes intramuscular electrodes that pass through the skin and are implanted into the muscles to be activated. An electrode is inserted through the skin and implanted in the muscle using a hypodermic needle and the electrode leads exit the skin and are connected to the external stimulator. A surface electrode is used as the return electrode. The percutaneous interface on the skin is protected by placing a junction connector over the skin surface where the electrodes exit and the percutaneous electrodes can then activate deep muscles, provide isolated and repeatable muscle contractions, and are less likely to produce pain during stimulation because they bypass the sensory afferents in the skin. In this review, one study used a four-channel percutaneous gluteal NMES system to improve gluteus tissue health in one patient with a SCI at level C4, in which the intramuscular electrodes were implanted bilaterally into the gluteus maximus. Another version of an NMES implant, in which the stimulation electrodes alongside the stimulator were fully implanted, was developed for standing and transfer in SCI. The appropriate muscle groups with this implant include the gluteus maximus, hamstrings, vastus lateralis, and erector spine. The implanted electrodes are connected by leads under the skin to the implanted stimulator, which eliminates the need for wiring outside of the body to an external stimulator. The electrodes can be made with larger and more durable leads because they do not pass through the skin and these may be powered by implanted batteries, in which case, revision surgery is only required every few years to replace the batteries.
Study limitations
Systematic reviews always present a number of limitations. These include publication bias (particularly against negative findings), language restrictions, and coding of key words. However, we adopted a well-structured search strategy that was approved by a clinical librarian, and supplemented all “explode” functions and utilized hand searches as well as contacting a specialist to minimize the potential for bias. A further limitation is the inclusion of a case report in the review, which was classified as providing a low level of evidence. Nevertheless, the aim and objectives of this current systematic review was to identify the updated evidence, and to make recommendations for future research, implementing electrical stimulation for PU management in SCI. Including such a case report study in our review has undoubtedly enabled provision of more thorough and broader evidence of ES, in the application of treating or preventing PUs in SCI.
Conclusion
In appraising ES as an intervention for PU prevention and treatment in SCI, there is a recognition of the challenges in selecting appropriate stimulation parameters, e.g. stimulation currents, stimulation frequency, length of time of stimulation, and outcome measures, which are not usually possible to validate and standardize.
The methodological quality of the studies included in this review was generally weak, in particular for those prevention studies, as most of them were case series without the control groups. There were only a small number of studies that assessed the long-term effect of ES on PU prevention. It has long been established that preventing a PU occurrence is crucial for the SCI population and the lack of Grade I evidence has undoubtedly limited the implementation of ES for PU prevention. Future research is recommended to conduct more rigorous long-term clinical studies, as well as improve the design of ES devices and determine standardized outcome measures in prevention of PUs.
A significant effect of ES on enhancement of PU healing is shown in limited Grade I evidence. The great variability in ES parameters, stimulating locations, and outcome measure leads to an inability to advocate any one standard approach for PU treatment. Future work is therefore recommended and urgently needed in the form of well-designed clinical studies using large sample populations on determining the optimal stimulation location and parameters to confirm the beneficial effect on the enhancement of PU healing in SCI.
Disclaimer statements
Contributors LQL designed the study, collected the data, analyzed and interpreted the data, wrote and revised the article in whole. JM collected the data, analyzed the data and revised the article. MT and SD interpreted the data and revised the article. AG designed the study, interpreted the data, wrote and revised the article.
Funding None.
Conflicts of interest None.
Ethics approval None.
Acknowledgment
We would like to thank Mr Paul Howell, a clinical librarian for helping online literature databases search. Authors also would like to thank Dr Sinead Mehigan, Head of Department of Adult, Child and Midwifery, School of Health and Education, Middlesex University, London, UK, for providing Endnote bibliographic software.
References
- 1.Anonymous. Spinal Cord Injury Statistics. [cited 2014 Apr 23]. Available at: http://www.apparelyzed.com/statistics.html .
- 2.National Spinal Cord Injury Statistical Center. [cited 2014 Apr 23]. Available at: https://www.nscisc.uab.edu .
- 3.McKinley WO, Jackson AB, Cardenas DD, DeVivo MJ. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil 1999;80(11):1402–10. [DOI] [PubMed] [Google Scholar]
- 4.Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Post MW. Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. J Rehabil Med 2007;39(5):393–8. [DOI] [PubMed] [Google Scholar]
- 5.Tam EW, Mak AF, Lam WN, Evans JH, Chow YY. Pelvic movement and interface pressure distribution during manual wheelchair propulsion. Arch Phys Med Rehabil 2003;84(10):1466–72. [DOI] [PubMed] [Google Scholar]
- 6.Ash D. An exploration of the occurrence of pressure ulcers in a British spinal injuries unit. J Clin Nurs 2002;11(4):470–8. [DOI] [PubMed] [Google Scholar]
- 7.Niazi ZB, Salzberg CA, Byrne DW, Viehbeck M. Recurrence of initial pressure ulcer in persons with spinal cord injuries. Adv Wound Care 1997;10(3):38–42. [PubMed] [Google Scholar]
- 8.National PU Advisory Panel and the European PU Advisory Panel (NPUAP/EPUAP). Prevention and treatment of PUs: clinical practice guideline. Washington, DC: NPUAP; 2009, p. 169. [Google Scholar]
- 9.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil 2004;85(11):1757–63. [DOI] [PubMed] [Google Scholar]
- 10.Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21(6):261–2, 264, 266. [DOI] [PubMed] [Google Scholar]
- 11.National Spinal Cord Injury Statistical Center. The 2005 annual statistical report for the model spinal cord injury care systems. Birmingham, AL: National Spinal Cord Injury Statistical Center; 2005. p. 119–22. [Google Scholar]
- 12.Marin J, Nixon J, Gorecki C. A systematic review of risk factors for the development and recurrence of pressure ulcers in people with spinal cord injuries. Spinal Cord 2013;51(7):522–7. [DOI] [PubMed] [Google Scholar]
- 13.Bogie KM, Reger SI, Levine SP, Sahgal V. Electrical stimulation for pressure sore prevention and wound healing. Assist Technol 2000;12(1):50–66. [DOI] [PubMed] [Google Scholar]
- 14.Jones ML, Mathewson CS, Adkins VK, Ayllon T. Use of behavioural contingencies to promote prevention of recurrent pressure ulcers. Arch Phys Med Rehabil 2003;84(6):796–802. [DOI] [PubMed] [Google Scholar]
- 15.Makhsous M, Rowles DM, Rymer WZ, Bankard J, Nam EK, Chen D, et al. . Periodically relieving ischial sitting load to decrease the risk of PUs. Arch Phys Med Rehabil 2007;88(7):862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogie KM, Nuseibeh I, Bader DL. Early progressive changes in tissue viability in the seated spinal cord injured subject. Paraplegia 1995;33(3):141–7. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson-Pell MW, Wilkie IC, Reswick JB, Barbenel JC. Pressure sore prevention for the wheelchair-bound spinal injury patient. Paraplegia 1980;18(1):42–51. [DOI] [PubMed] [Google Scholar]
- 18.Mawson AR, Biundo JJ Jr, Neville P, Linares HA, Winchester Y, Lopez A. Risk factors for early occurring pressure ulcers following spinal cord injury. Am J Phys Med Rehabil 1988;67(3):123–7. [DOI] [PubMed] [Google Scholar]
- 19.Rivkah Isseroff R, Dahle SE. Electrical stimulation therapy and wound healing: where are we now? Adv Wound Care 2011;1(6):238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan MA, Teasell RW, Wolfe DL, Keast D, Mortenson WB, Aubut JL, for the Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of therapeutic interventions for pressure ulcers after spinal cord injury. Arch Phys Med Rehabil 2009;90(2):213–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloth LC, Feedar JA. Acceleration of wound healing with high voltage, monophasic, pulsed current. Phys Ther 1988;68(4):503–8. [DOI] [PubMed] [Google Scholar]
- 22.Baker LL, Rubayi S, Villar F, Demuth SK. Effect of electrical stimulation waveform on healing of ulcers in human beings with spinal cord injury. Wound Repair Regen 1996;4(1):21–8. [DOI] [PubMed] [Google Scholar]
- 23.Levine SP, Kett RL, Cederna PS, Brooks SV. Electric muscle stimulation for pressure sore prevention: tissue shape variation. Arch Phys Med Rehabil 1990;71(3):210–5. [PubMed] [Google Scholar]
- 24.Levine SP, Kett RL, Cederna PS, Bowers LD, Brooks SV. Electrical muscle stimulation for pressure variation at the seating interface. J Rehabil Res Dev 1989;26(4):1–8. [PubMed] [Google Scholar]
- 25.Mittmann N, Chan BC, Craven BC, Isogai PK, Houghton P. Evaluation of the cost-effectiveness of electrical stimulation therapy for pressure ulcers in spinal cord injury. Arch Phys Med Rehabil 2011;92(6):866–72. [DOI] [PubMed] [Google Scholar]
- 26.Londen A, Herwegh M, Zee CH, Daffertshofer A, Smit CA, Niezen A, et al. . The effect of surface electric stimulation of the gluteal muscles on the interface pressure in seated people with spinal cord injury. Arch Phys Med Rehabil 2008;89(9):1724–32. [DOI] [PubMed] [Google Scholar]
- 27.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. . Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17(1):1–12. [DOI] [PubMed] [Google Scholar]
- 28.Liu LQ, Pengel LH, Morris PJ. CONSORT compliance and methodological quality of randomized controlled trials in solid organ transplantation, a 3-year overview. Transpl Int 2013;26(3):300–6. [DOI] [PubMed] [Google Scholar]
- 29.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52(6):377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding R, Karus D, Easterbrook P, Raveis VH, Higginson IJ, Marconi K. Does palliative care improve outcomes for patients with HIV/AIDS? A systematic review of the evidence. Sex Transm Infect 2005;81(1):5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clucas C, Sibley E, Harding R, Liu L, Catalan J, Sherr L. A systematic review of interventions for anxiety in people with HIV. Psychol Health Med 2011;16(5):528–47. [DOI] [PubMed] [Google Scholar]
- 32.Adegoke BO, Badmos KA. Acceleration of pressure ulcer healing in spinal cord injured patients using interrupted direct current. Afr J Med Med Sci 2001;30(3):195–7. [PubMed] [Google Scholar]
- 33.Griffin JW, Tooms RE, Mendius RA, Clifft JK, Vander Zwaag R, El-Zeky F. Efficacy of high voltage pulsed current for healing of PUs in patients with spinal cord injury. Phys Ther 1991;71(6):433–42; discussion 442–4. [DOI] [PubMed] [Google Scholar]
- 34.Gyawali S, Solis L, Chong SL, Curtis C, Seres P, Kornelsen I, et al. . Intermittent electrical stimulation redistributes pressure and promotes tissue oxygenation in loaded muscles of individuals with spinal cord injury. J Appl Physiol (1985) 2011;110(1):246–55. [DOI] [PubMed] [Google Scholar]
- 35.Houghton PE, Campbell KE, Fraser CH, Harris C, Keast DH, Potter J, et al. . Electrical stimulation therapy increases rate of healing of pressure ulcers in community-dwelling people with spinal cord injury. Arch Phys Med Rehabil 2010;91(5):669–78. [DOI] [PubMed] [Google Scholar]
- 36.Jercinovic A, Karba R, Vodovnik L, Stefanovska A, Kroselj P, Turk R, et al. . Low frequency pulsed current and PU healing. IEEE Trans Rehabil Eng 1994;2(4):225–33. [Google Scholar]
- 37.Karba R, Semrov D, Vodovnik L, Benko H, Savrin R. Direct current electrical stimulation for chronic wound healing enhancement. Part 1: clinical study and determination of electrical field distribution in the numerical wound model. In Bioelectrochem Bioenerg 1997;43:265–70. [Google Scholar]
- 38.Kim J, Ho CH, Wang X, Bogie K. The use of sensory electrical stimulation for PU prevention. Physiother Theory Pract 2010;26(8):528–36. [DOI] [PubMed] [Google Scholar]
- 39.Stefanovska A, Vodovnik L, Benko H, Turk R. Treatment of chronic wounds by means of electric and electromagnetic fields. Part 2. Value of FES parameters for pressure sore treatment. Med Biol Eng Comput 1993;31(3):213–20. [DOI] [PubMed] [Google Scholar]
- 40.Petrofsky JS. Health care benefits in patients who are involved in an electrical stimulation exercise program. J Neurol Orthop Med Surg 1992;13:249–54. [Google Scholar]
- 41.Bogie KM, Triolo RJ. Effects of regular use of neuromuscular electrical stimulation on tissue health. J Rehabil Res Dev 2003;40(6):469–75. [DOI] [PubMed] [Google Scholar]
- 42.Ferguson AC, Keating JF, Delargy MA, Andrews BJ. Reduction of seating pressure using FES in patients with spinal cord injury. A preliminary report. Paraplegia 1992;30(7):474–8. [DOI] [PubMed] [Google Scholar]
- 43.Levine SP, Kett RL, Gross MD, Wilson BA, Cederna PS, Juni JE. Blood flow in the gluteus maximus of seated individuals during electrical muscle stimulation. Arch Phys Med Rehabil 1990;71(9):682–6. [PubMed] [Google Scholar]
- 44.Liu LQ, Nicholson GP, Knight SL, Chelvarajah R, Gall A, Middleton FRI, et al. . Interface pressure and cutaneous hemoglobin and oxygenation changes under ischial tuberosities during sacral nerve root stimulation in spinal cord injury. J Rehabil Res Dev 2006;43(4):553–64. [DOI] [PubMed] [Google Scholar]
- 45.Liu LQ, Nicholson GP, Knight SL, Chelvarajah R, Gall A, Middleton FRI, et al. . Pressure changes under the ischial tuberosities of seated individuals during sacral nerve root stimulation. J Rehabil Res Dev 2006;43(2):209–18. [DOI] [PubMed] [Google Scholar]
- 46.Mawson AR, Siddiqui FH, Connolly BJ, Sharp CJ, Stewart GW, Summer WR, et al. . Effect of high voltage pulsed galvanic stimulation on sacral transcutaneous oxygen tension levels in the spinal cord injured. Paraplegia 1993;31(5):311–9. [DOI] [PubMed] [Google Scholar]
- 47.Recio AC, Felter CE, Schneider AC, McDonald JW. High-voltage electrical stimulation for the management of Stage III and IV pressure ulcers among adults with spinal cord injury: demonstration of its utility for recalcitrant wounds below the level of injury. J Spinal Cord Med 2012;35(1):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smit CAJ, Haverkamp GLG, de Groot S, Stolwijk-Swuste JM, Janssen TWJ. Effects of electrical stimulation-induced gluteal versus gluteal and hamstring muscles activation on sitting pressure distribution in persons with a spinal cord injury. Spinal Cord 2012;50(8):590–4. [DOI] [PubMed] [Google Scholar]
- 49.Smit CAJ, Zwinkels M, van Dijk T, de Groot S, Stolwijk-Swuste JM, Janssen TWJ. Gluteal blood flow and oxygenation during electrical stimulation-induced muscle activation versus pressure relief movements in wheelchair users with a spinal cord injury. Spinal Cord 2013;51(9):694–9. [DOI] [PubMed] [Google Scholar]
- 50.Trontelj K, Karba R, Vodovnik L, Savrin R, Strukelj MP. Treatment of chronic wounds by lower frequency pulsed electrical current. J Tissue Viability 1994;4(4):105–9. [Google Scholar]
- 51.Wu GA, Lombardo L, Triolo RJ, Bogie KM. The effects of combined trunk and gluteal neuromuscular electrical stimulation on posture and tissue health in spinal cord injury. PM R 2013;5(8):688–96. [DOI] [PubMed] [Google Scholar]
- 52.Bogie KM, Wang X, Triolo RJ. Long-term prevention of pressure ulcers in high-risk patients: a single case study of the use of gluteal neuromuscular electric stimulation. Arch Phys Med Rehabil 2006;87(4):585–91. [DOI] [PubMed] [Google Scholar]
- 53.Lippert-Grüner M. Gluteal neuromuscular stimulation in therapy and prophylaxis of recurrent sacral pressure ulcers. Spinal Cord 2003;41(6):365–6. [DOI] [PubMed] [Google Scholar]
- 54.Rischbieth H, Jelbart M, Marshall R. Neuromuscular electrical stimulation keeps a tetraplegic subject in his chair: a case study. Spinal Cord 1998;36(6):443–5. [DOI] [PubMed] [Google Scholar]
- 55.Pollack SF, Ragnarsson KT, Dijkers M. The effect of electrically induced lower extremity ergometry on an ischial pressure ulcer: a case study. J Spinal Cord Med 2004;27(2):143–7. [DOI] [PubMed] [Google Scholar]
- 56.Vanoncini M, Holderbaum W, Andrews BJ. Activation of lower back muscles via FES for pressure sores prevention in paraplegia: a case study. J Med Eng Technol 2010;34(3):224–31. [DOI] [PubMed] [Google Scholar]
- 57.Sussman C, Byl N. Electrical stimulation for wound healing. Wound care collaborative practice manual for physical therapists and nurses. New York: Aspen Publishers; 1998. [Google Scholar]
- 58.Bourguignon GJ, Bourguignon LYW. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1987;1(5):398–402. [DOI] [PubMed] [Google Scholar]