Abstract
Objective
Characterization of a non-invasive method of quantifying subepidermal moisture (SEM) surrounding stages III and IV pressure ulcers (PrUs) in spinal cord injury (SCI).
Design
Prospective, single-visit, single-rater, observational study, using repeated-measures analysis.
Method
Setting-inpatient units of one VA SCI Center.
Participants
Convenience sample of 16 subjects with SCI with stage III or IV PrUs over sacrum or ischium.
Interventions
Measurement with the MoistureMeter-D, a hand-held device using 300 MHz electromagnetic waves.
Outcome measures
Dielectric constant, a dimensionless number which increases with the moisture content. Each subject had a PrU site and a control site. Measurements were made at each site, on intact skin, at four points spaced angularly around the site, in triplicate.
Results
(1) Short-term, single-rater relative error was 2.5%. (2) Order effect: first readings were higher than second readings in 55 of 64 measurement sets. Order effect was significant for control sites (P < 0.0001) but not for PrU sites. (3) Angular effect: SEM varied by angle at the PrU sites (P < 0.01); 12 o'clock position the highest and 6 o'clock the lowest. (4) Ability to differentiate PrUs from intact skin: SEM at PrU sites was greater by 9.0% than control sites (P < 0.05). (5) Site effect: SEM was higher at sacral locations than ischial at control sites by 20% (P < 0.005).
Conclusions
SEM differentiates PrUs from intact skin. Future study designs must take into account order, angular, and site effects on this measure. This information will inform designers of future studies of SEM in healing of PrUs.
Keywords: Pressure ulcer, Edema, Spinal cord injuries, Instrumentation, Subepidermal moisture, Dielectric constant
Introduction
Over the last 6 years, there have been a series of papers exploring the use of subepidermal moisture (SEM) to predict pressure ulcer (PrU) risk in various populations. Bates-Jensen et al.1 examined SEM in a cohort of 31 nursing home (NH) residents, without spinal cord injury (SCI), and found that SEM differentiated between erythema and stage I PrUs. A second paper by these researchers extended these findings to a group that is poorly served by visual assessment of erythema, NH residents with dark skin tones. They investigated 66 NH residents (non-SCI) with dark skin tones and found that higher SEM was associated with increased future risk (1 week) of developing a PrU.2 The third paper of this series used an SEM device in an SCI population. They studied 34 veterans with SCI, and compared SEM and concurrent visual skin assessment (VSA) across nine anatomic locations for up to 6 weeks. They found that SEM differed significantly between normal skin and erythema/stage I PrU at the buttocks, and that SEM at the heels was lower than other sites regardless of skin condition.3
The impetus for this previous work is the belief that an easy-to-use, reliable, and valid device that measures SEM might detect an increased risk of PrU formation, which could lead to interventional measures to prevent further damage. In a larger sense, this work evolves from the realization that VSA has limitations for accurate prediction of PrU development or PrU healing. This was one of the conclusions of an expert panel that met in 2009 to develop a research agenda for PrUs in the SCI population under the sponsorship of the Veterans Administration (VA) SCI Quality Enhancement Research Initiative (QUERI).4 Our group has been interested in monitoring PrU healing, and developed an enhanced tool based on visual observations, that is reliable and valid in the SCI population.5,6 In order to add a biophysical measurement to visual assessment, we looked at SEM as a possible measure of PrU healing. Using SEM to monitor PrU healing might provide additional information above that of VSA, which could influence clinical decisions concerning wound healing, an application of SEM that has not yet been reported in the literature.
The purposes of this pilot study to report the findings of our initial experience with a device that measures SEM in a cohort of veterans with SCI with stages III–IV PrUs. The following research questions were addressed:
Q1. What is the short-term reproducibility of the device as determined by a single observer making three repeated measurements over the course of a few minutes?
Q2. Is there a trial order effect; i.e. are three repeated measurements taken in one position, over the course of a few minutes, statistically independent?
Q3. Does SEM surrounding a PrU vary according to angular orientation around the ulcer?
Q4. How effective is the device in differentiating PrUs from uninvolved skin?
Q5. Is there a difference between sacral and ischial locations for either control site SEM or in the ability of the device to differentiate PrUs from uninvolved skin?
Methods
Study design
This pilot was designed as a descriptive, single-visit, single-rater, observational study using a repeated-measures analysis. The setting was a single Veterans' Health Administration (VHA) Spinal Cord Injury/Disorders (SCI/D) Center.
The study was approved by the Institutional Review Board (IRB) and informed consent was obtained prior to study. Subjects signed an additional consent if they consented with photography.
Subjects
Sixteen subjects with SCI who had stage III or IV PrUs over the sacrum or ischium were included. Inclusion criteria were subjects having a single PrU in the sacral or ischial areas. Subjects were excluded if they had an acute medical illness other than a PrU.
Instrumentation
The MoistureMeter-D (Delfin Technologies Ltd, Kuopio, Finland) uses low-power 300 MHz electromagnetic waves to measure SEM. SEM is a non-calibrated number which correlates with the dielectric constant, which varies with the amount of free water in the tissue.7,8 The dielectric constant of air is 1.0, of dry skin 49, and of muscle 58.9 The depth of measurement is controlled by using different diameter probes. Available probes have diameters ranging from 10 to 55 mm, corresponding to effective measurement depths of 0.5–5 mm. We chose the probe for 1.5 mm effective measurement depth (Fig. 1) for its ability to include both epidermis and dermis, but not extend into the muscle.
Figure 1 .
MoistureMeter-D with probes of four diameters. The probe used in this study was the third from the left, for an effective measurement depth of 1.5 mm.
Site selection and identification
For each subject, a control site corresponding to the PrU was marked with a felt-tipped marker. For the ischial ulcers, the control site was over the contralateral uninvolved ischium, consisting of a region of uninvolved skin the same diameter and same relative location as the PrU on the involved side. For sacral PrU, the control site was located ∼12 cm proximal to the PrU, in the midline, also consisting of an area of uninvolved skin the same diameter as the sacral PrU.
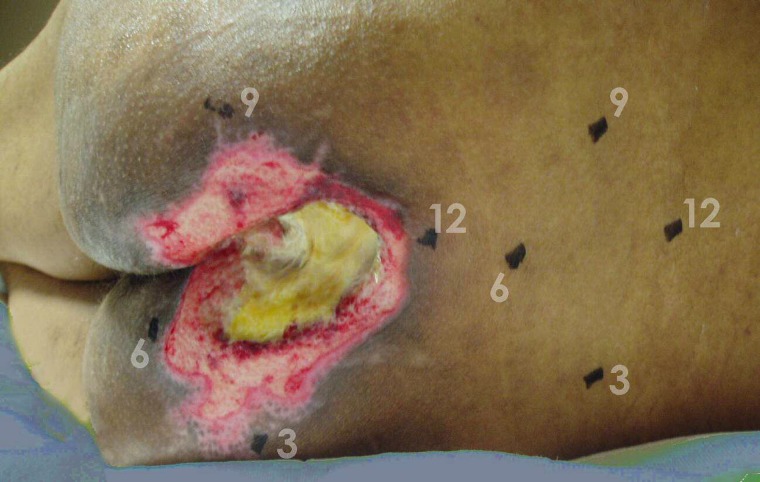
At each site, four measurement points were marked with a felt-tipped marker. These four measurement sites were located ∼1 cm from the PrU margin, which was sufficient to allow the entire area of the probe to be placed over intact skin. The four measurement points were spaced circumferentially around the wound at 90° intervals and aligned with the body axis (cranial, right, caudal, and left). The angular orientation of these measurement points will be referred to as 12, 3, 6, and 9 o'clock, respectively, to conform to the conventions of PrU assessment. The purpose of the marking was to improve the repeatability of the measurements and for photographic documentation. Photographs were made of subjects who signed an additional consent for photography. Fig. 2 illustrates a typical subject with a sacral PrU, illustrating the PrU and the control site after demarcation.
Figure 2 .
Subject with sacral pressure ulcer and corresponding control site. Figures represent the clock positions describing where measurements were taken.
Measurement protocol
All measurements were performed with the veteran in bed. Time of day, time since last weight shift, and time since the veteran had last been out of bed were not standardized nor recorded.
Measurements were taken by applying the probe to dry skin and applying firm pressure enough to slightly indent the skin. After approximately 8 seconds of data acquisition to allow for signal averaging, the MoistureMeter-D emitted an audio tone indicating that the measurement was complete. The liquid crystal screen then displayed a number which was recorded on a paper data form by an assistant. Each subject was identified with a sequential study number.
For each veteran, data collection started at the PrU site at the 12 o'clock measurement point. After each measurement, the probe was moved 90° to the next measurement point, going around the PrU site in a clockwise direction. This set of 4 measurements was then repeated two more times (trials), for a total of 12 measurements (3 trials of 4 angular orientations). The control site was then measured in the same manner. Each subject had 24 measurements, characterized by the site (PrU or control), the angular orientation, and the trial number of the measurement set.1–3
The probe was cleaned between veterans with alcohol which was the method recommended by the manufacturer and approved by the IRB.
Statistical analysis
Analyses were completed using SPSS version 11 (SPSS, Inc., Chicago, IL, USA) and Statistical Analysis Software (SAS) (SAS, Inc., Cary, NC, USA) using repeated-measures analysis. α was set a priori to 0.05. The possibility of confounding by age was examined by using a Pearson product-moment correlation between age and mean at the control site. Controlling for age was not required since this was not found to be significant.
Q1: Short-term repeatability. Each set of three trials was averaged. Relative error was computed by dividing the mean by the standard deviation. The mean relative error was computed for all 64 relative error values (16 subjects × 4 angular orientations) at the control site.
Q2: Trial order effect. Three sequential measurements (trials) were performed for each measurement point. A within-repeated-measures analysis was completed for each site with the repeated value equating to the three sequential measurements (trials 1–3).
Q3: Angular orientation effect. For each measurement point, the three trials were averaged to yield one value for each angular orientation and site. A second set of within-subject repeated measures was used to determine if measurements differed by angular orientation. For this research question, the repeated value was the angular orientation (12, 3, 6, and 9 o'clock).
Q4: Differentiating PrUs from uninvolved skin (site effect). After averaging the three trials at each angular orientation location for both the involved and the control site, a mixed-model repeated-measures analysis was completed to compare across site. The repeated factor was the angular orientation.
Q5: Difference between sacral and ischial location. An average value for the four angular orientations was computed for each subject and site. The sacral (n = 9) and ischial (n = 7) locations were then compared across sites using the Mann–Whitney U test, since the subgroup size was small and not normally distributed. Then, the site was compared to the location using the Wilcoxon signed-rank test.
Results
Demographic statistics
Table 1 describes the subjects by age in years and level of SCI, and their PrU by duration in months, location, and stage.
Table 1 .
Subject and wound descriptors
| Mean ± S.D. | Range | |
|---|---|---|
| Age of subject (years) | 60.6 ± 14.6 | 38–79 |
| Age of wound (months) | 7.0 ± 8.6 | 0.5–36 |
| Level of injury | Number (N = 16) | |
| High quadriplegia (C1–C4) | 2 | |
| Low quadriplegia (C5–C8) | 3 | |
| High paraplegia (T1–T6) | 6 | |
| Low paraplegia (T7–L3) | 5 | |
| Location of wound | ||
| Right ischium | 3 | |
| Left ischium | 4 | |
| Sacrum | 9 | |
| Stages of wound | ||
| Stage III | 4 | |
| Stage IV | 12 | |
Q1: Short-term repeatability. The mean relative error for all sets of three trials taken at the control site (n = 64) was 2.5% (confidence interval (CI), 2.0–2.9%). The range was from 0 to 10.7%.
Q2: Trial order effect. Trial 1 was higher than trial 2 in 55 of 64 measurement sets.
Table 2 shows the effect of trial order on SEM, averaged over subjects and all angular orientations by site, for each trial order by angular orientation and site. The data show that trial 1 was higher than subsequent trials.
Table 2 .
Effect of trial order by site
| Site | Trial number |
Trial order effect | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Ulcer (N = 16) | 51.6 | 51.0 | 51.0 | NS |
| Control (N = 16) | 47.5 | 46.8 | 46.4 | P < 0.0001 |
This effect was highly significant at the control site (P < 0.0001).
Q3: Angular orientation effect
Table 3 provides the mean SEM for each angular orientation and by site. At the PrU site, SEM differed significantly between the different angular orientations (P < 0.01), whereas at the control site, SEM variation was not statistically significant. The highest values were seen at the 12 o'clock angular orientation (cephalad) for the PrU site, and at 6 o'clock for the control site.
Table 3 .
Effect of angular orientation by site
| Angular orientation | Site |
|
|---|---|---|
| PrU | Control | |
| 12 o'clock | 57.2 ± 11.8 | 46.1 ± 7.9 |
| 3 o'clock | 48.8 ± 10.9 | 45.9 ± 11.8 |
| 6 o'clock | 45.2 ± 13.2 | 48.8 ± 9.2 |
| 9 o'clock | 52.9 ± 12.1 | 46.6 ± 7.4 |
| Directional effect? | Yes; P < 0.01 | No |
| Overall | 51.1 ± 8.2 | 46.9 ± 7.7 |
| P < 0.05 | ||
Q4: Differentiating PrUs from uninvolved skin (site effect)
As shown in the last row of Table 3, overall SEM at the PrU site was greater than at the control site by 9.0% ((51.1−46.9)/46.9) (P = 0.02).
Q5: Difference between sacral and ischial location
Table 4 indicates the mean SEM by site for the sacral and ischial locations, after averaging over all three trials, and all four angular orientations. SEM differed between PrU and uninvolved skin at the ischia but not the sacrum (53.0 vs. 41.1) (P < 0.0005). Furthermore, at the control site, SEM was greater for the sacral site than the ischial site (51.4 vs. 41.1) (P = 0.02).
Table 4 .
Effect of site and location
| Site | Location |
P | |
|---|---|---|---|
| Sacrum (n = 9) | Ischium (n = 7) | ||
| PrU (n = 16) | 49.6 | 53.0 | NS |
| Control (n = 16) | 51.4 | 41.1 | <0.005 |
| P | NS | 0.02 | |
Discussion
Literature review
Rationale
SEM is a biophysical measurement that correlates with edema. Edema is considered a factor that impairs wound healing and is included as such in multiple wound healing physiology review articles10–15 and wound care textbooks.16 The role of edema in wound healing is best established for venous ulcers, for which use of compression dressings to manage edema is a part of standard wound care.4,17,18 The role of edema is less defined in healing other types of wounds and more specifically, the role of edema in PrU healing is neither central nor well-established. Reflecting this lack of evidence, four of five published wound assessment scales do not include edema as a characteristic for assessment. The four that do not are the Pressure Ulcer Scale for Healing (PUSH Tool),19,20 the Sussman Wound Healing Tool,21 the Sessing Scale,22 and the Wound Healing Scale.23
Only the Bates-Jensen Wound Assessment Tool24 (formerly called the Pressure Sore Status Tool25) includes edema as an item. This tool instructs the examiner to rate edema surrounding a wound using the descriptors “minimal”, “non-pitting”, “pitting”, and “with crepitus”.
Ultimately, the value of measuring edema surrounding wounds needs to be established through a clinical trial of wound healing in which edema measurement was used to influence clinical decisions. Prior to undertaking such a trial, initial research is needed to first evaluate whether edema measures are psychometrically sound.
The purpose of this study was to gain initial experience with a device that measures SEM and, thereby, infers edema surrounding PrUs.
Edema assessment
Edema is defined as abnormally increased interstitial fluid, which manifests as swelling.26 Swelling refers to an increase in the size of the body part involved, typically determined visually. The traditional assessment of edema is by observation and palpation. Edema is conventionally graded on a scale from 1 to 4, and is further characterized as pitting or non-pitting. Pitting edema is defined as edema with the characteristic that briefly applied pressure leads to the formation of a small pit, presumably caused by a shift in the interstitial fluid as a result of the applied pressure. Non-pitting edema results when interstitial fluid cannot rapidly shift. Two examples of non-pitting edema are lymphedema, associated with obstruction of lymphatic vessels, and myxedema, which occurs in hypothyroidism.18 A related concept is induration, which represents a hardening of tissues on palpation, which might be caused by scarring or infiltration of the tissue by inflammatory cells or cancer.
The Bates-Jensen Wound Assessment Tool24 operationalizes assessment of edema and induration as follows:
Peripheral Tissue Edema & Induration: Assess tissues within 4 cm of wound edge. Non-pitting edema appears as skin that is shiny and taut. Identify pitting edema by firmly pressing a finger down into the tissues and waiting for 5 seconds, on release of pressure, tissues fail to resume previous position and an indentation appears. Induration is abnormal firmness of tissues with margins. Assess by gently pinching the tissues. Induration results in an inability to pinch the tissues. Use a transparent metric measuring guide to determine how far edema or induration extends beyond wound.
This tool grades edema as follows: 1 = no swelling or edema; 2 = non-pitting edema extends <4 cm around wound; 3 = non-pitting edema extends >4 cm around wound; 4 = pitting edema extends <4 cm around wound; 5 = crepitus and/or pitting edema extends >4 cm around wound.
Interestingly, this item only asks whether edema extends >4 cm, not the fraction of the wound circumference which is edematous. Some wound assessment items, such as amount of necrotic tissue, are reported by dividing the wound into quadrants to estimate the percentage of area involved. Others, such as undermining, are reported by using the construct of a clock face to estimate the fraction of the wound's circumference involved, and the angular orientation of the involved areas (i.e. 12 o'clock towards the head, 6 o'clock towards the feet).27 Edema has not been previously described in terms of angular orientation.
Besides observation and palpation, another clinical method of quantitating edema indirectly is by gross measurements of limb circumference. This method is recommended in the Clinical Practice Guideline of Prevention of Venous Thrombosis in Spinal Cord Injury.28 It has also been studied in association with lymphedema after mastectomy.29 Higher technology methods depend on interaction of the tissues with various forms of energy, including ultrasound, X-rays, magnetic fields, and radiofrequency electromagnetic waves. Ultrasound and X-rays can measure skin thickness and infer edema by comparison to adjacent, non-edematous skin.30 However, these methods are not water-specific, and cannot distinguish edematous skin (increased interstitial fluid) from indurated skin (inflammatory infiltrate). X-rays also have the disadvantage of exposing the veteran to ionizing radiation.
Magnetic resonance imaging (MRI) directly images water. MRI can not only measure skin thickness, but also can distinguish edema from other forms of tissue swelling. One study used MRI to evaluate PrUs in 37 persons with a spinal cord injury (SCI).31 MRI was able to determine the depth and extent of soft tissue involvement underlying PrUs, including fluid collections, heterotopic bone formation, and adjacent bone marrow edema that is a sign of osteomyelitis. Due to cost and scheduling constraints, MRI is not appropriate for routine wound monitoring.
Radiofrequency electromagnetic waves that are reflected from or transmitted through tissue can be used to determine tissue dielectric constant, which is strongly affected by water concentration.32,33 Various terms describe the process of measurement of tissue dielectric constant, including microwave dielectric spectroscopy,34 bioelectric impedance analysis,35 capacitive measurement,36 surface electrical capacitance,37 capacitance imaging,38 localized impedance,39 bioimpedancemetry,40 and time-domain reflectometry.41 These methods differ in their choice of frequency, and whether they use electrodes or antennas, but fundamentally use the same principle. Advantages of using radiofrequency waves to measure edema include water specificity, the potential for the device to be handheld, and the lack of ionizing radiation.
Likewise, a variety of terms have been used by the authors of studies using these devices to describe what they were measuring, including “free water content”,8 “tissue dielectric constant”,42 “skin tissue water”,43 “changes of tissue water”,44 “tissue water content”,45 “water content”,46 “tissue water”,47 “tissue edema”,48,49 “skin edema”,50 and “subepidermal moisture”.51,52 In this manuscript, the term “subepidermal moisture” (SEM) will be used for the measurement provided by the instrument, and “edema” for the clinical concept.
Two instruments that measure SEM are the MoistureMeter-D (Delfin Technologies Ltd,)53 and the DPM 9003 (Nova Technology Corporation, Portsmouth, NH, USA).54 While not approved by the Food and Drug Administration for making diagnoses, they are used in the cosmetics industry.55 Previous preliminary studies of measuring SEM using these devices were conducted on normal volunteers, burns, lymphedema, and stage I PrUs. Studies in normal volunteers have looked at changes in intact skin in response to hydration29 and in intact skin made edematous by being exposed to an irritant.30 A study of experimental burns in pigs showed that dielectric probes could be used to differentiate partial- and full-thickness burns as early as 8 hours after burn by examining increases in the water content of dermis and fat.39,56 This finding is relevant to healing in burns when considered with another study of experimental burns, which showed an inverse relationship between increases in tissue water and red blood cell content, indicating that edema negatively impacts local blood flow.57 Mayrovitz and Brown-Cross49 examined the arms of women with unilateral arm lymphedema, and found that using the 2.5 mm probe, the MoistureMeter-D differentiated between lymphedema and normal skin. Bates-Jensen et al. looked at 31 NH veterans, and showed that SEM can both differentiate erythema and stage I PrUs,1 and predict pressure damage.2
Discussion
Research question 1 addresses the short-term repeatability of the measurement by a single observer. The mean relative error was 2.5% (CI, 2.0–2.9%). This value does not fully account for the device variability, as only a single observer was used. A full measurement of instrumental precision would include measurements by multiple observers, which would increase the relative error.
Research question 2 looks at the order effect. While most clinical tests are done just once, some are done in sets, notably spirometry which is always done in triplicate. In this study, triplicate measurement was not done to suggest that clinicians would routinely have to perform triplicate repetitions, but rather to ascertain the repeatability of the test by a single observer, a part of establishing its reliability, and to look for an order effect, a source of systemic error.
When an instrumental measurement has error, one strategy for obtaining a more precise estimate of the truth is to make repeated measurements (trials), and then compute their mean value, as is done routinely in clinical spirometry. However, this strategy is valid only if the repeated trials are independent. If repeated trials are not independent, the most accurate measurement may be just a single trial. An analogy to this is the attempt to measure a runner's speed for an 800 m run. Repeated trials on the same day would show a decreasing trend due to fatigue, and thus lead to a falsely low estimate rather than an increase in precision.
The data show that trials using this device are not independent. The first trial was higher than the second at 59 of 64 measurement points. This result informs designers of future studies to be cautious about using repeated trials at a given physical site over short periods, and it calls for further research towards characterizing the time for the tissue to fully recover. Also, it suggests standardizing factors such as magnitude of applied pressure, and time between moving the patient from supine to a position that exposes the wound for measurement.
A mechanism that might explain this phenomenon is that the application of firm pressure during the first measurement expels water from the region, so that subsequent measurements will measure less tissue water. This mechanism is similar to the mechanism of pitting edema: the tissue pits (fails to spring back) because finger pressure expels water from the region, so that the tissue does not rebound to its original height.
Another hypothetical mechanism is that the amount of water in the skin results from a dynamic balance between transepidermal water loss (TEWL) and water supply from the vasculature.58 During the period of time that the probe is held against the skin, the probe occludes the stratum corneum, thereby blocking normal TEWL and allowing water to build up under the probe. Until the skin recovers, subsequent readings in the same location will differ. However, in this model, subsequent measurements would show higher amounts of water, rather than the lower amount seen in this study. The data support a model where water leaves the area such as under the influence of pressure.
The magnitude of this effect might be dependent on the amount of pressure applied, the diameter of the probe, and the nature of the tissue being measured. Further research to examine these dependencies is needed. Interestingly, this effect has not been seen in measurements of lymphedema (H.M., unpublished data). This observation makes sense, because lymphedema is generally non-pitting edema, which means that it does not exhibit rapid deformation as a result of applied pressure. Until this is established, research designs involving repeated measures at the same site should check their data for a temporal effect, which could introduce a systemic bias into their data analysis; this strategy should reduce measurement error caused by differences in application pressure, changes of the angle of application, and other uncontrolled factors.
This finding also suggests that measurement of SEM is sensitive to even brief applications of pressure. As such, SEM on body surfaces subject to pressure from the bed may change as the person is turned for access to the PrU. The amount of time spent by the subject in the lateral or prone positions prior to measurement is likely to be a confounding variable. Further research into the magnitude of the effect of positioning and time on tissue water content is needed.
Another implication of this finding is that the magnitude of pressure applied to the probe may influence the measurement. Different clinicians will have different ideas about what constitutes “applying firm pressure, just enough to slightly indent the skin”. While this analysis looked at only a single observer, further research must be done to determine differences between multiple observers (inter-rater agreement). Furthermore, the same observer may apply different pressures on different days (intra-rater agreement), depending on random factors such as fatigue, time of day, or time since last visit to the gymnasium. A study of multiple raters' consistency over time would be necessary to fully characterize the device's reliability in the setting of PrUs.
Research question 3 addresses the issue of variation with angular orientation. Table 3 shows that there is a significant directional dependence of SEM around PrUs (P < 0.01), although not around control sites. Around the PrUs, the average SEM at the 12 o'clock direction (57.2) was 38% higher than at the 6 o'clock position (45.2). In the control sites, there was no statistically significant difference, but the 6 o'clock position trended somewhat higher.
At the control sites, the lack of significant variation with angular orientation is not surprising, since the skin is intact. The trend at the control site of increased fluid towards the feet (6 o'clock) corresponds to our notion and clinical experience that edema generally increases with increasing distance from the heart, even if the subject has been in bed for a long period of time.
The physiological mechanism behind the significant variation with angular orientation at the PrU sites is unclear. A priori, we expected SEM to be greater at the 6 o'clock position even if the person is horizontal and gravity does not play a role, as it is farthest from the heart. However, the data show significantly increased SEM in the cephalad position. This must say something important about wound pathophysiology. An explanation for this finding might be related to the common observation that the red streaks of cellulitis generally advance in a cephalad direction, or that the direction of lymphatic flow is cephalad. Further research is needed to see if this phenomenon is observed just in PrUs, or in other situations where the lymphatic circulation is altered, such as in lymphedema or in venous ulcers. Future research should also examine whether this phenomenon differs between sacral and ischial ulcers. Subgroup analysis for this question was not performed due to the small sample size.
From a measurement perspective, a basic question is how to characterize the magnitude and extent of edema around wounds, given that SEM varies both with distance and angular orientation from the PrU center. It might be possible to define a construct for SEM that could be represented by a single number. Examples of this might be (1) the average of the values at all four directions as the best representation of the average for the wound; (2) the average of the values at 3 and 9 o'clock directions as a more parsimonious and less demanding representation of the wound average; or (3) a single reading at the 12 o'clock position, which would represent the worst-case value of the wound. Further research should be done in the setting of a longitudinal trial of wound healing.
Research question 4 addresses the ability of the device to differentiate between PrUs and uninvolved skin. The average SEM for the PrU sites (51.1) was greater than that of the control sites (46.9) by 9.0% (P < 0.05). This is suggestive that SEM is a measure that distinguishes PrUs from normal tissue. However, a more interesting and important question is whether SEM is predicts ulcer healing. This question cannot be answered by this study, which only looked at ulcers at a single point in time, but requires a longitudinal study of ulcer healing, comparing SEM with another variable, such as surface area, which corresponds in some way with healing.
Research question 5 addresses whether sacral and ischial locations differ. For intact skin, sacral was greater than ischial by 20%. This difference is not only highly significant (P < 0.005), but also is a large effect size (Cohen's d = 1.4), and is greater than the difference between intact skin and PrU of 9.0% found in question 4. This clearly shows that this instrument measures something that is not constant over the body (such as plasma sodium concentration), but rather something locally specific. This probably represents the effects of underlying tissue (fat/muscle/bone), and the thickness of the skin. A recent study using this device to measure skin water content in irritant-exposed skin showed a positive relationship between the water content as indicated by dielectric measurement, with ultrasound-measured skin thickness.30
Implications
Clinical
A device for measuring SEM is useful only if there is some benefit to measuring it more precisely than current clinical observation. Although it is too premature to make evidence-based recommendations regarding the use of this type of technology for PrU assessment, there are potential clinical implications in the field. These implications include: (1) edema assessment typically relies on palpation by the examiner's finger which is a relatively crude, observer-dependent approach; (2) even if the clinician continues to assess SEM manually, the results from this study may assist in identifying some of the issues related to assessment (e.g. variability of PrU circumferential sites); (3) a quantifiable means of edema assessment could facilitate documentation, become a measurement variable in PrU quality improvement initiatives for comparative effectiveness of PrU healing interventions (e.g. use of pressure redistribution devices), and improve communication among clinicians regarding PrU status; (4) a device may be a more reliable assessment of SEM in patients with dark skin tones in whom erythema cannot be easily detected; (5) it takes years, for even evidence-based innovations to be embedded into routine clinical practice and this study may assist in generating interest into future exploration; (6) to assess SEM using any method, albeit manually or digitally, it is important that all clinicians be trained using similar methods (e.g. orientation, amount of pressure) to facilitate assessor consistency; and (7) this study provides SEM data regarding full-thickness PrU which heretofore has not been addressed in the healthcare literature.
Research
Multiple opportunities for research in this area exist with evidentiary implications: (1) the ground-work regarding device characteristics, blatant and nuanced, must be learned and communicated to potential researchers so the appropriate methodology may be designed for future studies; (2) PrU research should include a more complete psychometrics of this device addressing the effects of multiple observers and varying time periods of observation; (3) research should expand the subject pool to include whether these findings apply to lymphedema and/or venous ulcers; (4) research should examine sacral and ischial wounds separately, to see how the physiology and pathophysiology at these sites differ; (5) the density of underlying tissue should be independently measured with a different technology such as ultrasound; (6) data may be used to design a longitudinal study of PrU healing in which clinical assessment of edema and measurement of SEM are compared with markers of healing such as surface area and other PrU variables;6,59 and (7) future research is needed to examine if edema or measuring SEM have a predictive value for measuring PrU healing. SEM might help differentiate a clean wound from an infected one, a chronic non-healing wound from one that is healing. While this study was limited to established wounds, another study2 looked use of SEM to predict skin breakdown in darkly complected individuals where it is difficult to recognize edema.
Limitations
This was a pilot study that did not attempt to report reliability or validity, which would have required multiple observers over longer time periods. The study was limited to a single observer and examined subjects at a single point in time. The applied pressure was uncontrolled. However, this study was not designed to be done under controlled, laboratory conditions, but rather in a clinical setting, as would be done if the device was being used as designed for clinical care. The study results apply to the device as used in clinical practice. Neither did the study control for the age of the veteran, age of the PrU, amount of time lying in bed, prior positioning in bed, body weight, previous surgery or ulcers in the same location, and the presence of medical conditions or drugs that affect edema. It did not try to answer questions about the role of edema in wound healing, which would require a longitudinal clinical trial. It did not try to answer whether the differences observed are clinically significant, which would also require a longitudinal clinical trial. The number of subjects was small.
Conclusions
This pilot study addressed characteristics of a method of quantifying SEM around PrUs, using a hand-held instrument which uses electromagnetic waves. The study revealed several characteristics about SEM that should guide design of future studies: (1) single observer repeatability was quite good. (2) A trial order effect was observed in a series of three measurements such that the first measurement was usually greater than subsequent ones. This suggests that a single trial at any measurement point is better than an average of repeated trials, which would introduce a systematic error. It also suggests that measurement is sensitive to the amount of pressure applied, which may vary greatly among different observers. (3) SEM around PrUs varies with angular orientation with the highest reading in the cephalad direction. This contrasted with the finding for control sites, where an insignificant variation existed with a trend towards higher readings in the caudal direction. It showed that SEM around PrUs is higher than at control sites overall, and SEM is greater at control sites in the sacral area than ischial areas, which suggests that SEM must be interpreted in reference to a specific location.
Disclaimer statements
Contributors JH, MD, PhD was the Principal Investigator, and the one who conceived of the project, obtained informed consent, obtained the measurements, and did the bulk of the writing. HM, PhD, of Nova Southeastern University loaned the MoistureMeter to JH, instructed JH in its use, reviewed the manuscript, and made editing suggestions. Shirley Groer, PhD was the Statistical Analyst and helped with the writing of the methods and results sections. She is acknowledged, but not included as an author because of her present position as a VARD Project Manager. Theresa Schwartz, RN helped Dr. JH to collect the data. She is acknowledged, but not included as an author because she did not participate in the writing. The views expressed are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Funding Funding was provided by VA RR&D and VA HSR&D.
Conflicts of interest None.
Ethics approval As this was not a therapeutic trial, it was not required to be registered. There were no ethical issues involved. The study was approved by the USF IRB and the VA R&D Committee.
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development (Rehabilitation Research and Development Service), and the use of facilities at the James A. Haley Veterans Hospital, Tampa, Florida. Dr Harrow was a recipient of a VA Rehabilitation Research and Development Career Development Award at the time the research was conducted. Many thanks to Shirley Groer, PhD for statistical analysis, to Susan Thomason, RN for assistance with revisions, and to Theresa Schwartz, RN for clinical and administrative assistance.
References
- 1.Bates-Jensen BM, McCreath HE, Pongquan V, Apeles NCR. Subepidermal moisture differentiates erythema and stage I pressure ulcers in nursing home residents. Wound Repair Regen 2008;16(2):189–97. [DOI] [PubMed] [Google Scholar]
- 2.Bates-Jensen BM, McCreath HE, Pongquan V. Subepidermal moisture is associated with early pressure ulcer damage in nursing home residents with dark skin tones. J Wound Ostomy Continence Nurs 2009;36(3):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guihan ML, Bates-Jenson BM, Chun S, Parachuri R, Chin AS, McCreath H. Assessing the feasibility of subepidermal moisture to predict erythema and stage 1 pressure ulcers in persons with spinal cord injury: a pilot study. J Spin Cord Med 2012;35(1):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henzel MK, Bogie KM, Guihan M, Ho CH. Pressure ulcer management and research priorities for veterans with spinal cord injury: consensus opinion from SCI QUERI Expert Panel on Pressure Ulcer Research Implementation. J Rehabil Res Dev 2011;48(3):xi–xxxii. [DOI] [PubMed] [Google Scholar]
- 5.Thomason SS, Luther S, Nelson A, Palacios P, Harrow J. Pressure ulcer healing in SCI: a novel evidence-based tool. J Wound Ostomy Continence Nurs 2008;35(3):S70. [Google Scholar]
- 6.Thomason SS, Luther SL, Powell-Cope GM, Harrow JJ, Palacios P. Validity and reliability of a pressure ulcer monitoring tool for persons with spinal cord impairment. J Spin Cord Med 2014;37(3):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delfin Technologies Ltd. [Internet]. Change in dielectric constant of SSF measured with the MoistureMeter-D 2007[cited 2007 Mar 12] [accessibility verified 2013 Dec 15]. Available from: http://www.delfintech.com/prod_moist_d_ssf.html/.
- 8.Alanen E, Nuutinen J, Nicklen K, Lahtinen T, Monkkonen J. Measurement of hydration in the stratum corneum with the MoistureMeter and comparison with the Corneometer. Skin Res Technol 2004;10(1):32–7. [DOI] [PubMed] [Google Scholar]
- 9.ITIS Foundation. Tissue properties [accessibility verified 2013 Dec 15]. Available from:http://www.itis.ethz.ch/itis-for-health/tissue-properties/database/dielectric-properties/.
- 10.de Laat EH, Scholte op Reimer WJ, van Achterberg T. Pressure ulcers: diagnostics and interventions aimed at wound-related complaints: a review of the literature. J Clin Nurs 2005;14(4):464–72. [DOI] [PubMed] [Google Scholar]
- 11.Kramer SA. Compression wraps for venous ulcer healing: a review. J Vasc Nurs 1999;17(4):89–97; quiz 8–9. [DOI] [PubMed] [Google Scholar]
- 12.Hunt TK, Hopf H, Hussain Z. Physiology of wound healing. Adv Skin Wound Care 2000;13(2 Suppl):S6–11. [PubMed] [Google Scholar]
- 13.Williams DT, Harding K. Healing responses of skin and muscle in critical illness. Crit Care Med 2003;31(8 Suppl):S547–57. [DOI] [PubMed] [Google Scholar]
- 14.Aller MA, Arias JL, Arias J. Post-traumatic inflammatory response: perhaps a succession of phases with a nutritional purpose. Med Hypotheses 2004;63(1):42–6. [DOI] [PubMed] [Google Scholar]
- 15.Puruhito. Pathophysiology of microcirculation in venous disease. Clin Hemorheol Microcirc 2000;23:239–42. [PubMed] [Google Scholar]
- 16.Sheffield P, Smith A, Fife C. Wound care practice. Flagstaff, AZ: Best Publishing Company; 2004. [Google Scholar]
- 17.Partsch H. Compression therapy of venous ulcers. Curr Probl Dermatol 1999;27:130–40. [DOI] [PubMed] [Google Scholar]
- 18.Trent JT, Falabella A, Eaglstein WH, Kirsner RS. Venous ulcers: pathophysiology and treatment options. Ostomy Wound Manage 2005;51(5):38–54; quiz 5–6. [PubMed] [Google Scholar]
- 19.Rodeheaver GT, Bartolucci AA, Franz RA, Sussman C, Ferrell BA, Cuddigan J, et al. . Pressure ulcer scale for healing: derivation and validation of the PUSH tool. Adv Wound Care 1997;10(5):96–101. [PubMed] [Google Scholar]
- 20.Sussman C. Presenting a draft pressure ulcer scale to monitor healing. Adv Wound Care 1997;10(5):92. [PubMed] [Google Scholar]
- 21.Sussman C, Swanson G. Utility of the Sussman Wound Healing Tool in predicting wound healing outcomes in physical therapy. Adv Wound Care 1997;10(5):74–7. [PubMed] [Google Scholar]
- 22.Ferrell BA, Artinian BM, Sessing D. The Sessing scale for assessment of pressure ulcer healing. J Am Geriatr Soc 1995;43(1):37–40. [DOI] [PubMed] [Google Scholar]
- 23.Krasner D. Wound Healing Scale, version 1.0: a proposal. Adv Wound Care 1997;10(5):82–5. [PubMed] [Google Scholar]
- 24.Bates-Jensen B. 2001 Bates-Jensen Wound Assessment Tool. [Internet] [cited 2007 Sep 29] [accessibility verified 2013 Dec 15]. Available from: http://borun.medsch.ucla.edu/modules/Pressure_ulcer_prevention/puBWAT.pdf .
- 25.Bates-Jensen BM, Vredevoe DL, Brecht ML. Validity and reliability of the Pressure Sore Status Tool. Decubitus 1992;5(6):20–8. [PubMed] [Google Scholar]
- 26.Cho S, Atwood JE. Peripheral edema. Am J Med 2002;113(7):580–6. [DOI] [PubMed] [Google Scholar]
- 27.Priebe M, Wuermser L, McCormack HE. Medical management of pressure ulcers. In: Lin V, (ed.) Spinal cord medicine: principles and practice. New York, NY: Demos Medical Publishing, Inc; 2003. p. 575. [Google Scholar]
- 28.Consortium for Spinal Cord Medicine. Prevention of thromboembolism in spinal cord injury. J Spinal Cord Med 1997;20(3):259–83. [DOI] [PubMed] [Google Scholar]
- 29.Mayrovitz HN, Sims N, Macdonald J. Assessment of limb volume by manual and automated methods in veterans with limb edema or lymphedema. Adv Skin Wound Care 2000;13(6):272–6. [PubMed] [Google Scholar]
- 30.Miettinen M, Monkkonen J, Lahtinen MR, Nuutinen J, Lahtinen T. Measurement of oedema in irritant-exposed skin by a dielectric technique. Skin Res Technol 2006;12(4):235–40. [DOI] [PubMed] [Google Scholar]
- 31.Hencey JY, Vermess M, van Geertruyden HH, Binard JE, Manchepalli S. Magnetic resonance imaging examinations of gluteal decubitus ulcers in spinal cord injury veterans. J Spinal Cord Med 1996;19(1):5–8. [DOI] [PubMed] [Google Scholar]
- 32.Lahtinen T, Nuutinen J, Alanen E. Dielectric properties of the skin. In: Klauenberg J, Miklavcic D, (eds.) Radio frequency radiation dosimetry. The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- 33.Alanen E, Lahtinen T, Nuutinen J. Penetration of electromagnetic fields of open-ended coaxial probe between 1 MHz and 1 GHz in dielectric skin measurements. Phys Med Biol 1999;44(7):N169–76. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi Y, Miura N, Shinyashiki N, Yagihara S. Free water content and monitoring of healing processes of skin burns studied by microwave dielectric spectroscopy in vivo. Phys Med Biol 2005;50(4):599–612. [DOI] [PubMed] [Google Scholar]
- 35.Desport JC, Preux PM, Guinvarc'h S, Rousset P, Salle JY, Daviet JC, et al. . Total body water and percentage fat mass measurements using bioelectrical impedance analysis and anthropometry in spinal cord-injured veterans. Clin Nutr 2000;19(3):185–90. [DOI] [PubMed] [Google Scholar]
- 36.Ollmar S, Nyren M, Nicander I, Emtestam L. Electrical impedance compared with other non-invasive bioengineering techniques and visual scoring for detection of irritation in human skin. Br J Dermatol 1994;130(1):29–36. [DOI] [PubMed] [Google Scholar]
- 37.Goretsky MJ, Supp AP, Greenhalgh DG, Warden GD, Boyce ST. Surface electrical capacitance as an index of epidermal barrier properties of composite skin substitutes and skin autografts. Wound Repair Regen 1995;3(4):419–25. [DOI] [PubMed] [Google Scholar]
- 38.Batisse D, Giron F, Leveque JL. Capacitance imaging of the skin surface. Skin Res Technol 2006;12(2):99–104. [DOI] [PubMed] [Google Scholar]
- 39.Aaron R, Shiffman CA. Using localized impedance measurements to study muscle changes in injury and disease. Ann N Y Acad Sci 2000;904:171–80. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov GG, Meshcheriakov GN, Kravchenko NR, Zaks IO, Moroz VV, Nikolaev DV, et al. . Bio-impedancemetry in the assessment of aqueous sectors of the body. Anesteziol Reanimatol 1999;1999(1):59–63. [PubMed] [Google Scholar]
- 41.Naito S, Hoshi M, Mashimo S. In vivo dielectric analysis of free water content of biomaterials by time domain reflectometry. Anal Biochem 1997;251(2):163–72. [DOI] [PubMed] [Google Scholar]
- 42.Mayrovitz HN. Assessing local tissue edema in postmastectomy lymphedema. Lymphology 2007;40(2):87–94. [PubMed] [Google Scholar]
- 43.Mayrovitz HN, Brown-Cross D, Washington Z. Skin tissue water and laser Doppler blood flow during a menstrual cycle. Clin Physiol Funct Imaging 2007;27:54–9. [DOI] [PubMed] [Google Scholar]
- 44.Nuutinen J, Ikäheimo R, Lahtinen T. Validation of a new dielectric device to assess changes of tissue water in skin and subcutaneous fat. Physiol Meas 2004;25:447–54. [DOI] [PubMed] [Google Scholar]
- 45.Harrow JJ, Mayrovitz HN. Initial assessment of tissue water content surrounding pressure ulcers in spinal cord injury veterans. Abstract In: Symposium on Advanced Wound Care & Medical Research Forum on Wound Repair; San Antonio, TX. Malvern, PA: HMP Communications; 2006 p. 73. [Google Scholar]
- 46.Miettinen M, Nuutinen J, Lahtinen MR, Lahtinen T. Water content at skin surface and in whole skin during irritant skin reaction. In: 13th Congress of European Academy of Dermatology and Venereology Meeting; 2004. [Google Scholar]
- 47.Papp A, Lahtinen T, Harma M, Nuutinen J, Uusaro A, Alhava E. Dielectric measurement in experimental burns: a new tool for burn depth determination? Plast Reconstr Surg 2006;117(3):889–98; discussion 99–901. [DOI] [PubMed] [Google Scholar]
- 48.Lahtinen T, Lampainen E, Nuutinen J. EdemaMeter – a new device to detect tissue edema in skin and subcutaneous tissues. Skin Res Technol 2003;9(2):182. [Google Scholar]
- 49.Mayrovitz HN, Brown-Cross D. Assessment of local tissue edema in arms of women with postmastectomy lymphedema. In: Symposium on Advanced Wound Care & Medical Research Forum on Wound Repair; San Antonio, TX. Malvern, PA: HMP Communications; 2006 p. 189. [Google Scholar]
- 50.Miettinen M, Llahtinen MR, Nuutinen J, Lahtinen T, Niinimäki M. Measurement of skin edema by a dielectric technique (MoistureMeter-D). Contact Dermatitis 2004;50(18):181–2. [Google Scholar]
- 51.Bates-Jensen BM, McCreath HE, Kono A, Apeles NC, Alessi C. Subepidermal moisture predicts erythema and stage 1 pressure ulcers in nursing home residents: a pilot study. J Am Geriatr Soc 2007;55(8):1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bates-Jensen BM, McCreath HE, Pongquan V, Apeles NC. Subepidermal moisture differentiates erythema and stage I pressure ulcers in nursing home residents. Wound Repair Regen 2008;16(2):189–97. [DOI] [PubMed] [Google Scholar]
- 53.Delfin Technologies. Reference Publications/MoistureMeter-D [accessibility verified 2013 Dec 15]. Available from: http://www.delfintech.com/en/reference_publications/.
- 54.Nova Technology Corporation. DPM 9003 [accessibility verified 2013 Dec 15]. Available at: http://www.novatechcorp.com/products.html.
- 55.Tagami H, Ohi M, Iwatsuki K, Kanamaru Y, Yamada M, Ichijo B. Evaluation of the skin surface hydration in vivo by electrical measurement. J Invest Dermatol 1980;75:500–7. [DOI] [PubMed] [Google Scholar]
- 56.Soo C, Garner WL. Dielectric measurement in experimental burns: a new tool for burn depth determination? Plast Reconstr Surg 2006;117(3):899–901. [DOI] [PubMed] [Google Scholar]
- 57.Papp A, Romppanen E, Lahtinen T, Uusaro A, Harma M, Alhava E. Red blood cell and tissue water content in experimental thermal injury. Burns 2005;31(8):1003–6. [DOI] [PubMed] [Google Scholar]
- 58.Zhai X, Yokota M, Maibach HI. In vitro human skin model to evaluate water permeability and determine wound dressings' occlusivity. Cutan Ocul Toxicol 2007;26(2):107–11. [DOI] [PubMed] [Google Scholar]
- 59.Thomason SS, Nelson AL, Harrow JJ, Luther S, Palacios P. Establishing sensitivity, reliability, and validity of a Pressure Ulcer Healing Tool for persons with spinal cord. Symposium on Advanced Wound Care, San Diego, April 24–27(2008, poster). [Google Scholar]