Abstract
The major heat shock protein, Hsp70, can protect against cell death by directly interfering with mitochondrial apoptosis pathways. However, Hsp70 also sensitizes cells to certain apoptotic stimuli like TNF. Little is known about how Hsp70 enhances apoptosis. We demonstrate here that Hsp70 promotes TNF killing by specifically binding the coiled-coil domain of IκB kinase γ (IKKγ) to inhibit IKK activity and consequently inhibit NF-κB-dependent antiapoptotic gene induction. An IKKγ mutant, which interacts with Hsp70, competitively inhibits the Hsp70–IKKγ interaction and relieves heat-mediated NF-κB suppression. Depletion of Hsp70 expression with RNA interference rescues TNF-mediated cell death. Although TNF may or may not be sufficient to trigger apoptosis on its own, TNF-triggered apoptosis was initiated or made worse when Hsp70 expression increased to high levels to disrupt NF-κB signaling. These results provide significant novel insights into the molecular mechanism for the pro-apoptotic behavior of Hsp70 in death-receptor-mediated cell death.
Keywords: Hsp70, NF-κB, IKKγ, TNF, apoptosis, death receptor signaling
Even though heat shock proteins are known to inhibit various types of apoptosis, some studies show that heat or elevated heat shock protein 70 (Hsp70) also potentiates cell death following specific stimuli. For instance, overexpression of HSF1 (heat shock transcription factor 1, one transcription factor that controls the heat shock response) enhances Fas-induced cell apoptosis (Xia et al. 2000). Elevated Hsp70 promotes TCR/CD3- and Fas/Apo-1/CD95-mediated Jurkat T-cell apoptosis (Liossis et al. 1997). Heat increases cell death following some chemotherapeutics and radiation (Dewey 1984), and heat shock also sensitizes AML cells and endothelial cells to apoptosis (Chant et al. 1996; DeMeester et al. 1998). Hsp70 inhibits cellular proliferation (Maheswaran et al. 1998). Moreover, it is also well established that heat shock or elevated Hsp70 alters the regulation of signaling cascades and transcription factors and potently sensitizes tumors to radiation (Curry et al. 1999). These results imply that heat or Hsp70 could disable a cell survival signal under the appropriate circumstances.
NF-κB is sequestered in the cytoplasm in an inactive complex with IκB proteins (Huxford et al. 1998; Malek et al. 2001). Activation of the NF-κB-mediated signal is initiated through degradation of phosphorylated IκB. Various stimuli activate the IκB kinase (IKK) complex, which in turn phosphorylates IκB, leading to NF-κB activation (Chen et al. 1995; Zandi et al. 1997). NF-κB is critical for survival of most cells through the induction of antiapoptotic genes (Beg et al. 1995; Beg and Baltimore 1996; Van Antwerp et al. 1996; Wang et al. 1998; Zong et al. 1999; Rudolph et al. 2000). IKK plays a central role in mediating NF-κB activation. IKK is composed of two catalytic subunits, IKKα and IKKβ, which can directly phosphorylate IκB. IKKγ (also called NEMO) is an absolutely essential regulatory component of the IKK complex that is necessary for NF-κB activation (Rothwarf et al. 1998; Yamaoka et al. 1998; Makris et al. 2002).
A number of studies have also shown that heat shock or elevated Hsp70 suppresses NF-κB activity (Feinstein et al. 1997; Guzhova et al. 1997; Curry et al. 1999; Andres et al. 2002; Malhotra and Wong 2002). Although these studies imply the possibility that Hsp70 impairs NF-κB signaling, the exact molecular basis of the Hsp70 and NF-κB interaction is still enigmatic.
This study therefore examined the mechanism by which Hsp70 interacts with NF-κB and might promote apoptosis. To do this, we studied several cell lines (Cos-1, Hela, 293 cells) that do not apparently undergo apoptosis when exposed to TNF alone. However, when these cells are exposed to TNF in combination with Hsp70 overexpression or heat shock, they die via apoptosis. Here, we demonstrate that Hsp70 associates directly and specifically with IKKγ and blocks the formation of the IKK complex—possibly by inhibiting oligomerization of IKKγ. This inhibits TNF-triggered NF-κB activation and subsequently prevents NF-κB-dependent anti-apoptotic gene expression. This work demonstrates how Hsp70 promotes rather than inhibits TNF-mediated cell death.
Results
Hsp70 inhibits NF-κB activity
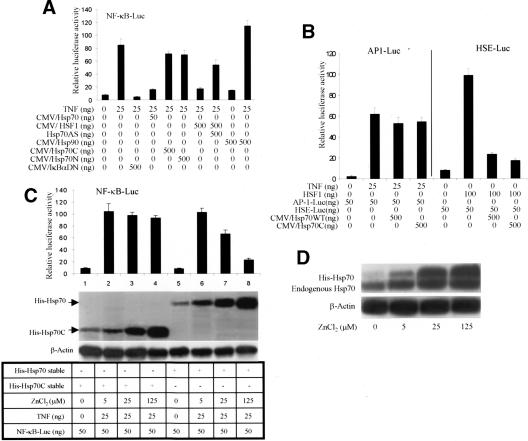
To determine whether Hsp70 itself specifically suppressed NF-κB activity, we cotransfected Cos-1 cells with an NF-κB reporter and the indicated constructs. In this transfection assay, Hsp70 and IκBαDN (IκBα dominant negative) suppressed NF-κB reporter gene activity, whereas Hsp70C (C terminus of Hsp70) and Hsp70N (N terminus of Hsp70) failed to do so (Fig. 1A). HSF1 also inhibited NF-κB activity. However, this inhibition was abrogated by cotransfection with Hsp70AS (antisense Hsp70), indicating that HSF1 did not directly affect NF-κB activity (Fig. 1A). Hsp90 did not affect NF-κB activity in resting cells but enhanced NF-κB activity when cells were treated with TNF (Fig. 1A).
Figure 1.
Hsp70 inhibits NF-κB activation. (A) Cos-1 cells were transfected with the indicated plasmids and the NF-κB reporter. Twenty-four hours after transfection, cells were treated with TNF (25 ng/mL) or vehicle for 15 min. Luciferase activity was measured 24 h later. (B) Effects of Hsp70 on Ap-1- and HSF-1-dependent transactivation. Cos-1 cells were cotransfected with an AP-1 or HSF-1 luciferase reporter along with the indicated vectors. Twenty-four hours later, cells were treated with TNF or vehicle for 15 min. After 24 h, luciferase activities were measured. (C) Hsp70- and Hsp70C-inducible cells were transiently transfected with an NF-κB-dependent luciferase reporter and then treated with different concentrations of ZnCl2 for 4 h to induce His-tagged Hsp70 and His-tagged Hsp70C expression. At 16 h after ZnCl2 treatment, cells were treated with TNF (25 ng/mL) or vehicle for 15 min. NF-κB activity was analyzed 24 h after TNF treatment. Hsp70 and Hsp70C expression levels were examined with an anti-His antibody. β-Actin served as the lane-loading control. (D) Immunoblots of exogenous and endogenous Hsp70 expression in Hsp70 stable cells were treated with ZnCl2 (for 4 h). Sixteen hours later, cell lysates were immunoblotted with anti-Hsp70 antibody. (E) Cos-1 cells were transfected with the indicated constructs and selected cells treated with TNF (25 ng/mL) for 15 min or heated for 30 min at 45°C. Twenty-four hours later, nuclear extracts were prepared and subjected to a gel shift assay using a 32P-labeled p65 probe. (NS) Nonspecific binding. (F) Hsp70- or Hsp70C-inducible Hela cells were treated with ZnCl2 (100 μM) or vehicle for 4 h. Sixteen hours later, cells were treated with TNF (25 ng/mL) or vehicle for 15 min. Cells were stained 15 min later with an anti-p65 antibody and a TRITC-conjugated second antibody. Nuclei were visualized with Hoechst. (G) Gel shift assay with in vitro translated Hsp70, HSF1, and p65 proteins and a 32P-labeled p65 probe. (NS) Nonspecific binding.
To demonstrate the specificity of the Hsp70 inhibition, we did similar experiments with AP-1- and HSF-dependent reporters. It was shown that Hsp70 did not significantly affect activation of AP-1, whereas both Hsp70 and Hsp70C efficiently inhibited HSF activity (Fig. 1B). This is consistent with findings that Hsp70 can inhibit HSF1 transcriptional activity (Abravaya et al. 1992; Shi et al. 1998). An additional control experiment showed that Hsp70 had similar effects on the refolding of β-gal and luciferase, as measured by their enzymatic activities (data not shown). Therefore, in all of the transfection assays shown, the elevated Hsp70 did not artificially decrease or increase the luciferase readings when normalized to β-gal.
To determine whether physiologically elevated levels of Hsp70 inhibit NF-κB activity, we established Hsp70-inducible cell lines using the nonintegrating plasmid pMEP4 under the control of a Zn2+-regulated metallothionein promoter. Hsp70 dose-dependently inhibited NF-κB activity (Fig. 1C). The modest reduction of NF-κB activity in Hsp70C-expressing cells (Fig. 1C, lanes 2–4) likely occurred because of Zn2+-mediated induction of endogenous Hsp70 (Fig. 1C,D). Cells treated with TNF alone, in the absence of ZnCl2, had a relative luciferase activity for NFκB-luc of 100 ± 8 (see results following), which is similar to the relative luciferase activity levels for the cells stably expressing His-Hsp70C in the presence of ZnCl2 (Fig. 1C, lanes 2–4) and for His-Hsp70 cells treated with low levels of ZnCl2 (Fig. 1C, lane 6).
Hsp70 did not directly interfere with the function of the NF-κB activation domain using a cotransfection assay with plasmid encoding Gal4 (DNA-binding domain)-p65 (activation domain) chimeric protein, a Gal-4 reporter, and Hsp70 construct (data not shown). Next, to analyze the effect of Hsp70 on NF-κB DNA-binding activity, we performed a gel mobility shift assay using a p65 probe and nuclear extracts isolated from Cos-1 cells. Heat, Hsp70, IκBαDN, and HSF1 inhibited NF-κB DNA binding (Fig. 1E, lanes 1–8). The effects of HSF1 were not a direct effect on the NF-κB activity because Hsp70 antisense (Hsp70AS) blocked the effect, showing that it is HSF1 induction of Hsp70 that inhibits NF-κB DNA binding (Fig. 1E, lane 8). Cellular localization data showed that Hsp70 inhibited TNF-induced translocation of p65 from the cytoplasm to the nucleus (Fig. 1F).
To test whether Hsp70 and HSF1 directly affect the NF-κB DNA-binding activity, we translated p65, Hsp70, and HSF1 proteins in vitro and performed the aforementioned gel mobility shift assays. Hsp70 and HSF1 failed to directly influence the NF-κB DNA binding (Fig. 1G). These negative data are shown because they help rule out the possibility that Hsp70 or HSF1 directly regulates NF-κB DNA-binding activity. In order to further rule out the possibility that Hsp70 directly interacts with NF-κB (p65), we constructed NLS/p65 plasmids with forced p65 nuclear localization that was independent of IκBα degradation. Cos-1 cells were cotransfected with NLS/p65, NF-κB reporter, and Hsp70 constructs. NF-κB reporter activity analysis showed that Hsp70 did not suppress NF-κB activity (data not shown). This confirmed the earlier findings that Hsp70 did not directly interact with NF-κB (p65).
Hsp70 inhibits TNF-induced phosphorylation of IκBα
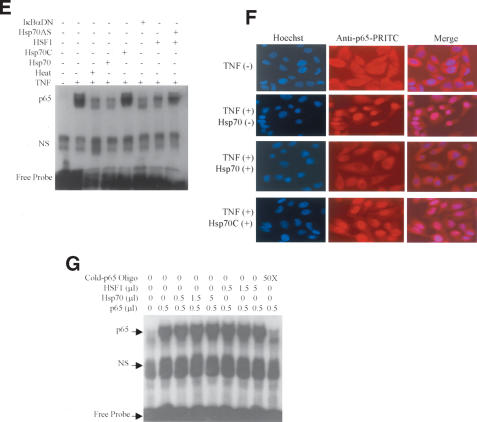
Because Hsp70 inhibited NF-κB activity without directly binding NF-κB, this indicated that Hsp70 impaired NF-κB signaling upstream of NF-κB (p65). Because activation of NF-κB requires phosphorylation of IκB, we examined the effects of Hsp70 on the phosphorylation of IκB using specific antibodies to examine phosphorylated and unphosphorylated IκBα proteins. The results showed that Hsp70 inhibited TNF-induced IκBα phosphorylation (Fig. 2A).
Figure 2.
Effects of Hsp70 on IκBα phosphorylation. (A) Hsp70- and Hsp70C-inducible Hela cells were induced with 100 μM ZnCl2 for 4 h. Sixteen hours later, cells were treated with TNF?25 ng/mL) for 15 min. Protein levels of phospho-IκBα,IκBα, and β-actin were examined 15 min after TNF treatment by Western blot. (B) Hsp70- and Hsp70C-inducible Hela cells were transiently transfected with Flag-IKKβ and exposed to ZnCl2 (0–125 μM) for 4 h. Sixteen hours later, the cells were treated with TNF (25 ng/mL) for 15 min. Fifteen minutes later, cells were lysed and Hsp70 and Hsp70C were immunoblotted with an anti-His antibody (top gels). An in vitro kinase assay was performed using GST–IκBα(1–56) as substrate (middle gels). IKKβ protein levels were determined with an anti-Flag antibody (lower gels).
These data suggested either that Hsp70 directly inhibited IκB activity, or that Hsp70 directly bound IκBα to mask its phosphorylation site, or that Hsp70 accelerated IκBα dephosphorylation. In order to differentiate between these possibilities, several experiments were performed. First, pull-down data, immunoprecipitation data, and two-hybrid experiments showed that Hsp70 did not interact with IκBα (see data below). Second, Hsp70- and Hsp70C-inducible Hela cells were transiently transfected with Flag-IKKα and Flag-IKKβ. The effect of Hsp70 on IKK activity was assayed using an in vitro kinase assay with exogenous GST–IκBα (1–56) as the substrate. Hsp70 reduced IKK activity in a dose-dependent manner (Fig. 2B). Neither Hsp70 nor Hsp70C altered IKKβ expression (Fig. 2B).
Hsp70 directly interacts with IKKγ
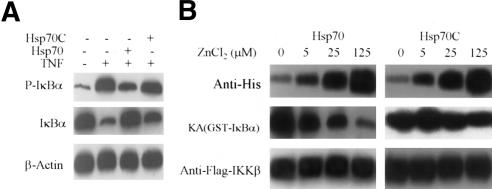
On the basis of these observations, we considered whether Hsp70 directly interacted with one or more components of the IKK complex and inhibited IKK activity. To determine which molecule(s) of the IKK complex might be the primary target of Hsp70, we searched for a direct interaction between the three IKKs and Hsp70 using a beads pull-down assay. 35S-labeled IKKγ was retained on Hsp70-, but not on Hsp70C-conjugated beads. IKKα and IKKβ did not associate with Hsp70 or with Hsp70C (Fig. 3A).
Figure 3.
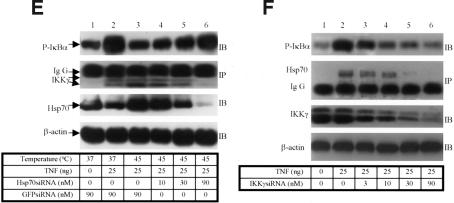
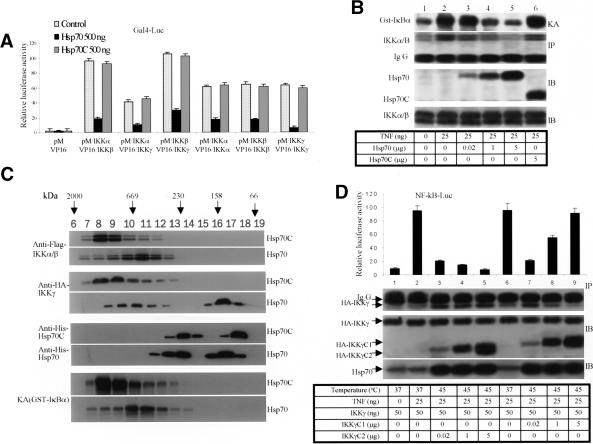
Hsp70 interacts with IKKγ. (A) Hsp70 or Hsp70C was immobilized on His beads and incubated with 35S-labeled IKKα, IKKβ, or IKKγ. After extensive washing, bound proteins were analyzed by SDS-PAGE. (B) Diagram of IKKγ and the truncation mutants used in this study. (C) His beads alone or His-Hsp70 immobilized on beads were incubated with 35S-labeled IKKγ and its truncation mutants. After extensive washing, the bound proteins were analyzed by SDS-PAGE. (D) Two-hybrid assays for protein–protein interactions in living cells. Cos-1 cells were transiently transfected with the Gal4-reporter together with the indicated constructs, and luciferase activity was measured 24 h later. (E) Interaction of endogenous Hsp70 and IKKγ. 293 cells were transfected with increasing amounts of Hsp70 siRNA (lanes 4–6) or control GFP–siRNA (lanes 1–3). After 48 h, cells were heated at 45°C for 30 min. Sixteen hours later, cells were treated for 15 min with TNF or vehicle. Cell lysates were immunoprecipitated with anti-Hsp70 antibody (IP) and then immunoblotted with anti-IKKγ antibody (IB). The IKK activities were detected using the whole-cell extracts with anti-phospho-IκBα antibody (IB, top gel). Hsp70 was detected with an anti-Hsp70 antibody (third gel from top). (F) Coimmunoprecipitation of endogenous IKKγ and Hsp70. 293 cells were transfected with increasing amounts of IKKγ siRNA (lanes 3–6). After 48 h, the cells were treated with vehicle or TNF (25 ng/mL) for 15 min. Cell lysates were immunoprecipitated with anti-IKKγ antibody and then immunoblotted with anti-Hsp70 antibody. The whole-cell lysates were immunoblotted with anti-IKKγ and anti-β-actin antibodies. IKK activities were detected using the whole-cell extracts with anti-phospho-IκBα antibody (top gel).
To map which region within IKKγ was responsible for this interaction, we constructed various deletions of IKKγ (Fig. 3B). The in vitro beads pull-down assay showed that only the IKKγ and IKKγC1, which contained the coiled-coil motif, interacted with Hsp70 (Fig. 3C), suggesting that Hsp70 bound to the coiled-coil motif of IKKγ.
To further confirm that Hsp70 associated with IKKγ within cells, we performed a two-hybrid assay in cultured mammalian cells. Hsp70 and Hsp70C CDNAs were in frame cloned into the pM vector containing the Gal-4 DNA-binding domain. Similarly, the IKKγ and its mutants, and IKKα, IKKβ, and IκBα CDNAs were in frame inserted into pVP16 plasmid containing the activation domain. The resulting constructs were cotransfected into Cos-1 cells with a Gal-4 reporter gene. The results confirmed the in vitro binding data showing Hsp70 interaction with IKKγ and IKKγC1 but not with IκBα, IKKα, or IKKβ (Fig. 3D). Hsp70C did not associate with any of the molecules tested (Fig. 3D).
To determine whether endogenous Hsp70 interacted with IKKγ at physiologically relevant levels, we exposed cells to heat and TNF, either with GFPsiRNA (control, Fig. 3E, lanes 1–3) or with Hsp70siRNA (Fig. 3E, lanes 4–6). The cells were lysed and immunoprecipitated with an anti-Hsp70 antibody. The amounts of IKKγ protein in the anti-Hsp70 immunoprecipitates were assessed with anti-IKKγ antibody (Fig. 3E). Portions of the total cell extracts were immunoblotted with β-actin antibody as a control for lane loading or for siRNA specificity. The results confirmed that detectable amounts of endogenous IKKγ were present in the anti-Hsp70 immunoprecipitates (Fig. 3E, second gel from top) but not in the antiserum control (Fig. 3E, lane 1). In addition, IKKγ specifically associated with endogenous Hsp70, as the Hsp70–IKKγ interaction was markedly decreased in Hsp70siRNA transfectants (Fig. 3E, second gel from top, lanes 4–6). IKK activity was analyzed next using an anti-phospho-IκBα (P-IκBα) antibody. As expected, elimination of Hsp70 by RNAi rescued heat-mediated inhibition of IκBα phosphorylation (Fig. 3E, top gel, lanes 3–6). Conversely, using a similar approach, the endogenous Hsp70 could also be detected in the anti-IKKγ immunoprecipitates (Fig. 3F, second gel from top, lanes 2–4) but not in the antiserum control (Fig. 3F, lane 1). IKK activity, as assessed by P-IκBα levels, decreased (Fig. 3E, top gel) with decreasing levels of IKKγ (Fig. 3F, third gel from top).
Hsp70 inhibits the formation of the IKK complex
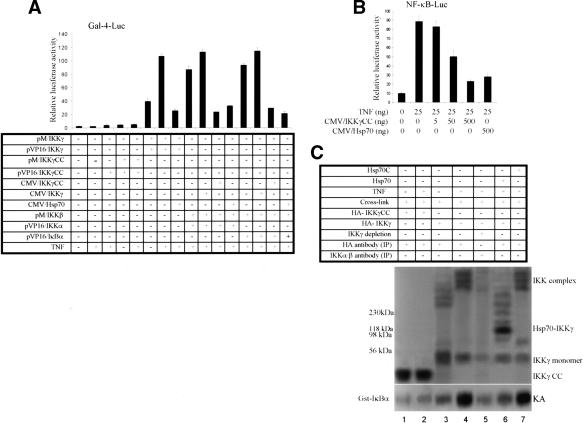
To define the interactions of the components of IKK and the effects of Hsp70 on the IKK complex in detail, we used the mammalian two-hybrid system to determine whether Hsp70 not only bound IKKγ, but also disrupted interactions between the three IKKs. IKKα, IKKβ, and IKKγ cDNAs were inserted in-frame into the pM plasmid containing Gal-4 DNA-binding domain and pVP16-vector-bearing activation domains, respectively. Cos-1 cells were cotransfected with Gal-4-dependent luciferase and IKK constructs. These data showed that IKKα,IKKβ, and IKKγ formed homocomplexes (IKKα/α, IKKβ/β, IKKγ/γ) and heterocomplexes (IKKα/β, IKKα/γ, IKKβ/γ), and these complexes were disrupted in Hsp70 overexpressing cells (Fig. 4A).
Figure 4.
Hsp70 disrupts formation of the IKK complex. (A) Two-hybrid experiment. Cos-1 cells were transfected with Gal4-Luc together with the indicated constructs in the figure and in Materials and Methods. Cells were subjected to luciferase analysis 24 h later. (B) Hsp70 disrupts the IKK complex. 293 cells were transfected with His-Hsp70 or His-Hsp70C. After transfection for 48 h, the cells were treated with vehicle or with TNF (25 ng/mL) for 15 min. After immunoprecipitation (IP) with anti-IKKγ antibody, the IKK activity was analyzed by in vitro kinase assay using GST–IκBα (1–56) as substrate (top gel). The level of IKKα/β present in the immunocomplexes was immunoblotted with anti-IKKα/β antibody (second gel from top). Expression of Hsp70, Hsp70C, and IKKα/β are shown using anti-His and IKKα/β antibodies from whole-cell extracts (third and fourth gels). (C) Hsp70 or Hsp70C stable Hela cells were transiently transfected with Flag-IKKα,IKKβ, and HA-IKKγ. After 48 h, cells were lysed and applied to a Superose 6 column. The fractions were subjected either to immunoblotting (top six gels) or in vitro kinase assays using the GST–IκBα (1–56) as substrate (lowest two gels). (D) 293 cells were transfected with the same amounts of HA-IKKγ (second gel) and increasing amounts of HA-IKKγC1 (lanes 7–9) or IKKγC2 (lanes 3–5) constructs. Twenty-four hours later, the cells were heated at 45°C for 30 min. After 16 h, the cells were treated for 15 min with TNF or vehicle. The cell lysates were immunoprecipitated with anti-Hsp70 antibody and then immunoblotted with anti-HA-IKKγ antibody (top gel). The total expression levels of IKKγ,IKKγC1, IKKγC2, and Hsp70 in the whole-cell lysates were immunoblotted with the appropriate antibodies. A portion of these cells was transiently transfected with an NF-κB-dependent reporter and Luciferase assays (top panel), performed as described in Materials and Methods 24 h after TNF treatment.
To determine the effects of Hsp70 on IKK complex assembly and IKK activity, we transfected 293 cells with His-Hsp70 or His-Hsp70C. The extracts were immunoprecipitated with an anti-IKKγ antibody. The amounts of IKKα and IKKβ proteins in the anti-IKKγ immunoprecipitates were detected with anti-IKKα/β antibody (Fig. 4B, second gel), and in vitro kinase assays were performed on the immunoprecipitated IKK complexes using exogenous GST–IκBα fusion protein as substrate. Transfection with Hsp70 (third gel from the top) abolished the robust activation of IKK in response to TNF stimulation (Fig. 4B, uppermost gel) and decreased the amounts of IKKα and IKKβ proteins in the anti-IKKγ immunoprecipitates (Fig. 4B, second gel from top). IKKα/β expressions were not changed (Fig. 4B, fourth gel from top).
These data suggested that Hsp70 might inhibit the assembly of the IKK complex. To test this possibility, we transiently transfected Hsp70- and Hsp70C-inducible Hela cells with Flag-IKKα, Flag-IKKβ, and HA-IKKγ constructs for 48 h. Following challenge with TNF, cells were extracted and subjected to gel filtration on a Superose 6 column. Each column fraction was immunoblotted with anti-Flag (IKKα/β), anti-HA (IKKγ), and anti-His (Hsp70, Hsp70C) antibodies. IKK activity was measured using an in vitro kinase assay. Hsp70 reduced the size of the IKK complex and markedly increased the amounts of IKKγ in the lower molecular weight fractions (fourth row) in comparison with those from Hsp70C (third row) transfected cells (Fig. 4C). Both the HA-IKKγ (fourth row) and His-Hsp70 (sixth row) were detected in the same column fractions migrating at ∼120 kDa, a finding consistent with stable Hsp70–IKKγ heterocomplexes. As shown earlier, Hsp70 inhibited IKK activity (Fig. 4C, bottom two rows).
Because these results suggested that the Hsp70–IKKγ interaction was critically involved in regulating NF-κB signaling, we confirmed whether disruption of IKKγ–Hsp70 interaction would be sufficient to overcome Hsp70-mediated NF-κB suppression. Because Hsp70 directly bound both IKKγ and IKKγC1, but not IKKγC2 (Fig. 3C,D), we used IKKγC1 to selectively disrupt the interaction between IKKγ and Hsp70 by competing for binding to Hsp70. Therefore, 293 cells were transfected with HA-IKKγ and increasing amounts of HA-IKKγC1 or HA-IKKγC2. Cells were exposed to TNF to activate NF-κB and exposed to heat shock (45°C) for 30 min to induce endogenous Hsp70, and cell extracts were immunoprecipitated with anti-Hsp70 antibody. Appreciable amounts of HA-IKKγ protein could be detected with anti-HA antibody in the anti-Hsp70 immunoprecipitates (Fig. 4D, top gel, lanes 3–5) but not in the antiserum control (Fig. 4D, top gel, lane 1). Another portion of these cells was transfected with an NF-κB reporter gene for NF-κB activity analysis. Immunoprecipitation showed that Hsp70 interacted with IKKγ and that IKKγC1 displaced IKKγ from Hsp70 immunocomplex (Fig. 4D, top gel, lanes 3–5,7–9). IKKγC1-mediated IKKγ release from the Hsp70–IKKγ complex was accompanied by gradual restoration of NF-κB activity (Fig. 4D, upper panel, lanes 7–9), whereas IKKγC2 had no such effect (Fig. 4D, upper panel, lanes 3–5).
Hsp70 precludes IKKγ oligomerization, which is required for NF-κB activation
These data showed that IKKγ formed homo-oligomers (Fig. 4A) and that the coiled-coil motif of IKKγ was the Hsp70-binding site (Fig. 3B,C), suggesting that the coiled-coil region serves as an effector domain to regulate the IKK complex via its oligomerization. If this conclusion is correct, the coiled-coil deletion mutant of IKKγ should not self-associate and should suppress NF-κB signaling. We tested this by transfecting Cos-1 cells with IKKγCC (the coiled-coil deletion mutant of IKKγ, Fig. 3B) and either Gal4- or NF-κB-dependent reporters. The IKKγCC failed to interact with itself or IKKα and IKKβ, even on TNF treatment (Fig. 5A). In addition, the IKKγCC, like Hsp70, inhibited TNF-mediated NF-κB activation (Fig. 5B). These effects of Hsp70 and IKKγCC on suppressing TNF-mediated NF-κB activation are specific because our previous data showed that neither Hsp70C nor Hsp70N affected NF-κB activity (Fig. 1A). These data suggested that IKKγ oligomerization via the coiled-coil motif was indispensable for IKK activity.
Figure 5.
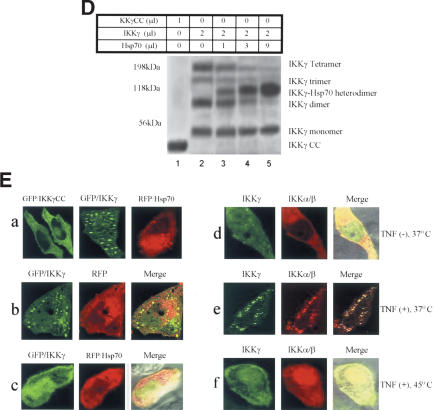
Hsp70 inhibits the IKKγ oligomerization required for NF-κB activation. (A) Two-hybrid experiment for protein interactions. Cos-1 cells were transiently transfected with a Gal-4-dependent reporter and the indicated constructs and then subjected to luciferase analysis 24 h later. (B) IKKγCC and Hsp70 inhibit TNF-induced (25 ng/mL) NF-κB activation. Cos-1 cells were transfected with NF-κB-Luc together with either IKKγCC or Hsp70 and then subjected to luciferase analysis 24 h later. (C) IKKγ formed oligomers. 293 cells expressing HA-IKKγ (lanes 3–7) or HA-IKKγCC (lanes 1,2) were labeled with 35S-methionine. Some of the cells were transfected with Hsp70 (lane 6) or Hsp70C (lane 7) expression vectors. Cells were treated with vehicle or TNF (25 ng/mL). Cells were lysed and the extracts were incubated with cross-linker (EGS). After cross-linking, one batch had IKKγ immunodepleted using IKKγ antibody (lane 5). IKKγ was immunoprecipitated with HA antibody (lanes 1–4,6,7), and the IKKγ immunodepleted extracts were immunoprecipitated with an anti-IKKα/β antibody (lane 5). The pellets were washed and analyzed by SDS-PAGE. IKK activity (KA) in the immunoprecipitates was determined with GST–IκBα as substrate (bottom gel). (D) Hsp70 inhibits the IKKγ oligomerization in vitro. 35S-labeled IKKγ and IKKγCC were incubated with or without purified Hsp70 and the cross-linking agent (EGS). The proteins were analyzed by SDS-PAGE. (E) IKKγ or IKK complexes can be visualized in vivo and are inhibited by Hsp70 or heat. GFP–IKKγ, GFP–IKKγCC, or RFP–Hsp70 were expressed alone (panel a) or together (panels b,c) in Hela cells. Nontransfected cells were exposed either to vehicle (panel d), TNF (25 ng/mL for 15 min; panel e), or TNF (25 ng/mL for 15min) plus heat (30 min at 45°C; panel f). They were then fixed and double immunostained with anti-IKKγ and anti-IKKα/β antibodies, and fluorescent signals were visualized by confocal microscopy.
To further examine the effects of Hsp70 on IKKγ oligomerization and its regulation of IKK activity, we transfected 293 cells with HA-IKKγCC or HA-IKKγ. Some of the cells were cotransfected with Hsp70 or Hsp70C. All cells were metabolically labeled with 35S-methionine and some were then briefly exposed to TNF. In vivo chemical cross-linking experiments in 293 cell extracts were performed using the homobifunctional cross-linker ethyleneglycol-bis-succinimidylsuccinate (EGS), then were immunoprecipitated with anti-HA antibody or with anti-IKKα/β antibody (for IKKγ-depleted extracts). Some extracts were immunodepleted with an anti-IKKγ antibody prior to immunoprecipitation. To analyze the oligomeric state of IKKγ, we analyzed the cross-linked, immunoprecipitated complexes by SDS-PAGE. We readily detected the different IKKγ oligomeric states with masses of >250 kDa (Fig. 5C, lane 3), which was not the case for IKKγCC transfected cells (Fig. 5C; lanes 1,2). The IKKγ multimers containing the IKKα/β complexes may be necessary for basal NF-κB activity (Fig. 5C, lane 3). TNF treatment resulted in formation of the high-molecular-weight IKK complex (Fig. 5C, lanes 4,7), whereas immunodepletion of IKKγ and transfection with Hsp70 markedly decreased the amount of the high-molecular-weight IKK complex (Fig. 5C, lanes 5,6). To determine whether the catalytic activity of the IKK complex required IKKγ oligomerization, we retrieved IKK complexes from the transfected cells by immunoprecipitation with anti-HA/IKKγ antibody or anti-IKKα/β antibody and examined them for in vitro kinase activity. Only IKKγ transfected cells that were treated with TNF had high IKK activity (Fig. 5C, lanes 4,7). IKK activity was greatly decreased in TNF-treated cells by IKKγCC transfection (Fig. 5C, lane 2), by Hsp70 cotransfection (Fig. 5C, lane 6), and following IKKγ depletion (Fig. 5C, lane 5). These results indicate that inducible IKK activity is critically dependent on IKKγ or its oligomerization. These in vivo data also suggest that overexpression of Hsp70 but not Hsp70C blocks formation of the IKK complex and favors formation of Hsp70–IKKγ heterodimers (Fig. 5C, lane 6).
Therefore, we next determined whether Hsp70 would block IKKγ oligomerization in vitro and whether IKKγ oligomerization was dependent on any other proteins. 35S-labeled HA-IKKγ protein and purified Hsp70 were cross-linked with EGS followed by anti-HA antibody immunoprecipitation. Cross-linked, immunoprecipitated IKKγ yielded additional species with molecular weights corresponding to IKKγ dimers, trimers, and tetramers, with these multimers being dose-dependently inhibited by addition of Hsp70 (Fig. 5D). This occurred because Hsp70 associated with monomeric IKKγ to form heterodimers (Fig. 5D, lanes 3–5). These in vitro experiments support the idea that Hsp70 inhibited formation of the IKK complex by blocking oligomerization of IKKγ. Although these data show that Hsp70 is sufficient to prevent IKKγ oligomerization in vitro, future studies will be needed to determine whether Hsp70 blocks IKKγ oligomerization in vivo.
To further confirm the effects of Hsp70 on IKKγ or IKK complex in living cells, we transfected GFP/IKKγ (GFP, green fluorescent protein), GFP/IKKγCC, and RFP/Hsp70 (RFP, red fluorescent protein) into Hela cells as reporters and examined their subcellular localization using confocal microscopy. Expression of GFP/IKKγ demonstrated multifocal, punctate regions of staining. They could be macromolecular foci composed of either IKKγ oligomeric complexes and/or complexes of IKKγ with other proteins. This contrasted with IKKγCC expression, which showed diffuse, uniform fluorescence (Fig. 5E, panel a). These findings were observed in >95% of the cells in which GFP/IKKγ or GFP/IKKγCC was expressed. Cotransfection of GFP/IKKγ and RFP/Hsp70 resulted in a dramatic redistribution of IKKγ from discrete macromolecular foci to uniform fluorescence (Fig. 5E, panel c)—the findings being observed in >60% of cells in which both GFP/IKKγ and RFP/Hsp70 were coexpressed (in some cells, the levels of Hsp70 expression may be not sufficient for the redistribution of IKKγ). In nontransfected cells, double labeling with antibodies to IKKγ and IKKα/β again showed macromolecular foci in TNF-treated cells (Fig. 5E, panel e), whereas there were no IKKγ/IKK foci in control (no TNF treatment, Fig. 5E, panel d) or heat-shocked (Fig. 5E, panel f) cells (Fig. 5E)— the findings again being observed in >95% of cells. These data represent a clear in vivo demonstration of the ability of Hsp70 to inhibit formation of these IKKγ immunostained foci. The data are consistent with Hsp70 interfering with the formation of IKKγ oligomers (dimers, trimers, tetramers), and/or with Hsp70 interfering with the association of IKKγ with other proteins that make up the IKK macromolecular complex.
Hsp70 promotes TNF-mediated cell death
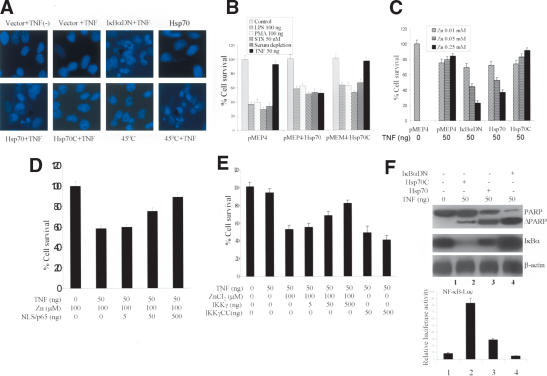
Because Hsp70 suppression of NF-κB activity could adversely affect cell survival, we investigated the effects of Hsp70 on cell survival following treatment with several stressors. We compared the apoptotic behavior of control Hela cells to IκBαDN (IκBαDN-dominant negative used to block NF-κB activation) and Hsp70 stable Hela cells. In many of the experiments, the cells were exposed to TNF for 4 h and/or heating to 45°C for 30 min. Cell death was detected by examination of the nuclear morphology using Hoechst staining. Normal cell survival was observed for cells with vector alone, vector + TNF, Hsp70C + TNF, or Hsp70, or when heated alone (Fig. 6A). Cell death, manifested by nuclei with intensely condensed and occasionally fragmented morphology, was observed with cells treated with TNF plus any of the following three treatments: overexpressing IκBαDN, overexpressing Hsp70, or heating (Fig. 6A).
Figure 6.
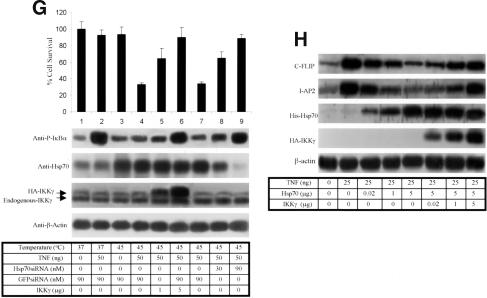
Effects of Hsp70 and NF-κB on cell survival. (A) Hsp70-, Hsp70C-, and IκBαDN-inducible Hela cells were treated with ZnCl2 (100 μM) for 4 h. Sixteen hours later, the cells were treated with vehicle or TNF (50 ng/mL) for 4 h. Nuclei were stained with Hoechst. (B) Hsp70- or Hsp70C-inducible Hela cells had Hsp70 (middle panel) or Hsp70C (right panel) induced with ZnCl2 (100 μM) for 4 h. Sixteen hours later, all cells were treated with the indicated agents. Cell viability was assessed 24 h later using MTT. (C) IκBαDN-, Hsp70-, or Hsp70C-inducible Hela cells, or cells with empty vector (pMEP4), were treated with increasing concentrations of ZnCl2 for 4 h. Sixteen hours later, cells were treated with vehicle or TNF (50 ng/mL). Cell viability was assessed 24 h later using MTT. (D) NF-κB rescues Hsp70–TNF-induced cell apoptosis. Hsp70-inducible Hela cells were transiently transfected with an NF-κB/p65 expression vector. Twenty-four hours later, the cells were treated with ZnCl2 (100 μM) for 4 h to induce Hsp70. TNF (50 ng/mL) or vehicle was then added and cell viability assessed 24 h later using MTT. (E) IKKγ overcomes TNF–Hsp70-induced cell apoptosis. Hsp70-inducible Hela cells were transiently transfected with IKKγ or IKKγCC or empty vector. Twenty-four hours later, the cells were treated with vehicle or with ZnCl2 (100 μM) for 4 h to induce Hsp70. Sixteen hours later, TNF (50 ng/mL) or vehicle was added and cell viability assessed 24 h after that using MTT. (F) IκBαDN-, Hsp70-, or Hsp70C-inducible Hela cells were treated with ZnCl2 (100 μM) for 4 h. Sixteen hours later, cells were then treated with vehicle or TNF (50 ng/mL). Twenty-four hours later, Western blots were performed on cell extracts with antibodies to PARP, IκBα, and β-actin. NF-κB activity was assessed in cells under the same conditions using a luciferase assay (bottom panel). (G) The effects of Hsp70–IKKγ interactions on IKK activity and cell survival. HA-IKKγ or Hsp70 siRNA (lanes 8,9) or GFPsiRNA (lanes 1–4,6,7) were transiently transfected into 293 cells. After 48 h, the cells were treated either with vehicle, with TNF (50 ng/mL), and/or with heat (30 min at 45°C). Cells were lysed immediately, and the lysates immunoblotted with anti-phospho-IκBα (top gel), anti-Hsp70 (second gel down), anti-IKKγ (third gel down), and β-actin antibodies. The survival of similarly treated cells was determined 24 h later using MTT. (H) Hsp70 inhibits FLIP and IAP-2 expression. 293 cells were transiently transfected with His-Hsp70- or HA-IKKγ-expressing constructs. Forty-eight hours later, cells were treated with vehicle or TNF, and FLIP and IAP-2 expression were assayed by immunoblots 24 h later.
Quantitative evaluation of Hela cell survival showed that treatment of Hela cells with LPS, PMA, staurosporine (STS), and serum deprivation decreased cell survival from 100% to 40% or less. In contrast, nearly 95% of the cells survived when treated with TNF alone (Fig. 6B, left panel). Following expression of Hsp70, however, cell survival following LPS, PMA, STS, and serum deprivation increased to 60%, whereas TNF treatment plus Hsp70 decreased survival from 95% (Fig. 6B, left panel) to ∼50% (Fig. 6B, middle panel). Expression of Hsp70C combined with TNF treatment was associated with nearly normal 95% cell survival (Fig. 6B, right panel), similar to the cell survival with TNF treatment alone (Fig. 6B, left panel).
In a second experiment designed to examine the effects of NF-κB and dose-dependent changes of Hsp70 and Hsp70C on cell survival, treatment of control Hela cells (empty vector) with TNF plus 0.01–0.25 mM ZnCl2 decreased cell survival from 100% to roughly 80% (Fig. 6C). In cells stably expressing IκBαDN, increasing induction of IκBαDN with increasing doses of ZnCl2 decreased cell survival from 73% down to 22% after TNF treatment; and, in cells stably expressing Hsp70, increasing induction of Hsp70 with increasing doses of ZnCl2 decreased cell survival from 75% down to 35% after TNF treatment. Importantly, cells stably expressing Hsp70C—which does not block NF-κB activation—had near normal survival with increasing doses of ZnCl2 from 78% up to 95%. It is notable that for both the Hela cells stably expressing the vector pMEP4 and for the cells stably expressing Hsp70C, increasing doses of ZnCl2 actually improved cell survival by a small but significant amount in both cases. The explanation for this is not clear, because increasing ZnCl2 doses induce endogenous Hsp70 to a modest degree (Fig. 1D). It is possible that the ZnCl2 induces additional heat shock or other stress proteins that protect the Hela cells by a small amount in all of the experiments shown. In the case of the vector and Hsp70C-expressing cells, the small protective effects of these ZnCl2-induced stress proteins are observed; however, in the IκBαDN and Hsp70-expressing cells, the combined lethal effects of increasing amounts of IκBαDN and Hsp70 plus TNF treatment lead to significant cell death and the small protective effects of ZnCl2 are overwhelmed.
These results suggest that elevated Hsp70 sensitized cells to TNF killing by interfering with NF-κB signaling. If this were the case, activation of NF-κB would be expected to decrease apoptosis following combined exposure to Hsp70 and TNF. Hsp70 zinc-inducible cells were transiently transfected with NLS/p65 construct. Indeed, increasing levels of p65 improved cell survival following zinc induction of His-Hsp70 and TNF treatment (Fig. 6D). To provide further evidence for this, we reversed the improved cell survival obtained with increasing levels of IKKγ by the IKKγ coiled-coil deletion mutant, IKKγCC (Fig. 6E).
To examine the mechanism by which Hsp70 inhibition of NF-κB signaling leads to cell death, we determined whether elevated Hsp70 favored TNF-mediated caspase activation via inhibiting the NF-κB pathway. The effects of Hsp70 and IκBαDN on caspase-3 processing were examined. The results showed that Hsp70 and IκBαDN, but not Hsp70C, decreased TNF-induced NF-κB activity (Fig. 6F, lower panel). In addition, Hsp70 and IκBαDN significantly enhanced TNF-induced PARP cleavage (Fig. 6F, topmost gel).
Enforced expression of p65/NF-κB or IKKγ did not entirely rescue Hsp70-mediated cell death (Fig. 6C,D). This might be due to the low efficiency of transient transfection in Hela cells (∼30%, data not shown). Additionally, Hsp70AS did not appear to block Hsp70 function efficiently (Fig. 1A,E). We therefore performed additional studies in 293 cells, where much higher rates of transfection could be obtained (∼90%–95% rate of transfection, data not shown). The 293 cells were transfected with IKKγ and Hsp70 siRNA or control GFPsiRNA for 2 d and then exposed to heat (30 min at 45°C) and/or TNF (50 ng for 15 min). IKKγ activity was detected using an anti-P-IκBα antibody. Cell survival was measured using MTT 24 h after heat and/or TNF treatment. Treatment with GFPsiRNA alone, TNF plus GFPsiRNA, or heat shock plus GFPsiRNA did not affect cell survival (Fig. 6G, lanes 1–3). The combination of TNF treatment and heat shock, however, decreased cell survival to ∼30% (Fig. 6G, lanes 4,7). Overexpression of HA-IKKγ (confirmed with HA-IKKγ antibody; Fig. 6G, third gel from top, lanes 5,6) enhanced IκBα phosphorylation (Fig. 6G, top gel, lanes 5,6) and increased cell survival to near control levels of 90% (Fig. 6G, top panel, lanes 5,6). Similarly, transfection of cells with Hsp70 siRNA almost reversed heat-shock-mediated IKK inhibition (Fig. 6G, top gel, lanes 7–9) and increased cell survival levels to near control levels of 90% despite combined heat shock and TNF treatment (Fig. 6G, top panel, lanes 7–9). Lanes 4 and 7 in Figure 6G are replicates of each experiment and are not duplicates.
Because Hsp70 inhibits NF-κB transactivation and promotes caspase activation during TNF signaling (Fig. 6F), it is proposed that Hsp70 would block endogenous NF-κB-dependent antiapoptotic gene expression. Indeed, the results show that expression levels of cFLIP and IAP-2, two antiapoptotic proteins (Fig. 6H, upper two gels), decreased in TNF-treated 293 cells with increasing levels of His-Hsp70 protein. This Hsp70-mediated decrease of cFLIP and IAP-2 expression was IKKγ dependent because increasing IKKγ levels significantly reversed this (Fig. 6H, last three lanes).
Discussion
This study demonstrates how Hsp70 negatively regulates NF-κB, thus establishing the first direct, mechanistic link between Hsp70 and the NF-κB signaling cascade. The Hsp70 decrease of NF-κB activity tips the balance from cell survival to cell death following TNF/death receptor stimulation (Fig. 7). The ability of Hsp70 to inhibit NF-κB signaling may contribute to the enhanced sensitivity of heated cells to chemotherapeutic agents and radiation treatment, and it helps explain at least some of the previous reports that demonstrate the pro-apoptotic effects of heat shock and Hsp70.
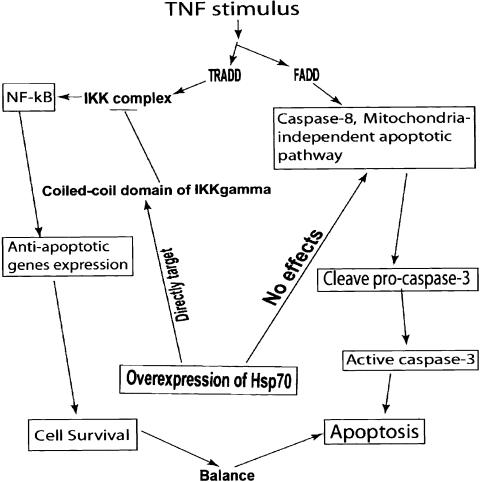
Figure 7.
Proposed pro-apoptotic role of Hsp70 in TNF-mediated apoptosis. TNF simultaneously activates caspase-8 and NF-κB (Micheau and Tschopp 2003). Hsp70 directly targets the coiled-coil domain of IKKγ and impairs TNF-triggered NF-κB activation. This occurs by binding of Hsp70 to IKKγ, which prevents formation of the IKK complex and blocks TNF-triggered NF-κB activation. Additionally, Hsp70 cannot block caspase-8-mediated cell apoptosis (Beere et al. 2000). Therefore, once Hsp70 levels are sufficient to significantly block the formation of the IKK complex, this plays an important role in shifting cells from survival to death in response to TNF challenge.
Hsp70 inhibits NF-κB activation by binding IKKγ
Previous studies have shown that Hsp70 and heat shock modulate NF-κB function, but the mechanism by which Hsp70 inhibits NF-κB remained unclear (Feinstein et al. 1997; Guzhova et al. 1997; Curry et al. 1999; Andres et al. 2002; Malhotra et al. 2002). In the present study, we clarify the mechanism by which Hsp70 inhibits NF-κB. Our data demonstrate that Hsp70 directly binds to IKKγ and this inhibits formation of the IKK complex and blocks NF-κB activation.
IKKγ contains several distinct domains that are involved in regulating IKK activity (Rothwarf et al. 1998; May et al. 2000; Yamamoto et al. 2001). The interaction of IKKγ with IKKα and IKKβ is critical for the assembly of the high-molecular-weight IKK complex that activates NF-κB, and IKKγ appears to function as an adaptor protein to increase the interactions of key factors required for NF-κB activation (Yamaoka et al. 1998). IKKγ is an essential component of the IKK signalsome, as demonstrated in IKKγ-deficient or IKKγ mutant cells, which are unable to trigger the NF-κB response to a wide array of stimuli (Rothwarf et al. 1998; May et al. 2000; Rudolph et al. 2000; Makris et al. 2002). The large 700–900-kDa IKK complex does not form in cells lacking IKKγ (Yamaoka et al. 1998). A variety of proteins that interact with IKKγ, including RIP, A20, Tax, CIKS, vCLAP, and CYLD are involved in regulating NF-κB signaling (Jin et al. 1999; Leonardi et al. 2000; Poyet et al. 2000, 2001; Zhang et al. 2000; Brummelkamp et al. 2003). We postulate and verify the possibility that Hsp70 binds IKKγ to hamper IKK activation. Our results show that Hsp70–IKKγ interaction plays a key role in NF-κB signaling.
It has been well characterized that the coiled-coil domain of IKKγ is responsible for IKKγ oligomerization, which is critical for activating IKK activity (Poyet et al. 2000). The results of this study show that Hsp70 specifically binds the coiled-coil domain of IKKγ. These findings agree, at least in part, with those of Agou et al. (2002) who have shown that IKKγ (NEMO) binding via the coiled-coil domain to Hsp70 prevents incorrect interdomain pairing reactions. In the present study, we suggest that excess Hsp70 binding to IKKγ may prevent IKKγ self-association that is critical for the formation of the high-molecular-weight IKK complex. Hsp70 binding to the coiled-coil domain of IKKγ might lead to a chaperone-dependent change in the conformation of IKKγ. In this case, IKKγ does not form oligomers when bound to Hsp70, and it is rendered inaccessible to the IKKs and prevents assembly of the IKK complex. Because IKK activity is markedly impaired in cells that express IKKγCC or Hsp70, and enforced oligomerization of IKKγ was able to activate NF-κB (Poyet et al. 2000), these data indicate that IKKγ oligomerization is absolutely essential for TNF-induced NF-κB activation (Tegethoff et al. 2003).
Although strong evidence is provided that Hsp70 targeting to IKKγ plays a negative role in NF-κB signaling, the data do not rule out the possibility that Hsp70 may interact with other components of the IKK complex. The gel filtration data show that Hsp70 is detected in fractions in addition to the IKKγ fractions (200–500 kDa). Future studies will be required to examine the possibility that Hsp70 could have other targets in this pathway. Interestingly, we found that IKKγ can be directly visualized in macromolecular foci in living cells, which is in line with previous reports (Poyet et al. 2000; Heussler et al. 2002). Moreover, overexpression of Hsp70 or heat treatment significantly suppresses these macromolecular foci. These findings could suggest that Hsp70 inhibits these foci by preventing IKKγ oligomerization or Hsp70 suppresses these foci by inhibiting IKKγ binding to other proteins in the IKK complex.
Although Hsp70 did not bind directly to either IKKα or IKKβ (Fig. 3A), Hsp70 still influenced the formation of IKKα and IKKβ hetero- and homocomplexes (Fig. 4A). It is likely that Hsp70 indirectly regulates the assembly of the IKK complex via interacting with IKKγ. Because IKKγ is a component of the IKKα and IKKβ hetero- and homocomplexes (Yamaoka et al. 1998; Mercurio et al. 1999), the formation of these complexes may be IKKγ dependent. Several reports demonstrate that IKKγ is required to facilitate the interactions of the IKK complex as a whole and/or that it influences individual components of the IKK complex (Mercurio et al. 1999; Poyet et al. 2000; Yamamoto et al. 2001). The exact mechanism remains to be elucidated.
The finding that transfection of Hsp70 siRNA into 293 cells restores IKK activity is strong evidence, along with the other experiments shown, that Hsp70 directly regulates IKK activity. However, it is notable that complete elimination of Hsp70 expression with Hsp70 siRNA (Fig. 3E, lane 6) did not increase IKK activity over the nonheat-shock control, where moderate amounts of Hsp70 were present (Fig. 3E, lane 2). This suggests that other heat shock proteins could also down-regulate IKK activity. Indeed, it has recently been shown that Hsp27 binds IKKβ and inhibits NF-κB activity (Park et al. 2003). The situation is even more complex because Hsp90 can also bind the kinase domain of IKKα or IKKβ to form part of the ∼900-kDa IKK complex (Chen et al. 2002). However, Hsp90 binding increased TNF-mediated NF-κB activation, as shown in the present study (Fig. 1A). Therefore, although heat shock down-regulates NF-κB activity (Fig. 4D), this is likely due to a complex interaction of Hsp70–IKKγ and Hsp27–IKKβ to down-regulate IKK activity, whereas Hsp90–IKKα and Hsp90–IKKβ interactions up-regulate IKK activity. Because heat shock down-regulates NF-κB activation (Fig. 4D), the effects of Hsp70 and Hsp27 on down-regulation must overwhelm the up-regulation by Hsp90. In addition, the current studies demonstrate that although low levels of Hsp70 do not appear to affect IKK activity a great deal, high-level Hsp70 expression significantly blocks IKK activity and markedly decreases NK-κB activity.
Although the data show that Hsp70 specifically binds the coiled-coil motif of IKKγ, the regions of Hsp70 responsible for this binding are less clear. Although the ATP-binding domain of Hsp70 might be expected to bind IKKγ, our data show that neither the N-terminal nor the C-terminal domains of Hsp70 significantly affect NF-κB activity (Fig. 1A). This result is similar to a recent study in which it was shown that full-length Hsp70 binds Apaf-1, whereas neither Hsp70C nor Hsp70N could be shown to interact with Apaf-1 (Ravagnan et al. 2001). The results of our study and that of Ravagnan et al. (2001) could suggest that full-length Hsp70 is essential for the interaction with some molecules such as IKKγ and Apaf-1. This is not surprising because the ATP-binding domains and the peptide-binding domains of Hsp70 are functionally coupled each other and probably essential for the complete repertoire of physiological effects of the molecule.
Hsp70 promotes apoptosis by blocking NF-κB-dependent gene expression
The discovery that Hsp70 suppressed NF-κB activation provides the first clear explanation for the pro-apoptotic effect of Hsp70 on cell survival. TNF applied to the three types of cells examined in this study activated NF-κB but did not produce apparent apoptosis, a finding consistent with the recent report (Micheau and Tschopp 2003). TNF applied to the same three cell types overexpressing Hsp70 in this study, however, led to the failure to activate NF-κB, decreased expression of NF-κB antiapoptotic genes such as c-FLIP and IAP-2, and activated caspase-3-dependent cleavage of PARP. These findings are in stark contrast with the effects of Hsp70 on mitochondrial-mediated apoptosis, in which Hsp70 inhibits cell apoptosis by interfering with Apaf-1 and activation of caspase-3-mediated apoptotic pathways (Beere et al. 2000). Hsp70 appears to facilitate apoptosis that is initiated by TNF activation of its death receptors, with very high levels of Hsp70 protein being required to sensitize cells to TNF killing. These results are also generally consistent with a study that demonstrated increased cell death following inhibition of antiapoptotic genes (Goyal et al. 2000).
The death-promoting effect reported here for Hsp70 is at variance with the commonly described protective effect of Hsp70. However, some reports show that heat shock can also increase susceptibility to death, as occurs for NK or LAK cells (Jaattela 1990; Fujieda et al. 1995). In acute myeloid leukemia, apoptosis correlated with increased Hsp70 levels (Chant et al. 1996). A pro-apoptotic function of Hsp70 itself has been described after TCR/CD3 or CD95 activation in Jurkat cells overexpressing Hsp70 (Liossis et al. 1997). Hsp70 was found to accelerate the caspase-activated DNase and DNA fragmentation in TCR-stimulated T-cell apoptosis (Liu et al. 2003). It has been known for some time that heat produces radiosensitization, in which prior heat shock increases cell death in tumors produced by radiation (Dewey and Freeman 1980; Dewey 1994). The adenovirus E1A sensitizes tumor cells to lysis by macrophages through nitric oxide- and TNF-α-dependent mechanisms, despite up-regulation of Hsp70 (Miura et al. 2003). These data, together with our results, demonstrate that Hsp70 potentiates TNF-mediated cell apoptosis. Although Hsp70 generally prevents cell death, Hsp70 can promote cell death when it is overexpressed in a cell that is also exposed to TNF and possibly other death-receptor molecules.
There is a report that TNF mediates susceptibility to heat-induced apoptosis by TNF-induced inhibition of Hsp70 expression (Schett et al. 2003). However, this is inconsistent with the finding that TNF receptor I is required for induction of macrophage heat shock protein 70 (Heimbach et al. 2001). In addition, TNF induces Hsp70 expression in cardiac myocytes (Nakano et al. 1996). Therefore, although TNF could down-regulate Hsp70 in selected cells in certain circumstances (Schett et al. 2003), our data and other studies do not find that TNF inhibits HSF1/Hsp70 induction in most cells (Schett et al. 1998; Heimbach et al. 2001). Instead, our data show that TNF-induced cell death is mediated or at least potentiated by Hsp70 down-regulation of NF-κB signaling in most cells. The differences in these results might relate to differences in NF-κB-mediated gene expression in different cell types, where NF-κB mediates cell survival in neuronal and most other cells, whereas NF-κB may mediate cell death in at least some cell types.
Although most studies, including the present one, have shown that TNF and heat shock work synergistically to kill cells, the potential for sensitizing cells to TNF killing by manipulating Hsp70 may not be applicable to all cells, as evidenced by at least one report (Jaattela et al. 1998). The discrepancy between these studies could be due to several experimental variables that have not been controlled for. For instance, recent data show that Hsp70 only temporarily protects against TNF-mediated cell apoptosis and this protection is lost after 16 h (Gabai et al. 2002). Although one study has shown that Hsp70 inhibited TNF-induced ME180 cell death (Jaattela et al. 1998), another has found that Hsp70 did not impede TNF-mediated ME180 cell apoptosis (Ravagnan et al. 2001). It is certainly possible that elevated Hsp70 might protect cells from TNF-induced cell death, particularly during early stages. However, very high levels of Hsp70 do not favor cell survival when cells are exposed to TNF challenge, at least in this study (Fig. 6A,C,G). In addition, the current study shows that the Hsp70 effect on IKKγ is dose related, with lower Hsp70 levels seemingly having little effect on IKK activity or NF-κB activation (Figs. 3E, 4D). Only very high levels of Hsp70 protein decrease IKK activity (Fig. 4B), and the highest Hsp70 protein levels are required for the greatest TNF-induced apoptosis (Fig. 6B,C,G). It is worth emphasizing that even in the current study, Hsp70 enhances TNF-induced apoptosis, but this did not occur for other stimuli (Fig. 6B). Instead, Hsp70 provided modest protection against LPS-, PMA-, STS-, and serum deprivation-induced cell death (Fig. 6B). Taken together, these findings indicate that the overexpression of Hsp70 is capable of either promoting or inhibiting apoptosis, depending on the nature of the stimulus. Finally, even our data suggest that zinc induction of other heat shock and other stress proteins may protect, at least to some degree, against TNF-induced cell death (Fig. 6C).
Our unique findings help explain a number of important and puzzling phenomena that heat shock or elevated Hsp70 potentiate TNF-, FasL- and radiation-mediated cell death. It is likely that this Hsp70–NF-κB interaction is involved in a variety of disease conditions. Clinical studies over a number of years have shown that there is often an advantage for using heat combined with radiation or cytotoxic drugs to enhance tumor cell killing (Connor et al. 1977; Miller et al. 1977; Dewey and Freeman 1980; Dewey 1984; Curry et al. 1999). We have identified the structural requirement for the interaction of Hsp70 with IKKγ and shown that the coiled-coil domain of IKKγ is necessary for TNF-triggered, Hsp70-dependent decrease of NF-κB activation. The coiled-coil domain of IKKγ thus appears to be an attractive target for drug development. Drugs like Hsp70 that target the coiled-coil domain of IKKγ might decrease NF-κB activation during death-receptor stimulation and be clinically useful for enhancing tumor cell death or controlling inflammation.
Materials and methods
Cell culture
Hela, Cos-1 (ATCC), and 293 (Invitrogen) cells were grown in DMEM medium (Gibco) supplemented with 10% (v/v) fetal calf serum and 2 mM L-glutamine (Gibco). Hela Zn2+-inducible cell lines were generated by transfection with pMEP4, pMEP4/His-Hsp70, pMEP4/His-Hsp70C, and pMEP4/IκBαDN constructs. The transfected cells were selected with hygromycin (Gibco) for 2 wk. The selected clones were incubated in medium with Zn2+ for 4 h to induce gene expression that was confirmed by Western blot 24 h later.
Cell viability assays
The MTT assay was used to assess cell viability and was performed according to the directions in the manufacturer's instruction manual (Sigma).
Plasmids and reagents
The following constructs were used in various experiments in this study: pCMV/p65, pCMV4/IκBαDN (IκBα dominant negative), pCR-Flag/IKKα, pCR-Flag/IKKβ, pRC-HA/IKKα, pRC-Flag/IKKβ, pRC-HA/IKKγ, GST/IκBα (1–56), and pcDNA3/HSF1 (see Acknowledgments). Other plasmids were obtained commercially: pBluescript/Hsp90, pAG153/Hsp70 (ATCC); pSV/β-galactosidase (Promega); Cal4-Luc, NF-κB-Luc (Stratagene); and pMEP4 (Invitrogen). The mammalian two-hybrid system and pEGFP and pDsRed2 were obtained from Clontech and pCruz HA was obtained from Santa Cruz.
The other plasmids were constructed by PCR or appropriate restrictive-enzyme-digested methods: pcDNA3/Hsp90, pcDNA3/Hsp70 antisense, pcDNA3/IκBαDN, pcDNA3/His-Hsp70N (amino acids 1–420), pcDNA3/His-Hsp70C (amino acids 420–640), pMEP4/IκBαDN, pMEP4/His-Hsp70, pMEP4/His-Hsp70C (amino acids 420–640), pM/IKKα, pVP16/IKKα, pM/IKKβ, pVP16/IKKβ, pM/IKKγ, pVP16/IKKγ, pM/IKKγCC (deleted amino acids 260–320, see Fig. 3B), pVP16/IKKγCC, pM/IκBα, pVP16/IκBα, pCruz/IKKγ, pCruz/IKKγN (amino acids 1–100), pCruz/IKKγC1 (amino acids 250–419), pCruz/IKKγC2 (amino acids 320–419), pCruz/IKKγCC, pM/p65AD, NLS/p65, GFP/IKKγ, GFP/IKKγCC, RFP/HSP70, pRSET/Hsp70, pRSET/Hsp70C (amino acids 420–640).
Antibodies used included anti-His, phospho-IκBα (Cell Signaling), β-actin and TRITC-conjugated IgG, Flag (Sigma), HA, IκBα, IKKα/β (which recognized both IKKα and IKKβ), IKKγ, p65, c-FLIP, IAP-2 (Santa Cruz), and PARP (Oncogene). Chemicals used included STS, TNF, LPS, PMA, protein G agarose beads, and Hoechst (Sigma). The TNT kit that was used for in vitro translation and the Luciferase kits were from Promega. The SuperFect transfection kit was from Qiagen. The gel filtration column kit was from Amersham Pharmacia Biotech. The [γ32P]ATP (3000 mCi/nmole) and [35S]methionine were from Dupont/NEN and the cross-linker EGS was from Pierce Inc.
Transfection and gene silencing assays
Hsp70 siRNA, IKKγ siRNA, and GFP siRNAs were generated as follows. The recombinant dice enzyme was used to cleave in vitro transcribed dsRNA into 22-bp siRNA according to the instructions supplied by the Dicer siRNA Generation Kit (Gene Therapy Systems, Inc).
The following primers were used for Hsp70: 5′-primer, 5′-GCGTAATACGACTCACTATAGGGAGAATGCCCCCAGCTACGTGGCCTTC-3′; and 3′-primer, 5′-GCGTAATACGACTCACTATAGGGAGATAAAGCTTGGCGTCGCGCAGAG C-3′.
The following primers were used for IKKγ: 5′-primer, 5′-GCGTAATACGACTCACTATAGGGAGAATGTGCACCTGCCTTCAGAACAG-3′; and 3′-primer, 5′-GCGTAATACGACTCACTATAGGGAGATAACTGGAAGTCCGCCTTGGTAG-3′.
The following primers were used for GFP: 5′-primer, 5′-GCGTAATACGACTCACTATAGGGAGAATGAAGCTGACCTGAAGTTCATC-3′; and 3′-primer, 5′-GCGTAATACGACTCACTATAGGGAGATAATGATCGCGCGCTTCTCGTTG-3′.
Mammalian two-hybrid assays
IKKα, IKKβ, IKKγ, IKKγ mutants, IκBα, Hsp70, Hsp70N, Hsp70C, and other constructs were in-frame inserted into the mammalian two-hybrid vectors pVP16 and pM, respectively. Cos-1 cells were cotransfected with these constructs and the Gal4-Luc reporter gene and equal amounts of β-gal plasmid as an internal control. Luciferase activity was measured 24 h after transfections.
Reporter gene assays
Hela cell stable cell lines, 293 cells, and Cos-1 cells were seeded into 24-well plates. On the following day, cells were transfected using the SurpFect kit. Each well was transfected with 50 ng NF-κB-, AP-1-, or HSF-1-dependent luciferase reporters, 25 ng pSV/β-galactosidase as an internal control, and the desired constructs as described in the figures or in their legends. Twenty-four hours posttransfection, the cells were stimulated for 15 min with TNF. Twenty-four hours later, the cell extracts were subjected to luciferase assays.
Electrophoretic mobility shift assay
EMSA was performed as previously described (Lu et al. 2002). The 32P-labeled oligonucleotide AGTTGGGGACTTTCCCAGG was used as the probe. Probe (2.5 ng), nuclear proteins (5 μg), or the indicated amounts of in vitro translated proteins were used as described in the figure legends.
In vitro protein interactions
Three microliters of 35S-labeled-IKKα, IKKβ, IKKγ, and IKKγ mutants and 0.25 μM of Hsp70 or Hsp70C previously immobilized on beads were incubated in binding buffer for 3 h at 4°C. The mixtures were washed five times with wash buffer to remove nonspecifically bound proteins and then analyzed by SDS-PAGE and autoradiography.
Gel filtration chromatography
Gel filtration chromatography was carried out on a Superose 6 column. Hsp70 or Hsp70C stably expressing cells were transiently transfected with Flag-tagged IKKα, Flag-tagged IKKβ, and HA-tagged IKKγ. Cells (2 × 107) were washed with PBS. Cells were collected, and S100 extracts were prepared as previously described (Poyet et al. 2000). The cell extracts were loaded onto a Superose 6 column. The column was precalibrated with the molecular mass marker proteins dextran (2000 kDa), thyroglobulin (670 kDa), β-amylase (230 kDa), and bovine serum albumin (66 kDa). Proteins were eluted from the column and analyzed by SDS-PAGE followed by Western blotting for the different IKK subunits and Hsp70 and Hsp70C using appropriate antibodies. IKK activity of each fraction was measured using an in vitro kinase assay.
Immunoprecipitation, immunoblotting, and kinase assays
Immunoprecipitation studies were performed as described by Chen et al. (2002). Briefly, cells were collected and lysed in 500 μL lysis buffer. Cleared cell extracts were incubated in 2 μg/500 μL of the appropriate antibodies and 10 μL of protein-G agarose beads for 3 h at 4°C. After the incubation, the precipitated complexes were washed five times and samples were boiled for 5 min in loading buffer, applied to SDS-PAGE, and analyzed by Western blotting using appropriate antibodies. IKKα/β was immunoprecipitated from cell extracts with the appropriate antibodies, as indicated in the figure legends. In vitro kinase assay of the immunocomplexes was performed as described (Chen et al. 2002).
Chemical cross-linking of proteins
293 cells were cultured in DMEM without methionine for 1 h and then labeled with 500 μCi of 35S-methionine per 60 mm dish for 6 h. The cells were lysed in lysis buffer. Cross-linking using EGS and immunoprecipitation were performed as recently described (Tegethoff et al. 2003). Cross-linking of in vitro translated IKKγ was performed using the same methods.
Confocal microscopy
Microscopy was performed using a Zeiss LSM-510 confocal microscope.
Acknowledgments
We thank Dr. G. Ghosh, Dr. H. Nakano, Dr. D.V. Goeddel, Dr. A. Lin, and Dr. S.S. Makarov for providing essential plasmids and Dr. Christianna Sample for providing technical assistance with the confocal microscopy. We thank Dr. D.L. Vaux for critically reading the manuscript and for valuable suggestions. We also thank the National Institutes of Health (NS38743, NS42774, NS43252) and the American Heart Association (Bugher Award) for supporting these studies. We also thank the reviewers of this manuscript and the Genes & Development journal for providing many helpful and insightful comments that have substantially improved the final version of this work.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1188204.
References
- Abravaya K., Myers, M.P., Murphy, S.P., and Morimoto, R.I. 1992. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes & Dev. 6: 1153-1164. [DOI] [PubMed] [Google Scholar]
- Agou F., Ye, F., Goffinont, S., Courtois, G., Yamaoka, S., Israel, A. and Veron, M. 2002. NEMO trimerizes through its coiled-coil C-terminal domain. J. Biol. Chem. 277: 17464-17475. [DOI] [PubMed] [Google Scholar]
- Andres D., Diez-Fernandez, C., Castrillo, A., and Cascales, M. 2002. Relationship between the activation of heat shock factor and the suppression of nuclear factor–κB activity in rat hepatocyte cultures treated with cyclosporine A. Biochem. Pharmacol. 64: 247-256. [DOI] [PubMed] [Google Scholar]
- Barkett M. and Gilmore, T.D. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18: 6910-6924. [DOI] [PubMed] [Google Scholar]
- Beere H.M., Wolf, B.B., Cain, K., Mosser, D.D., Mahboubi, A., Kuwana, T., Tailor, P., Morimoto, R.I., Cohen, G.M., and Green, D.R. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2: 469-475. [DOI] [PubMed] [Google Scholar]
- Beg A.A. and Baltimore, D. 1996. An essential role for NF-κBin preventing TNF-α-induced cell death. Science 274: 782-784. [DOI] [PubMed] [Google Scholar]
- Beg A.A., Sha, W.C., Bronson, R.T., Ghosh, S., and Baltimore, D. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κ B. Nature 376: 167-170. [DOI] [PubMed] [Google Scholar]
- Brummelkamp T.R., Nijman, S.M., Dirac, A.M., and Bernards, R. 2003. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-κB. Nature 424: 797-801. [DOI] [PubMed] [Google Scholar]
- Chant I.D., Rose, P.E., and Morris, A.G. 1996. Susceptibility of AML cells to in vitro apoptosis correlates with heat shock protein 70 (hsp 70) expression. Br. J. Haematol. 93: 898-902. [DOI] [PubMed] [Google Scholar]
- Chen Z., Hagler, J., Palombella, V.J., Melandri, F., Scherer, D., Ballard, D., and Maniatis, T. 1995. Signal-induced site-specific phosphorylation targets I κ B α to the ubiquitin–proteasome pathway. Genes & Dev. 9: 1586-1597. [DOI] [PubMed] [Google Scholar]
- Chen G., Cao, P., and Goeddel, D.V. 2002. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9: 401-410. [DOI] [PubMed] [Google Scholar]
- Connor W.G., Gerner, E.W., Miller, R.C., and Boone, M.L. 1977. Prospects for hyperthermia in human cancer therapy. Part II: Implications of biological and physical data for applications of hyperthermia to man. Radiology 123: 497-503. [DOI] [PubMed] [Google Scholar]
- Curry H.A., Clemens, R.A., Shah, S., Bradbury, C.M., Botero, A., Goswami, P., and Gius, D. 1999. Heat shock inhibits radiation-induced activation of NF-κB via inhibition of I-κB kinase. J. Biol. Chem. 274: 23061-23067. [DOI] [PubMed] [Google Scholar]
- DeMeester S.L., Buchman, T.G., Qiu, Y., Dunnigan, K., Hotchkiss, R.S., Karl, L.E., and Cobb, J.P. 1998. Pyrrolidine dithiocarbamate activates the heat shock response and thereby induces apoptosis in primed endothelial cells. Shock 10: 1-6. [DOI] [PubMed] [Google Scholar]
- Dewey W.C. 1984. Interaction of heat with radiation and chemotherapy. Cancer Res. 44: 4714s-4720s. [PubMed] [Google Scholar]
- ———. 1994. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperthermia 10: 457-483. [DOI] [PubMed] [Google Scholar]
- Dewey W.C. and Freeman, M.L. 1980. Rationale for use of hyperthermia in cancer therapy. Ann. N. Y. Acad. Sci. 335: 372-378. [DOI] [PubMed] [Google Scholar]
- Feinstein D.L., Galea, E., and Reis, D.J. 1997. Suppression of glial nitric oxide synthase induction by heat shock: Effects on proteolytic degradation of IκB-α. Nitric Oxide 1: 167-176. [DOI] [PubMed] [Google Scholar]
- Fujieda S., Noda, I., Saito, H., Hoshino, T., and Yagita, M. 1995. Heat shock enhances the susceptibility of tumor cells to lysis by lymphokine-activated killer cells. Arch. Otolaryngol. Head Neck Surg. 121: 1009-1014. [DOI] [PubMed] [Google Scholar]
- Gabai V.L., Mabuchi, K., Mosser, D.D., and Sherman, M.Y. 2002. Hsp72 and stress kinase c-jun N-terminal kinase regulate the bid-dependent pathway in tumor necrosis factor-induced apoptosis. Mol. Cell. Biol. 22: 3415-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal L., McCall, K., Agapite, J., Hartwieg, E., and Steller, H. 2000. Induction of apoptosis by Drosophila reaper, hid and grim through inhibition of IAP function. EMBO J. 19: 589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I.V., Darieva, Z.A., Melo, A.R., and Margulis, B.A. 1997. Major stress protein Hsp70 interacts with NF-κB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones 2: 132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harhaj E.W. and Sun, S.C. 1999. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J. Biol. Chem. 274: 22911-22914. [DOI] [PubMed] [Google Scholar]
- Heimbach J.K., Reznikov, L.L., Calkins, C.M., Robinson, T.N., Dinarello, C.A., Harken, A.H., and Meng, X. 2001. TNF receptor I is required for induction of macrophage heat shock protein 70. Am. J. Physiol. Cell Physiol. 281: C241-C247. [DOI] [PubMed] [Google Scholar]
- Heussler V.T., Rottenberg, S., Schwab, R., Kuenzi, P., Fernandez, P.C., McKellar, S., Shiels, B., Chen, Z.J., Orth, K., Wallach, D., et al. 2002. Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. Science 298: 1033-1036. [DOI] [PubMed] [Google Scholar]
- Huxford T., Huang, D.B., Malek, S., and Ghosh, G. 1998. The crystal structure of the IκB&apha;/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell 95: 759-770. [DOI] [PubMed] [Google Scholar]
- Jaattela M. 1990. Effects of heat shock on cytolysis mediated by NK cells, LAK cells, activated monocytes and TNFs α and β. Scand. J. Immunol. 31: 175-182. [DOI] [PubMed] [Google Scholar]
- Jaattela M., Wissing, D., Kokholm, K., Kallunki, T., and Egeblad, M. 1998. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 17: 6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D.Y., Giordano, V., Kibler, K.V., Nakano, H., and Jeang, K.T. 1999. Role of adapter function in oncoprotein-mediated activation of NF-κB. Human T-cell leukemia virus type I Tax interacts directly with IκB kinase γ. J. Biol. Chem. 274: 17402-17405. [DOI] [PubMed] [Google Scholar]
- Leonardi A., Chariot, A., Claudio, E., Cunningham, K., and Siebenlist, U. 2000. CIKS, a connection to Iκ B kinase and stress-activated protein kinase. Proc. Natl. Acad. Sci. 97: 10494-10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Kang, J., Friedman, J., Tarassishin, L., Ye, J., Kovalenko, A., Wallach, D., and Horwitz, M.S. 1999. Identification of a cell protein (FIP-3) as a modulator of NF-κB activity and as a target of an adenovirus inhibitor of tumor necrosis factor α-induced apoptosis. Proc. Natl. Acad. Sci. 96: 1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liossis S.N., Ding, X.Z., Kiang, J.G., and Tsokos, G.C. 1997. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J. Immunol. 158: 5668-5675. [PubMed] [Google Scholar]
- Liu Q.L., Kishi, H., Ohtsuka, K., and Muraguchi, A. 2003. Heat shock protein 70 binds caspase-activated DNase and enhances its activity in TCR-stimulated T cells. Blood 102: 1788-1796. [DOI] [PubMed] [Google Scholar]
- Lu A., Ran, R., Parmentier-Batteur, S., Nee, A., and Sharp, F.R. 2002. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J. Neurochem. 81: 355-364. [DOI] [PubMed] [Google Scholar]
- Makris C., Roberts, J.L., and Karin, M. 2002. The carboxyl-terminal region of IκB kinase γ (IKKγ) is required for full IKK activation. Mol. Cell. Biol. 22: 6573-6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek S., Chen, Y., Huxford, T., and Ghosh, G. 2001. IκBβ, but not IκBα, functions as a classical cytoplasmic inhibitor of NF-κB dimers by masking both NF-κB nuclear localization sequences in resting cells. J. Biol. Chem. 276: 45225-45235. [DOI] [PubMed] [Google Scholar]
- Malhotra V. and Wong, H.R. 2002. Interactions between the heat shock response and the nuclear factor–κ B signaling pathway. Crit. Care Med. 30: S89-S95. [PubMed] [Google Scholar]
- Malhotra V., Eaves-Pyles, T., Odoms, K., Quaid, G., Shanley, T.P., and Wong, H.R. 2002. Heat shock inhibits activation of NF-κB in the absence of heat shock factor-1. Biochem. Biophys. Res. Commun. 291: 453-457. [DOI] [PubMed] [Google Scholar]
- May M.J., D'Acquisto, F., Madge, L.A., Glockner, J., Pober, J.S., and Ghosh, S. 2000. Selective inhibition of NF-κB activation by a peptide that blocks the interaction of NEMO with the IκB kinase complex. Science 289: 1550-1554. [DOI] [PubMed] [Google Scholar]
- Mercurio F., Murray, B.W., Shevchenko, A., Bennett, B.L., Young, D.B., Li, J.W., Pascual, G., Motiwala, A., Zhu, H., Mann, M., et al. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19: 1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O. and Tschopp, J. 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114: 181-190. [DOI] [PubMed] [Google Scholar]
- Miller R.C., Connor, W.G., Heusinkveld, R.S., and Boone, M.L. 1977. Prospects for hyperthermia in human cancer therapy. Part I: Hyperthermic effects in man and spontaneous animal tumors. Radiology 123: 489-495. [DOI] [PubMed] [Google Scholar]
- Miura T.A., Morris, K., Ryan, S., Cook, J.L., and Routes, J.M. 2003. Adenovirus E1A, not human papillomavirus E7, sensitizes tumor cells to lysis by macrophages through nitric oxide- and TNF-α-dependent mechanisms despite up-regulation of 70-kDa heat shock protein. J. Immunol. 170: 4119-4126. [DOI] [PubMed] [Google Scholar]
- Mosser D.D., Caron, A.W., Bourget, L., Denis-Larose, C., and Massie, B. 1997. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell. Biol. 17: 5317-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Knowlton, A.A., Yokoyama, T., Lesslauer, W., and Mann, D.L. 1996. Tumor necrosis factor-α-induced expression of heat shock protein 72 in adult feline cardiac myocytes. Am. J. Physiol. 270 (4 Pt 2): H1231-H1239. [DOI] [PubMed] [Google Scholar]
- Park K.J., Gaynor, R.B., and Kwak, Y.T. 2003. Heat shock protein 27 association with the I κ B kinase complex regulates tumor necrosis factor α-induced NF-κ B activation. J. Biol. Chem. 278: 35272-35278. [DOI] [PubMed] [Google Scholar]
- Poyet J.L., Srinivasula, S.M., Lin, J.H., Fernandes-Alnemri, T., Yamaoka, S., Tsichlis, P.N., and Alnemri, E.S. 2000. Activation of the Iκ B kinases by RIP via IKKγ/NEMO-mediated oligomerization. J. Biol. Chem. 275: 37966-37977. [DOI] [PubMed] [Google Scholar]
- Poyet J.L., Srinivasula, S.M., and Alnemri, E.S. 2001. vCLAP, a caspase-recruitment domain-containing protein of equine Herpesvirus-2, persistently activates the Iκ B kinases through oligomerization of IKKγ. J. Biol. Chem. 276: 3183-3187. [DOI] [PubMed] [Google Scholar]
- Ravagnan L., Gurbuxani, S., Susin, S.A., Maisse, C., Daugas, E., Zamzami, N., Mak, T., Jaattela, M., Penninger, J.M., Garrido, C., et al. 2001. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 3: 839-843. [DOI] [PubMed] [Google Scholar]
- Rothwarf D.M., Zandi, E., Natoli, G., and Karin, M. 1998. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 395: 297-300. [DOI] [PubMed] [Google Scholar]
- Rudolph D., Yeh, W.C., Wakeham, A., Rudolph, B., Nallainathan, D., Potter, J., Elia, A.J., and Mak, T.W. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes & Dev. 14: 854-862. [PMC free article] [PubMed] [Google Scholar]
- Saleh A., Srinivasula, S.M., Balkir, L., Robbins, P.D., and Alnemri, E.S. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2: 476-483. [DOI] [PubMed] [Google Scholar]
- Schett G., Redlich, K., Xu, Q., Bizan, P., Groger, M., Tohidast-Akrad, M., Kiener, H., Smolen, J., and Steiner, G. 1998. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J. Clin. Invest. 102: 302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schett G., Steiner, C.W., Xu, Q., Smolen, J.S., and Steiner, G. 2003. TNFα mediates susceptibility to heat-induced apoptosis by protein phosphatase-mediated inhibition of the HSF1/hsp70 stress response. Cell Death Differ. 10: 1126-1136. [DOI] [PubMed] [Google Scholar]
- Tegethoff S., Behlke, J., and Scheidereit, C. 2003. Tetrameric oligomerization of IκB kinase γ (IKKγ) is obligatory for IKK complex activity and NF-κB activation. Mol. Cell. Biol. 23: 2029-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp D.J., Martin, S.J., Kafri, T., Green, D.R., and Verma, I.M. 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274: 787-789. [DOI] [PubMed] [Google Scholar]
- Wang C.Y., Mayo, M.W., Korneluk, R.G., Goeddel, D.V., and Baldwin Jr., A.S. 1998. NF-κB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281: 1680-1683. [DOI] [PubMed] [Google Scholar]
- Xia W., Voellmy, R., and Spector, N.L. 2000. Sensitization of tumor cells to fas killing through overexpression of heat-shock transcription factor 1. J. Cell Physiol. 183: 425-431. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Kim, D.W., Kwak, Y.T., Prajapati, S., Verma, U., and Gaynor, R.B. 2001. IKKγ/NEMO facilitates the recruitment of the IκB proteins into the IκB kinase complex. J. Biol. Chem. 276: 36327-36336. [DOI] [PubMed] [Google Scholar]
- Yamaoka S., Courtois, G., Bessia, C., Whiteside, S.T., Weil, R., Agou, F., Kirk, H.E., Kay, R.J., and Israel, A. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93: 1231-1240. [DOI] [PubMed] [Google Scholar]
- Zandi E., Rothwarf, D.M., Delhase, M., Hayakawa, M., and Karin, M. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91: 243-252. [DOI] [PubMed] [Google Scholar]
- Zhang S.Q., Kovalenko, A., Cantarella, G., and Wallach, D. 2000. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12: 301-311. [DOI] [PubMed] [Google Scholar]
- Zong W.X., Edelstein, L.C., Chen, C., Bash, J., and Gelinas, C. 1999. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-κB that blocks TNFα-induced apoptosis. Genes & Dev. 13: 382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]